Abstract
The genus Eustigmaeus Berlese, 1910 represents the unique phytophagous group within the superfamily Raphignathoidea. Four species within this genus have been known to inhabit mosses and feed on them as larvae, nymphs, and adults. However, the interactions with mosses have remained poorly understood. In order to reveal the diversity and host-plant use of the moss-feeding species, we conducted an extensive field study in Japan. This study revealed an array of moss-feeding species inhabiting various moss species, with 10 morphologically distinctive species newly documented in Japan. Through DNA barcoding based on cytochrome c oxidase subunit I (COI) sequences, these morphospecies were recovered as distinct entities. Notably, the host-plant use of four species was elucidated. Among these, Eustigmaeus sp. 9 exhibited polyphagy, while three species (Eustigmaeus spp. 1–3) demonstrated varying degrees of host specificity, each using moss species from the Hypnales, Philonotis, and Dicranidae, respectively. While a few moss-feeding species were frequently found in the same geographic area, more than one species rarely co-occurred within the same moss colonies. Eustigmaeus offers a unique study system, with its diverse moss-feeding species and indications of specific host plant use. Consequently, the moss-feeding Eustigmaeus serves as a valuable model for exploring the macroevolutionary patterns underlying diversification in moss-feeding arthropods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obligate phytophagy has independently evolved several times within the Acariformes (Lindquist 1998). The obligate phytophagous mites typically exhibit a suite of adaptations to their host plants, including specialized mouthparts (De Lillo et al. 2001). Particularly, two superfamilies, Tetranychoidea and Eriophyoidea, have achieved enormous diversity in their interactions with vascular plants (Zhang et al. 2011). These groups have been relatively well-studied, primarily owing to their economic impact as agricultural pests (Lindquist and Oldfield 1996; Bolland et al. 1998). In contrast, the biology of other obligate phytophagous mite groups within Heterostigmata, Raphignathoidea, and Eupodoidea, remains poorly understood (Krantz and Lindquist 1979; Lindquist 1998). We herein focus on an obligate phytophagous group in Raphignathoidea that inhabits mosses (Bryophyta).
The genus Eustigamaeus Berlese, 1910 (Prostigmata: Stigmaeidae) is the unique phytophagous group within the family Stigmaeidae under the superfamily Raphignathoidea, which primarily comprises predatory species (Fan and Zhang 2005). Eustigmaeus mites are characterized by their reddish coloration and sluggish movement, with a reproduction strategy involving arrhenotokous parthenogenesis (Gerson 1972). Their ranges encompass all ecozones except the Antarctic (Fan et al. 2016). Among the more than 120 known species, at least 70 species have been collected from substrates including mosses (Fan et al. 2016; Beron 2020; Bizarro et al. 2020; Khaustov 2021a, b; Khaustov et al. 2023). Notably, Gerson (1972) collected 11 species inhabiting mosses, and the rearing experiments clarified that four of these species, E. frigidus (Habeeb, 1958), E. rhodomelus (Koch, 1841), E. clavatus (Canestrini and Fanzago, 1876), E. schusteri (Summers and Price 1961), were moss-feeders. These mites complete their entire life cycle on mosses (Gerson 1972), utilizing moss patches as sources of food for their larvae, nymphs, and adults and even as mating sites. Their feeding strategy involves piercing the cell walls of moss leaves with their chelicerae and extracting the cell contents (Gerson 1972). After Gerson’s (1972) work, some Eustigmaeus species were inferred to be moss-feeding based on their habitats and the presence of green gut contents (Flechtmann 1985; Swift 1994). However, the diversity of moss-feeding species and their host plant use remains veiled. Moss gametophytes, including leaves and stems, exhibit diverse morphology, habitat preferences, and chemistry across different lineages (Ueno et al. 2009). This diversity may create opportunities for specialized host-plant use, akin to some bryophyte-feeding insects (Imada et al. 2011; Imada and Kato 2016; Imada 2021; Kato et al. 2022). In fact, Gerson (1972) noted variations in the performance of E. frigidus on different moss species. The moss-feeding species can thus be more diverse than previously thought and potentially be host specific.
Revealing moss-mite interactions is inherently challenging, primarily due to the minuscule size of mites and the intricate structure of moss colonies. Moss colonies often form complex life forms, including cushions, mats, and tufts, where various moss species coexist. However, our extensive fieldwork across Japan, together with the meticulous host-plant identification through the observation of mite habit, has revealed a diversity of moss-feeding species of Eustigmaeus and their varying degrees of host specificity.
