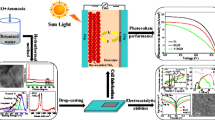
Abstract
Fe3O4-reduced graphene oxide (Fe3O4-RGO) binder-free counter electrode (CE) is prepared by using an easy and low-cost electrophoretic deposition method and controlling the hydrogen evolution process followed by an electrochemical reduction process for dye-sensitized solar cell (DSSC). X-ray diffraction, X-ray photoelectron spectroscopy, energy dispersive spectrometer, Raman spectroscopy, field emission scanning electron microscopy, and transmission electron microscopy (TEM) indicate clearly the formation of Fe3O4-RGO nanocomposite. TEM images show that the Fe3O4 nanoparticles with diameters in the range of 10–30 nm are uniformly deposited on RGO. The layer-by-layer deposition of iron oxide species anchored on graphene nanosheets during the EPD on FTO provides a unique film for DSSC. To evaluate the chemical catalysis and stability of prepared CEs toward I3 − reduction and the interfacial charge transfer properties, Fe3O4-RGO nanocomposite and RGO are characterized by cyclic voltammetry, Tafel polarization, and electrochemical impedance spectroscopy. Under AM 1.5 irradiation (100 mW cm−2), the DSSC based on the Fe3O4-RGO shows a power conversion efficiency of 5.91%, which is comparable with the Pt CE, suggesting that the Fe3O4-RGO nanocomposite is an effective CE material for low-cost DSSC. The proposed approach can prepare a thin film of Fe3O4-RGO at short time with suitable performance in DSSC.

Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dye-sensitized solar cell (DSSC) is a dawn for the future photovoltaic technology due to their low cost, simple fabrication processes, and high efficiency [1]. The DSSC has several parts such as photoanode, dye, electrolyte, and counter electrode (CE). CE collects the electrons from the external circuit and then injects them into the electrolyte to catalyze the reduction of triiodide (\( {\mathrm{I}}_3^{-} \)) to iodide (I−) at CE/electrolyte interface. An efficient CE should possess the outstanding characteristics of good conductivity for the electrons transporting, highly catalytic activity for the reaction of \( {\mathrm{I}}_3^{-} \) to I−, and excellent chemical stability [2,3,4]. Gratzel et al. [5] used TiO2 nanoparticles based electrodes sensitized with a series of Ruthenium-dyes as working electrodes, a Pt CE, and an \( {\mathrm{I}}^{-}/{\mathrm{I}}_3^{-} \) redox electrolyte. In recent complementary researches, certain forms of carbon [6, 7] and metal oxides [8] are considered as candidates for CE in DSSCs. Iron oxides because of their abundance, low cost, and environmentally friendly nature have allocated more attention to themselves [9]. Among all known iron oxides, Fe3O4 due to the presence of Fe cations in two valence states exhibits most interesting properties [10,11,12,13]. Iron compounds have been widely enlarged for catalysis [14], lithium ion batteries [15], supercapacitors [16], and drug delivery [17]. Among the various carbon materials, graphene renders splendid attributes such as high electrical conductivity, high chemical stability, high surface area, and high electrocatalytic activity toward \( {\mathrm{I}}_3^{-} \) reduction [18]. Hence, graphene nanosheets can be exploited as noteworthy matrix for the growing and anchoring of various categories of functional substances. In order to endow more conductivity, the removal of oxygen-containing functional groups from GO sheets [19] is achieved by several ways such as chemical or electrochemical [20] and graphene sheets are produced. The electrochemical reduction is foregone due to its low cost, fast, simplicity, and environmentally safety [21].
Electrophoretic deposition (EPD) affords the possibility of fabrication of thin film on the conductive substrates [22]. EPD relies on simple device (DC power supply) and two electrodes and provides a short time process for preparation of uniform layers with high quality [23]. In the field of DSSCs, Lee et al. [24] prepared graphene/carbon nanotube composites CE by doctor blade method and DSSC assembled with this CE exhibited an efficiency of 4.0%. Gao et al. [25] fabricated nickel phosphide-embedded graphene CE by the hydrothermal reaction of red phosphorus, nickel chloride, and graphene oxide in a mixture of ethylene glycol-water. DSSC with this CE exhibited a power conversion efficiency of 5.70%. Lin et al. [26] prepared a composite film of MoS2/graphene CE and DSSC fabricated with this CE exhibited an efficiency of 5.98%.
