Abstract
A single extremely halophilic strain was isolated from salt brine produced when a fresh water lake flooded a large salt mine located beneath the lake. The water that entered this mine contained less than 0.34 M NaCl, but over time, this sealed brine became saturated by Cenozoic age salt (121–125 million-year BCE). The isolated strain requires at least 1.7 M NaCl for survival and grows optimally in 3.1 M NaCl. Therefore, it could not have survived or been present in the waters that flooded this salt mine. The strain grows at a pH range from 6.5 to 9.0 and has a wide tolerance to temperatures from 25 ℃ to at least 60 ℃. The comparison of 16S rRNA and rpoB′ genes revealed that strain 1–13-28T is related to Halorubrum tebenquichense DSM 14210T showing 98.6% and 98.1% similarities, respectively. Phylogenetic analyses based on 16S rRNA, rpoB′ genes and 122 concatenated archaeal genes show that the strain 1–13-28T consistently forms a cluster with Halorubrum tebenquichense of the genus Halorubrum. Strain 1–13-28T contained sulfated mannosyl glucosyl diether, and the polar lipid profile was identical to those of most Halorubrum species. Based on the overall combination of physiological, phylogenetic, polar lipids and phylogenomic characteristics, strain 1–13-28T (= ATCC 700083T = CGMCC 1.62627T) represents a newly identified species within the genus Halorubrum for which the name Halorubrum hochsteinianum is proposed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
On November 20, 1980, Lake Peignur (Pen–ur) was a 3-m-deep, placid, fresh water lake, in Iberia Parish, Louisiana, USA. On November 22, 1980, it was a roiling salt water impoundment, 260 m deep, belching whole barges from the deep water and 35.5 hectares larger. The events of November 21st, responsible for these changes are relevant to the discovery and provenance of this species. Early in the morning of 21, November, the drill bit of an oil rig in lake Peignur seized. As the seven-man crew worked to free it, the entire rig began listing toward the water. The crew abandoned the rig and watched in horror as the 46-m-tall derrick disappeared into the 3-m-deep lake. Meanwhile, over 430 m below the surface, 50 salt miners realized that their mine had somehow been fatally opened to the lake above and fresh water was filling the entire cavity. They also evacuated, but the last men out were standing hip deep in surging fresh water at the mine’s highest level (< 100 m below ground). As the day progressed the world’s largest man-made whirlpool sucked down 11 barges, a tug boat, parking lots, 35 hectares of lake front and a portion of a mansion (Gold 1981). Water flowed in torrents from the fresh water river that normally fed the lake and backward from the natural river that emptied into the Gulf of Mexico creating a 50-m-high waterfall (the largest ever in Louisiana). A 122-m-tall geyser of muddy fresh water also erupted from the main mine shaft of the now doomed mine. Lake Peignur, at 61 m deep, is still the deepest lake in the state. Most important, for this study, all the billions of gallons of water that flowed into the mine at the time of the disaster contained less than 2.0% w/v salt. Shortly after the disaster, the main shaft and one of the two flooded air shafts were permanently sealed, one shaft remained open.
Seven years later, RHV and graduate student J. Hansel Huval gained access to this shaft and were able to obtain a sample of the now saturated salt brine. This brine sample contained a single extremely halophilic microbe that was given the designation of JI-1 (Jefferson Island 1) later changed to 1–13-28. The organism has been kept in liquid nitrogen in the personal collection of RHV. As various tests were performed, lab personnel began to notice that the organism, while being a Halorubrum, appeared to represent a unique species. In order to meet the proposed minimal standards for describing new species of the Halobacteriaceae (Oren et al. 1997), a full polyphasic study of the strain has been conducted. The results of this examination show clearly that strain 1–13-28T represents a newly described species of Halorubrum for which the species name Halorubrum hochsteinianum is proposed.
The genus Halorubrum contains the largest number of species among the current genera within the class Halobacteria. This genus was assigned to the family Halorubraceae (type genus of this family) within the order Haloferacales. At the time of writing, Halorubrum consists of 39 validly published species names (LPSN, https://lpsn.dsmz.de/search?word=Halorubrum). They were isolated from diverse hypersaline environments such as marine solar salterns, soda lakes, salt lakes, brine of salted brown alga Laminaria, salt-fermented foods and commercial rock salt (Amoozegar et al. 2017). The major polar lipid profiles of the species of Halorubrum were phosphatidylglycerol (PG), phosphatidyl glycerol phosphate methyl ester (PGP-Me), phosphatidyl glycerol sulfate (PGS) and a glycolipid (sulfated diglycosyl diether, S-DGD) (Oren. 2018).