Materials and methods
Sampling
To reveal the diversity of moss-feeding Eustigmaeus species, extensive fieldwork was conducted mainly in Western Japan, with additional sampling in some locations in Central and Eastern Japan, from March 2021 to May 2022. This approach was informed by preliminary assessments conducted between April and December 2020, which revealed that Eustigmaeus mites were typically found within moss colonies of varying sizes situated in consistently moist microhabitats, e.g., areas along riverbanks, streams, pavement roads, and mountainous pathways. Consequently, our focus was on moss colonies thriving in such moisture-rich environments. Furthermore, moss patches where the leaves of the moss were fully spread out and in a wet condition were targeted, as these were the preferred habitats of the mites.
Mosses were sampled by hand and carefully placed in plastic containers (NK200, Risupack Inc.). Each container was sealed in a plastic bag to prevent the potential mixing of mites originating from different moss patches. To ensure the purity of the samples, great care was taken to collect moss colonies composed of a single moss species from the same patch. Even in such diligent collection, occasionally, a moss colony that appeared to consist of a single moss species might contain a few other species. A colony size of 300–1000 cm3 (in field conditions) was sampled for each moss species. At a locality, a patch was defined as a spatially isolated moss colony composed of one or more moss species, assigning each patch a unique “patch code” (Table 1). Occasionally, a moss patch was ill-defined in case the specific type of substrate or microhabitat where the moss colonies overlain was continuous at a moderately large spatial scale; patches were then distinguished when each cluster was several meters apart and a patch code was assigned to each of them. In case the cooccurrence of multiple moss species within a patch was evident (e.g., patch code AH shown in Fig. 1e), each species was segregated by allocating them into different plastic containers (Table 1). When the same moss species was encountered in different patches within a locality, the colonies were placed in separate plastic containers. After completing the sampling, the characteristics of the habitats and substrates at each patch were documented. Subsequently, the sealed samples were transported to our laboratory, where they were stored in darkness at 4 °C until they were subjected to microscopic analysis.
Confirmation of moss-feeding habit and determination of host plant
In addition to four moss-feeding species, Eustigmaeus contains six insect parasite species (Chaudhri 1965; Abonnenc 1970; Zhang and Gerson 1995; Reeves et al. 2008; Majidi et al. 2019), and the rest of the known species in the genus have unknown feeding habits. Additionally, some Eustigmaeus species have been collected from habitats other than moss colonies (Fan et al. 2016). Thus, not all moss-inhabiting species of Eustigmaeus are moss-feeders. The following method ensures not only the confirmation of the moss-feeding habit of the species, but also the determination of their precise host plants within moss colonies, which can sometimes consist of multiple moss species.
For the collected living mites, the moss-feeding habit was determined and their host plants were identified by closely observing them on moss shoots. Initially, a moss colony enclosed in a plastic container was placed under a stereomicroscope (Leica M125C), and a mite individual within the colony was searched with tweezers. Before observation, we ensured that the mites were active by incubating the moss colonies at a temperature of 20 ± 5 °C for at least 12 h, with the light condition as “Light 2” of the incubator (SANYO MLR-350). If an immobile mite was found on a moss shoot, whether there were characteristic feeding traces on the moss leaves was verified. Specifically, we observed if the mite was actively feeding on the leaf cells on a moss shoot. We identified transparent cell patterns, which were typically a small part of a whole leaf but continuous to some extent, as feeding marks caused by Eustigmaeus mites. This identification was based on Gerson’s (1972) description and figures and our observations of their feeding behavior (Fig. 2). We determined whether the mite species exhibited moss-feeding habit, either through the presence of feeding marks on moss leaves or their active feeding behavior. The damaged moss shoots were then recorded as the host plant taxa. To precisely determine the moss-mite associations to the species level, each individual mite and its associated host-plant shoot(s) were recorded and preserved as paired voucher specimens. For selected mite specimens, genomic DNA was extracted for DNA barcoding and subsequent phylogenetic analysis before they were mounted in Hoyer’s medium for morphological identification.
Behavioral observation. a Leaf of Rhynchostegium riparioides with characteristic feeding mark caused by Eustigmaeus mite. Cells in the infested area (arrow) are transparent due to the suction feeding of cell contents. b Two individuals of Eustigmaeus sp. 1 on Rhynchostegium riparioides shoot. Scale bar = 200 μm
The mounted mite specimen was identified at the species level. Only adult females of the mites were captured for identification, although some were later found to be nymphs (Table S1). On sampling, we obtained one to around 20 pairs of mites per colony with the shoots of host mosses. The number of collected mite specimens per colony is indicated in Table 1. Any remaining mite individuals in the moss colony were extracted using the Berlese-Tullgren funnel. Most of these mite individuals were stored in 70% ethanol for future morphological identification, while a few were preserved in 99% ethanol for DNA extraction.
