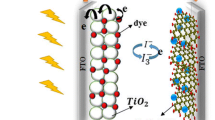
Abstract
This work is focused on fabricating a counter electrode (CE) for application in a dye-sensitized solar cell (DSSC). Cobalt manganese oxide (CoMn2O4) nanoparticles (NPs) and its nanocomposite with graphene were prepared by a hydrothermal method. The nanocomposites of cobalt manganese oxide with graphene as CoMn2O4/(graphene)x (where x = 0.2, 0.4, and 0.6) were synthesized as an electrode material, and a cell was fabricated for the x = 0.6 nanocomposite to investigate the activity for use in DSSCs. These nanocomposites were characterized by x-ray diffraction (XRD), scanning electron microscopy (SEM), energy-dispersive x-ray spectroscopy (EDX), and Fourier transform infrared (FTIR) spectroscopy. SEM analysis revealed the nature of the particles, and the average grain size was in good agreement with XRD results. EDX showed the ratio of the respective samples. The XRD pattern showed the hexagonal structure of CoMn2O4 NPs with an average size of 39.45 nm. The FTIR spectrum indicated O–H stretching bonds and the vibrational bending of MnO at the interstitial sites. For electrochemical analysis, cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were performed. Through CV analysis, an anodic peak current (Ipa) was observed at 41.42 µA, while the cathodic peak current (Ipc) was observed at −37.13 µA, and peak-to-peak separation (ΔEpp) = 0.31 V for CoMn2O4 NPs. For graphene, Ipa = 180.45 µA, Ipc = −230 µA, and ΔEpp = 0.25 V, while Ipa = 100.23 µA, Ipc = 80.65 µA, and ΔEpp = 0.16 V for the CoMn2O4/graphene (x = 0.6) nanocomposite, which also showed excellent electrocatalytic properties. The charge transfer mechanism on the surface of the electrode was found to have rapid oxidation–reduction behavior and can be used as an alternative to platinum as the CE in the DSSC. The CV analysis of the CoMn2O4/graphene (x = 0.6) nanocomposite-based DSSC showed that the Ipa current was observed at 26.85 mA, while the Ipc current was observed at −27.25 mA in the two-electrode system. The Rs values for CoMn2O4/graphene with x = 0.2, 0.4, and 0.6 were 142.80 Ω, 141.33 Ω, 135.18 Ω, and 131.18 Ω, respectively, as an electrode material. The exchange current density J = 2.13 × 10−3 A/cm2 was found using the charge transfer resistance value (Rct = 6.03 Ω cm2) and was verified by the Tafel curve.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Universal energy demands are growing with the passage of time. Large energy resources such as coal, gas, and oil are being rapidly depleted. In addition, their long-term effect on the atmosphere necessitates the development of sources of renewable energy.1 The huge amount of carbon dioxide (CO2) in our atmosphere cannot be absorbed by plants due to the burning of gas, oil, coal, and other fossil fuels. The earth's temperature is rising due to the significant increase of CO2 in the environment.2 In a recent study, it was observed that human energy consumption in 1 year is 1.1 × 1011 kWh. The solar energy supply in 1 h is 1.78 × 1014 kWh, and by optimizing the efficiency of solar cells, we can simply handle the energy crisis. Si-based solar cells cover the majority of the market in photovoltaics. However, the inflexibility, high-energy processing, and loss of efficiency at higher temperatures are leading to shortfalls in Si solar cells. Therefore, dye-sensitized solar cells (DSSCs) have emerged from the idea of “counterfeit photosynthesis," mimicking the activity of plants, transforming sunlight into helpful energy. DSSC technology is based on photosynthesis, just like flowers and plants can transform sunlight into energy and can work even in a cloudy environment or diffuse sunlight. For DSSC fabrication, Michael Gratzel sought to replace chlorophyll with a light-absorbing dye where the electrons in molecules are excited to a higher energy state through incident sunlight. The energy produced is captured by an electrolyte and then a catalyst like a nearby area of the leaf during photosynthesis.3 DSSCs are used for converting solar energy,4 and their working principle is based on an electrochemical junction. DSSCs work on the principle of four basic steps,5 i.e., light absorption, charge separation, charge collection, and dye regeneration. Their invention was a great milestone because it enabled high efficiency of up to 14.3% to be achieved.6 In early times, flat electrodes received great attention in photo-electrochemical studies, but those systems had an intrinsic issue.7 In