Materials and methods
Isoation and cultivation of the isolate
The saturated salt brine was sampled using a sterilized Nansen style water sampler lowered into the shaft on a 150-m-long line. The water sample was placed in Casamino Acids Medium (CAS) (Vreeland et al. 1980) supplemented with 20% (w/v) NaCl and incubated at 35 ℃. After several weeks, the inoculated medium developed a bright red color and extensive growth. Multiple colony isolations on solid CAS (with 20%) provided only a single isolate which received the laboratory culture collection designation 1-13-28T.
The strain was purified using multiple single colony isolations on solid CAS medium supplemented with 20% (w/v) NaCl. The cultures were incubated at 35 ℃. At first isolation, the culture required several weeks of incubation which has been found to be typical of newly reanimated microorganisms (Vreeland et al. 2000); however, further transfers result in more rapid reproduction. The culture was maintained at 4 ℃ on agar slopes of CAS medium with 20% (w/v) NaCl and contained in 0.3 mL CAS plus 10–20% glycerol, in hermetically sealed sperm straws under liquid nitrogen (− 195.8 ℃). This culture has now been subjected to a full polyphasic taxonomic analysis in order to meet the proposed minimal standards for the taxonomy of new species of halophilic archaea (Oren et al. 1997).
Phylogenetic analysis
The genomic DNA of strain 1-13-28T was extracted and purified using the genomic DNA extraction kit (Beijing ComWin Biotech Co., Ltd.), according to the protocol described previously (Cui et al. 2011). The 16S rRNA gene was amplified by PCR using the forward primer 20F (5'-ATTCCGGTTGATCCTGCCGG-3') and reverse primer 1452R (5'-AGGAGGTGATCCAGCCGCAG-3'), then cloned and sequenced as described previously (Cui et al. 2009). The primer pair HrpoB2 1420F and HrpoA 153R were used to amplify the rpoB′ gene (Minegishi et al. 2010). The 16S rRNA and rpoB′ gene sequences were aligned with the ClustalW program integrated in MEGA 6 software (Tamura et al. 2013) and the phylogenetic trees were reconstructed using the maximum-likelihood (ML) (Felsenstein 1981), neighbor-joining (NJ) (Saitou and Nei 1987) and maximum-parsimony (MP) (Fitch 1971) algorithms in the MEGA 6 software. The 16S rRNA and rpoB′ gene sequence similarities between strain 1-13-28T and the current members of the genus Halorubrum were calculated by the pairwise aligner (https://www.ezbiocloud.net/tools/pairAlign).
Genome sequencing and analysis
The complete genome of strain 1-13-28T was sequenced and assembled as described previously (Sun et al. 2022). A Genome-based phylogenetic tree was reconstructed by IQ-TREE (Nguyen et al. 2015) with standard model based on an alignment of 122 conserved archaeal protein marker genes provided by the Genome Taxonomy Database (GTDB) (Parks et al. 2018). The overall genome-related indexes, including average nucleotide identity (ANI), in silico DNA–DNA hybridization (isDDH) and average amino acid identity (AAI) values were determined by the online ANI calculator (Richter et al. 2016), genome-to-genome distance calculator (Meier-Kolthoff et al. 2013), and AAI calculator (Luo et al. 2014), respectively. The Kyoto Encyclopedia of Genes and Genomes (KEGG) database (Kanehisa et al. 2006) was used to analyze the general metabolic pathways of the strain. Functional annotation of these genomes was performed through the online Rapid Annotation using Subsystem Technology (RAST) server (Aziz et al. 2008).
