Abstract
Immunotherapies that target programmed cell death protein 1 (PD-1) signals are standard therapies for advanced-stage lung cancer, and the expression of programmed death-ligand 1 (PD-L1) in cancer tissue predicts immunotherapy efficacy. Although programmed death-ligand 2 (PD-L2) is expressed in cancer cells and macrophages, similar to PD-L1, its significance in lung cancer is unclear. Double immunohistochemistry analyses using anti-PD-L2 and anti-PU.1 antibodies were carried out on tissue array sections from 231 cases of lung adenocarcinoma, and PD-L2 expression in macrophages was evaluated. High PD-L2 expression in macrophages was associated with longer progression-free survival (PFS) and cancer-specific survival (CSS) and observed more often in females, non-heavy smokers, and patients with epidermal growth factor receptor (EGFR) mutations and those at a lower disease stage. Significant correlations were found more frequently in patients with EGFR mutations. Cell culture studies revealed that cancer cell-derived soluble factors induced PD-L2 overexpression in macrophages, suggesting the involvement of the JAK-STAT signaling pathway. The present findings suggest that PD-L2 expression in macrophages predicts PFS and CSS in lung adenocarcinoma without immunotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is a leading cause of cancer-related deaths globally [1, 2], and despite the recent advances made in screening, diagnosis, and treatment approaches, many cases remain undiagnosed until the advanced stage. For many years, cytotoxic anticancer agents have played a critical role in therapies for patients with unresectable lung cancer [1, 2]. However, starting in the mid-2010s, immune checkpoint therapies targeting the programmed cell death protein 1 (PD-1) axis began to be used as a novel treatment strategy [3,4,5].
Interactions between PD-1 on T cells and its ligands, programmed death-ligand 1 (PD-L1) and programmed death-ligand 2 (PD-L2), in cancer and immune cells are known to regulate the anticancer immune response and play important roles in cancer cell escape from the immune system [6]. In recent years, immunotherapies consisting of monoclonal antibodies directed against PD-1 or PD-L1 that block the PD-1 axis have emerged as standard therapy for advanced lung cancer [3,4,5,6]. The use of immunohistochemistry (IHC) with anti-PD-L1 antibodies to monitor PD-L1 expression in cancer cells may provide a potential predictive biomarker regarding the efficacy of anti-PD-1/PD-L1 therapy for patients with non-small-cell lung cancer (NSCLC) [7, 8]. However, some PD-L1-positive patients have been found to respond poorly to immunotherapies, which suggests that interactions involving not only PD-1 and PD-L1, but also PD-L2, affect this response [9].
A number of retrospective studies have reported an association between the expression of PD-L1 in cancer cells and a poor clinical course for patients with lung adenocarcinoma [10, 11]. In addition, we previously reported an association between the expression of PD-L2 in cancer cells and longer progression-free survival (PFS) in such patients [10]. PD-L1 and PD-L2 are known to be expressed in not only cancer, but also stromal cells, including myeloid cells [12, 13]. The components of myeloid cells detected in the cancer stroma or microenvironment are referred to as tumor-associated macrophages (TAMs). TAMs are known to exert protumor activities associated with invasion, metastasis, neovascularization, and immunosuppression, and an association has been found between an elevated number of TAMs and a worse clinical prognosis in lung and other types of cancer [14].
Using double IHC, we previously performed a detailed analysis of the expression of PD-L1 in TAMs in patients with NSCLC [13]. Our findings revealed an association between PD-L1 overexpression in TAMs and worse cancer-specific survival (CSS). Furthermore, PD-L1 expression in macrophages was found to be dependent on activation of the Janus kinase/signal transducer and activator of transcription proteins (JAK-STAT) signaling pathway. However, the significance of PD-L2 expression in TAMs remains unclear.
Given this background, in the present study, we conducted a detailed investigation of PD-L2 expression in TAMs in patients with NSCLC using double IHC. Based on the results, we investigated the mechanisms underlying the regulation of PD-L2 expression in macrophages.