Although other methods such as hydrothermal, infiltration, etc. are used extensively to prepare nanostructured materials, other useful method could be considered. Herein, we demonstrate for the first time the preparation of binder-free reduced graphene oxide (RGO)-Fe3O4 nanocomposite film on fluorine doped tin oxide (FTO) by one step simple and low-cost EPD without using binders as CE in DSSC. Fe3O4-reduced graphene oxide was prepared in two steps: (i) electrophoretic deposition from suspension of graphene oxide (GO) in the presence of FeCl2 and FeCl3 on FTO, (ii) electrochemically reduction of prepared electrode in 0.5 M NaNO3 electrolyte. The performance of Fe3O4-RGO nanocomposite CE is investigated in the improvement of activity and reversibility of the catalytic reduction of \( {\mathrm{I}}_3^{-} \) into I− in DSSC.
Experimental
Fabrication of various counter electrodes
In order to synthesis GO, a modified Hummer’s method was followed [27]. FTO (sheet resistance of 15 Ω/□) was first cleaned with double distilled water, HCl, ethanol, and acetone and dried in air. For EPD, a glass tube was equipped with two electrodes of FTO (anode) and Pt foil (cathode) placed in parallel with a distance of 1 cm. To deposit GO on FTO, a suspension of GO with 2 mg mL−1 density was prepared by adding 20 mg of the GO to 10 mL double distilled water and was dispersed by sonication. The deposition was carried out by applying a constant DC voltage of 10 V between two electrodes by using a power supply during 1 min. GO sheets with a negative charge migrate to the anode and deposit on it. After deposition, GO film was reduced electrochemically at − 1.1 V (vs. Ag|AgCl|KCl (3 M)) in 0.5 M NaNO3 for 700 s to remove the functional groups on GO and then was dried in an oven at 80 °C for 1 h. To fabricate nanocomposite CE, the suspension of iron salts-GO with 2 mg mL−1 density was prepared in two steps: first 10 mg of the GO was added to 10 mL double distilled water and dispersed by ultrasonic irradiation for 1 h. Then, FeCl2 (3.33 mg) and FeCl3 (6.66 mg) were added into GO suspension and dispersed by ultrasonic for 1 h. The deposition was carried out by applying a constant DC voltage of 5.5 V between FTO and Pt electrode for 2 min. This voltage can prevent the hydrogen and oxygen evolution raised from hydrolysis of water. The gas evolution can prevent the formation of adherent film on FTO surface. Addition of metal ions to the GO suspension creates positive charge on dispersed species [28] which can migrate to the cathode and deposit on FTO. After EPD, the as-prepared film was converted to RGO-Fe3O4 nanocomposite by electrochemical reduction in 0.5 M NaNO3 by applying constant voltage of − 1.1 V for 700 s and dried in oven at 80 °C during 1 h. To compare the overall performance of the conventional DSSCs, a Pt-coated FTO was also prepared with Pt paste by doctor blade technique to an area (0.25 cm2) of the FTO substrate and treatment at 450 °C for 30 min.
Assembly of DSSC
The TiO2 photoanode was prepared as follows: FTO conductive glass was first treated with 50 mM TiCl4 aqueous solution at 70 °C for 30 min. TiO2 particles were deposited onto pre-cleaned FTO glass by using a doctor blade technique. A film of 20 nm sized TiO2 particles was deposited onto the FTO glass electrode. The film was dried at 120 °C for 2 min. Then, layer of 200 nm TiO2 particles was coated on the top of the first TiO2 layer. The resulting TiO2 film was annealed at 500 °C for 30 min. The annealed film was then immersed overnight (24 h) in 0.4 mM dye cis-bis (isothiocyanato) bis (2 20-bipyridyl-4,40-dicar-boxylato) ruthenium(II) bis-tetrabutyl ammonium (N719) (From Dyesol) solution. Then the photoanode was rinsed with ethanol and dried in air. The photoanode and Fe3O4-RGO CE were assembled into cell with a Surlyn. The iodide electrolyte was injected into the cell through a hole on the CE.