Phenotypic determination
Cell morphology and motility in exponentially growing liquid cultures were observed using a microscope equipped with phase-contrast optics (Nikon, Ci-L). The minimum salt concentration preventing cell lysis was determined by suspending washed cells in serial solutions containing NaCl ranging from 0 to 150 g/L and the stability of the cells was examined by light microscopy. The salt concentration growth range and the optimal NaCl concentration for growth were determined in modified NHM medium (without MgCl2·6H2O, 0.1 g/L MgSO4·7H2O) containing 5%, 8%, 10%, 12%, 15%, 18%, 20%, 23%, 25%, 28% and 30% (w/v) of NaCl. The temperature range for growth was determined after incubation in NHM broth at 10, 15, 20, 25, 30, 37, 40, 43, 45, 50, 55 and 60 ℃. The pH range for growth was determined in modified NHM medium at pH 5.0, 5.5, 6.0, 6.5, 7.0, 7.5, 8.0, 8.5, 9.0, 9.5 and 10.0, using the following buffers: CPBS (pH 5.0–6.0), PBS (pH 6.0–7.5), Tris–HCl (pH 7.5–9.0) at a concentration of 100 mM and 50 mM CHES-NaOH (pH 9.0–10.0). Under alkaline conditions with Tris–HCl and CHES-NaOH, MgCl2·6H2O was not added and MgSO4·7H2O was at a concentration of 0.1 g/L in modified NHM medium. Tests for catalase and oxidase activities, and for the hydrolysis of starch, gelatin, casein and Tween 80 were performed as described by Gonzalez et al. (1978). Other phenotypic tests such as nutrition, biochemical activities, and antibiotic sensitivity were performed according to the proposed minimal standards for the description of new taxa in the order Halobacteriales (Oren et al. 1997).
Chemotaxonomic characterization
The polar lipids of strain 1-13-28T was analyzed following aerobic growth in the CAS + 20% NaCl medium, at 37 ℃. The polar lipids were extracted and analyzed by two-dimensional TLC (Cui et al. 2010). Specific sprayed detection reagents, including phosphate stain reagent for phospholipids (Vaskovsly and Kostetsky 1968) and α-naphthol stain for glycolipids (Siakotos and Rouser 1965), were used, and the general detection reagent, sulfuric acid–ethanol (1:2, by vol.), was used to detect the total polar lipids. Isoprenoid quinones were extracted, purified by TLC and analyzed by high-performance liquid chromatography (Waino et al. 2000).
Results and discussion
Phylogeny
The complete 16S rRNA gene sequence of strain 1–13-28 T was found to be 1470 bp. Comparative sequence analysis revealed that strain 1-13-28T was affiliated with the genus Halorubrum. The 16S rRNA gene similarities among strain 1-13-28T and the type strains of the genus Halorubrum were 93.8–98.6%, lower than the suggested threshold (98.65%) for separating two prokaryotic species (Kim et al. 2014), and the most closely related species was Halorubrum tebenquichense (Table S1).
The rpoB' gene of strain 1-13-28T was extracted from the genome sequence and was found to be 1830 bp in length. The rpoB' gene sequence similarities between strain 1-13-28T and the current members of the genus Halorubrum were 87.3–98.1%, and the most closely related strain was Halorubrum tebenquichense DSM 14210T (Table S1).
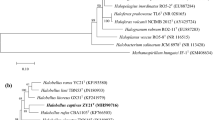
Phylogenetic trees based on 16S rRNA genes (Fig. 1a), rpoB′ genes (Fig. 1b) and 122 archaeal marker genes (Fig. 1c) showed that strain 1-13-28T nests quite completely within the genus Halorubrum consistently related to Halorubrum tebenquichense (Lizama et al. 2002).
Maximum-likelihood phylogenetic tree reconstructions based on 16S rRNA gene sequences (a), rpoB′ gene sequences (b) and 122 concatenated conserved archaeal marker gene sequences (c), showing the relationship among strain 1-13-28T and related members of the genus Halorubrum. Bootstrap values (based on 1000 replicates) greater than 70% are shown at branches. Bar, 0.02 substitutions per nucleotide position
Genomic features
The complete genome sequence of strain 1-13-28T, consisting of 2 contigs with N50 value of 2,962,004 and a mean coverage of 999× , contains one circular chromosome and one circular plasmid, with lengths of 2,962,004 bp (chromosome) and 101,418 bp (plasmid), respectively. The DNA G + C content of the strain was 69.1% (genome) (Table S2). The complete genome of strain 1-13-28T contains two rRNA operons (two 5S rRNA, two 16S rRNA and two 23S rRNA gene) and 48 tRNA genes. The 16S rRNA gene sequence of strain 1-13-28T derived from complete genome sequencing showed 100% similarity to that from conventional Sanger sequencing.