DSSC photoanodes, the effective electron injection is influenced by the absorbed dye's first monolayer. But the amount of light harvested from just a single monolayer is very low. When nonporous TiO2 is used, the effectiveness of the surface area can be enhanced 1000-fold. An interesting characteristic of nanocrystalline TiO2 films is that the transport of charges by photo-generated electrons that pass through the particles and grain boundaries is comparatively efficient.8 The Rct value of thermally exfoliated graphene (TEG) films is 11.7 Ω cm2, which is close to that of platinum electrodes at 6.5 Ω cm2.9 Another important part of a DSSC is the electrolyte,10 which catalyzes the process of regeneration of tri-iodide to iodide ions. Major requirements for making DSSCs with high efficiencies include high electrical conductivity, good catalytic activity, and cost-effectiveness. Low efficiency results from only a few dyes absorbing light in a wide spectral range. These cells are stable for a very short period of time upon fabrication. This issue can be resolved by using a solid electrolyte or a polymer gel electrolyte.11 On the other hand, platinum-sputtered film on fluorine-doped tin oxide (FTO) can function as a conventional counter electrode (CE), but its high cost limits the commercialization of DSSCs.12,13 It became a third-generation technology using the nanoscale properties of the device.14
For cost-effective and efficient CEs, scientists have been working to develop alternative electrode materials based on nanocomposites. The materials for the CE must exhibit several synergistic effects, such as nanoparticles (NPs) having a high surface area and high electronic conduction. The problem with this architecture is the high cost of platinum being used as a CE. To solve this problem, scientists have made several efforts to develop a cost-effective and comparatively efficient CE as an alternative to platinum.15 To date now, polymers,16 hybrid materials, and alloys have been successfully used as CEs. Nanocomposite based on graphene is considered to be an effective electrocatalyst for energy conversion devices. Graphene is an integral constituent of graphite; it is a two-dimensional aromatic monolayer made of carbon atoms.17 It possesses high electric conductivity, a large surface area, structure flexibility, chemical tolerance, and reassembly properties.18 These properties show that it can be an effective matrix for metals and metal oxides for enhancing their electrochemical performances.11
Our research investigated the potential of CoMn2O4/graphene nanocomposite as a CE material for DSSCs. This work aimed to address the limitations of conventional platinum CEs, which are expensive and can be scarce. The characterization of our CoMn2O4/graphene nanocomposite revealed a significant increase in electron mobility compared to traditional materials. This improvement can be attributed to the synergistic effects between CoMn2O4 and graphene. CoMn2O4 provides the necessary electrocatalytic properties for the counter electrode reaction in DSSCs, while graphene's excellent conductivity facilitates efficient electron transport within the electrode. The observed increase in electron mobility suggests a promising avenue for enhancing the overall efficiency of DSSCs. By enabling faster regeneration of the redox shuttle at the counter electrode, this CoMn2O4/graphene nanocomposite has the potential to outperform traditional platinum CEs while offering a more cost-effective solution. Future research could explore further optimization of the CoMn2O4/graphene ratio and morphology to achieve even greater efficiency gains. Additionally, investigating the applicability of this nanocomposite in other types of electrochemical devices beyond DSSCs could be a valuable area for further exploration. The characterization has shown increasing trends in electron mobility, which will be used to advance the efficiency of solar cells in place of platinum as a CE. Also, this work addresses the low-cost CoMn2O4/graphene-based nanocomposite as an electrode.
Experimental Procedures for Synthesis of CoMn2O4 NPs and CoMn2O4/Graphene Nanocomposites
Materials Used
Cobalt acetate [Co(CH3COOH), 98%], manganese acetate [Mn(CH3COOH), 89%], lithium perchlorate (LiClO4), lithium iodide (LiI), iodine (I2), sodium hydroxide (NaOH), fluorine-doped tin oxide (FTO) glass, and hydrogen peroxide (H2O2, 3 wt.%) were obtained from Sigma-Aldrich. Graphite powder was used as a precursor material.