Materials and methods
Samples
Paraffin-embedded tissue samples were collected from patients who had been diagnosed with lung adenocarcinoma at Kumamoto University Hospital, Kumamoto, Japan, between 2010 and 2013. All specimens were reviewed by two experienced pathologists. Supplementary Table 1 shows the clinicopathological data for all 231 enrolled cases. A tissue microarray previously prepared by our group [10] was used for the purposes of the present study.
IHC analysis
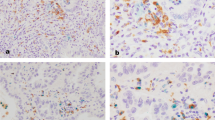
Paraffin-embedded sections were subjected to IHC using a routine protocol [10, 13, 15, 16]. Briefly, an anti-PD-L2 antibody (clone D7U8C; Cell Signaling Technology, Danvers, MA, USA) and anti-PU.1 antibody (clone EPR3158Y; Abcam, Cambridge, UK) were used as the primary antibodies, and horseradish peroxidase (HRP)-labeled anti-rabbit (for anti-PD-L2) antibody (Nichirei, Tokyo, Japan) as the secondary antibody. Positive signals were visualized by using 3,3′-diaminobenzidine. Next, for the double IHC, sections were heat-treated with ethylenediaminetetraacetic acid (1 mM, pH 8.0) buffer. After anti-PU.1 antibody was reacted, all sections were treated with HRP-labeled anti-rabbit antibody. Subsequently, HistoGreen substrate (#AYS-E109; Linaris, Dossenheim, Germany) was used to visualize positive signals. All immunostained sections were then evaluated by two experienced investigators (Y.S. and Y.K.) who had been blinded to the patients’ characteristics and outcomes. The PD-L2 expression level in macrophages was scored as low or high based on the proportion of positive cells (< 50% vs. ≥ 50% positive cells in PU.1-positive TAMs, respectively). The number of CD8-positive T cells in the specimens has been determined elsewhere [13].
Cell culture of human macrophages and lung cancer cell lines
The NCI-H358, H23, and H1975 cell lines were kindly gifted by Tomoya Yamaguchi (Kumamoto University, Kumamoto, Japan), and the PC-9 and A549 cell lines were obtained from the Japanese Collection of Research Bioresources Cell Bank (Osaka, Japan). Human monocyte-derived macrophages were prepared in accordance with protocols approved by the Kumamoto University Hospital Review Board (approval No. 1169), as previously described [10, 13, 15, 16]. The following inhibitors (each at a final concentration of 10 nM) were used: Stat1 (Fludarabine; Wako, Osaka, Japan), Stat3 (WP1066; Santa Cruz, Dallas, TX, USA), Stat5 (573108; Merck KGaA, Darmstadt, Germany), JNK (SP600125; Santa Cruz), ERK (FR180204; Santa Cruz), and JAK (Ruxolitinib; ChemScene LLC, Monmouth Junction, NJ, USA).
Cell enzyme-linked immunosorbent assay (ELISA)
The macrophages were cultured in a 96-well microplate and stimulated for 1 day using a conditioned medium (CM) of lung adenocarcinoma cells. After fixation with 1% paraformaldehyde, the cells were reacted with anti-PD-L2 antibody (clone 24F.10C12; BioLegend, San Diego, CA, USA). Tetramethylbenzidine developing solution (BioLegend) was added to visualize positive signals, as described elsewhere [10].
Statistical analyses
Prism (GraphPad Software, San Diego, CA, USA), JMP7 (SAS Institute, Chicago, IL, USA), and EZR [17] were used to perform the statistical analyses.
