Abstract
In this contribution, we report the optical and photo-electrochemical characterizations of the ferrite spinel CaFe2O4. The oxide was synthesized by sol–gel route using Polyvinylpyrrolidone organic polymer (PVP K30). The phase was confirmed by X-ray diffractometry (XRD) and a crystallite size of 51 nm was determined from the Williamson-Hall plot. The analysis by UV–Vis diffuse reflectance provided an indirect optical transition of 1.75 eV, assigned to the crystal field splitting of 3d orbital of Fe3+ in six-fold coordination. The electrochemical properties were investigated in Na2SO4 (0.1 M) to confirm the stability of the spinel and to evaluate its photocatalytic performance. p-type semiconductor was shown from the capacitance measurement with a flat band potential (Efb) of 0.48 VAg/AgCl and holes density (NA) of 3.66 × 1019 cm−3. The Electrochemical Impedance Spectroscopy (EIS) showed a depressed semicircle at high frequencies with the existence of a double-layer capacitance (Cdl) followed by a Warburg diffusion (Wr). The bulk resistance in the dark (27 Ω cm2) decreases down to 22.9 Ω cm2 under illumination confirming the semi-conductivity. The conduction band (−1.10 V), made up of Fe3+: eg orbital, is more cathodic than the O2/O2·− level (~ −0.5 V). The photo-catalysis was assessed through the oxidation of Drimarene Green X-3G (DGX) a recalcitrant and hazardous dye upon visible irradiation (16 mW cm−2), through the radicals O2·−. A conversion yield of ~ 45% is achieved at free pH, a catalyst dose of 1 g/l and DGX solution 10 mg/l within 4 h of illumination. The photodegradation obeys second-order kinetic with a photocatalytic half-life of 48.87 h.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the most important concerns of our time is the energy and water supply [1, 2]. Indeed, the growing demography and accelerated industrialization, lead to the release of large quantities of all kinds of pollutants. (metals, pesticides, dyes, drugs, etc…) [3,4,5]. The presence of these pollutants in the aquatic environment even at concentrations represents a serious threat to the ecosystem and human health. Therefore, our planet requires quick strategies to save the environment from this pollution [6,7,8]. One of the main sources of pollution comes from liquid dyestuffs waste with a global production equivalent of 800,000 tons per year, including dyes discharged from the food, detergent and textile industries [9, 10]. They are classified as hazardous compounds with harmful effects on living organisms and inhibit considerably the photosynthesis by reducing the penetration of light into the water [11, 12]. To meet these challenges and avoid any harm to the environment, it is necessary to proceed to their elimination before they are discharged into waterways. Among the alternatives for removing these harmful organics, one can cite biosorption [13], chemical oxidation [14], burning [15], biodegradation and membrane separation [16]. However, these methods remain rather limited because they are expensive and often difficult to implement; in addition, incomplete oxidation needs additional organic processing [17]. To overcome these drawbacks, advanced oxidation processes (AOPs) are used as a "green" strategy in water research to get rid of organic matter and prevent pollution [18]. In this respect, the solar photocatalysis has emerged as a clean alternative for its efficacy under soft conditions [19, 20]. Semiconductors such as TiO2, SnO2 and BaTiO3 are photo-electrochemically stable and have been used in environmental photocatalytic applications [21,22,23]. However, their photo-responses are restricted to UV light due to the wide optical gap (Eg) and are therefore of little practical use with respect to solar emissions which account for only about 5% of sunlight [24, 25]. In this regard, continued interest in photocatalysis has led to significant experimental work on narrow band gap semiconductors like delafossites [26], perovskites [27] and spinels [28] which gave satisfactory results in the H2 production and oxidation of organics [29,30,31]. In the spinels, the electrons are excited in a conduction band (CB) made up of 3d orbitals with energy high enough to reduce dissolved oxygen to O2·− radicals and water to gaseous hydrogen. [32]. Importantly, the energy of the valence band (VB), consisting also of 3d character, is raised, thus shifting the spectral photoresponse to the visible light region. The spinels are among our most researched photocatalysts due to their low cost, friendly environmental characteristics, narrow band gap and magnetic properties [33, 34].