The ANI and isDDH values between strain 1-13-28T and Halorubrum tebenquichense DSM 14210T were 93.6% and 54.7%, respectively, which are below the threshold values proposed for species delimitation (95–96% for ANI and 70% for isDDH) (Goris et al. 2007; Richter et al. 2009). Besides, the two values between strain 1-13-28T and other species of the genus Halorubrum are 76.8–93.6% and 22.8–54.7%, respectively (Table 1), the ANI and isDDH values are lower than the recommended cutoff values. The AAI values between strain 1-13-28T and the Halorubrum members were 70.5–93.7%, higher than the genus boundary of 65% AAI (Konstantinidis et al. 2017). These results suggested that strain 1-13-28T belongs to the genus Halorubrum.
Genome annotation results showed that 2743 genes were annotated to COG database, 2085 genes were annotated to GO database and 2768 genes were annotated to KEGG database. The subsystem category distribution of the novel isolate annotated by RAST server was presented in Fig. S1, which indicated that amino acids and derivatives, and protein metabolism were the richest RAST subsystems for strain 1-13-28T. Functional annotation conducted through KEGG database showed that strain 1-13-28T possessed the genes involved in glycolysis and pyruvate oxidation pathways, corresponding to its capability to utilize glucose and pyruvate for growth. On the contrary, no genes associated with fructose or galactose utilization, casein, starch, or Tween 80 hydrolysis were observed in this strain, as confirmed by phenotypic test results.
Phenotypic characteristics
The cells of strain 1-13-28T were motile, thin rods approximately 1.0–1.5 μm in length (Fig. S2) and lysed in NaCl concentrations below 10% (w/v), the organism grows up to 28% (w/v) NaCl with an optimum growth concentration of 18% (w/v). The organism grows in pH from 6.0 to 9.5 with an optimum pH of 6.5. The optimum growth temperature is 40 ℃ with a tolerance of 25–60 ℃. Magnesium is not required but the organism grows optimally in 0.005 M Mg2+ and grows at Mg2+ concentration up to 1.0 M. Strain 1-13-28T is primarily aerobic but grows anaerobically in the presence of nitrate with reduction to nitrite. Anaerobic growth does not occur in the presence of arginine or DMSO. Strain 1-13-28T utilizes d-glucose, d-mannose, maltose, acetate, pyruvate, dl-lactate, succinate, l-alanine, l-arginine, l-glutamate and l-ornithine as sole sources of carbon and energy. Starch, Tween 80, or casein are not hydrolyzed, while gelatin could be hydrolyzed. H2S is produced from thiosulfate and Indole is not formed. Catalase is positive and oxidase is negative. The cells are sensitive to novobiocin (30 ug/disc), and bacitracin (0.04 i.u./disc), but insensitive to all other antimicrobials tested. The distinct phenotypic characteristics differentiating strain 1-13-28T from the most closely members of Halorubrum are colonial pigmentation, NaCl, temperature and pH range for growth, reduction of nitrate to nitrite, utilization of specific carbon sources, hydrolysis of gelatin, and H2S formation (Table 2).
Chemotaxonomic characteristics
Two-dimensional thin-layer chromatography (TLC) revealed that strain 1-13-28T contained phosphatidic acid (PA), phosphatidylglycerol (PG), phosphatidylglycerol phosphate methyl ester (PGP-Me), phosphatidylglycerol sulfate (PGS), and a glycolipid (GL) (Fig. S3). One-dimensional TLC showed that the glycolipid (GL) was chromatographically identical to sulfated mannosyl glucosyl diether (S-DGD-3) detected in Halorubrum saccharovorum CGMCC 1.2147T. It was reported that S-DGD-3 was identified in most Halorubrum species (Chen et al. 2016; Han and Cui 2015). Polar lipids of strain 1-13-28T were identical to those of most Halorubrum neutrophilic species (Oren 2018). The major respiratory quinones of the strain were menaquinone MK-8 and MK-8(H2). These two menaquinones were also detected in other Halorubrum species (Oren 2018).