Results and discussion
Formation mechanism
EPD was applied to suspension containing the iron salts and GO and a thin film was formed on FTO. Film containing GO nanosheets and iron ions could be converted to Fe3O4-RGO through the electrochemical reduction by applying the potential of E app = − 1.1 V (vs. Ag|AgCl|KCl (3 M) by AUTOLAB 302 N) to the electrode in 0.5 M NaNO3. In negative potential, OH− ions can be produced during the following reactions [29]:
Then, the generated OH− ions contribute in the formation of Fe3O4 nanoparticles through the formation of iron hydroxide and dehydration of it [30,31,32]
In other hand, applying negative potential to the electrode causes the reduction of GO to RGO nanosheets through removing some functional groups.
Structural analysis
Figure 1 shows the X-ray diffraction (XRD, by GBC MMA, Cu Kα radiation) pattern of the Fe3O4-RGO nanocomposite deposited on FTO. Several diffraction peaks were observed. These peaks were assigned to be (220), (311), (422), (511), and (440) corresponding to the Fe3O4 nanoparticles. The appeared peaks in XRD pattern of Fe3O4 nanoparticles are in good agreement with that of magnetite (JCPDS File Card no. 88-0315). Wang et al. prepared Fe3O4 by hydrothermal and reported the same XRD pattern [29]. The appearance of peaks with weak intensity for Fe3O4 nanoparticles may be probably due to the small amount of available nanoparticles for analysis. Also, the intensity of peaks may be small due to the low degree of crystallinity.
All peaks in the XRD pattern of Fe3O4 nanoparticles are also in good agreement with Wang and coworker reported in the literature [29]. The appearance of peaks with weak intensity for Fe3O4 nanoparticles may be probably due to the small amount of available nanoparticles for analysis. The diffraction peaks in 2θ = 27°, 34°, and 66° are attributed to the FTO glass substrate [29, 33].
The Fourier transform infrared spectroscopy (FTIR, by Vector 22- Bruker, in a region between 4000 and 400 cm−1 by using KBr pellet technique) spectra of GO, RGO, and RGO-Fe3O4 nanocomposite films are shown in Fig. 2a. In FTIR spectrum of GO, the characteristic adsorption signals include a broad peak at approximately 3500 cm−1 assigned to O-H stretching of carboxyl groups, an intense peak at approximately 1720–1740 cm−1 attributed to C=O stretching of carbonyl or carboxyl groups, and a weak peak at approximately 1590–1620 cm−1 for C=C vibration of non-oxidized graphite [34]. The absorption peak around 630 cm−1 shows stretching vibration of Fe-O in Fe3O4 nanoparticles [35].
Raman spectroscopy (by Senterra-Bruker, excited by a laser with a wavelength of 785 nm) is another technique used complementary to FTIR in order to reveal the structural information of graphene. In Fig. 2b, Raman spectra of RGO and Fe3O4-RGO nanocomposite film display two separated peaks around 1310 and 1580 cm−1 attributed to D band and G band, respectively. The D and G band can be related to sp3 defects in the graphene and to the in-plane vibrations of the graphitic structure, respectively [36]. The intensity ratios (r = I D/I G) for RGO is 1.9 and Fe3O4-RGO nanocomposite film is 2.1. The origin of the higher I D/I G ratio in Fe3O4-RGO nanocomposite than that in RGO is due to the presence of localized sp3 defects within the sp2 carbon network [37]. The Raman spectrum of Fe3O4-RGO nanocomposite has same typical peaks of the Fe-O stretching vibration, around 600 cm−1 which is also observed by the FTIR, indicating the Fe3O4-RGO nanocomposite synthesized [36].
Formation of Fe3O4 nanoparticles on RGO is also confirmed with X-ray photoelectron spectroscopy (XPS, by X-Ray 8025, Bestec, Germany, Mg Kα radiation) because it is sensitive to the presence of Fe2+ and Fe3+ cations [38]. Figure 2c displays the Fe2p XPS spectrum of the nanocomposite. The broad peaks around 710.9 and 724.5 eV are attributed to the Fe2p3/2 and Fe2p1/2, indicating the formation of Fe3O4 [39].