Synthesis Procedure
Synthesis of Cobalt Manganese Oxide Nanoparticles
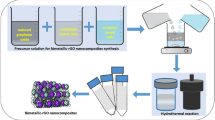
CoMn2O4 NPs were synthesized by the hydrothermal method, as shown in Fig. 1.19 First, 40 ml of an aqueous solution containing 0.3 M each of the cobalt acetate and manganese acetate solutions was prepared in a beaker.20 Next, 20 ml of 2 M NaOH solution was added dropwise to the solution, and the pH value was adjusted to 10. Then, 0.5 ml of H2O2 was added, and the solution was stirred for 30 min. During constant stirring, the color of the solution changed from greenish blue to brownish black. The reaction mixture was transferred to a stainless-steel autoclave, and the autoclave was kept in an oven for 24 h at 170°C. The mixture was then washed with distilled water several times, and the product was dried at 70°C for 6 h.21
Synthesis of Cobalt Manganese Oxide/Graphene Nanocomposite
The synthesis of the CoMn2O4/graphene nanocomposite was executed by a hydrothermal process. CoMn2O4 NPs and graphene were taken with ratios of x = 0.2 wt.%, 0.4 wt.%, and 0.6 wt.%. Next, 40 ml each of manganese acetate and cobalt acetate solutions with molarity 0.3 M were taken, and graphene was added with the above-given wt.% and sonicated for 2 h in cold water to form a uniform dispersion. The dispersed solution was then transferred to a stainless-steel autoclave and kept in an oven for 24 h at 170°C. The resulting product was washed several times with distilled water, then dried at 70°C for 7 h.22 The above procedure was followed for bare CoMn2O4 NPs.23
Electrolyte Preparation for Electrochemical Measurement
The electrochemical analysis was performed using an electrolyte of 0.1 M LiClO4, 1 mM LiI, and 1 mM I2 in deionized (DI) water. The configuration consisted of a three-electrode system with active material as a working electrode, Ag/AgCl as a reference electrode, and platinum as a CE for electrochemical measurement over a voltage window in the range of 0 V to 0.7 V at a scan rate of 30 mV s−1.24,25
Extraction of Natural Dye and Fabrication of the DSSC
Preparation of Natural Dye
Fresh black currant and cherry fruits were used as a precursor for dye. Equal numbers of cherries and black currants were washed with distilled water to remove surface impurities and dried at room temperature for 2 h. Then after grinding them in a mortar and pestle, the extracted juice was mixed with 30 ml of ethanol and then boiled for 30 min at room temperature in the dark. The prepared solution was then filtered, resulting in a liquid dye. Figure 2 shows the systematic representation of this procedure.
Preparation of the DSSC and Injecting Electrolyte
FTO glass was cut into pieces 1 × 1 inches and used as substrates for both side plates the of DSSC. Both FTO glasses were sonicated in ethanol for 2 h for impurity removal and activation of the glass. The CoMn2O4/graphene nanocomposite was first dissolved in ethanol and sonicated for 1 h to form a uniform dispersion. Then this slurry was coated on the conductive side of the FTO glass using the doctor blade method.26 The same conventional method was adopted for the photoanode of TiO2. The active area was approximately 1 cm2. After preparing the electrodes, slurries were placed in open air to dry. The electrode was then placed in the oven at 50ºC for 10 min to dry the solvent.27 After this, the photoanode was placed on a hot plate and heated at 100ºC for 15 min for solvent evaporation. For dye sensitization, the substrate was dipped in black currant juice extracted earlier. This layer is responsible for the oxidation–reduction process. Liquid electrolyte was prepared by dissolving 0.4 M lithium iodide and 0.04 M iodine in 0.3 M acetonitrile with a volume ratio of 1:1. The two electrodes were attached to each other with binder clips. The electrolyte was injected dropwise into the cell. The step-by-step fabrication process is shown in Fig. 3.
Structural Characterization
X-ray diffraction (XRD) was conducted using a PANalytical diffractometer with a CuKα source. Scanning electron microscopy (SEM) was carried out using a TESCAN MAIA3 ultra-high-resolution scanning electron microscope. Fourier transform infrared (FTIR) spectroscopy was conducted using the IRTracer-100 FTIR spectrometer (Shimadzu) within the range of 4000–500 cm−1. Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were performed using a Gamry potentiostat/galvanostat with a three-electrode configuration.
Results and Discussion
Morphological Analysis of Cobalt Manganese Oxide/Graphene Nanocomposite
SEM was used to study the surface morphology of CoMn2O4 NPs and the composite (x = 0.6) as shown in Fig. 4a and b, respectively. SEM confirmed the shape of CoMn2O4 NPs as spherical. NP images were taken with 500-nm resolution, from which nearly 40-nm-diameter particles were found, as shown in Fig. 4a. Figure 4b reveals the formation of smooth graphene flakes and the fractured edges of the graphene layer structure over the entire cross section. It can be clearly observed that the prepared CoMn2O4/graphene nanocomposite shows a shiny layer, and CoMn2O4 NPs are lying on the graphene sheets, which shows that the composite has been successfully synthesized. CoMn2O4/graphene combinations frequently exhibit synergistic effects. Nanoparticles may enhance catalytic or charge storage capabilities, and graphene enhances mechanical strength and electrical conductivity.
Elemental Composition Analysis
The energy-dispersive x-ray (EDX) spectrum shows the elemental composition of the prepared CoMn2O4 NPs and its composite. Figure 5 presents the EDX spectra, which provide information about Co and Mn along with the characteristic x-ray peaks. It also shows the atomic percentages (wt.%) of Co, Mn, and graphene over (Co + Mn) and the composition ratio of Co/Mn/G. The analysis indicates that the average Mn wt.% and Co wt.% continuously decreased from 24.25% to 6.64% and from 39.73% to 8.43%, respectively, during composite formation. Usually at.% and wt.% for particular materials are different in calculations. However, the change in EDX spectra occurs due to the embedded layers of reduced graphene oxide (rGO) affecting grain size and impurities, which might bring a change in the Co and Mn atomic ratios.