Ecological implications
It was impossible to calculate how much underground 125-million-year-old (lower Cretaceous age) salt was dissolved in the mine following the Lake Peignur disaster. The very limited accessibility to the underground brine made it equally impossible to conduct a microbiological survey of the saturated salt brine in that underground man-made impoundment. However, the initial sample contained at least one organism able to reproduce on the high salt medium. The physiology of that organism would never have allowed it to survive in the water that initially flooded the mine to become the saturated brine sampled 7 years later. Consequently, the organism must have been present, viable, and trapped somewhere within the salt layers. Since that sample was obtained numerous studies have shown that long-term microbial viability in salt is indeed possible (McGenity et al. 2000; Mormile et al. 2003; Vreeland et al. 1980, 2000, 2007; Stan-Lotter et al. 2002). Park et al. (2009) performed an extensive survey of DNA sequences isolated from salt crystals of several different ages. During that survey, several hundred different sequences were amplified, cloned, and sequenced. The authors demonstrated that in several instances the amplified archaeal DNA contained a unique 55 BP sequence that was not present in samples after 125 MYA. A secondary structural analysis of these 16S rRNA’s showed a structural alteration of the resulting 16S rRNA molecule. When this sequence was artificially removed in computer analyses the resulting sequences and secondary molecular structure matched that of both the Halorubrum and its related genus Haloarcula. In the reverse analysis, artificially adding the 55 BP to the 16S rRNA sequences from recently isolated members of these genera yielded a secondary structure identical to the ancient one. Adding it to the sequences of other haloarchaea yielded a grotesquely altered likely non-functional secondary molecule. These authors hypothesized that this 55 BP sequence was present prior to 121 MYA and its loss led to the development of Halorubrum and Haloarcula. Both genera are now isolated from brine sources across the world. Finding a unique species of one of these two genera, in brine naturally created from many tons of ancient 121 MYA salt is certainly not proof of this hypothesis, but it does provide some support. Its potential age aside, the overall characteristics of this strain, its DNA relatedness and its lipid profile support the conclusion that it represents a new species within the genus Halorubrum for which the proposed species name is Halorubrum hochsteinianum sp. nov.
Description of Halorubrum hochsteinianum sp. nov.
Halorubrum hochsteinianum (hoch.stei.ni.a'num. N.L. neut. n. hochsteinianum, named in honor of Dr. Lawrence Hochstein).
Cells are motile, thin rods approximately 1.0 to 1.5 µm in length × 0.5 µm wide. Gram stain negative. Cells lyse in NaCl concentrations below 10% M NaCl (w/v). The species grows up to 28% (w/v) NaCl. Tolerates any pH from 6.0 to 9.5 with a temperature tolerance of 25–60 ℃. The optimum growth conditions on CAS or NHM media are 28% (w/v) NaCl, pH of 6.5 and temperature at 40 ℃. Magnesium is not required but the organism grows optimally in 0.005 M Mg2+ and grows at Mg2+ concentrations up to 1.0 M. Aerobic but grows anaerobically in the presence of nitrate with reduction to nitrite. Anaerobic growth does not occur in the presence of arginine or DMSO. Utilizes d-glucose, d-mannose, maltose, acetate, pyruvate, dl-lactate, succinate, l-alanine, l-arginine, l-glutamate and l-ornithine as sole sources of carbon and energy. Starch, Tween 80, or casein are not hydrolyzed, while gelatin could be hydrolyzed. H2S is produced from thiosulfate and Indole is not formed. Catalase is positive and oxidase is negative. Phospholipid and glycolipids include: phosphatidic acid (PA), phosphatidylglycerol (PG), phosphatidylglycerol phosphate methyl ester (PGP-Me), phosphatidylglycerol sulfate (PGS), and sulfated mannosyl glucosyl diether (S-DGD-3). The G + C content of the DNA is 69.4% (Genome). The type strain is 1–13-28T (= ATCC 700083T = CGMCC 1.62627T) and was isolated from flooded salt mine beneath Lake Peignur, Iberia Parish, LA 29.981 N, 91.983 W. The GenBank/EMBL/DDBJ accession numbers for the 16S rRNA gene, and whole-genome sequence of strain 1-13-28T are ON606013, and CP098415-CP098416, respectively.
Data availability
The sequences determined in this study have been deposited in the NCBI Genbank database.