As identification of the host plant taxa based on a few moss shoots was technically difficult, a handful of moss shoots that occurred near the host shoot were used as a reference specimen. Several shoots were randomly chosen from the reference specimen and identified based on the morphology. The identified moss species was designated as the host plant. All mite specimens used in this study were deposited in the Zoological Collection of Kyoto University (KUZ) (Tables S1–S3). Reference specimens of mosses were also deposited at the herbarium of National Museum of Nature and Science (TNS) (Table S1).
Morphological identification
To distinguish species among the collected mites, we assessed 52 morphological characters in adult females that are commonly used for the species-level taxonomy of Eustigmaeus (Figs. S1 and S2; Table S4). The examined characters classified into idiosomal size, dorsal shield ornamentation, presence or absence of eye, dorsal seta length and shape, the number of seta pairs on dorsal shields, the distance between dorsal setae, endopodal shield state, the number of endopodal seta pairs, the number of aggenital and pseudanal seta pairs, were primarily extracted from the keys in Fan and Zhang (2005), Stathakis et al. (2016), and Khaustov et al. (2023). Humeral, endopodal, and aggenital shield ornamentations were also examined as their variations among congeners have been reported (Stathakis et al. 2016; Khaustov 2019; Khaustov et al. 2023). For each species, we examined one to four individuals from every collection site where that species was found (Table S4). Specimens were mounted in Hoyer’s medium.
Each species was assigned into four species groups according to the definitions by Summers and Price (1961), Chaudhri (1965), Wood (1972), and Kaźmierski (2000). Eustigmaeus species with flat, arcuate or sabre-shaped dorsal setae and one pair of aggenital seta were classified into “segnis” group; species with bushy dorsal setae and three pairs of aggenital setae were classified into “pectinatus” group; species with flat, arcuate or sabre-shaped dorsal setae and three pairs of aggenital setae, three or four pairs of paseudoanal setae, and genu II with setae k were classified into “pinnatus” group; species with varied shapes of dorsal setae (but not flat, arcuate, sabre-shaped or bushy) and two or three pairs of aggenital setae were classified into “maculatus” group. The species group assignment is indicated in Tables S1 and S2.
DNA extraction
Mite samples for DNA extraction were preserved in 99% ethanol and stored at -20 °C. Genomic DNA was extracted from a whole body of a mite specimen using a NucleoSpin® Tissue (Takara) following the manufacturer’s protocol with some modifications to retrieve the mite body. The specimen was placed at the bottom of the 1.5 ml microcentrifuge tube and left it for 30 min for dehydration; 180 μl of buffer T1 and 25 μl of Proteinase K (100 μg/ml or 20 mg/ml) were then added. Particularly for a specimen with hard sclerotization, the body was crushed with a pestle. The solution was incubated at 56 °C for 24 h, and briefly vortexed, 200 μl of buffer B3 was then added, vortexed again for 5 s, and incubated at 70 °C for 10 min. The mixture was then centrifuged for 5 min, 11,000 g at room temperature. The supernatant was then transferred to a new microcentrifuge tube. The mite body that remained in the tube was retrieved and stored in 70% ethanol, which was subsequently mounted on a microscope slide with Hoyer’s medium as a voucher specimen. The parts of the body of the voucher specimen were sometimes lacking but still preserved the most of morphological details, even in the case the body was crushed, allowing us to use it for identification. For the rest of the protocol for DNA extraction, we followed the manufacturer’s protocol.