Results
Increased PD-L2 expression in TAMs was associated with a better prognosis in lung adenocarcinoma, especially among EGFR-mutation–positive patients
As we previously reported, similar to PD-L1, PD-L2 is expressed by not only tumor, but also immune cells, including macrophages [10]. However, it is difficult to distinguish PD-L2-positive macrophages from PD-L2-positive tumor cells using single IHC analysis because tumor cells and macrophages are sometimes strongly positive for PD-L2 expression. Therefore, we evaluated PD-L2 expression specifically in TAMs using double IHC with an anti-PU.1 antibody, macrophage-specific markers, and an anti-PD-L2 antibody (Fig. 1a). Anti-PU.1 antibody is capable of being used to label pan-macrophages [18]. In this study, the expression of PD-L2 in macrophages was scored as low or high based on the proportion of positive cells (< 50% vs. ≥ 50% positive cells, respectively). PD-L2 expression was low in 99 (42.9%) of the 231 patients examined, and high in 132 (57.1%). High PD-L2 expression levels were associated with female sex, Brinkman Index < 600, early pathological stage, and the presence of EGFR mutations, as shown in Supplementary Table 2. In addition, no significant association was found between PD-L2 expression in TAMs and the CD8-positive area in cancer tissues (Fig. 1b).
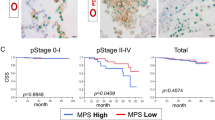
Next, as shown in Fig. 2, we investigated the effects of the TAM-PD-L2 interaction on the prognosis of patients with adenocarcinoma using Kaplan–Meier survival curves. The results revealed that PFS and CSS were significantly longer in the high than in the low TAM-PD-L2 group. No significant differences in PFS or CSS were found by subgroup analysis of the pathological stage. However, among the EGFR-mutation-positive cases, PFS and CSS were significantly longer in the high than in the low TAM-PD-L2 group. By contrast, no significant differences in PFS or CSS were seen in the wild-type EGFR cases.
Next, the results of univariate Cox regression analysis indicated associations among shorter PFS and male sex, heavy smoking, advanced pathological stage, and low TAM-PD-L2. As shown in Supplementary Table 3, advanced pathological stage was the only independent prognostic factor according to the results of multivariate analysis. Furthermore, shorter CSS was associated with male sex, heavy smoking, advanced pathological stage, wild-type EGFR, and low TAM-PD-L2, and advanced pathological stage and wild-type EGFR were identified as prognostic factors in the multivariate analysis (Supplementary Table 4). The results of a chi-square test revealed that the low TAM-PD-L2 group tended to have lower PD-L2 expression levels in tumor cells (Supplementary Table 5).
PD-L2 expression in macrophages is dependent on the JAK-STAT signaling pathway
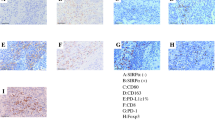
Next, the mechanisms underlying the expression of PD-L2 in macrophages in the same experimental system were investigated using cultured cells. First, to determine whether PD-L2 expression in macrophages is affected by cancer cell-derived factors, a CM of lung adenocarcinoma cells was added to human monocyte-derived macrophages, and immunocytostaining and cell ELISA were used to analyze the expression of PD-L2 in macrophages. The results indicated that the addition of a CM of PC9, H358, and H1975 cancer cells increased PD-L2 expression in macrophages (Fig. 3a). Based on the results of phospho-receptor tyrosine kinase array assays, transcription factors such as STAT3, STAT5, and c-Jun appear to be involved in the expression of PD-L1 in macrophages [13]; therefore, we investigated which pathways contribute to the expression of PD-L2 in macrophages using inhibitors of these molecules. As no direct inhibitors of c-Jun are currently available, we used inhibitors of its upstream kinases, JNK and ERK, and also examined a STAT1 inhibitor. The STAT3 inhibitor strongly suppressed the expression of PD-L2, and the STAT1, STAT5, JNK, and ERK inhibitors slightly suppressed PD-L2 expression (Fig. 3b). Because JAK signaling is located upstream of STAT3, we also decided to investigate whether the JAK inhibitor suppressed the expression of PD-L2 in macrophages. The results revealed that both the STAT3 and JAK inhibitors significantly suppressed PD-L2 expression in a concentration-dependent manner (Fig. 3c).