Therefore, our research program was partially directed toward the spinel family AB2O4 [35]. Based on the above contents, we report in this contribution the characterizations of CaFe2O4 synthesized by chemical route. It has remarkable magnetic and catalytic properties due to its ability to absorb half of the solar spectrum and convert it into chemical and/or electrical energy (Eg ~ 2 eV) [36].
Optimization by such operational factors as catalyst dose, dye concentration and photocatalyst recycling potential. This research aimed to study the photoelectrochemical properties and photocatalytic activity of BaFe2O4 nanoparticles and to explore the possibility of using them in the photocatalysis of DGX under visible light irradiation.
Many dyes are weakly biodegradable and therefore not easy to oxidize by classical techniques. The morphology of the catalyst (porosity, roughness and specific surface area) has a considerable influence on the photocatalytic performance, and decreasing the crystallite size improves the dye photo-oxidation because of enhanced surface areas. The spinel is a low polaron hopping semiconductor, and the lifetime of the electron/hole (e−/h+) pairs must be long enough to reach the liquid interface and to contribute to photo-electrochemical reactions. For this, we have chosen the hydrothermal route for preparing fine powders with an enhanced surface-to-volume ratio.
Experimental
Synthesis of material
CaFe2O4 was prepared by sol–gel route using polyvinylpyrrolidone K30 (PVP K30) as a combustion agent. Ca(NO3)2, 6H2O (Fluka, 98%) and Fe(NO3)3, 9H2O (Biochem, 98%) previously stored in a desiccator under static vacuum, were dissolved separately in ethanol under stirring; an excess of 5% citric acid was added to the solutions. Then the solutions were mixed in 200 ml of water and heated at 60 °C for 2 h. PVP K30 dissolved in water was added dropwise to the mixing solution. After evaporation at 100 °C (20 h), the mixture turned into a black gel, which was burned at 300 °C and the auto combustion gave a xerogel. The latter was ground in an agate mortar and heat-treated in the air at 850 °C (8 h, 5 °C/min) in an alumina crucible to remove the carbonaceous residue that remained after the combustion. The fine brown powder was stored in an airtight bottle until use. Distilled water was used in all preparations.
Characterization methods
The X-ray diffraction (XRD) was carried out with a PANalytical X-ray diffractometer (Cu Kα line, λ = 1.54056 Å) at a scanning rate of 0.01°/s in the range (10–90°).
The diffuse reflectance of the spinel was plotted with a Jasco 650 Ultraviolet–Visible spectrophotometer which goes from 190 to 900 nm using BaSO4 as a reference. The powder was pressed into dense pellets (∅ = 13 mm, thickness ~ 0.8 mm) under 300 MPa and fired at 900 °C, yielding a compactness of 92% with good mechanical properties.
The electrochemical study was realized in a Pyrex cell, which permitted the introduction of the reference electrode, Pt counter electrode and the working electrode (WE). The potentials were given against Ag/AgCl, which refers to a saturated potassium chloride (KCl) electrolyte. The electrodes are immersed in Na2SO4 (0.1 M) solution and piloted by a PGZ 301 Potentiostat at a rate of 10 mV s−1. The capacity characteristic (C) was studied at 10 kHz and the electrochemical impedance spectroscopy (EIS) was measured in the range (10−2–105 Hz), once the open circuit potential (OCP) was stabilized.
Photocatalytic study
The photocatalytic oxidation of DGX was carried out in a double-walled Pyrex reactor connected to a thermo-stated bath (25 °C). The CaFe2O4 powder with a mass of 300 mg was suspended in 300 ml of DGX solution (10 mg/l, free pH) by magnetic stirring; the adsorption experiment was realized in the dark for 90 min to reach the adsorption equilibrium. Then, a tungsten-halogen lamp (200 W, 16 mW/cm2) irradiated the reactor; 5 ml aliquots were periodically taken and the photocatalyst particles were separated by centrifugation (4500 rpm for 15 min). The remaining DGX concentrations were analyzed by measuring the absorbance with a UV–Vis spectrophotometer (Shimadzu 1800, λmax = 660 nm). The degradation efficiency was calculated from the relationship:
Here absads and abs are the equilibrium concentrations in the dark and the absorbance after time t of DGX, respectively.
