Abstract
In the present work, a novel, simple, and sensitive clozapine (CLZ) sensor was developed based on nickel oxide nanoparticle (NiO)-decorated graphene quantum dot (GQD)-modified glassy carbon electrode (NiO/GQD/GCE). NiO/GQD/GCE was prepared by simple electrodeposition, the electrochemical behavior of CLZ at the surface of the prepared electrode was studied by cyclic voltammetry (CV) and differential pulse voltammetry (DPV), and an improved reversibility and increased peak current with negative shift in the oxidation potential were observed at the proposed electrode. The effect of some experimental parameters has been examined, and based on the results, an electron transfer–chemical reaction–electron transfer mechanism has been proposed for CLZ electrooxidation. The differential pulse voltammetric response of the NiO/GQD/GCE was linear to the concentration of CLZ in the range of 3 × 10−9 to 1 × 10−6 M, and the detection limit was found to be 0.55 nM (S/N = 3). The method has been successfully used for the selective determination of the CLZ amount in the pharmaceutical preparations and human serum samples with good accuracy and precision.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Clozapine, a tricyclic dibenzodiazepine derivative, is an effective antipsychotic drug that is used to treat schizophrenic patients with good efficiency [1, 2]. Dose-dependent effects of CLZ (clozapine) confirm importance of its determination in biological and pharmaceutical preparation [3, 4]. Several analytical methods have been reported for the determination of CLZ including chromatography, mass spectrometry, spectrophotometry, and electrochemical methods [5,6,7,8,9,10,11,12]. Among these, electrochemical methods have attracted high attention due to excellent characteristics such as simple operation, high sensitivity, and low costs. Several electrochemical studies were performed for the determination of CLZ. The chemically modified electrodes (CMEs) offer significant advantages such as low charge transfer resistance, low over potential, high selectivity and sensitivity, and high stability in the design and development of electrochemical sensors. Various bare and modified electrodes used for CLZ detection include unmodified glassy carbon electrode and sepiolite-modified carbon paste electrode (CPE) [12], electrochemically pretreated glassy carbon electrode [13], CNT-SDS-modified CPE [14], horseradish peroxidase (HRP)-immobilized CPE [15], PVC membrane electrode [16], 16-mercaptohexadecanoic acid self-assembled monolayer-modified gold electrode [17], TiO2NP-modified CPE [18], poly(2-hydroxy-5-[(4-sulfophenyl)azo]benzoic acid) film-modified glassy carbon electrode (GCE) [19], silicate nanotube-modified electrode [20], and MWCNT/New Coccine-doped PPY-modified GCE [21].
Carbon nanomaterials such as graphene and graphene quantum dot attracted considerable attention for sensing applications due to ideal characteristics such as large surface-to-volume ratio, good surface grafting, biocompatibility, low toxicity, and special electrical property. Some reports are available about electrochemical application of graphene quantum dot (GQD)-modified electrodes [22,23,24].
Electrocatalytic activity of the GQD-modified electrode could be improved by compositing a conductive GQD matrix with transition metal oxide nanoparticles. Transition metal oxides are an important class of semiconductors applied in solar cells, electronics, and catalysis and can improve electrochemical responses [25,26,27,28,29]. Among the various types of transition metal oxides, nickel-containing nanoparticles gained special interest in electrochemistry due to its low cost, high catalytic activity, and biocompatibility [30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47]. Recently, we used graphene oxide and graphene quantum dot as an electrode modifier for the construction of sensors and biosensors [48,49,50]. To the best of our knowledge, no study has been reported about application of nickel oxide (NiO)/GQD nanoparticles as an electrode modifier in electrochemical sensors. In the present study, a rapid, simple, and sensitive electrochemical sensor was developed for the determination of CLZ based on desired characteristics of GQD and nickel oxide nanoparticles. To aim this goal, a glassy carbon electrode was electrochemically modified with NiO/GQD nanocomposite, and then, the electrochemical behavior of CLZ was studied on the surface of the prepared NiO/GQD/GCE using a cyclic voltammetric method. The oxidation current of CLZ at the surface of NiO/GQD/GCE increased noticeably compared to that of the bare GCE and GQD-modified GCE indicating that the composite materials combine electrocatalytic activity of transition metal oxides with electrical conductivity of GQD. Furthermore, the modified electrode showed good reproducibility, high stability, and wide linear range. Additionally for sensitive determination of CLZ, differential pulse voltammetry was used, and after optimization of effective parameters, this electrode was applied for the determination of CLZ in pharmaceutical formulations and biological fluids with good sensitivity and selectivity.