X-ray Diffraction
Figure 6 shows the XRD patterns of CoMn2O4 NPs and CoMn2O4/graphene nanocomposite prepared by hydrothermal method. JCPDS card no. (00-018-0410) matched with CoMn2O4 NPs. In Fig. 6a, the diffraction peaks for CoMn2O4 NPs on 2θ are at 33.26°, 36.4°, and 44°, corresponding to the (103), (211), and (220) crystal planes, respectively. The peak at 36.4° with Miller indices (211) showed the hexagonal phase of CoMn2O4 with a grain size of 39.45 nm. In Fig. 6b, the (002) plane at 2θ value of 26° represents graphene of CoMn2O4/graphene nanocomposite. The average crystallite size for the NPs was calculated using the Scherer formula. Highly crystalline CoMn2O4 NPs might exhibit improved charge transfer properties. Changes in the lattice structure at the interface between CoMn2O4/graphene nanocomposite understanding and these interactions are crucial for optimizing charge transfer pathways.
Fourier Transformation Infrared Spectroscopy
Figure 7 presents different vibrational modes for bare CoMn2O4 and its composite with x = 0.6 from the FTIR spectrum. Pure CoMn2O4 usually has unique absorption bands that are linked to its metal–oxygen bonds. These bands correspond to the stretching vibrations of the Co–O bonds and the Mn–O bonds. These peaks indicate the spinel structure of CoMn2O4, which is used to confirm the presence of cobalt and manganese oxides. Two major absorption bands were noted below 1000 cm−1, and these bands are the same for all manganese-based metal oxides. Other small peaks were detected around 700 cm−1 and 500 cm−1 that may belong to the bending vibration of manganese oxide at octahedral sites28 and cobalt oxide at tetrahedral sites, respectively. Small and broad peaks observed around 3100 cm−1, 2922 cm−1, and 2950 cm−1 correspond to the stretching vibration of O–H, which may be present due to functional groups on graphene oxide. The pronounced peak corresponds to the C=C stretching vibrations of graphene's aromatic rings, indicating the presence of sp2 hybridized carbons. Secondly, a sharp downward peak in the CoMn2O4/graphene-based nanocomposite at 2360 cm−1 shows the presence of carbon in the composites in the case of bare CoMn2O4, this downward peak is smaller. This peak origination indicates the incorporation of graphene in the composite since a minute change occurs, but the graphene presence shows a signature which has been detected in FTIR spectra. A small peak at 1780 cm−1 indicates a C=O bond in the nanocomposite, and the peak at 1580 cm−1 belongs to the bending vibration of C=C.
Cyclic Voltammetry and Electrochemical Impedance Spectroscopy for an Electrode Material
CV characterization is an extensive technique used to study the electrocatalytic properties of the synthesized electrode material. Figure 8a presents the CV curve of bare and nanocomposite ratios x = 0.2, 0.4, and 0.6 at a scan rate of 30 mV/s with a potential window ranging from −0.8 V to 1 V. CoMn2O4 NPs show an oxidation peak at 0.5 V and a reduction peak at 0.1 V. Graphene shows an oxidation peak at 0.7 V and a reduction peak at 0.4 V. For x = 0.2, the oxidation peak appeared at 0.7 V, and the reduction peak at 0.42 V. For the ratio x = 0.4, the oxidation peak appeared at 0.7 V, and the reduction peak at 0.5 V. For x = 0.6, the oxidation peak appeared at 0.53 V, and a reduction peak appeared at 0.5 V. Two essential parameters for evaluating the electrocatalytic activity of electrode materials are peak-to-peak separation ΔEpp and the absolute value of oxidation and reduction currents29 shown in Eqs. 1 and 2.30,31 The trend of oxidation and reduction values indicate an enhancement of electrochemical performance when graphene is added to the pure CoMn2O4 NPs. More active sites for electrochemical reactions are provided by the nanoparticles' greatly enhanced surface area. Cobalt manganese oxide and graphene nanocomposite can maintain good electrical contact and conductivity and offer more charge transfer in a more effective way.
The electrocatalytic properties of electrode material are shown in Table I.