Results and discussion
Photocatalyst characterization
XRD analysis
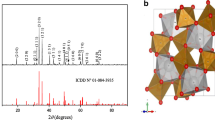
After the synthesis and calcination steps, the phase purity and crystal structure of CaFe2O4 were confirmed by XRD analysis. The diffractogram is shown in Fig. 1. The oxide crystallizes in an orthorhombic symmetry with the lattice parameters: a = 9.2379 Å, b = 10.6997 Å and c = 3.0202, in agreement with the Pnma space group (N° 62) according to ICDD card No. 01-072-1199. The corrected peaks are characteristic of high purity and agree well with those previously reported [37, 38]. Except for CaFe2O4, no other diffraction peaks associated with the hematite Fe2O3 [39, 40], CaO or other impurities appear in the diffractogram. The structure of the spinel CaFe2O4, shown in Fig. 1 (Inset), was built thanks to the Vista software. The average crystallite size (D) was obtained from the width of the peak (β) by using the Williamson-Hall (W–H) relationship:
K is the form factor and п the lattice strain of CaFe2O4 powder. The β cosθ line variance as a function of 4sinθ is shown in Fig. 2. The slope and y-intercept respectively yield a lattice strain (п) of 5 × 10−5 and a crystal size (D) of 51 nm. Assuming compact and spherical crystallites, a specific surface area of ~ 28 m2 g−1 is calculated from the relation (= 6/ρexp D) where ρexp is the experimental density (4.19 g cm−3), measured by picnometry; this value is relatively higher than that determined in previous works [41, 42], and indicates a certain porosity of the powder. It is known that a large specific surface could provide a large number of active sites in a photocatalytic process [43].
UV–Vis analysis
The photo activity of the semiconductor is closely related to its light absorption. The optical behavior of the spinel was determined by UV visible spectrophotometry in the range of 190–900 nm. CaFe2O4 has an absorption edge (λo) equal to 599 nm corresponding to a gap (λo = 1240/Eg) with a marked absorption enhancement in the visible-light region, the gap energy (Eg) is determined from the relation:
α is the absorption coefficient and its reciprocal value corresponds to the penetration depth of the monochromatic light. For the direct and indirect transition of a semiconductor, k is equal to 0.5 and 2. The intersection of the straight lines (αhν)2 and (αhν)1/2 with the energy-axis (Fig. 3) give direct (2.07 eV) and indirect (1.75 eV) transitions, the latter conforms with the brick red color of the powder.
Electrochemical properties
The current potential J(E) profiles plotted in Na2SO4 electrolyte (Fig. 4) show that the CaFe2O4 has good electrochemical stability over the entire potential range indicated by the low dark current Jd (< ~ 1 mA/cm2). The peak at ~ −0.3 V is due to the reduction of dissolved oxygen (2H2O + O2 + 2e− → H2O2 + 2OH−); it has been observed that the height of the peak decreases under N2 bubbling [44]. The current increase linearly at lower potentials, due to the charge of the double layer at the electrode/solution interface. Then, the current saturation returns to a diffusion-limited process. The increased current above ~ 0.6 V is assigned to the formation of O2 with a low over-voltage while the decrease below −0.9 V is due to the evolution of H2 as indicated by the bubbles observed on the electrode surface.