PCR amplification and sequencing
The mitochondrial cytochrome c oxidase subunit I (COI) region was selected for DNA barcoding and validation of the morphological species. The COI region was amplified by a nested PCR, using primer sets for the initial PCR were COX1_16F and COX1_1324R; those for the second PCR were COX1_25Fshort_T and COX1_1282R_T (Klimov et al. 2018). For the initial PCR, the total reaction volume was 20 μl. This contained 8.05 μl of distilled water, 10 μl of KOD One PCR Master Mix -Blue- (TOYOBO), 0.8 μl of each primer of COX1_16F and COX1_1324R, 0.35 μl of DNA template. For the second PCR, the total PCR reaction volume was 20 μl: it contained 7.5 μl of distilled water, 10 μl of KOD One PCR Master Mix -Blue- (TOYOBO), 0.8 μl of each primer of COX1_25Fshort_T and COX1_1282R_T, 0.9 μl of the initial PCR product. The thermal cycler conditions for the first and second PCRs followed Klimov et al. (2018). The second PCR products were purified either with ExoSAP-IT or with NucleoSpin® Gel and PCR Clean-up (Takara). Sequencing was done in both directions. Sequencing reactions were conducted by Eurofins Genomics Inc. (Tokyo, Japan). After trimming the primer and low-quality regions in the obtained sequence data, 883–1163 bp of 33 OTUs were obtained. Neither indels nor stop codons were found. The sequence data were deposited on International Nucleotide Sequence Databases (INSD) through the DNA Data Bank of Japan under the accession numbers from LC789942 to LC789974.
Molecular phylogenetic analysis
A phylogenetic analysis focusing on the species within the genus Eustigmaeus was conducted to infer the phylogenetic relationships of the moss-feeding species. To determine the outgroup of Eustigmaeus, there was no available inference on the phylogenetic position of each genus in Stigmaeidae with molecular data, so we preliminary reconstructed the phylogenetic tree, including 77 sequences of 10 genera in Stigmaeidae, which based on 700–1300 bp length of COI sequences from INSD (Table S2). Two sequences of unidentified species of Homocaligus (Homocaligidae) were used as the outgroup (Table S2). The sequences were aligned with ClustalW in MEGA11 (Tamura et al. 2021). A maximum-likelihood (ML) phylogeny was reconstructed with IQ-TREE 1.6.12 (Nguyen et al. 2015). As a result, Stigmaeus akimovi (OP960167) and Stigmaeus dignus (MW367928) were selected as the outgroup due to their highest log-likelihood value.
In addition to the 33 newly obtained sequences of Eustigmaeus spp., sequences of Eustigmaeus spp., Villersia sp., Ledermuelleriopsis spp., Cheylostigmaeus spp., and Postumius sp., were downloaded from INSD and included in the ingroup (Table S2). The analyzed Eustigmaeus species were assigned into the four species groups as described in the “Morphological identification” section (Table S2). Their morphological traits were based on the original descriptions or redescriptions. The alignment of sequences for in- and outgroups followed the same method as described above. ML phylogeny was reconstructed with IQ-tree 1.6.12 (Nguyen et al. 2015). The best-fitting substitution model was estimated by Modelfinder (Kalyaanamoorthy et al. 2017), and K3Pu + F + I + G4, TN + F + R2, GTR + F + I + G4 were then selected for 1st, 2nd, 3rd positions, respectively. UF-bootstrap (Hoang et al. 2018) and SH-like approximate likelihood ratio test (SH-aLRT; Guindon et al. 2010) with 1000 replicates were performed. Clades with support values of both SH-aLRT ≥ 80% and UFboot ≥ 95% were considered reliable (Minh et al. 2022). The resultant tree was visualized with Figtree v1.4.4 (Rambaut 2018). The genetic distances were calculated by Kimura 2-parameter model (Kimura 1980) with MEGA 11, with pairwise deletion of missing data (Tamura et al. 2021).
Species delimitation analysis
To examine the species delimitation based on genetic distance and to determine if it aligns with the boundary of newly recognized morphospecies, the species delimitation tests, Automatic Barcoding Gap Discovery (ABGD; Puillandre et al. 2012) and Assemble Species by Automatic Partitioning (ASAP; Puillandre et al. 2021), were applied to the COI dataset of OTUs comprising Clade A (Fig. 4). The ABGD analysis was performed in a webserver (https://bioinfo.mnhn.fr/abi/public/abgd/abgdweb.html) using the K2P model, with default prior value of maximum intraspecific distance (Pmin = 0.001, Pmax = 0.1), relative gap width (X) was specified to 1.0, with1000 steps. The ASAP analysis is a hierarchical clustering algorithm based on the pairwise genetic distance. This method does not require a user to define any prior value and provides a score for each partition which helps the user identify the best one. The ASAP analysis was carried out online (https://bioinfo.mnhn.fr/abi/public/asap/asapweb.html) using a default setting with the K2P model. As the great sequence length variation was present in the datasets (ranging from 792 to 1216 bp), the input sequences were trimmed to 740 bp to remove the effects of missing data.