The standard electrochemical rate constant of a redox reaction is negative, which correlates with the peak-to-peak separation of ΔEpp.32 Therefore, the small ΔEpp and the large oxidation and reduction current values are like high electrolytic activity. The value of ΔEpp of CoMn2O4/graphene nanocomposite with x = 0.6 was lower than that of platinum, the bare material, graphene, and their nanocomposites with x = 0.2, and 0.4. The redox reaction rate of I3−/I− is inversely proportional to ΔEpp, and a decrease in ΔEpp means an increase in the rate of the redox reaction. These results show that the rate of regeneration of iodide from triiodide increased with CEs.33
The EIS measurement was used to determine the charge transfer mechanism response and the impedance relative to frequency. Figure 8b shows the Nyquist plots of CoMn2O4 and CoMn2O4/graphene nanocomposite (x = 0.2, 0.4, and 0.6), which describe the real and imaginary impedances of the material. The material resistance relates to the real impedance. Graphene has a relatively high conductivity as compared to CoMn2O4 NPs alone, which states that it has the property of better conduction and a low impedance value. With an increase in the concentration of graphene in CoMn2O4 NPs, the impedance values decreased due to the conducting nature of graphene. High impedance values lead to high charge transfer resistance and low charge mobility in the electrolyte solution due to the desired material on the working electrode. The solution resistance Rs has the combined resistance of electrolyte and internal electrode, which is determined through the intercept on the real impedance axis. The Rs values for CoMn2O4, x = 0.2, 0.4, and 0.6 are 142.80 Ω, 141.33 Ω, 135.18 Ω, and 131.18 Ω, respectively.
J–V Characteristics of CoMn2O4 /Graphene as Electrode Material
Figure 9 shows the J–V response of material as an electrode. This technique is utilized by applying biased voltage using a two-probe system with a light source and checking the current against that bias. The black line shows the behavior of the bare material in the dark (without light), i.e., approximately 10−8 µA/cm−2. All other lines represent the bare material and nanocomposite with respective ratios of responses in the light region as an electrode material. The bare material and nanocomposite ratios x = 0.2 and 0.4 have shown ohmic (linear) behavior with current density values of 16 µA/cm−2, 18 µA/cm−2, and 22 µA/cm−2, respectively. The lower concentrations have shown a low magnitude in current density because of the low concentration of graphene. The higher current density was attributed to higher electron mobility. The behavior for the ratio of x = 0.6 after 3 V has slight bending, which might be due to the minority carriers. Overall, the linearity observed in the highest ratio, which indicates that the material is an efficient material for DSSC, has slight bending, which might be due to the minority carriers.
CV and EIS of CoMn2O4/Graphene (x = 0.6) Nanocomposite-Based DSSC
Figure 10a shows the CV curve of the CoMn2O4/graphene nanocomposite (x = 0.6)-based DSSC in the potential window from −3.5 to 3.5 at a scan rate of 30 mV/s. The anodic peak current was observed at Ipa = 26.85 mA, while the cathodic peak current was observed at Ipc = 27.26 mA. A smaller ∆Epp indicates better electrocatalytic activity, as it implies faster electron transfer kinetics at the FTO substrate surface. The CoMn2O4/graphene nanocomposite (x = 0.6)-based DSSC shows ΔEpp = 0.21 V, i.e., very small (Table II), which reveals the faster electron transfer kinetics of the DSSC. The reason behind the chosen ratio of x = 0.6 is that the charge transfer mechanism is enhanced, as determined from the above material characterizations. It also resulted in improved catalytic activity, low charge transfer resistance, and an increase in overall efficiency of DSSCs. A number of segments were performed, and the overlapping of oxidation and reduction peaks confirmed the stability of the DSSC.
Figure 10b represents the Nyquist plot of the DSSC. The impedance data was recorded in the frequency domain of 0 kHz to 10 kHz. The point intersecting the real axis (x-axis) is 8 Ω. The given plot has two parts: a semicircle region and a small straight line. The semicircle portion diameter is due to the charge transfer resistance, while in the low-frequency region, the small linear part signifies the Warburg impedance because of diffusion processes. The inset of Fig. 10b illustrates an equivalent circuit fitted for the DSSC by a spectrum analyzer. The large frequency semicircle is attributed to the charge transfer (recombination) resistance in parallel with the total capacitance. Charge transfer resistance Rct = 6.03 Ω was measured from the EIS as shown in Table III. The semicircle arc represents the characteristics of the diffusion-limited electron transfer process on the photoanode and counter electrode surfaces. Higher chemical capacitance is likely to increase the electron strength in the conduction band of the CoMn2O4/graphene counter electrode, and this higher value would possibly decrease charge recombination resistance, ultimately enhancing the charge transfer ability. Figure 10c represents Bode plots. The graph between impedance and frequency has a slope and two steps. The Bode plotting scheme is for electron lifetime and the phase differences at different frequencies. At low frequencies, the value of impedance is high because all types of polarization are active, and more time is required for polarization as compared to an applied alternating-current signal.