The photocurrent onset potential (Eon) below which the photocurrent appears is determined according to the Gartner model [45]:
Here α (cm−1) is the absorption coefficient and W the space charge region; the potential Eon (−0.15 V) is deduced from the cross point of the line Jph2 (E) to E-axis (Fig. 5). In a first approximation. The interfacial capacity (C) is measured to obtain the flat band potential (Efb) which is crucial to accurately determine the “potential/energy” of the electronic band and to build the band diagram in order to evaluate the photoactivity of spinel:
The extrapolation of the line (C−2) plot to infinite capacity (Fig. 6) gives the potential Efb (0.48 V), while the negative slope implies p-type conduction of CaFe2O4 with holes as dominant carriers. The flattening region below ~ 0.5 V is attributed to the accumulation zone of (h+/e−) pairs, where their recombination predominates. The discrepancy between the potentials Eon and Efb indicates the presence of surface states within the gap region [46].
The holes density NA (3.66 × 1019 cm−3) was computed from the slope value (m = 4.82 × 1010 (F−2 cm4/V)) where the dielectric measurement of (ε ~ 80) at 293 K was taken from our previous work [47]. such value extends the depletion width, an advantageous property in photocatalysis:
ΔU is the interfacial band bending (Efb − E), equal to ~ 0.5 V. The term (ΔU εεo/e) is constant and the length (W ~ 11 nm) is controlled by the doping density NA. Good photoactivity is expected when the condition (αλ−1 < W) is fulfilled; this condition requires a semiconductor moderately doped (NA small) with a Fermi level of ~ 0.5 above the valence band. αλ is the extinction coefficient of the spinel for the monochromatic light (λ).
The chemical stability is confirmed by the potentiodynamic potential plot measured in the Na2SO4 (0.1 M) solution, which is a common technique for checking the corrosion process. The slope dlogJd/dE (Fig. 6 inset) gives a corrosion potential (Ecor) of −0.07 V and a small exchange density (Jex) of 0.01 mA cm−2, and therefore the oxide shows excellent chemical inertness.
The EIS graph, i.e., imaginary (Zim) versus real (Zreal) part of the complex impedance brings insights into the reactions occurring at the solid/liquid interface and the data were recorded at the open circuit potential after its stabilization in the region (10–2 − 105 Hz). The EIS representation exhibits two semicircles in the dark and under visible light (Fig. 7), assigned to the charge transfer resistance (Rct1) and grains boundaries. A Warburg diffusion (Wr) is evidenced by the straight line inclined at 45° at low frequencies. and these two elements are in parallel with the double layer capacity (Cdl) in the electrode/electrolyte interface. The Rct1 can be derived from the high-frequency intercept (27.1 Ω cm2) which decreases down to 22.9 Ω cm2 under illumination, thus corroborating the semiconducting character of the spinel. Then for the second semi-circle associated with the charge transfer resistance (Rct2), the center is localized below the real axis and this comes with the existence of a constant phase element (CPE) which represents a deviation from a pure capacitive behavior. The offset from the origin (RS) is equal to the electrolytic resistance of the Na2SO4 solution (0.1 M), attributed to the ionic molar conductivities at an infinite solution (Λ∞) of SO42− (~ 160 Ω−1 cm2 mol−1) and Na+ (~ 50 Ω−1 cm2 mol−1) [48]. The EIS data are fitted to an equivalent electrical circuit (Fig. 7, Inset) and the electrochemical parameters are summarized in Table 1. The Bode representation is given in SM 1.
DGX photodegradation
To assess the photocatalytic activity of CaFe2O4, DGX was used as a model pollutant under visible irradiation. Firstly, the reaction was performed in the absence of a catalyst to explore the role of photolysis. The result showed that the seft-degradation of DGX is insignificant and is relatively stable. Before the irradiation of light, the mixture of DGX solution/photocatalyst was kept in the dark under stirring for 90 min to reach the adsorption–desorption equilibrium. Then, it was deduced from the degradation experiments that the light/catalyst system plays a crucial essential role in the photodegradation of the dye. Fig. 8 shows the evolution of the DGX concentration (10 mg/l) as a function of time under visible light illumination for a catalyst dose of 1 g/l. The maximum absorption peak at 553 nm, and 45% of the dye was photocatalytically removed within 4 h at 25 °C at free pH.