Energy dispersive X-ray spectroscopy (EDX, by Oxford instrument, with operating voltage of 5 kV) is used to elemental analysis of prepared film and the recorded spectrum is shown in Fig. 2d. The presence of Fe, O, C, and N in EDX analysis confirms the graphene and iron compound in the sample. The high percent of oxygen arises from oxide and functional groups on the graphene present in Fe3O4-RGO nanocomposite. Also N can be incorporated to the graphene during the GO formation or the electrochemical reduction of GO into RGO in NaNO3 solution and N may be added into the structure during the Hummer’s method to synthesize of GO. The presence of Sn and Si elements attribute to the FTO substrate.
Surface morphology and hydrophilicity
To investigate the morphology and structure of the products, field emission scanning electron microscopy (FESEM, by Sigma-Zeiss, Germany) and transmission electron microscopy (TEM, by Tecnai 20, FEI) images were recorded. The FESEM images of RGO are depicted in Fig. 3a, b, and shows the RGO with crumpled and rippled like morphology [40]. Figure 3c, d reveals a homogeneous dispersion of Fe3O4 nanoparticles on the surface of graphene sheets. The Fe3O4 nanoparticles distributed between the graphene sheets can prevented the agglomeration of them [35]. Also, the further agglomeration of Fe3O4 nanoparticles could be reduced at such condition and increases catalytic active sites.
Figure 4 presents the TEM images of Fe3O4-RGO nanocomposite at two different magnifications. Fe3O4 nanoparticles are anchored uniformly on the surface of graphene nanosheets. The size of nanoparticles loaded on graphene nanosheets is in the range of 10–30 nm. A sandwich-like structure could be formed during the formation of layer-by-layer deposition of Fe3O4 nanoparticles anchored RGO on FTO during the EPD process where graphene nanosheets containing iron oxide species immigrate to electrode surface and deposit on it.
Contact angle is usefulness for better performance electrolyte/electrode interface and simple charge transfer [41,42,43]. To measure the contact angle, a drop of water is produced on a solid and the angle formed between the solid/liquid interface and the liquid/vapor interface is calculated. The surface with low contact angle has more hydrophilicity and water wet the surface. Images of water contact angle measurements are shown in Fig. 5. The measured water contact angles of RGO and Fe3O4-RGO films are 54.3 and 23.8, respectively. The formation of Fe3O4 on RGO sheets increases the hydrophilic properties of nanocomposite film and improves its attachment on the FTO. RGO film with less hydrophilic nature than Fe3O4-RGO has low trend to attach to the surface of FTO especially with increasing the time of reduction and removing oxygen-containing functional groups. During the electrochemical reduction, more functional groups are removed leads to increase in hydrophobicity and film detaches from electrode surface. Therefore, an optimum reduction time is necessary for electrochemical reduction process to keep the film hydrophilic. It seems that the decrease in hydrophobicity of nanocomposite can improve the performance of DSSC during the electrochemical studies.
Electrochemical measurements
Power conversion efficiency (PCE) of DSSCs is strongly dependent on the electron transfer and electrocatalytic activity of CE. Better catalytic activity and faster electron transfer lead to higher PCE for DSSCs. The catalytic activity of CE for the \( {\mathrm{I}}^{-}/{\mathrm{I}}_3^{-} \) redox couple in DSSC was studied by cyclic voltammetry (CV) in acetonitrile solution containing 50 mM LiI, 10 mM I2, and 0.5 M LiClO4, using a three-electrode system at a scan rate of 50 mV s−1 (by AUTOLAB 302 N, the Netherlands). The CV curve shows two pairs, the right peaks are assigned to the redox reaction between \( {\mathrm{I}}_2/{\mathrm{I}}_3^{-} \), as illustrated in Eq. 6. The other redox pair is associated with that of \( {\mathrm{I}}_3^{-}/{\mathrm{I}}^{-} \), as presented in Eq. 7 [44, 45].