Tafel Polarization Curve
Figure 11 presents a Tafel plot of CoMn2O4/graphene nanocomposite-based DSSC. The Tafel polarization curve is a plot between the logarithm of current density and the electrode’s potential. It usually measures the kinetics of electrochemical reactions, which helps us check the reaction mechanism. The Tafel curve for dye-sensitized solar cells gives us two points: an anodic peak (on the right side) and a cathodic peak (on the left side of graph). In Fig. 11, the anodic current density gives us a maximum value at positive potential, while the cathodic peak gives a maximum value at negative potential. Anodic and cathodic peaks are due to the oxidation and reduction of the electrolyte, respectively. The exchange current density was measured using the formula
where J is the current density, R is a gas constant equal to 8.314 J/mol K, T represents room temperature (25°C), F is the Faraday constant equal to 96,485 C/mol, and n is the number of electrons involved in the redox reaction (n = 2). The obtained exchange current density of our cell was 2.13103A/cm2.
The data extracted from the cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), and Tafel plot offer valuable insights into the electrochemical behavior of the CoMn2O4/graphene nanocomposite DSSC. The CV plot in Fig. 10a shows well-defined oxidation and reduction peaks, indicating good reversibility of the redox processes at the electrode surface. The small peak-to-peak separation (ΔEpp) of 0.21 V suggests fast electron transfer kinetics, further supported by the high values of anodic and cathodic peak currents (Ipa and Ipc). This observation aligns with the desired characteristic of efficient dye regeneration in DSSCs. The Nyquist plot of the DSSC in Fig. 10b reveals two key features. One is a semicircle at high frequencies, attributed to the charge transfer resistance (Rct) at the electrode–electrolyte interface. The low measured value of Rct (6.03 Ω) signifies efficient electron transfer between the electrolyte and the electrode. The second is a linear portion at lower frequencies indicative of Warburg impedance, which reflects the diffusion of ions within the electrolyte. The inset of Fig. 10b depicts the equivalent circuit model used to fit the EIS data. The presence of a large capacitance in parallel with Rct suggests a high charge storage capacity at the electrode surface. This can potentially suppress charge recombination and enhance overall device performance. The Tafel plot in Fig. 11 provides information about the reaction kinetics at the electrode. The presence of well-defined peaks at positive and negative potentials confirms the oxidation and reduction processes occurring within the DSSC. By combining the information from all three techniques, we can conclude that the CoMn2O4/graphene nanocomposite exhibits promising properties for a counter electrode in DSSCs. The CV data suggests fast electron transfer kinetics and good reversibility of the redox processes. The EIS results confirm low charge transfer resistance and high capacitance, indicating efficient charge transfer and potentially reduced recombination. While the specific Tafel slopes are not provided, the plot confirms the occurrence of oxidation and reduction reactions.
Conclusion
The CoMn2O4 NPs and CoMn2O4/graphene nanocomposite were successfully prepared by the hydrothermal method. SEM and EDX revealed the particle nature and presence of all elemental compositions in their respective ratios. FTIR spectra gave information about the vibrational bending of bonds present in the bare CoMn2O4 and nanocomposite materials. The XRD pattern showed the hexagonal phase of CoMn2O4/rGO with a grain size of 39.45 nm. The CV curve of CoMn2O4 NPs shows two peaks for both oxidation and reduction. For CoMn2O4/graphene nanocomposite x = 0.6, the anodic peak was observed at Ipa = 100.23 µA, and the cathodic peak was observed at Ipc = 80.65 µA; peak-to-peak separation was ΔEpp = 0.03 V, which is relatively less than that of platinum. Among NPs and all nanocomposites, CoMn2O4/graphene nanocomposite x = 0.6 shows better electrocatalytic properties and attained high anodic and cathodic peaks, a relatively greater current than the platinum electrode, and less peak-to-peak separation than platinum. Unique characteristics of graphene and crystalline nanoparticles emphasize their significance in DSSC enhancement. The DSSC has shown a charge transfer resistance of 6.03 Ω from the EIS plot. The CV curve for the DSSC has shown oxidation and reduction peaks, which supports the good stability of the cell. Further, the exchange current density J = 2.13 × 10-3 A/cm2 was calculated by the Tafel curve. All these points indicate that the composite material can be used as an alternative to platinum as a CE in DSSCs.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
M.S. Khan, I. Murtaza, A. Shuja, M.A. Asghar, C.G. Nuñez, R. Abid, A. Haider, and M. Faraz, Unveiling the electrochemical advantages of a scalable and novel aniline-derived polybenzoxazole-reduced graphene oxide composite decorated with manganese oxide nanoparticles for supercapacitor applications. J. Energy Storage 73, 109109 (2023).