PD-L2 expression mechanisms in macrophages. a Human monocyte-derived macrophages were stimulated with a conditioned medium of cancer cell lines, and PD-L2 expression was tested by IHC (left) and cell ELISA (right). b Macrophages were stimulated with a conditioned medium with or without five inhibitors for signal molecules. c Macrophages were stimulated with a conditioned medium under various concentrations of STAT3 and JAK inhibitors. *p < 0.05
Discussion
To the best of our knowledge, this is the first study to assess the clinical significance of PD-L2 expression in patients with lung cancer using double IHC analysis of macrophage-specific markers. The results of the present study revealed that the expression of PD-L2 is associated with a better clinical course in patients with lung adenocarcinoma, especially that involving EGFR mutations. In the clinicopathological factors, early stage was most predictable factor for better clinical course. In a previous study, we investigated the clinical significance of PD-L1 in cancer cells and macrophages in lung adenocarcinoma using the same cohort as that in the present study [10]. Because it is difficult to distinguish macrophages from cancer cells in adenocarcinoma, PD-L1 was over-evaluated for the determination of the tumor proportion score in approximately 10% of the cases. In the same study, the expression of PD-L2 in cancer cells was also evaluated; it was detected in half of the cases and associated with longer PFS. The longest PFS among all cases of lung adenocarcinoma was seen in PD-L1-negative and PD-L2-positive patients. Notably, no association was found between PD-L1 and PD-L2 expression in cancer cells. The results of the present study identified a significant correlation between PD-L1 and PD-L2 expression in macrophages. These findings suggest a discrepancy in PD-L1/L2 expression between cancer cells and macrophages.
Although the details of the mechanisms underlying PD-L1 overexpression in cancer cells and macrophages are well known, those underlying the expression of PD-L2 in macrophages have not been elucidated in detail [19]. Oncogenic signaling pathways, such as JAK-STAT, RAS-ERK, and PI3K-AKT-MTOR, are known to be involved in the expression of PD-L1 in cancer cells [20]. The primary regulators of PD-L1 overexpression in monocyte-derived macrophages are STAT1 and STAT3 signals [21]. STAT3 signaling is known to be critical for both PD-L1 and PD-L2 overexpression in lymphoma-associated macrophages [12]. The results of the present study revealed that STAT, JNK, and ERK signaling affect the expression of PD-L2 in human macrophages, and this pattern is similar to that of PD-L1 expression in macrophages. However, despite the significant correlation identified between PD-L1 and PD-L2 expression in macrophages, no extensive overlap was observed in human lung adenocarcinoma specimens. Therefore, further studies are needed to elucidate the mechanisms underlying lung cancer-associated macrophages.
Although the significance of PD-L2 expression in macrophages remains unclear, animal studies have reported its involvement in immune suppression. In a mouse MC38 colon cancer model, Umezu et al. [22] observed post-immunotherapy PD-L2 overexpression using an anti-PD-L1 antibody in macrophages; moreover, cancer cell growth was synergistically reduced by anti-PD-L1 and anti-PD-L2 antibodies via activation of the immune system. Similar observations were seen in an E0771 breast cancer model [23]. In nasopharyngeal carcinoma, stromal PD-L2 expression was linked to a more favorable clinical course, and macrophages were suggested to express PD-L2 [24]. In another study on pancreatic cancer, high levels of PD-L2 expression were found to predict a poor clinical course, and cancer cell-derived interleukin-6 to amplify PD-L2 expression in macrophages [25]. Therefore, an increasing body of evidence suggests the importance of PD-L2 expression in macrophages.
Macrophage activation is known to be a heterogeneous due to the complex mechanisms, and M1/M2 concept has been described in many studies [26]. However, recent studies based on single cell RNA-sequence in human cancer samples indicated M1/M2 concept cannot explain the heterogeneity of macrophage activation [27]. Since it is unclear whether this concept would be applicable to humans, we did not test if PD-L2 overexpression was associated to M1/M2 balance in the present study.