Results
Species identification and phylogenetic relationship
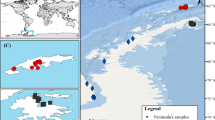
The moss-feeding species of Eustigmaeus were collected from 36 localities in Japan (Fig. 3; Table 1), which were all identified as the member of the “maculatus” group and distinguished into 10 species based on a combination of morphological characters such as dorsal shield ornamentation, dorsal seta length and shape, and the number of aggenital setae pairs (shown in Figs. S1 and S2; Table S4). These species were all likely to be new to Japan because their morphological features were clearly distinct from those of the four Eustigmaeus species—E. anauniensis (Canestrini, 1889), E. lirellus (Summers and Price 1961), E. segnis (Koch, 1836), E. arcuatus (Chaudhri 1965)—recorded from Japan (Beron 2020). In the following, they are tentatively referred to as ‘Eustigmaeus sp. 1’, although this treatment does not necessarily indicate that they are undescribed species. The COI sequence of all morphospecies except Eustigmaeus sp. 5 was obtained. For the widely distributed species, Eustigmaeus sp. 1, Eustigmaeus sp. 2, Eustigmaeus sp. 3, and Eustigmaeus sp. 9, the sequences were based on individuals from localities that largely cover their distribution (Table S2). However, the sequence of the Eustigmaeus sp. 4 sampled from LC 29 was not obtained.
Sampling localities of moss-feeding Eustigmaeus species. Locality codes correspond to those in Table 1
The resultant phylogeny recovered four clades (labeled Clade A–D). Of these, Clade D was robustly supported, but the other clades were less supported, and the phylogenetic relationships between these clades could not be fully resolved. Clade A (SH-aLRT = 87.2%, UFBoot = 80%) included all examined “maculatus” species and one Villersia species. Clade B contained a part of “pectinatus” species. Clade C was composed of the other part of “pectinatus” species, “pinnatus” species, and Ledermuelleriopsis species. Clade D consisted of “segnis” species. A clade consisting of Cheylostigmaeus and Postumius species was resolved as a sister of the four clades.
Within Clade A, the Japanese moss-feeding species did not form a monophyletic group. The respective monophyly was well-supported for four morphological species from Japan (Eustigmaeus sp. 1, Eustigmaeus sp. 2, Eustigmaeus sp. 3, and Eustigmaeus sp. 7), but weakly supported for Eustigmaeus sp. 9 (SH-aLRT = 80.8%, UFBoot = 70%). Three of the four singletons of morphospecies respectively formed a clade with a different named species from abroad. Pairwise genetic distances were varied among closely related species, e.g., 1.6% between Eustigmaeus sp. 8 and Eustigmaeus pseudolacunus and 6.1% between Eustigmaeus sp. 6 and Eustigmaeus grandis (Table S5). Eustigmaeus sp. 9 formed a well-supported clade (SH-aLRT = 97.3%, UFBoot = 98%) with Eustigmaeus rhodomelus (OL863247 and OP960172). The non-monophyly of Eustigmaeus rhodomelus may be related to its genetic differences among the four sequences because they were taken from a geographically wide range (Table S2).
The pairwise genetic distance analysis between 80 OTUs showed that intraspecific divergence was below 3.7%, while interspecific divergence exceeded 7.8% in the morphological species (Table S5). The intra- and inter-specific distances between Japanese moss-feeding species are summarized in Table 2. The partitioning hypotheses inferred by ABGD and ASAP are presented in Fig. 4. The ABGD detected 20 (P = 0. 006805–0.024626), 24 (P = 0.002707–0.006774), and 27 (P = 0.001000–0.002719) groups. The ASAP assigned the best score (asap-score = 2.50) to the 20 groups hypothesis. In the 20-grouping hypothesis, nine morphospecies were classified into nine discrete groups. Eustigmaeus rhodomelus from Russia (OL863247 and OP960172) and Eustigmaeus pseudolacunus were assigned to the same group as Eustigmaeus sp. 9 and Eustigmaeus sp. 8, respectively. In the 24-grouping hypothesis, a group composed of Eustigmaeus sp. 9 and Eustigmaeus rhodomelus (OL863247 and OP960172) was split into four, and Eustigmaeus sp. 2 group was separated into two. In the 27-grouping hypothesis, in addition to the subdivision in the 24-grouping hypothesis, a group of Eustigmaeus sp. 1 was classified into four groups.