The kinetics of the DGX photodegradation onto CaFe2O4 was also considered.
Kinetics are usually represented according to pseudo-first- and second-order models respectively [49]:
Here v is the photocatalytic rate of the degradation (mg/l min), k1 is the first-order apparent constant of the degradation (min−1), k2 is the second order apparent constant of the degradation (l/mg min), C is the concentration (mg/l) and t the irradiation time (min). The integration of equations. (7 and 8) together with the determination (C=Co when t = 0) gives the following equations
Based on Fig. 9 and Table 2, we can conclude that the kinetic of photocatalysis on CaFe2O4 is well described by the second order, as indicated by the straight lines with higher correlation coefficients (R2 = 0.985). The former can describe the disappearance of the DGX with an apparent kinetic constant kapp. This value allows us to extract the initial rate, and the photocatalytic half-life time (t1/2) of 48.87 h is calculated using the following relationship:
Photocatalytic mechanism
In order to improve the photocatalytic performance, it was interesting to develop the catalyst powder by sol–gel method with the goal reduction to length the electrons have to cross to reach the interface. For better elucidating the photocatalytic degradation of DGX on CaFe2O4 nanocomposites under visible irradiation, a plausible mechanism has been suggested on the basis of by energy diagram. Based on the electrical (Ea = 0.17 eV) optical (Eg = 2.07 eV) and photo-electrochemical (Efb = 0.48 V) characterizations, where Ea is the activation energy [34], the band edge positions of the synthesized spinel are expressed as by the relations:
These relationships allow us to infer potential values of the valence band (0.65 V) and conduction band (−1.10 V), where e is the elementary charge. These values indicate that both bands derive mainly from Fe3+: 3d orbital in six-fold coordination (t2g3 → eg2) [47]. Once irradiation of the semiconductor CaFe2O4 by energetic photons (hν > Eg), the (e−/h+) pairs generated within the diffusion length of the semiconductor are separated by the interfacial electric field (Eq. 10). CB-CaFe2O4 (−1.10 V) is located above the level O2/O2·− (−0.5 V), clarifying that the electron can contribute in the DGX photo-oxidation.
Thus, the electrons react with dissolved oxygen to produce reactive O2·−. On the other hand, it is unlikely that the hole in the valence band oxidizes H2O to generate ·OH because the potential of VB−CaFe2O4 (0.65 V) is anodic of the couple H2O/·OH (2 V). Based on the above-mentioned contents, the photocatalytic degradation of DGX is suggested as follows:
CaFe2O4 with its environmentally friendly characteristics has been tested for hydrogen production under visible irradiation. The results are promising and will be communicated very soon.
Conclusions
Conclusively, this work was dedicated to the synthesis of the spinel CaFe2O4 by a facile sol–gel process using a Polyvinylpyrrolidone organic polymer (PVP K30), followed by an exhaustive characterization through optical and electrochemical studies. These properties have confirmed the stability and p-type semi-conductivity of spinel, which can exploit a large part of solar radiation into chemical energy. The photo-electrochemistry enabled us to construct the energy diagram with the accurate positions of the conduction and the valence bands, a precursor to photocatalysis. Drimarene Green is a dangerous and persistent dye, released into the natural aquatic environment by uncontrolled industrial effluents. It was successfully oxidized under visible irradiation in the presence of CaFe2O4. Experimental results revealed that 45% DGX degradation by O2·− radicals under ideal conditions, for less than 4 h of exposure under visible light irradiation. The photocatalytic oxidation followed the pseudo second-order kinetic with a photocatalytic half-life of 48.87 h.
Data availability
Not applicable.