Experiment
Instrumentation and reagents
Electrochemical experiments were carried out using the AUTOLAB PGSTAT 30 electrochemical analysis system and GPES 4.9 software package (Eco Chemie, the Netherlands). A three-electrode system was used, composed of a modified GCE as the working electrode, a saturated calomel electrode (SCE) as the reference electrode, and a platinum wire as the auxiliary electrode.
A digital pH/mV meter (Metrohm, pH Lab 827) was used for pH measurements, and an ultrasonic bath (KODO model JAC1002) was used for cleaning the electrode surface.
Clozapine (C18H19ClN4) was purchased from Sigma. CLZ tablets (25 mg, Tehran Shimi Co., Tehran, Iran) were purchased from the local pharmacies. For the preparation of stock solutions of CLZ, certain amount of this drug was weighed and dissolved in 0.1 mol L−1 HNO3 solution and protected from light during investigation. Other chemicals were of analytical grade, purchased from Merck, and their solutions were prepared by dissolving appropriate amounts of them in twice-distilled water. Fresh human blood serum samples were obtained from Azerbaijan hospital (Urmia, Iran). GQDs were synthesized by pyrolyzing citric acid and dispersing the carbonized products into alkaline solutions [48].
Preparation of the modified electrode
Before modification, the GCE (2 mm in diameter) was polished with 0.05 mm alumina slurry on a polishing cloth and thoroughly rinsed with double-distilled water. Then, it was successively sonicated in double-distilled water for 5 min and was allowed to dry at room temperature. So the three-electrode system was transferred into GQD solution, and GQD electrodeposited by 60 repetitive cycles in the potential range from 0.0 to 1.0 V at a scan rate of 100 mV/s. Finally, a thin film of GQDs was obtained at the surface of GCE. The GQD/GCE electrode was immersed in 0.1 M acetate buffer solution with pH 4.0 (AcB) containing 10 mM Ni(NO3)2 and then a potential of −1.1 V for 210 s under stirring conditions applied to the electrode. After that, a linear sweep potential from 0.1 to 0.7 V with a scan rate of 0.1 V s−1 was applied by CV to the resultant Ni/GQD-modified electrode in 0.1 M NaOH solution until reproducible scans were obtained. In this step, electrodissolution of Ni and formation of a passive NiO layer on the GQD/GCE surface take place and NiO/GQD/GCE was prepared. The prepared modified electrode was washed and used daily.
Procedure for real sample preparation
The serum samples were centrifuged, filtered, and diluted at 1:100 with 0.1 M phosphate buffer solution of pH 2 and applied as real samples. The serum samples were drug free, and CLZ was not detected in healthy serum samples. For recovery assessment, serum samples were spiked with known amounts of CLZ stock solution.
For the determination of CLZ in the pharmaceutical formulation, five tablets each containing 25 mg CLZ were accurately weighed and powdered. The accurately weighed quantity of this powder equivalent to 32.68 mg CLZ was dissolved in 100 mL of 0.1 KNO3 by sonication for about 15 min and then centrifuged. The resulting supernatant was used as the real sample with a nominal CLZ concentration of 1 mM. Other dilute solutions of clozapine were prepared using this prepared sample and PBS (pH 2), so that the CLZ concentration lies in the range of calibration curve. Then, the sample was transferred into the voltammetric cell. The accumulation of CLZ on the surface of the working electrode was performed by stirring for 300 s, and then, the stripping voltammograms were recorded between 0.3 and 0.7 V (vs. Ag/AgCl). Finally, keeping the dilution factor in consideration, the concentration of CLZ in the pharmaceutical formulations was determined.
Results and discussion
Morphological analysis
Figure 1 shows the SEM images of GCE before (a) and after the modification of GQD (b) and NiO/GQD (c). Results present the presence of numerous round-shaped prepared GQDs on the electrode surface (Fig. 1b). The obtained image for NiO/GQD/GCE (Fig. 1c) illustrates well-dispersed and well-deposited NiO nanoparticles on the surface of GQD-modified GCE with the average diameter of about 70–80 nm.