Phylogenetic relationships for moss-feeding Eustigmaeus species reconstructed by maximum-likelihood analysis based on mitochondrial COI gene sequences. Sequences newly obtained in this study are shown in bold. Nodal value (%) consists of SH-aLRT value and UF-boot value; the values are not shown for some terminal nodes. Symbol near the tip indicates species group assignment. Bars on the right represent the results of species delimitation analyses
Distribution, habitat, and host plant use
Although the species sampled from more than seven localities, Eustigmaeus sp. 1, Eustigmaeus sp. 2, Eustigmaeus sp. 3, and Eustigmaeus sp. 9, were widely distributed in Japan, the geographic ranges of the other species (i.e., Eustigmaeus sp. 4, Eustigmaeus sp. 5, Eustigmaeus sp. 6, Eustigmaeus sp. 7, Eustigmaeus sp. 8, and Eustigmaeus sp. 10) were not clarified in this study because they were collected only from one or two localities (Fig. 3; Table 1). The distributions of Eustigmaeus sp. 1, Eustigmaeus sp. 2, Eustigmaeus sp. 3, and Eustigmaeus sp. 9 were overlapped with each other. Notably, multiple species were frequently sampled from the same locality or closely located locality (Fig. 3; Table 1). For example, Eustigmaeus sp. 1, Eustigmaeus sp. 3, Eustigmaeus sp. 7, Eustigmaeus sp. 9 were sampled from closely located localities (LC 33–36), and distances between them were less than 10 km.
The moss-feeding species were found in a moss colony that was constantly wet retaining high humidity and water supply. Such moss patches were on various substrates in a diverse ecological setting (Fig. 1; Table 1). Some mite species tended to be sampled from a specific type of habitat. In the case of frequently-sampled Eustigmaeus sp. 1, Eustigmaeus sp. 2, Eustigmaeus sp. 3, and Eustigmaeus sp. 9, we observed the following patterns. Eustigmaeus sp. 1 was consistently sampled from mosses on wet rocks and boulders around the stream and river across all nine patches. Eustigmaeus sp. 2 was predominantly collected from rock faces and the concrete walls in wet environments, observed in nine out of 13 patches. Eustigmaeus sp. 3 was found on rock faces along pavement roads or mountain paths in eight out of 10 patches. Eustigmaeus sp. 9 exhibited a wider range of habitat variability, including open areas with relatively low surrounding humidity, at patch code AP and AS (Table 1).
Table 1 shows a list of 10 moss-feeding species and their respective host plants, encompassing 26 species in total. These host plants belong to the Bryopsida, comprising 18 genera, 11 families, and five orders. Based on the field observations, different host plant use among the frequently-sampled species was suggested: Eustigmaeus sp. 1, Eustigmaeus sp. 2, Eustigmaeus sp. 3, and Eustigmaeus sp. 9. Eustigmaeus sp. 1 exclusively inhabited Hypnales mosses at all nine patches, with a majority of samples found on Rhynchostegium riparioides. Eustigmaeus sp. 2 predominantly used Philonotis species across 12 out of 13 patches. While Eustigmaeus sp. 3 was exclusively discovered from the dicranid moss lineages, Pottiales and Dicranales, we found two examples (patch codes AB, AE) that an individual was on other lineages of mosses shoots with feeding mark that coexisted in a Dicranidae moss colony. Eustigmaeus sp. 3 may feed on them as well. Eustigmaeus sp. 9 was found in several distantly related moss species, suggesting its polyphagous nature.