References
Asif M, Muneer T (2007) Energy supply, its demand and security issues for developed and emerging economies. Renew Sustain Energy Rev 11:1388–1413. https://doi.org/10.1016/j.rser.2005.12.004
Wang M, Webber M, Finlayson B, Barnett J (2008) Rural industries and water pollution in China. J Environ Manage 86:648–659. https://doi.org/10.1016/j.jenvman.2006.12.019
Lezier V, Gusarova M, Kopytova A (2017) Water supply of the population as a problem of energy efficiency on the example of the Tyumen region of Russia. IOP Conf Ser: Earth Environ Sci. https://doi.org/10.1088/1755-1315/90/1/012069
Baaloudj O, Assadi I, Nasrallah N et al (2021) Simultaneous removal of antibiotics and inactivation of antibiotic-resistant bacteria by photocatalysis: a review. J Water Process Eng 42:102089. https://doi.org/10.1016/j.jwpe.2021.102089
Baaloudj O, Nasrallah N, Bouallouche R et al (2022) High efficient Cefixime removal from water by the sillenite Bi12TiO20: photocatalytic mechanism and degradation pathway. J Clean Prod 330:129934. https://doi.org/10.1016/j.jclepro.2021.129934
Ayangbenro AS, Babalola OO (2017) A new strategy for heavy metal polluted environments: a review of microbial biosorbents. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph14010094
Chawla L, Cushing DF (2007) Education for strategic environmental behavior. Environ Educ Res 13:437–452. https://doi.org/10.1080/13504620701581539
Sharma S, Sharma V, Chatterjee S (2021) Microplastics in the mediterranean sea: sources, pollution intensity, sea health, and regulatory policies. Front Mar Sci. https://doi.org/10.3389/fmars.2021.634934
Kishor R, Purchase D, Saratale GD et al (2021) Ecotoxicological and health concerns of persistent coloring pollutants of textile industry wastewater and treatment approaches for environmental safety. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2020.105012
Chen HL, Burns LD (2006) Environmental analysis of textile products. Cloth Text Res J 24:248–261. https://doi.org/10.1177/0887302X06293065
Raj S (2008) Bio-decolourization of textile effluent containing Reactive Black-B by effluent-adapted and non-adapted bacteria. Afr J Biotechnol 7:3309–3313
Jothi D (2015) Application of enzyme extracted from aloe vera plant in chemical pretreatment of cotton knitted textile to reduce pollution load. World J Eng Technol 03:37–44. https://doi.org/10.4236/wjet.2015.33b007
Park D, Yun YS, Park JM (2010) The past, present, and future trends of biosorption. Biotechnol Bioprocess Eng 15:86–102. https://doi.org/10.1007/s12257-009-0199-4
Mantzavinos D, Psillakis E (2004) Enhancement of biodegradability of industrial wastewaters by chemical oxidation pre-treatment. J Chem Technol Biotechnol 79:431–454. https://doi.org/10.1002/jctb.1020
Gadde B, Bonnet S, Menke C, Garivait S (2009) Air pollutant emissions from rice straw open field burning in India, Thailand and the Philippines. Environ Pollut 157:1554–1558. https://doi.org/10.1016/j.envpol.2009.01.004
López C, Mielgo I, Moreira MT et al (2002) Enzymatic membrane reactors for biodegradation of recalcitrant compounds: application to dye decolourisation. J Biotechnol 99:249–257. https://doi.org/10.1016/S0168-1656(02)00217-1
Zhang D, Ghysels S, Ronsse F (2020) Effluent recirculation enables near-complete oxidation of organics during supercritical water oxidation at mild conditions: a proof of principle. Chemosphere 250:126213. https://doi.org/10.1016/j.chemosphere.2020.126213
Astas S (2008) Use of selected advanced oxidation processes (AOPs) for wastewater treatment—a mini review. Global NEST J 10:376–385