M.A. Khan, N. Ahmed, M.S. Khan, H. Shahid, I. Murtaza, N. Abbas, K. Javed, A. Gulab, and S. Khan, Silver nanowires/polypyrrole nanostructured composite as an alternative electrocatalytic material for dye-sensitized solar cells. Optik 268, 169734 (2022).
A. Agrawal, S.A. Siddiqui, A. Soni, and G.D. Sharma, Advancements, frontiers and analysis of metal oxide semiconductor, dye, electrolyte and counter electrode of dye sensitized solar cell. Sol. Energy 233, 378 (2022).
A.A. Kebede, T. Kalogiannis, J. Van Mierlo, and M. Berecibar, A comprehensive review of stationary energy storage devices for large scale renewable energy sources grid integration. Renew. Sustain. Energy Rev. 159, 112213 (2022).
A.S. Rasal, M.-H. Wu, A.V. Ghule, G. Gatechew, A.A. Kashale, I.-W.P. Chen, and J.-Y. Chang, Advancing the stability and efficiency of quantum dot-sensitized solar cells through a novel, green, and water-based thixotropic biopolymer/ordered nanopores silica designed quasi-solid-state gel electrolytes. Chem. Eng. J. 446, 137293 (2022).
K. Paramasivaganesh, D. Sakthilatha, A. Sankar, P.S. Karthik, M. Shkir, F. Maiz, W.K. Kim, and S. Gedi, Hydrothermal production of low-cost CeNi2S4-reduced graphene oxide composites as an efficient counter electrode for high performance dye-sensitized solar cells. Inorg. Chem. Commun. 142, 109701 (2022).
M.S. Khan, I. Murtaza, A. Shuja, S. Fahad, M.W. Khan, J. Ahmmad, S.M. Wabaidur, and M.Z. Ansari, Energy on-the-go: V2O5-pBOA-Graphene nanocomposite for wearable supercapacitor applications. Electrochim. Acta 486, 144119 (2024).
M. Baruah, S.L. Ezung, S. Sharma, U.B. Sinha, and D. Sinha, Synthesis and characterization of Ni-doped TiO2 activated carbon nanocomposite for the photocatalytic degradation of anthracene. Inorg. Chem. Commun. 144, 109905 (2022).
S.S. Nemala, P. Kartikay, K.S. Aneja, P. Bhargava, H.M. Bohm, S. Bohm, and S. Mallick, Novel high pressure exfoliated graphene-based semitransparent stable DSSCs for building integrated photovoltaics. ACS Appl. Energy Mater. 1(6), 2512 (2018).
K. Kakiage, Y. Aoyama, T. Yano, K. Oya, J.-I. Fujisawa, and M. Hanaya, Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 51(88), 15894 (2015).
F. Zhang, K. Yang, G. Liu, Y. Chen, M. Wang, S. Li, and R. Li, Recent advances on graphene: synthesis, properties and applications. Compos. A Appl. Sci. Manuf. 160, 107051 (2022).
B. O’regan and M. Grätzel, A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353(6346), 737 (1991).
Y.-J. Huang, P.K. Sahoo, D.-S. Tsai, and C.-P. Lee, Recent advances on Pt-free electro-catalysts for dye-sensitized solar cells. Molecules 26(17), 5186 (2021).
P. Joshi, Y. Xie, M. Ropp, D. Galipeau, S. Bailey, and Q. Qiao, Dye-sensitized solar cells based on low cost nanoscale carbon/TiO2 composite counter electrode. Energy Environ. Sci. 2(4), 426 (2009).
Z. Yang, M. Liu, C. Zhang, W.W. Tjiu, T. Liu, and H. Peng, Carbon nanotubes bridged with graphene nanoribbons and their use in high-efficiency dye-sensitized solar cells. Angew. Chem. Int. Ed. 52(14), 3996 (2013).
T. Xu, D. Kong, H. Tang, X. Qin, X. Li, A. Gurung, K. Kou, L. Chen, Q. Qiao, and W. Huang, Transparent MoS2/PEDOT composite counter electrodes for bifacial dye-sensitized solar cells. ACS Omega 5(15), 8687 (2020).
Y. Gu, R. Ping, F. Liu, G. Zhang, M. Liu, and J. Sun, Novel carbon nitride/metal oxide nanocomposites as efficient and robust catalysts for coupling of CO2 and epoxides. Ind. Eng. Chem. Res. 60(16), 5723 (2021).
M.S. Khan, I. Murtaza, A. Shuja, H.R. Khan, R. Abid, C.G. Nuñez, S. Fahad, H. Tariq, and A. Naveed, Tailored NiO-pBOA-GNP ternary nanocomposite: Advances in flexible supercapacitors and practical applications for wearable technology and environmental monitoring. J. Energy Storage 86, 111128 (2024).