Electrochemical characterization of CLZ on the various electrodes
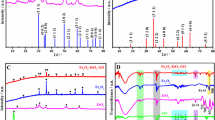
The electrochemical behavior of CLZ was studied at the surface of a bare and GQD- and NiO/GQD-modified glassy carbon electrode using CV. As shown in Fig. 2a, bare GCE do not show any obvious peak for CLZ, but GQD/GCE and NiO/GQD/GCE show one oxidation peak (I) and two reduction peaks (I′ and II′). As presented, CLZ has a well-defined anodic peak with weak cathodic peaks observed in the reverse scan at the surface of the modified electrodes. The peak current along with reversibility of the electrochemical reaction increased at NiO/GQD/GCE compared to other electrodes. This improvement in the electrochemical behavior at the surface of a NiO/GQD composite-modified electrode was attributed to the fast heterogeneous electron transfer kinetics, larger accessible surface, and numerous active sites of the surface that get up from the synergic coupling between the excellent catalytic effect of NiO nanoparticles and high density of edge plane sites of GQDs.
Based on the CV studies, there is a single anodic peak corresponding to the two-electron oxidation of the clozapine followed by two low current cathodic peaks. This behavior resembles an electron transfer–chemical reaction–electron transfer (ECE) mechanism in CLZ oxidation as reported earlier [18, 51] in which the oxidation product (P1) is chemically transformed to P2 which can be oxidized easier than the initial one. Theoretically for a reversible system, the ratio of anodic peak current to the cathodic peak is unity. When a slow chemical reaction follows the initial electrochemical oxidation, the current ratio increases in low scan rates (as seen in Fig. 2). The first reduction peak (I′) in the reverse scan is related to the reduction of P1, and the second one (II′) is related to the reduction of the final oxidation product (CLZox) which is subsequently reoxidized (peak II) in the second anodic scan (Fig. 2b). Therefore, appearance of this oxidation peak decreases the height of the main anodic peak. By increasing the scan rate, the height of the main oxidation and reduction peaks (I, I′) increases, but the height of the new observed peaks does not increase (data not shown). This is due to the slow chemical rate constant and the decreased voltammogram recording time which causes a decreased chemical conversion of P1 to P2. These results are well reported in the similar literature [18, 51].
Effect of pH
The influence of pH value on the electrochemical behavior of 2 μM CLZ at the NiO/GQD/GCE was also investigated with the phosphate buffer solution at different pH values by cyclic voltammetry. As seen in Fig. 3a, by decreasing solution pH, the peak current increases remarkably and reaches to the maximum value at pH 2. Moreover, the value of Ipa/Ipc and the peak-to-peak separation decrease illustrating the increased reversibility at lower pH values. So, as the peak current and the peak shape for CLZ are in the best form in PBS with pH 2, consequently pH 2 was selected as the optimal condition for other experiments.
On the other hand, oxidation peak potential of CLZ shifted linearly to more positive values with decreasing pH which means that the voltammetric behavior of CLZ is a proton transfer process under experimental conditions. According to Fig. 3b, over the pH range of 2–7, a linear relationship was obtained between E and pH, which can be expressed by the following equation:
A slope of −0.038 V per pH value is close to the Nernstian one of −0.03 V per pH and implies that one proton and two electrons are involved in the oxidation process.
Based on the obtained results, a possible mechanism to explain the electrochemical oxidation of CLZ on the NiO/GQD/GCE can be expressed as follows:
Analytical measurements
For the sensitive determination of trace amounts of CLZ, DPV was used as an electrochemical method. CV studies confirmed that CLZ oxidation is diffusion controlled, but detailed studies showed that at lower concentrations of clozapine, the electrode process exhibits adsorptive characteristics and accumulation leads to the enhanced sensitivity. Therefore, in continuation, the effect of experimental parameters on the sensor results was studied.