Figure 6a shows the CV curves for different electrodes. The redox peak current densities of \( {\mathrm{I}}^{-}/{\mathrm{I}}_3^{-} \) on RGO CE are substantially small. To activate the catalytic reaction on RGO CE, more active sites are necessary. The formation of Fe3O4 on graphene can increase the active sites and the redox peak current densities of \( {\mathrm{I}}^{-}/{\mathrm{I}}_3^{-} \) on RGO-Fe3O4 CE are higher than that of RGO CE, which indicates the ability of Fe3O4-RGO CE in electrocatalytic redox reaction. Besides, little changes in redox peaks shift were observed after 20 CV cycles at Fe3O4-RGO CE, as shown in Fig. 6b. These results further verify the good electrochemical stability of the Fe3O4-RGO CE in \( {\mathrm{I}}^{-}/{\mathrm{I}}_3^{-} \) system. Also, the prepared composite exhibits the good mechanical attachment and film could be only removed by mechanical scratch.
Electrochemical properties characterization of CEs fabricated on FTO substrate: a CV curves of various CEs in I3 −/I− electrolyte; b the stability evaluation of cyclic voltammograms for the Fe3O4-RGO CE; c CV curves of Fe3O4-RGO CE for I−/I3 − redox species at different scan rates 10, 20, 30, 40, 50, 60, 70, 80, 90, 100 mV s−1
The stacking CV curves of Fe3O4-RGO CE were recorded at various scan rates (Fig. 6c). While increasing the scan rate, the cathodic peaks gradually shift to more negative potentials and the anodic peaks shift positively. This shows some kinetic limitation in electrode processes at higher scan rates.
In order to further confirm the CE electrochemical characteristics, electrochemical impedance spectroscopy (EIS) analysis and Tafel polarization were carried out with the symmetrical cells fabricated with two similar electrodes under the dark condition. EIS recorded in a frequency range of 0.01 Hz to 20 kHz at open circuit potential (OCP) by using a potentiostat/galvanostat Palmsense3 (PS Trace software version 4.2.2, Netherlands) [46]. In typical Nyquist plot, the high frequency semicircle displays the charge transfer resistance (R ct) at CE/electrolyte interface. The lower R ct for CEs manifests its larger catalytic ability [47]. Nyquist plots of RGO and Fe3O4-RGO are shown in Fig. 7a. A semicircle is observed in the high frequency for two CEs which represent the R ct at the CE/electrolyte interface. The R ct value on the RGO symmetric cell was ~ 832 Ω cm2. Whereas, after formation of Fe3O4 on RGO, the R ct value (340 Ω cm2) decreased. In accordance with the CV, the electrocatalytic activity of Fe3O4-RGO is better than that RGO due to its smaller R ct. By fitting the EIS with the equivalent circuit in Zview software, R s, R ct, W, and CPE values are evaluated, and the results are summarized in Table 1.
A typical Tafel curve can be divided into three zones: low potential, middle potential, and high potential attributed to the polarization zone, Tafel zone, and diffusion zone, respectively. [48]. Tafel polarization curves for RGO and Fe3O4-RGO are shown in Fig. 7b. The slope of a Fe3O4-RGO curve is higher than that of RGO in the Tafel zone, indicating large exchange current density on the electrode surface. This implies that the better catalytic activity is obtained by Fe3O4-RGO which is also in good agreement with the EIS and CV.
Photovoltaic performance
The current density-photovoltage (J-V) curves of DSSCs under simulated AM 1.5 illumination (I = 100 mW cm−2) with a solar simulator (Sharif Solar, Iran) are shown in Fig. 8. The J-V curve of a reference DSSC with Pt CE is also presented. The photovoltaic parameters including V oc, J sc, Fill factor (FF), and PCE are listed in Table 2. FF and PCE were calculated by the following equations [49]:
Where J max (mA cm−2) is current density, V max (V) is voltage, P in is the incident light power, and J sc (mA cm−2) and V oc (V) are the and open-circuit voltage in the J-V curves at the point of maximum power output, respectively [49].
The DSSC with the RGO CE shows a PCE of 3.30%, whereas the DSSC with Fe3O4-RGO CE exhibits a higher PCE (5.91%). The DSSC with Fe3O4-RGO CE exhibited the good performance with the J sc of 24.45 mA cm−2, V oc of 0.755 V, FF of 0.32, and PCE of 5.91% under illumination of AM1.5 simulated sunlight (Almost similar to the results reported in the past [50,51,52]). The DSSC of Fe3O4-RGO CE yields a PCE of 5.91% in comparison with 8.6% for Pt CE.