Spasiano D, Marotta R, Malato S et al (2015) Solar photocatalysis: materials, reactors, some commercial, and pre-industrialized applications: a comprehensive approach. Appl Catal B 170–171:90–123. https://doi.org/10.1016/j.apcatb.2014.12.050
Malato S, Fernández-Ibáñez P, Maldonado MI et al (2009) Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal Today 147:1–59. https://doi.org/10.1016/j.cattod.2009.06.018
Suria S, Pewarna T, Jadi S, Nanohablur B (2012) 11 Khalil. 41:1011–1016
Liu D, Fessenden RW, Hug GL, Kamat PV (1997) Dye capped semiconductor nanoclusters: role of back electron transfer in the photosensitization of SnO2 nanocrystallites with cresyl violet aggregates. J Phys Chem B 101:2583–2590. https://doi.org/10.1021/jp962695p
Pare B, Jonnalagadda SB, Tomar H et al (2008) ZnO assisted photocatalytic degradation of acridine orange in aqueous solution using visible irradiation. Desalination 232:80–90. https://doi.org/10.1016/j.desal.2008.01.007
Zyoud AH, Zubi A, Hejjawi S et al (2020) Removal of acetaminophen from water by simulated solar light photodegradation with ZnO and TiO2 nanoparticles: catalytic efficiency assessment for future prospects. J Environ Chem Eng 8:104038. https://doi.org/10.1016/j.jece.2020.104038
Abo R, Kummer NA, Merkel BJ (2016) Optimized photodegradation of Bisphenol A in water using ZnO, TiO2 and SnO2 photocatalysts under UV radiation as a decontamination procedure. Drink Water Eng Sci 9:27–35. https://doi.org/10.5194/dwes-9-27-2016
Yin L, Shi Y, Lu L et al (2016) A novel delafossite structured visible-light sensitive AgFeO2 photocatalyst: preparation, photocatalytic properties, and reaction mechanism. Catalysts. https://doi.org/10.3390/catal6050069
Kanhere P, Chen Z (2014) A review on visible light active perovskite-based photocatalysts. Molecules 19:19995–20022. https://doi.org/10.3390/molecules191219995
Chandrasekaran S, Bowen C, Zhang P et al (2018) Spinel photocatalysts for environmental remediation, hydrogen generation, CO2 reduction and photoelectrochemical water splitting. J Mater Chem A 6:11078–11104. https://doi.org/10.1039/c8ta03669a
Brahimi R, Bessekhouad Y, Bouguelia A, Trari M (2007) CuAlO2/TiO2 heterojunction applied to visible light H2 production. J Photochem Photobiol, A 186:242–247. https://doi.org/10.1016/j.jphotochem.2006.08.013
Bessekhouad Y, Trari M (2002) Photocatalytic hydrogen production from suspension of spinel powders AMn2O4(A=Cu and Zn). Int J Hydrogen Energy 27:357–362. https://doi.org/10.1016/S0360-3199(01)00159-8
Saadi S, Bouguelia A, Trari M (2006) Photoassisted hydrogen evolution over spinel CuM2O4 (M=Al, Cr, Mn, Fe and Co). Renew Energy 31:2245–2256. https://doi.org/10.1016/j.renene.2005.10.014
Edwards PP, Egdell RG, Fragala I et al (1984) A study of the spinel materials LiTi2O4 and Li4 3Ti5 3O4 by photoelectron spectroscopy. J Solid State Chem 54:127–135. https://doi.org/10.1016/0022-4596(84)90140-3
Cherifi K, Rekhila G, Omeiri S et al (2019) Physical and photoelectrochemical properties of the spinel ZnCr2O4 prepared by sol gel: application to Orange II degradation under solar light. J Photochem Photobiol, A 368:290–295. https://doi.org/10.1016/j.jphotochem.2018.10.003
Kenfoud H, Nasrallah N, Meziani D, Trari M (2021) Photoelectrochemical study of the spinel CaFe2O4 nanostructure: application to Basic Blue 41 oxidation under solar light. J Solid State Electrochem. https://doi.org/10.1007/s10008-021-04952-8
Lee SH, Takagi H, Louca D et al (2010) Frustrated magnetism and cooperative phase transitions in spinels. J Phys Soc Jpn 79:1–14. https://doi.org/10.1143/JPSJ.79.011004