References
Amoozegar MA, Siroosi M, Atashgahi S, Smidt H, Ventosa A (2017) Systematics of haloarchaea and biotechnological potential of their hydrolytic enzymes. Microbiology 163(5):623–645. https://doi.org/10.1099/mic.0.000463
Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA et al (2008) The RAST Server: rapid annotations using subsystems technology. BMC Genomics 9:75. https://doi.org/10.1186/1471-2164-9-75
Chen S, Liu HC, Zhou J, Xiang H (2016) Halorubrum pallidum sp. nov., an extremely halophilic archaeon isolated from a subterranean rock salt. Int J Syst Evol Microbiol. 66(8):2980–2986. https://doi.org/10.1099/ijsem.0.001129
Cui HL, Zhou PJ, Oren A, Liu SJ (2009) Intraspecific polymorphism of 16S rRNA genes in two halophilic archaeal genera, Haloarcula and Halomicrobium. Extremophiles 13:31–37. https://doi.org/10.1007/s00792-008-0194-2
Cui HL, Gao X, Yang X, Xu XW (2010) Halorussus rarus gen. nov., sp. nov., a new member of the family Halobacteriaceae isolated from a marine solar saltern. Extremophiles 14:493–499. https://doi.org/10.1007/s00792-010-0329-0
Cui HL, Yang X, Mou YZ (2011) Salinarchaeum laminariae gen. nov., sp. nov.: a new member of the family Halobacteriaceae isolated from salted brown alga Laminaria. Extremophiles 15(6):625–631. https://doi.org/10.1007/s00792-011-0393-0
Felsenstein J (1981) Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol 17:368–376. https://doi.org/10.1007/BF01734359
Fitch WM (1971) Toward defining the course of evolution: minimum change for a specific tree topology. Syst Biol 20:406–416. https://doi.org/10.2307/2412116
Gold M (1981) Who pulled the plug on Lake Peigneur? Sci 81:56–63
Gonzalez C, Gutierrez C, Ramirez C (1978) Halobacterium vallismortis sp. nov. an amylolytic and carbohydrate-metabolizing, extremely halophilic bacterium. Can J Microbiol. 24(6):710–715. https://doi.org/10.1139/m78-119
Goris J, Konstantinidis KT, Klappenbach JA, Coenye T, Vandamme P, Tiedje JM (2007) DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int J Syst Evol Microbiol 57:81–91. https://doi.org/10.1099/ijs.0.64483-0
Han D, Cui HL (2015) Halorubrum laminariae sp. Nov., isolated from the brine of salted brown alga Laminaria. Antonie van Leeuwenhoek. 107(1):217–223. https://doi.org/10.1007/s10482-014-0319-9
Kanehisa M, Goto S, Hattori M, Aoki-Kinoshita KF, Itoh M, Kawashima S et al (2006) From genomics to chemical genomics: new developments in KEGG. Nucleic Acids Res 34:D354–D357. https://doi.org/10.1093/nar/gkj102
Kim M, Oh HS, Park SC, Chun J (2014) Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int J Syst Evol Microbiol 64:346–351. https://doi.org/10.1099/ijs.0.059774-0
Konstantinidis KT, Rosselló-Móra R, Amann R (2017) Uncultivated microbes in need of their own taxonomy. ISME J 11(11):2399–2406. https://doi.org/10.1038/ismej.2017.113
Lizama C, Monteoliva-Sanchez M, Suarez-Garcıa A, Rosello-Mora R, Aguilera M et al (2002) Halorubrum tebenquichense sp. nov., a novel halophilic archaeon isolated from the Atacama Saltern. Chile Int J Syst Evol Microbiol 52:149–155. https://doi.org/10.1099/00207713-52-1-149
Luo C, Rodriguez-R LM, Konstantinidis KT (2014) MyTaxa: an advanced taxonomic classifier for genomic and metagenomic sequences. Nucleic Acids Res 42(8):e73. https://doi.org/10.1093/nar/gku169
McGenity TJ, Gemmell RT, Grant WD, Stan-Lotter H (2000) Origins of halophilic microorganisms in ancient salt deposits. Environ Microbiol 2(3):243–250. https://doi.org/10.1046/j.1462-2920.2000.00105.x
Meier-Kolthoff JP, Auch AF, Klenk HP, Göker M (2013) Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics 14:60. https://doi.org/10.1186/1471-2105-14-60
Minegishi H, Kamekura M, Itoh T, Echigo A, Usami R, Hashimoto T (2010) Further refinement of the phylogeny of the Halobacteriaceae based on the full-length RNA polymerase subunit B’ (rpoB’) gene. Int J Syst Evol Microbiol 60(10):2398–2408. https://doi.org/10.1099/ijs.0.017160-0