Conclusions
We successfully synthetized Pt-free counter electrodes-based RGO and Fe3O4-RGO nanocomposite films on FTO glass substrates, which were formed by a simple and low-cost EPD technique followed with an electrochemical reduction step. It seems that a sandwich-like structure may be formed by deposition of layer-by-layer Fe3O4 nanoparticles anchored RGO nanosheets on FTO during the EPD process. XRD, XPS, and Raman studies confirm the formation of Fe3O4 nanoparticles and the FESEM and TEM images show the presence of Fe3O4 nanoparticles dispersed on the surface of the RGO. The electrochemical tests reveal that binder-free Fe3O4-RGO nanocomposite CE exhibits good electrocatalytic activity on reducing \( {\mathrm{I}}_3^{-} \) ions and resulting enhancement of performance DSSC. The Fe3O4-RGO nanocomposite CE achieves a PCE of 5.91%, which is higher than that of the RGO CE (3.30%). According to results, the proposed deposition method can prepare a thin film of Fe3O4-RGO without using binder on FTO at short time with suitable performance as CE material in DSSC.
References
Hagfeldt A, Boschloo G, Sun L, Kloo L, Petterssn H (2010) Dye-sensitized solar cells. Chem Rev 110:6595–6663
Yang W, Ma X, Xu X, Li Y, Raj S, Ning G, Wang A, Chen SH (2015) Sulfur-doped porous carbon as metal-free counter electrode for high-efficiency dye-sensitized solar cells. J Power Sources 282:228–234
Wu M, Ma T (2014) Recent progress of counter electrode catalysts in dye-sensitized solar cells. J Phys Chem C 118:16727–16742
Jinbiao J, Jihuai W, Yongguang T, Jinghao H, Min Z, Jianming L (2015) Transparent nickel selenide used as counter electrode in high efficient dye-sensitized solar cells. J Alloys Compd 640:29–33
O’Reagen B, Gratzel M (1991) A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353:737–74+0
Li P, Wu J, Lin J, Huang M, Huang Y, Li Q (2009) High-performance and low platinum loading Pt/carbon black counter electrode for dye-sensitized solar cells. Sol Energy 83:845–849
Velten J, Mozer AJ, Li D, Officer D, Wallace G, Baughman R, Zakhidov A (2012) Carbon nanotube/graphene nanocomposite as efficient counter electrodes in dye-sensitized solar cells. Nanotechnology 23:085201
Dao VD, Larina LL, Lee JK, Jung KD, Huy BT, Choi HS (2015) Graphene-based RuO2 nanohybrid as a highly efficient catalyst for triiodide reduction in dye-sensitized solar cells. Carbon 81:710–719
Yao Y, Miao S, Liu S, Ma LP, Sun H, Wang S (2012) Synthesis, characterization, and adsorption properties of magnetic Fe3O4@graphene nanocomposite. Chem Eng J 184:326–332
Kyotani T, Tsai L, Tomita A (1997) Formation of platinum nanorods and nanoparticles in uniform carbon nanotubes prepared by a template carbonization method. Chem Commun 4756:701–702
Flahaut E, Peigney A, Laurent C, Marlière C, Chastel F, Rousset A (2000) Carbon nanotube–metal–oxide nanocomposites: microstructure, electrical conductivity and mechanical properties. Acta Mater 48:3803–3812
Van der Zaag P, Bloemen P (2000) On the construction of an Fe3O4-based all-oxide spin valve. J Magn Magn Mater 211:301–308
Raj K, Moskowitz B, Casciari R (1995) Advances in ferrofluid technology. J Magn Magn Mater 149:174–180
Tahir AA, Upul Wijayantha KG, Saremi-Yarahmadi S, Maznar M, Mckee V (2009) Nanostructured α-Fe2O3 thin films for photoelectrochemical hydrogen generation. Chem Mater 21:3763–3772
Deosarkar MP, Pawar SM, Bhanvase BA (2014) In situ sonochemical synthesis of Fe3O4-graphene nanocomposite for lithium rechargeable batteries. Chem Eng Process Process Intensif 83:49–55