Effect of accumulation potential and accumulation time
For a 5 μM clozapine solution, there is no change in the anodic peak current with increasing accumulation time, indicating that the accumulation had no effect and the diffusion controlled the surface kinetic process. However, at lower concentrations of clozapine (40 nM), by increasing accumulation time up to 5 min, the obtained peak current and hence the sensitivity of the sensor increase (Fig. 4). Therefore, for achieving a lower detection limit, 5 min was chosen as the accumulation time. Additionally, the effect of accumulation potential was studied, and results showed that by decreasing potential to negative values, the oxidation signal increase may be due to the protonation and positive charge of CLZ in this experimental condition (the pKa of clozapine was about 4.5). But, to avoid unwanted signal related to reducible compounds which may be present in the real samples, −0.1 V was selected as the best potential for CLZ accumulation (Fig. 4).
Hence, 5 min accumulation at −0.1 V was used as the optimum condition for analytical measurements.
Interference study
The determination of clozapine in biological fluids is important due to the dose-dependent effects of this drug. Some compounds present in biological matrices, like glucose (GL), uric acid (UA), ascorbic acid (AA), and dopamine (DP), can oxidize in a suitable potential. Therefore, developing a selective and sensitive method is important to the determination of CLZ in the presence of these compounds. Our studies revealed that no signal related to these materials was observed in the diluted serum sample in the desired potential range. This result may be due to the two matters: different oxidation potential of the compound or lower concentration of that than the sensor’s detection limit. CV studies showed that only uric acid oxidizes at the CLZ oxidation potential and the other three compounds oxidize at potentials different from CLZ oxidation and subsequently have no interference in our detection. In the case of uric acid, detailed DPV studies showed that for 50 nM CLZ, the presence of 100-fold concentration of uric acid hardly can cause 5% variation in the resulted signal. Thus, 50-fold concentration of uric acid in the real samples is permitted. For other compounds, there is no interference until 700-fold concentration.
The interference effect of CLZ was also studied in diluted serum samples. For this, the concentrations of AA, UA, DA, and GL were kept constant and the concentration of CLZ was increased (Fig. 5). Results showed that CLZ could be distinguished and determined selectively at the surface of the NiO/GQD-modified electrode in the presence of these interferences in the real sample.
Linear range and limit of detection
Differential pulse voltammetry was considered a sensitive technique for CLZ determination. Under the optimized condition, determination of different concentrations of CLZ was performed (Fig. 6). The results showed that the anodic peak current increases linearly by increasing CLZ concentration from 3 to 1000 nM and the regression equation was Ip (μA) = (0.092 ± 0.008) + (42.2 ± 0.5) C (μM) (R2 = 0.999). The detection limit for CLZ was calculated to be 0.55 nM. Table 1 summarizes the comparison of NiO/GQD/GCE responses for CLZ in terms of linear range, sensitivity, and detection limit with other modified electrodes reported earlier. It can be seen that NiO/GQD-modified GCE has better analytical performance than other reported works.
Regeneration and repeatability of electrodes
In order to study the repeatability of the proposed CLZ sensor, a solution of 50 nM CLZ was evaluated for successive five times with the same NiO/GQD/GCE under the same conditions. The obtained relative standard deviation (RSD) for the results was 2.84%. The reproducibility of the method was studied by five modified electrodes which were prepared at different days by the same fabrication procedure and used for the determination of 50 nM CLZ solution The RSD for the between-electrode peak currents was obtained as 3.93%. Other results showed that the response of the electrode retained 94.4% of its initial value after 30 days when the sensor was stored under ambient condition, which indicates suitable storage stability of the proposed sensor.
Analysis of real samples
To demonstrate the analytical applicability of the proposed sensor for drug determination in pharmaceutical preparations, the prepared NiO/GQD/GCE was applied for the analysis of CLZ under the optimized condition in pharmaceutical tablets using the DPV method. After the preparation of pharmaceutical samples as mentioned in the “Procedure for real sample preparation” section, a solution containing approximately 30 nM CLZ prepared by PBS and then CLZ concentration was analyzed by a standard addition method. The voltammetric responses and corresponding calibration plots of peak currents vs. concentrations are shown in Fig. 7. Using the standard addition method, the clozapine content was determined to be 99.02 mg with a RSD of 2.53% (n = 3), which is very close to the labeled amount of 100 mg. It is concluded that the tablet matrix does not have any interferences in the electrochemical analysis of CLZ. Moreover, for accuracy studies, the recoveries of the spiked tablet solutions at concentration levels were evaluated (Table 2). The recovery results are in the range of 98.6–102.7%, with the relative standard deviation between 1.89 and 2.11%, which are acceptable levels according to the US FDA Guidance for industry (bioanalytical method validation) [53]. The modified electrode was also used to analyze CLZ in human blood serum samples using the standard addition method. The calculated recoveries for serum samples were in the range of 99.5–104%, and the relative standard deviation was between 1.53 and 2.12%. The results indicated high accuracy and selectivity of the proposed sensor for CLZ analysis in complex matrices.