Mathubala G, Manikandan A, Antony SA, Ramar P (2016) Photocatalytic degradation of methylene blue dye and magneto- optical studies of magnetically recyclable spinel NixMn1-xFe2O4 (x=0.0–1.0) nanoparticles. J Mol Struct 1113:79–87. https://doi.org/10.1016/j.molstruc.2016.02.032
Sharma N, Shaju KM, Subba Rao GV, Chowdari BVR (2003) Iron-tin oxides with CaFe2O4 structure as anodes for Li-ion batteries. J Power Sources 124:204–212. https://doi.org/10.1016/S0378-7753(03)00670-0
Candeia RA, Bernardi MIB, Longo E et al (2004) Synthesis and characterization of spinel pigment CaFe 2 O 4 obtained by the polymeric precursor method. Mater Lett 58:569–572. https://doi.org/10.1016/S0167-577X(03)00563-9
Mekatel E, Trari M, Nibou D et al (2019) Preparation and characterization of Α-Fe2O3 supported clay as a novel photocatalyst for hydrogen evolution. Int J Hydrogen Energy 44:10309–10315. https://doi.org/10.1016/j.ijhydene.2019.03.007
Abdi A, Trari M (2013) Investigation on photoelectrochemical and pseudo-capacitance properties of the non-stoichiometric hematite α-Fe2O3 elaborated by sol-gel. Electrochim Acta 111:869–875. https://doi.org/10.1016/j.electacta.2013.08.076
Ikenaga NO, Ohgaito Y, Suzuki T (2005) H2S absorption behavior of calcium ferrite prepared in the presence of coal. Energy Fuels 19:170–179. https://doi.org/10.1021/ef049907z
Miller D, Siriwardane R (2018) CaFe2O4 oxygen carrier characterization during the partial oxidation of coal in the chemical looping gasification application. Appl Energy 224:1–24
Li D, Song H, Meng X et al (2020) Effects of particle size on the structure and photocatalytic performance by alkali-treated TiO(2). Nanomaterials (Basel, Switzerland). https://doi.org/10.3390/nano10030546
Lahmar H, Benamira M, Douafer S et al (2020) Photocatalytic degradation of methyl orange on the novel hetero-system La2NiO4/ZnO under solar light. Chem Phys Lett 742:137132. https://doi.org/10.1016/j.cplett.2020.137132
Jiang Z, Huang S, Qian B (1994) Semiconductor properties of Ag2O film formed on the silver electrode in 1MNaOH solution. Electrochim Acta 39:2465–2470
Kenfoud H, Nasrallah N, Baaloudj O et al (2022) Enhanced photocatalytic reduction of Cr(VI) by the novel hetero-system BaFe2O4/SnO2. J Phys Chem Solids 160:110315. https://doi.org/10.1016/j.jpcs.2021.110315
Kenfoud H, Nasrallah N, Baaloudj O et al (2020) Photocatalytic reduction of Cr(VI) onto the spinel CaFe2O4 nanoparticles. Optik 223:165610. https://doi.org/10.1016/j.ijleo.2020.165610
Sahmi A, Bensadok K, Trari M (2017) Photoelectrochemical properties of CaWO4 synthetized by chemical route: application to the phenobarbital electro-photocatalysis. J Photochem Photobiol, A 349:36–41. https://doi.org/10.1016/j.jphotochem.2017.08.070
Hemmi A, Belmedani M, Mekatel E et al (2021) Kinetic and mechanism studies of tetracycline photodegradation using synthesized ZnAl2O4. React Kinet Mech Catal 134:1039–1054. https://doi.org/10.1007/s11144-021-02114-5
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
DB: writing—original draft preparation and investigation, HK: writing—review and editing, MT: Writing and supervising.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
We would like to declare on behalf of my co-authors that the work described was original research that has not been published previously, and not under consideration for publication elsewhere, in whole or in part.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Berdous, D., Kenfoud, H. & Trari, M. Physico-chemical properties of the spinel CaFe2O4 synthesized by sol–gel route: application to drimarene green X-3G photodegradation. Reac Kinet Mech Cat 136, 507–522 (2023). https://doi.org/10.1007/s11144-022-02337-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-022-02337-0