In the present study, PD-L2 expression in macrophages was investigated using double IHC. As a result, high PD-L2 expression was found to predict a better clinical course. These data suggest that PD-L2 overexpression in macrophages is regulated by cancer cell-derived factors. STAT, JNK, and ERK signaling appear to be involved in the overexpression of PD-L2, but the detailed mechanisms still need to be elucidated. Although PD-L2 appears to be involved in immune suppression in cancer tissues, similar to PD-L1, the functional significance of PD-L2 in cancer therapy needs to be clarified in future studies.
Availability of data and materials
The data presented in this study are available in this article and its supplementary materials.
Abbreviations
- PD-1:
-
Programmed cell death protein 1
- PD-L1:
-
Programmed cell death 1 ligand 1
- JAK:
-
Janus kinase
- STAT:
-
Signal transducer and activator of transcription
- IHC:
-
Immunohistochemistry
- PFS:
-
Progression-free survival
- CSS:
-
Cancer-specific survival
- EGFR:
-
Epidermal growth factor receptor
- NSCLC:
-
Non-small-cell lung cancer
- TAMs:
-
Tumor-associated macrophages
- ELISA:
-
Enzyme-linked immunosorbent assay
- CM:
-
Conditioned medium
References
Hoffman PC, Mauer AM, Vokes EE (2000) Lung cancer. Lancet 355(9202):479–485
Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS (2021) Lung cancer. Lancet 398(10299):535–554
Reck M, Rodríguez-Abreu D, Robinson AG et al (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375(19):1823–1833
Hellmann MD, Paz-Ares L, Caro RB et al (2020) Atezolizumab for first-line treatment of PD-L1 selected patients with NSCLC. N Engl J Med 383(14):1328–1339
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12(4):252–264
Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science. 2020;367(6477):eaax0182.
Koomen BM, Badrising SK, Heuvel MM, Willems SM (2020) Comparability of PD-L1 immunohistochemistry assays for non-small-cell lung cancer: a systematic review. Histopathology 76(6):793–802
Herbst RS, Soria JC, Kowanetz M et al (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515(7528):563–567
Shinchi Y, Komohara Y, Yonemitsu K et al (2019) Accurate expression of PD-L1/L2 in lung adenocarcinoma cells: a retrospective study by double immunohistochemistry. Cancer Sci 110(9):2711–2721
Kozuma Y, Takada K, Toyokawa G, Kohashi K, Shimokawa M, Hirai F, Tagawa T, Okamoto T, Oda Y, Maehara Y (2018) Indoleamine 2,3-dioxygenase 1 and programmed cell death-ligand 1 co-expression correlates with aggressive features in lung adenocarcinoma. Eur J Cancer 101:20–29
Horlad H, Ma C, Yano H et al (2016) An IL-27/Stat3 axis induces expression of programmed cell death 1 ligands (PD-L1/2) on infiltrating macrophages in lymphoma. Cancer Sci 107(11):1696–1704
Shinchi Y, Ishizuka S, Komohara Y et al (2022) The expression of PD-1 ligand 1 on macrophages and its clinical impacts and mechanisms in lung adenocarcinoma. Cancer Immunol Immunother 71(11):2645–2661
Komohara Y, Fujiwara Y, Ohnishi K, Takeya M (2016) Tumor-associated macrophages: potential therapeutic targets for anti-cancer therapy. Adv Drug Deliv Rev 99:180–185
Matsubara E, Komohara Y, Shinchi Y et al (2021) CD163-positive cancer cells are a predictor of a worse clinical course in lung adenocarcinoma. Pathol Int 71(10):666–673
Matsubara E, Komohara Y, Esumi S et al (2022) SPP1 derived from macrophages is associated with a worse clinical course and chemo-resistance in lung adenocarcinoma. Cancers 14(18):4374
Y, Kanda. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–8.