Li L, Gao P, Gai S, He F, Chen Y, Zhang M, Yang P (2016) Ultra small and highly dispersed Fe3O4 nanoparticles anchored on reduced graphene for supercapacitor application. Electrochim Acta 190:566–573
Ding Y, Shen SZ, Sun H, Sun K, Liu F, Qi Y, Yan J (2015) Design and construction of polymerized-chitosan coated Fe3O4 magnetic nanoparticles and its application for hydrophobic drug delivery. Mater Sci Eng C 48:487–498
Houmad M, Zaari H, Benyoussef A, El Kenz A, Ez-Zahraouy H (2015) Optical conductivity enhancement and band gap opening with silicon doped graphene. Carbon 94:1021–1027
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field effect in atomically thin carbon films. Science 306:666–669
An SJ, Zhu Y, Lee SH, Stoller MD, Emilsson T, Park S, Velamakanni A, An J, Ruoff R (2010) Thin film fabrication and simultaneous anodic reduction of deposited graphene oxide platelets by electrophoretic deposition. J Phys Chem Lett 1:1259–1263
Pei S, Cheng H-M (2012) The reduction of graphene oxide. Carbon 50:3210–3228
Huo J, Zheng M, Tu Y, Wu J, Hu L, Dai S (2015) A high performance cobalt sulfide counter electrode for dye-sensitized solar cells. Electrochim Acta 159:166–173
Sarkar P, De D, Uchikochi T, Besra L (2012) Electrophoretic deposition (EPD): fundamentals and novel applications in fabrication of advanced ceramic microstructures. Electrophor Depos Nanomater 52:190
Battumur T, Mujawar SH, Truong QT, Ambade SB, Lee DS, Lee W, Han SH (2012) Graphene/carbon nanotubes composites as a counter electrode for dye-sensitized solar cells. Curr Appl Phys 12:49–53
Dou YY, Li GR, Song J, Gao XP (2012) Nickel phosphide-embedded graphene as counter electrode for dye-sensitized solar cells. Phys Chem Chem Phys 14:1339–1342
Yue G, Lin JY, Tai SY, Xiao Y, Wu J (2012) A catalytic composite film of MoS2/graphene flake as a counter electrode for Pt-free dye-sensitized solar cells. Electrochim Acta 85:162–168
William J, Hummers S, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339
Wu Z, Pei S, Ren W, Tang D, Gao L, Liu B, Cheng H (2009) Field emission of single-layer graphene films prepared by electrophoretic deposition. Adv Mater 21:1756–1760
Lian P, Zhu X, Xiang H, Li Z, Yang W, Wang H (2010) Enhanced cycling performance of Fe3O4-graphene nanocomposite as an anode material for lithium-ion batteries. Electrochim Acta 56:834–840
Salamon J, Sathishkumar Y, Ramachandran K, Soo Y, Jin D, Rhan A, Gnana G (2015) One-pot synthesis of magnetite nanorods/graphene composites and its catalytic activity toward electrochemical detection of dopamine. Biosens Bioelectron 64:269–276
Cao K, Jiao L, Liu H, Liu Y, Wang Y, Guo Z, Yuan H (2015) 3D hierarchical porous α-Fe2O3 nanosheets for high-performance lithium-ion batteries. Adv Energy Mater 5:1–9
Izaki M, Shinoura O (2001) Room-temperature deposition of defect-free magnetite film by chemical reaction from an aqueous solution. Adv Mater 13:142–145
Peng S, Zhu P, Thavasi V, Mhaisalkar SG, Ramakrishna S (2011) Facile solution deposition of ZnIn2S4 nanosheet films on FTO substrates for photoelectric application †. Nano 3:2602–2608
Nethravathi C, Rajamathi M (2008) Chemically modified graphene sheets produced by the solvothermal reduction of colloidal dispersions of graphite oxide. Carbon 46:1994–1998
Ghasemi S, Ahmadi F (2015) Effect of surfactant on the electrochemical performance of graphene/iron oxide electrode for supercapacitor. J Power Sources 289:129–137
Zhang M, Jia MQ, Jin YH (2012) Fe3O4/reduced graphene oxide nanocomposite as high performance anode for lithium ion batteries. Appl Surf Sci 261:298–305