Mormile MR, Biesen MA, Gutierrez MC, Ventosa A, Pavlovich JB, Onstott TC, Fredrickson JK (2003) Isolation of Halobacterium salinarum retrieved directly from halite brine inclusions. Environ Microbiol 5(11):1094–1102. https://doi.org/10.1046/j.1462-2920.2003.00509.x
Nguyen LT, Schmidt HA, Haeseler AV, Minh BQ (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32:268–274. https://doi.org/10.1093/molbev/msu300
Oren A (2018) Halorubrum. In: Trujillo ME, Dedysh S, DeVos P, Hedlund B, Kämpfer P, Rainey FA, Whitman Bergey;s WB (eds) Manual of systematics of archaea and bacteria. Wiley, New York. https://doi.org/10.1002/9781118960608.gbm00487.pub2
Oren A, Ventosa A, Grant WD (1997) Proposed minimal standards for description of new taxa in the order Halobacteriales. Int J Syst Evol Microbiol 47:233–238. https://doi.org/10.1099/00207713-47-1-233
Park JS, Vreeland RH, Cho BC, Lowenstein TK, Timofeeff MN et al (2009) Haloarchaeal diversity in 23, 121 and 419 MYA salts. Geobiology 7(5):515–523. https://doi.org/10.1111/j.1472-4669.2009.00218.x
Parks DH, Chuvochina M, Waite DW, Rinke C, Skarshewski A et al (2018) A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life. Nat Biotechnol 36(10):996–1004. https://doi.org/10.1038/nbt.4229
Richter M, Rosselló-Móra R (2009) Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci U S A 106:19126–19131. https://doi.org/10.1073/pnas.0906412106
Richter M, Rosselló-Móra R, Olive GF (2016) JSpeciesWS: a web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinformatics 32(6):929–931. https://doi.org/10.1093/bioinformatics/btv681
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4(4):406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Siakotos AN, Rouser G (1965) Analytical separation of nonlipid water soluble substances and gangliosides from other lipids by dextran gel column chromatography. J Am Oil Chem Soc 42:913–919. https://doi.org/10.1007/BF02632444
Stan-Lotter H, Pfaffenhuemer M, Legat A, Busse HJ, Radax C, Gruber C (2002) Halococcus dombrowskii sp. nov. an archaeal isolate from a Permo-Triassic alpine salt deposit. Int J Syst Evol Microbiol 52:1807–1814. https://doi.org/10.1099/00207713-52-5-1807
Sun YP, Wang BB, Zheng XW, Wu ZP, Hou J, Cui HL (2022) Description of Halosolutus amylolyticus gen. nov., sp. nov., Halosolutus halophilus sp nov and Halosolutus gelatinilyticus sp nov, and genome-based taxonomy of genera Natribaculum and Halovarius. Int J Syst Evol Microbiol. https://doi.org/10.1099/ijsem.0.005598
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 30(12):2725–2729. https://doi.org/10.1093/molbev/mst197
Vaskovsky VE, Kostetsky EY (1968) Modified spray for the detection of phospholipids on thin-layer chromatograms. J Lipid Res 9(3):396
Vreeland RH, Litchfield CD, Martin EL, Elliot E (1980) Halomonas elongata, a new genus and species of extremely salt-tolerant bacteria. Int J Syst Bacteriol 30:485–495. https://doi.org/10.1099/00207713-30-2-485
Vreeland RH, Rosenzweig WD, Powers DW (2000) Isolation of a 250 million year old bacterium from a primary salt crystal. Nature 407(6806):897–900. https://doi.org/10.1038/35038060
Vreeland RH, Jones J, Monson A, Rosenzweig WD, Lowenstein TK, Timofeeff M et al (2007) Isolation of live cretaceous (121–112 million years old) halophilic archaea from primary salt crystals. Geomicrobiol J 24:275–282. https://doi.org/10.1080/01490450701456917
Wainø M, Tindall BJ, Ingvorsen K (2000) Halorhabdus utahensis gen. nov., sp. nov., an aerobic, extremely halophilic member of the archaea from great salt lake. Utah Int J Syst Evol Microbiol 50:183–190. https://doi.org/10.1099/00207713-50-1-183
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by Oren.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vreeland, R.H., Sun, YP., Wang, BB. et al. Halorubrum hochsteinianum sp. nov., an ancient haloarchaeon from a natural experiment. Extremophiles 28, 1 (2024). https://doi.org/10.1007/s00792-023-01320-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00792-023-01320-4