Kovaleva OV, Rashidova MA, Samoilova DV, Podlesnaya PA, Mochalnikova VV, Gratchev A (2020) Immunosuppressive phenotype of esophagus tumors stroma. Anal Cell Pathol 2020:5424780
Wang Y, Du J, Gao Z, Sun H, Mei M, Wang Y, Ren Y, Zhou X. Evolving landscape of PD-L2: bring new light to checkpoint immunotherapy. Br J Cancer. 2022; Online ahead of print.
Zerdes I, Matikas A, Bergh J, Rassidakis GZ, Foukakis T (2018) Genetic, transcriptional and post-translational regulation of the programmed death protein ligand 1 in cancer: biology and clinical correlations. Oncogene 37(34):4639–4661
Matsusaka K, Fujiwara Y, Pan C et al (2021) α1-acid glycoprotein enhances the immunosuppressive and protumor functions of tumor-associated macrophages. Cancer Res 81(17):4545–4559
Umezu D, Okada N, Sakoda Y, Adachi K, Ojima T, Yamaue H, Eto M, Tamada K (2019) Inhibitory functions of PD-L1 and PD-L2 in the regulation of anti-tumor immunity in murine tumor microenvironment. Cancer Immunol Immunother 68(2):201–211
Yonemitsu K, Pan C, Fujiwara Y, Miyasato Y, Shiota T, Yano H, Hosaka S, Tamada K, Yamamoto Y, Komohara K (2022) GM-CSF derived from the inflammatory microenvironment potentially enhanced PD-L1 expression on tumor-associated macrophages in human breast cancer. Sci Rep 12(1):12007
Li A, Wu W, Deng S et al (2022) Expression of programmed death ligand-2 is associated with prognosis in nasopharyngeal carcinoma microenvironment. J Cancer 13(15):3606–3614
Davidson C, Taggart D, Sims AH, Lonergan DW, Canel M, Serrels A (2022) FAK promotes stromal PD-L2 expression associated with poor survival in pancreatic cancer. Br J Cancer 127(10):1893–1905
Komohara Y, Fujiwara Y, Ohnishi K, Takeya M (2016) Tumor-associated macrophages: potential therapeutic targets for anti-cancer therapy. Adv Drug Deliv Rev 99(Pt B):180–185
Cassetta L, Pollard JW (2018) Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov 17(12):887–904
Komohara Y, Kurotaki D, Tsukamoto H et al (2023) Involvement of protumor macrophages in breast cancer progression and characterization of macrophage phenotypes. Cancer Sci 114(6):2220–2229
Acknowledgements
We thank Mr. Takenobu Nakagawa for the technical assistance.
Funding
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (Nos. 16H05162 and 20H03459).
Author information
Authors and Affiliations
Contributions
EM: Data curation, Formal Analysis, Investigation, Software, Writing-original draft; YS: Investigation, Software, Visualization; YF: Conceptualization, Methodology, Validation; MS: Supervision; YK: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Writing-review & editing. All authors read and approved the final version of the manuscript to be submitted.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The study was approved by the Institutional Review Board of Kumamoto University (#1174), and conducted in accordance with the Declaration of Helsinki. Human macrophages were obtained from healthy donors in accordance with protocols approved by the Kumamoto University Hospital Review Board (approval No. 1169. No animal studies or registry/registration of this study was performed.
Consent to participate
Patient consent for inclusion in this study was waived by the Institutional Review Board of Kumamoto University (#2059) because the CSS and PFS data were obtained from previous reports [10, 15, 16]. Although all of the retrospective patient data were automatically included in the study, the patients were given the opportunity to refuse participation by opting out.
Consent for publication
Informed consent for publication was obtained from the relevant participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Matsubara, E., Shinchi, Y., Komohara, Y. et al. PD-L2 overexpression on tumor-associated macrophages is one of the predictors for better prognosis in lung adenocarcinoma. Med Mol Morphol 56, 250–256 (2023). https://doi.org/10.1007/s00795-023-00361-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00795-023-00361-0