Notably, at certain localities, different mite species within a pair specifically occurred on different mosses growing in immediate proximity. At LC 4, although both Eustigmaeus sp. 4 and Eustigmaeus sp. 5 were present in the same patch on a concrete wall (Fig. 1e; Table 1), Eustigmaeus sp. 4 was only collected from Gollania ruginosa and Eustigmaeus sp. 5 was mainly found from Rosulabryum capillare. Similarly, the recorded host-plant species were distinctive between a pair of Eustigmaeus sp. 2 and Eustigmaeus sp. 9 at patch code P in LC 13, Eustigmaeus sp. 9 occurred on Bryum cyclophyllum and Philonotis falcata but Eustigmaeus sp. 2 used Philonotis turneriana. In other instances, a pair of mite species used different moss species from different patches within the same locality. At LC 8, Eustigmaeus sp. 6 was sampled from Brachythecium rivulare on the wet concrete wall and nearby along a pavement road, and Eustigmaeus sp. 1 inhabited Rhynchostegium riparioides on a wet rock in a stream (Fig. 1a; Table 1). Additionally, Eustigmaeus sp. 7 was collected from Pseudohygrohypnum eugyrium on a wet boulder by a river (Fig. 1f) but Eustigmaeus sp. 9 used Bryum recurvulum on a wet concrete beside a pavement road at LC 35 (Table 1). We noted only two cases where multiple Eustigmaeus species coexisted within the same colony of a particular moss species (i.e., Eustigmaeus sp. 2 and Eustigmaeus sp. 9 from Pohlia sp. at LC 2, Eustigmaeus sp. 3 and Eustigmaeus sp. 9 from Brachythecium helminthocladum at LC 26). Eustigmaeus sp. 2 and Eustigmaeus sp. 9 both used Philonotis falcata at LC 13 but they were derived from different patches. With these two exceptions, Eustigmaeus species sharing the same host plant did not coexist within the same colony in the field (e.g., Eustigmaeus sp. 2 and Eustigmaeus sp. 9 shared Philonotis falcata, Eustigmaeus sp. 1 and Eustigmaeus sp. 7 shared Entodon luridus, Eustigmaeus sp. 5 and Eustigmaeus sp. 9 shared Bryum paradoxum, Eustigmaeus sp. 1 and Eustigmaeus sp. 9 shared Rhynchostegium riparioides, and Eustigmaeus sp. 6 and Eustigmaeus sp. 8 shared Brachythecium rivulare) (Table 1).
Discussion
Diversity of moss-feeding species
The field sampling and microscopic observation among 10 species have provided a glimpse into the previously overlooked diversity of moss-feeding species in Eustigmaeus. These 10 species have been identified as distinct entities based on the morphological identification, phylogenetic analyses, and genetic distance-based species delimitation methods (Fig. 4; Table S4), except for Eustigmaeus sp. 5, for which DNA sequence data was not obtained. Although ABGD provided three partitioning hypotheses to the species within Clade A (20, 24, and 27 groups), the 20-grouping hypothesis that ASAP gave the best score (threshold value was 5%) was thus consistent with the morphological identification. However, the taxonomic position of these species warrants further taxonomic studies. It is important to highlight that six of 10 species have only been collected from one or two localities, suggesting that the true extent of diversity within moss-feeding Eustigmaeus mites remains largely unexplored.
The 10 species discovered in this study, as well as the four species previously reported as moss-feeders (Gerson 1972), all fall within the “maculatus” group (Summers and Price 1961; Fan and Zhang 2004). The phylogeny represented the “maculatus” species formed a clade with a Villersia species. Although the species in Villersia are characterized by the presence of setae sce and d2 on independent platelets [seta d2 of Villersia jamaliensis is not on independent platelet but included in this genus by Khaustov (2019)], other characteristics are generally in common with “maculatus” species in Eustigmaeus (Summers 1966; Wood 1972). Given the morphological similarities and the result of phylogenetic analysis, it is inferred that the autapomorphy of Villersia is merely a variation within the “maculatus” group and this genus is reasonably considered as a member of this group. Currently, the species that matches the definition of the “maculatus” group counts more than 50 species. Revealing the prevalence of moss-feeding habit within this group is crucial for understanding the evolution of this habit. Additionally, to trace the origin of the moss-feeding habit found in Eustigmaeus, it is necessary to solve the phylogenetic relationships among different species groups and related taxa. It is also crucial to accumulate knowledge regarding their feeding habits, an aspect that is largely lacking in the existing body of information.
Ecology of moss-feeding species
Extensive field sampling of Eustigmaeus species and accurate host determination accumulated knowledge on their host plant records and geographic ranges. For frequently-sampled species, Eustigmaeus sp. 1, Eustigmaeus sp. 2, Eustigmaeus sp. 3, the geographic ranges were overlapped, as did the period of occurrence for adult females, while they differ in their utilization of host plants (Fig. 3; Table 1). The recorded host plant lineages, Hypnales, Philonotis, Dicranidae, are phylogenetically and morphologically distant from each other (Liu et al. 2019). Furthermore, these species exhibited different preferences for environmental conditions in the current study (Table 1). These findings suggest that the distinct niches for each mite species are shaped not only by the inherent characteristics of the moss plant body, such as chemistry and gametophyte morphology, but also by various factors influenced by the environment, including abiotic factors and predator fauna. These factors possibly play a role in determining the host plant use of the three Eustigmaeus species.