Conclusion
In the present work, the NiO/GQD nanocomposite-modified glassy carbon electrode was fabricated by simple electrodeposition and used to study electrochemical behavior and oxidation mechanism of clozapine. The oxidation process obeys an ECE mechanism and was pH dependent and diffusion controlled. The prepared sensor was applied for the determination of trace amount of clozapine using the differential pulse voltammetric method. This prepared sensor showed better analytical applicability compared to other modified electrodes and was used successfully to determine CLZ in the human serum samples without any complicated and time-consuming pretreatments.
References
Kane J, Honigfeld J, Singer H, Meltzer HY (1988) Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry 45(9):789–796
Casey DE (1998) Effects of clozapine therapy in schizophrenic individuals at risk for tardive dyskinesia. J Clin Psychiatry 59:31–37
Lieberman JA (1998) Maximizing clozapine therapy: managing side effects. J Clin Psychiatry 59:38–43
Meltzer HY (1998) Suicide in schizophrenia: risk factors and clozapine treatment. J Clin Psychiatry 59:15–20
Zheng MM, Wang ST, Hu WK, Feng YQ (2010) In-tube solid-phase microextraction based on hybrid silica monolith coupled to liquid chromatography-mass spectrometry for automated analysis of ten antidepressants in human urine and plasma. J Chromatogr A 1217(48):7493–7501
Wohlfarth A, Toepfner N, Hermanns-Clausen M, Auwärter V (2011) Sensitive quantification of clozapine and its main metabolites norclozapine and clozapine-N-oxide in serum and urine using LC-MS/MS after simple liquid–liquid extraction work-up. Anal Bioanal Chem 400(3):737–746
Zhang G, Terry AV Jr, Bartlett MG (2007) Simultaneous determination of five antipsychotic drugs in rat plasma by high performance liquid chromatography with ultraviolet detection. J Chromatogr B 856(1-2):20–28
Shen YL, Wu HL, Ko WK, Wu SM (2002) Simultaneous determination of clozapine, clozapine N-oxide, N-desmethylclozapine, risperidone, and 9-hydroxyrisperidone in plasma by high performance liquid chromatography with ultraviolet detection. Anal Chim Acta 460(2):201–208
Vardakou I, Dona A, Pistos C, Alevisopoulos G, Athanaselis S, Maravelias C, Spiliopoulou C (2010) Validated GC/MS method for the simultaneous determination of clozapine and norclozapine in human plasma. Application in psychiatric patients under clozapine treatment. J Chromatogr B 878(25):2327–2332
Sastry CSP, Rekha TV, Satyanarayana A (1998) Spectrophotometric determination of clozapine in pharmaceuticals. Microchim Acta 128(3-4):201–205
Mohamed AA, Al-Ghannam SM (2004) Spectrophotometric determination of clozapine based on its oxidation with bromate in a micellar medium. Farmaco 59(11):907–911
Hernandez L, Gonzalez E, Hernandez P (1988) Determination of clozapine by adsorptive anodic voltammetry using glassy carbon and modified carbon paste electrodes. Analyst 113(11):1715–1718
Farhadi K, Karimpour A (2007) Electrochemical behavior and determination of clozapine on a glassy carbon electrode modified by electrochemical oxidation. Anal Sci 23(4):479–483
Manjunatha JG, Swamy BEK, Mamatha GP, Gilbert O, Srinivas MT, Sherigara BS (2011) Electrochemical studies of clozapine drug using carbon nanotube-SDS modified carbon paste electrode: a cyclic voltammetry study. Pharma Chem 3:236–249
Blankert B, Dominguez O, El Ayyas W, Arcos J, Kauffmann JM (2004) Horseradish peroxidase electrode for the analysis of clozapine. Anal Lett 37(5):903–913
Al Attas AS (2009) Novel PVC membrane selective electrode for the determination of clozapine in pharmaceutical preparations. Int J Electrochem Sci 4:9–19