X. Yue, G. Wang, J. Wang, L. Fan, J. Hao, S. Wang, M. Yang, and Y. Liu, Electrochromic and electrochemical properties of Co3O4 nanosheets prepared by hydrothermal method. Coatings 12(11), 1682 (2022).
P. Wei, M. Abid, H. Adun, D. Kemena Awoh, D. Cai, J.H. Zaini, and O. Bamisile, Progress in energy storage technologies and methods for renewable energy systems application. Appl. Sci. 13(9), 5626 (2023).
P. Kumar, J. Kaur, and A.K. Tiwari, Synthesis and characterization of manganese dioxide agglomerated nanoparticles for supercapacitor application. IOP Conf. Ser. Mater. Sci. Eng. 1248(1), 012052 (2022).
Rusi and S. Majid, Green synthesis of in situ electrodeposited rGO/MnO2 nanocomposite for high energy density supercapacitors. Sci. Rep. 5(1), 16195 (2015).
K. Narasimharao, G. Venkata Ramana, D. Sreedhar, and V. Vasudevarao, Synthesis of graphene oxide by modified hummers method and hydrothermal synthesis of graphene-NiO nano composite for supercapacitor application. J. Mater. Sci. Eng. 5(284), 2169 (2016).
S. Kukunuri, S. Karthick, and S. Sampath, Robust, metallic Pd 17 Se 15 and Pd 7 Se 4 phases from a single source precursor and their use as counter electrodes in dye sensitized solar cells. J. Mater. Chem. A 3(33), 17144 (2015).
Z. Wan, C. Jia, and Y. Wang, In situ growth of hierarchical NiS2 hollow microspheres as efficient counter electrode for dye-sensitized solar cell. Nanoscale 7(29), 12737 (2015).
A.U. Agobi, A.J. Ekpunobi, A.I. Ikeuba, and H. Louis, the effects of graphene oxide load on the optical, structural and electrical properties of ternary nanocomposites (Polyvinyl alcohol/copper/graphene oxide) for electronic and photovoltaic application. Results Opt. 8, 100261 (2022).
C.-H. Tsai, C.-J. Shih, W.-S. Wang, W.-F. Chi, W.-C. Huang, Y.-C. Hu, and Y.-H. Yu, Fabrication of reduced graphene oxide/macrocyclic cobalt complex nanocomposites as counter electrodes for Pt-free dye-sensitized solar cells. Appl. Surf. Sci. 434, 412 (2018).
S.A. Hosseini, D. Salari, A. Niaei, F. Deganello, G. Pantaleo, and P. Hojati, Chemical-physical properties of spinel CoMn2O4 nano-powders and catalytic activity in the 2-propanol and toluene combustion: effect of the preparation method. J. Environ. Sci. Health Part A 46(3), 291–297 (2011).
F. Gong, H. Wang, X. Xu, G. Zhou, and Z.-S. Wang, In situ growth of Co0.85Se and Ni0.85Se on conductive substrates as high-performance counter electrodes for dye-sensitized solar cells. J. Am. Chem. Soc. 134(26), 10953 (2012).
L.H. Kharboot, N.A. Fadil, T.A.A. Bakar, A.S.M. Najib, N.H. Nordin, and H. Ghazali, A review of transition metal sulfides as counter electrodes for dye-sensitized and quantum dot-sensitized solar cells. Materials 16(7), 2881 (2023).
Z. Ku, X. Li, G. Liu, H. Wang, Y. Rong, M. Xu, L. Liu, M. Hu, Y. Yang, and H. Han, Transparent NiS counter electrodes for thiolate/disulfide mediated dye-sensitized solar cells. J. Mater. Chem. A 1(2), 237 (2013).
R.G. Compton and C.E. Banks, Understanding voltammetry (Singapore: World Scientific, 2018).
S.-Y. Jang, Y.-G. Kim, D.Y. Kim, H.-G. Kim, and S.M. Jo, Electrodynamically sprayed thin films of aqueous dispersible graphene nanosheets: highly efficient cathodes for dye-sensitized solar cells. ACS Appl. Mater. Interfaces 4(7), 3500 (2012).
Acknowledgments
We are thankful to Professor Ahmed Shuja Syed and Shah Fahad, Centre for Advanced Electronics & Photovoltaic Engineering (CAEPE), International Islamic University Islamabad, for SEM and J–V analysis of the CoMn2O4/rGO electrode material of our samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khan, M.S., Haider, F., Majeed, A. et al. Synergistic Effects and Characterization of CoMn2O4/Graphene in DSSC Counter Electrodes. J. Electron. Mater. (2024). https://doi.org/10.1007/s11664-024-11388-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11664-024-11388-z