Li X, Huang X, Liu D, Wang X, Song S, Zhou L, Zhang H (2011) Synthesis of 3D hierarchical Fe3O4/graphene composites with high lithium storage capacity and for controlled drug delivery. J Phys Chem C 115:21567–21573
Sun G, Dong B, Cao M, Wei B, Hu C (2011) Hierarchical dendrite-like magnetic materials of Fe3O4, γ-Fe2O3, and Fe with high performance of microwave absorption. Chem Mater 23:1587–1593
Li X, Si Z, Lei Y, Tang J, Wang S, Su S, Song S, Zhao L, Zhang H (2010) Direct hydrothermal synthesis of single-crystalline triangular Fe3O4 nanoprisms. Cryst Eng Comm 12:2060
Bhuvaneswari S, Pratheeksha PM, Anandan S, Rangappa D, Gopalan R, Rao TN (2014) Efficient reduced graphene oxide grafted porous Fe3O4 composite as a high performance anode material for Li-ion batteries. Phys Chem Chem Phys 16:5284
Liu L, Zhao F, Liu J, Yang F (2013) Preparation of highly conductive cathodic membrane with graphene (oxide)/PPy and the membrane antifouling property in filtrating yeast suspensions in EMBR. J Memb Sci 437:99–107
Mane RS, Chang J, Ham D, Pawar BN, Ganesh T, Cho BW, Lee JK, Han SH (2009) Dye-sensitized solar cell and electrochemical supercapacitor applications of electrochemically deposited hydrophilic and nanocrystalline tin oxide film electrodes. Curr Appl Phys 9:87–91
Zhu Y, Xu X, Zhang L, Chen J, Cao Y (2012) High efficiency inverted polymeric bulk-heterojunction solar cells with hydrophilic conjugated polymers as cathode interlayer on ITO. Sol Energy Mater Sol Cells 97:83–88
Lee TH, Do K, Lee YW, Jeon SS, Kim C, Ko J, Im S (2012) High-performance dye-sensitized solar cells based on PEDOT nanofibers as an efficient catalytic counter electrode. J Mater Chem 22:21624
Bi H, Cui H, Lin T, Huang F (2015) Graphene wrapped copper–nickel nanospheres on highly conductive graphene film for use as counter electrodes of dye-sensitized solar cells. Carbon 91:153–160
Hauch A, Georg A (2001) Diffusion in the electrolyte and charge-transfer reaction at the platinum electrode in dye-sensitized solar cells. Electrochim Acta 46:3457–3466
Zhang J, Ma M, Tang Q, Yu L (2016) Multistep electrochemical deposition of hierarchical platinum alloy counter electrodes for dye-sensitized solar cells. J Power Sources 303:243–249
Liao Y, Pan K, Wang L, Pan Q, Zhou W, Miao X, Fu H (2013) Facile synthesis of high-crystallinity graphitic carbon/Fe3C nanocomposites as counter electrodes for high-efficiency dye-sensitized solar cells. ACS Appl Mater 5:3663–3670
Xiao Y, Wang C, Han G (2015) Effects of thiourea concentration on electrocatalytic performances of nickel sulfide counter electrodes for use in dye-sensitized solar cells. Mater Res Bull 61:326–332
Chang Q, Ma Z, Wang J, Yan Y, Shi W, Chen Q (2015) Graphene nanosheets@ZnO nanorods as three-dimensional high efficient counter electrodes for dye sensitized solar cells. Electrochim Acta 151:459–466
Wang H, Sun K, Tao F, Stacchiola DJ, Hu YH (2013) 3D honeycomb-like structured graphene and its high efficiency as a counter-electrode catalyst for dye-sensitized solar cells. Angew Chemie - Int Ed 52:9210–9214
Wang H, Wei W, Hu YH (2014) NiO as an efficient counter electrode catalyst for dye-sensitized solar cells. Top Catal 57:607–611
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghasemi, S., Hosseini, S.R. & Kazemi, Z. Electrophoretic preparation of graphene-iron oxide nanocomposite as an efficient Pt-free counter electrode for dye-sensitized solar cell. J Solid State Electrochem 22, 245–253 (2018). https://doi.org/10.1007/s10008-017-3741-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-017-3741-x