Huang F, Qu S, Zhang S, Liu B, Kong J (2007) Sensitive detection of clozapine using a gold electrode modified with 16-mercaptohexadecanoic acid self-assembled monolayer. Talanta 72(2):457–462
Mashhadizadeh MH, Afshar E (2013) Electrochemical investigation of clozapine at TiO2 nanoparticles modified carbon paste electrode and simultaneous adsorptive voltammetric determination of two antipsychotic drugs. Electrochim Acta 87:816–823
Nigović B, Spajić J (2011) A novel electrochemical sensor for assaying of antipsychotic drug quetiapine. Talanta 86:393–399
Qu S, Pei S, Zhang S, Song P (2013) Preparation of silicate nanotubes and its application for electrochemical sensing of clozapine. Mater Lett 102:10356–10358
Shahrokhian S, Kamalzadeh Z, Hamzehloei A (2013) Electrochemical determination of clozapine on MWCNTs/New Coccine doped PPY modified GCE: an experimental design approach. Bioelectrochemistry 90:36–43
Zhao J, Chen GF, Zhu L, Li GX (2011) Graphene quantum dots-based platform for the fabrication of electrochemical biosensors. Electrochem Commun 13(1):31–33
Roushani M, Abdi Z (2014) Novel electrochemical sensor based on graphene quantum dots/riboflavin nanocomposite for the detection of persulfate. Sensors Actuators B Chem 201:503–510
Tan F, Cong L, Li X, Zhao Q, Zhao H, Quan X, Chen J (2016) An electrochemical sensor based on molecularly imprinted polypyrrole/graphene quantum dots composite for detection of bisphenol A in water samples. Sensors Actuators B Chem 233:599–606
Hernandez-Santos D, Gonzalez-Garcia MB, Garcia AC (2002) Metal-nanoparticles based electroanalysis. Electroanalysis 14(18):1225–1235
Katz E, Willner I, Wang J (2004) Electroanalytical and bioelectroanalytical systems based on metal and semiconductor nanoparticles. Electroanalysis 16(12):19–44
Rafiee B, Fakhari AR (2013) Electrocatalytic oxidation and determination of insulin at nickel oxide nanoparticles-multiwalled carbon nanotube modified screen printed electrode. Biosens Bioelectron 46:130–135
El-Khatib KM, Abdel Hameed RM (2011) Development of CuO/carbon Vulcan XC72 as non-enzymatic sensor for glucose determination. Biosens Bioelectron 26(8):3542–3548
Lin KC, Lin YC, Chen SM (2013) A highly sensitive nonenzymatic glucose sensor based on multi-walled carbon nanotubes decorated with nickel and copper nanoparticles. Electrochim Acta 96:164–172
Shamsipur M, Najafi M, Hosseini MR (2010) Highly improved electrooxidation of glucose at a nickel(II) oxide/multi-walled carbon nanotube modified glassy carbon electrode. Bioelectrochemistry 77(2):120–124
Nikahd B, Khalilzadeh MA (2016) Liquid phase determination of bisphenol A in food samples using novel nanostructure ionic liquid modified sensor. J Mol Liq 215:253–257
Yousef Elahi M, Heli H, Bathaie SZ, Mousavi MF (2007) Electrocatalytic oxidation of glucose at a Ni-curcumin modified glassy carbon electrode. J Solid State Electrochem 11:273–282
Noorbakhsh A, Salimi A (2009) Amperometric detection of hydrogen peroxide at nano-nickel oxide/thionine and celestine blue nanocomposite-modified glassy carbon electrodes. Electrochim Acta 54(26):6312–6321
Jafarian M, Forouzandeh F, Danaee I, Gobal F, Mahjani MG (2009) Electrocatalytic oxidation of glucose on Ni and NiCu alloy modified glassy carbon electrode. J Solid State Electrochem 13(8):1171–1179
Ojani R, Raoof JB, Norouzi B (2011) Performance of glucose electrooxidation on Ni–Co composition dispersed on the poly(isonicotinic acid) (SDS) film. J Solid State Electrochem 15(6):1139–1147
Liu BD, Luo LQ, Ding YP, Si XJ, Wei YL, Ouyang XQ, Xu D (2014) Differential pulse voltammetric determination of ascorbic acid in the presence of folic acid at electro-deposited NiO/graphene composite film modified electrode. Electrochim Acta 142:336–342
Cao X, Xu YJ, Wang N (2011) Facile synthesis of NiO nanoflowers and their electrocatalytic performance. Sensors Actuators B Chem 153:434–438
Chekin F, Bagheri S, Arof AK, Bee Abd Hamid S (2012) Preparation and characterization of Ni(II)/polyacrylonitrile and carbon nanotube composite modified electrode and application for carbohydrates electrocatalytic oxidation. J Solid State Electrochem 16(10):3245–3251
Luo L, Li F, Zhu L, Ding Y, Zhang Z, Deng D, Lu B (2013) Nonenzymatic glucose sensor based on nickel (II)oxide/ordered mesoporous carbon modified glassy carbon electrode. Colloids Surf B 102:307–311
Wolfart F, Maciel A, Nagata N, Vidotti M (2013) Electrocatalytical properties presented by Cu/Ni alloy modified electrodes toward the oxidation of glucose. J Solid State Electrochem 17(5):1333–1338
Yi W, Yang D, Chen H, Liu P, Tan J, Li H (2014) A highly sensitive nonenzymatic glucose sensor based on nickel oxide–carbon nanotube hybrid nanobelts. J Solid State Electrochem 18(4):899–908
El-Refaei SM, Saleh MM, Awad MI (2014) Tolerance of glucose electrocatalytic oxidation on NiO x/MnO x/GC electrode to poisoning by halides. J Solid State Electrochem 18(1):5–12
Yu Z, Li H, Zhang X, Liu N, Zhang X (2015) NiO/graphene nanocomposite for determination of H2O2 with a low detection limit. Talanta 144:1–5
Wang L, Tang Y, Wang L, Zhu H, Meng X, Chen Y, Sun Y, Yang XJ, Wan P (2015) Fast conversion of redox couple on Ni(OH)2/C nanocomposite electrode for high-performance nonenzymatic glucose sensor. J Solid State Electrochem 19(3):851–860
Soomro RA, Ibupoto ZH, Sirajuddin AMI, Willander M (2015) Controlled synthesis and electrochemical application of skein-shaped NiO nanostructures. J Solid State Electrochem 19(3):913–922
Fouladgar M, Ahmadzadeh S (2016) Application of a nanostructured sensor based on NiO nanoparticles modified carbon paste electrode for determination of methyldopa in the presence of folic acid. Appl Surf Sci 379:150–155
Li X, Wen H, Fu Q, Peng D, Yu J, Zhang Q, Huang X (2016) Morphology-dependent NiO modified glassy carbon electrode surface for lead(II) and cadmium(II) detection. Appl Surf Sci 363:7–12
Ahour F, Ahsani MK (2016) An electrochemical label-free and sensitive thrombin aptasensor based on graphene oxide modified pencil graphite electrode. Biosens Bioelectron 86:764–769
Ahour F, Shamsi A (2017) Electrochemical label-free and sensitive nanobiosensing of DNA hybridization by graphene oxide modified pencil graphite electrode. Anal Biochem 532:64–71
Ahour F, Taheri M (2018) Anodic stripping voltammetric determination of copper (II) ions at a graphene quantum dot-modified pencil graphite electrode. J Iran Chem Soc 15(2):343–350
Kauffmann J-M, Vire J-C, Patriarche GJ (1979) Electrochemical oxidation of derivatives of dibenzodiazepin, dibenzothiazepin and dibenzoxazepin. Anal Letters 12(11):1217–1234
Arvand M, Ghasempour M (2012) Voltammetric determination of clozapine in pharmaceutical formulations and biological fluids using an in situ surfactant modified carbon ionic liquid electrode. Electroanalysis 24(3):683–690
U.S. FDA Guidance for Industry, Bioanalytical Method Validation, 2001
Funding
We gratefully acknowledge the partial financial support from the Nanotechnology Research Center and Faculty of Chemistry, Urmia University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shamsi, A., Ahour, F. & Sehatnia, B. Nickel oxide nanoparticles decorated graphene quantum dot as an effective electrode modifier for electrocatalytic oxidation and analysis of clozapine. J Solid State Electrochem 22, 2681–2689 (2018). https://doi.org/10.1007/s10008-018-3982-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-018-3982-3