We rarely observed multiple Eustigmaeus species coexisting within the same colony of a given moss species in the field, even though some mite species shared the same host plant (Table 1). Phytophagous mites are known to undergo long-distance dispersal mainly through aerial dispersal (Li and Margolies 1993; Lawson et al. 1996; Osakabe et al. 2008; Michalska et al. 2010; Galvão et al. 2012; Majer et al. 2021), which may also be the case for Eustigmaeus. Consequently, if the distributions of Eustigmaeus species overlap, it is possible for them to disperse to host plants already occupied by other mite species. This scenario could lead to frequent interactions between the established mite species and newly arrived species on mosses. Therefore, interspecific interactions such as resource competition (López-Olmos and Ferragut 2023) and reproductive interference (Takafuji et al. 1997), which prevent the coexistence of species on a host plant, may also play a key role in determining the host plant use among Eustigmaeus species.
Moss-feeding Eustigmaeus species demonstrate a preference for constantly moist moss colonies (Table 1). This preference may be linked to the poikilohydry of moss. Mosses are poikilohydric plants, meaning that their bodies dehydrated when exposed to dry conditions and rehydrate when water becomes available (Proctor 2008). However, as Eustigmaeus mites rely on fluid feeding, it is likely that they cannot suck out the cell contents from dehydrated moss cells. Consequently, mosses growing in periodically drying environments may not be suitable as host plants, and the mites may instead favor mosses in constantly wet environments. Such mosses would provide a stable source of food for the mites, as they would remain hydrated and available at all times. Therefore, drying environment may strikingly restrict the host plant use of Eustigmaeus over the natures of moss gametophyte.
The current study highlights the genus Eustigmaeus as a rare group that contains diverse moss-feeding species, with some species exhibiting specific host plant use. Mosses are seldom consumed by phytophagous arthropods (Gerson 1969, 1982) and some studies have shown that mosses are typically unsuitable food resources for generalist consumers in arthropods, likely due to their chemical defense mechanisms (Markham et al. 2006; Parker et al. 2007; Haines and Renwick 2009; Duhin et al. 2022). Moreover, known moss-feeding insects are either host-specific but less diverse (Imada 2021), diverse but not host-specific (Pyszko et al. 2020; Ruan et al. 2020), or for which information about host plants is scarce (Gerson 1982). The association between Eustigmaeus mites and mosses thus presents a promising model for studying the macroevolutionary patterns of diversification in moss-feeding arthropods. However, the complex associations between Eustigmaeus species and mosses are only partially understood. Thus, further information on the diversity of moss-feeding species and their host plant use around the world is necessary to fully elucidate these relationships.
Data availability
The sequence data were deposited on International Nucleotide Sequence Databases (INSD) through the DNA Data Bank of Japan under the accession numbers from LC789942 to LC789974.
References
Abonnenc E (1970) Notes sur les acariens parasites des Phlébotomes. Cah ORSTOM Entomol Médicale Parasitol 8:89–94
Beron P (2020) Acarorum Catalogus VII. Trombidiformes, Prostigmata, Raphignathoidea Fam. Barbutiidae, Caligonellidae, Camerobiidae, Cryptognathidae, Dasythyreidae, Dytiscacaridae, Eupalopsellidae, Homocaligidae, Mecognathidae, Raphignathidae, Stigmaeidae, Xenocaligonellididae. Pensoft Publishers and the National Museum of Natural History, Sofia
Bizarro GL, Noronha ACS, Silva GL, Ferla NJ, Johann L (2020) A new species of Eustigmaeus Berlese (Acari: Stigmaeidae) from Brazil. Acarologia 60:825–830. https://doi.org/10.24349/acarologia/20204403
Bolland HR, Gutierrez J, Flechtmann CHW (1998) World catalogue of the spider mite family (Acari: Tetranychidae). Brill Academic Publishers, Leiden
Chaudhri WM (1965) New mites of the genus Ledermuelleria. Acarologia 7:467–486
De Lillo E, Di Palma A, Nuzzaci G (2001) Morphological adaptations of mite chelicerae to different trophic activities (Acari). Entomologica 35:125–180. https://doi.org/10.15162/0425-1016/735
Duhin A, Machado RAR, Turlings TCJ, Röder G (2022) Early land plants: plentiful but neglected nutritional resources for herbivores? Ecol Evol 12:1–14. https://doi.org/10.1002/ece3.9617
Fan Q-H, Zhang Z-Q (2004) Revision of raphignathoid mites (Acari: Prostigmata) in the collection of H Habeeb. Zootaxa 763:1–28. https://doi.org/10.11646/zootaxa.763.1.1
Fan Q-H, Zhang Z-Q (2005) Raphignathoidea (Acari: Prostigmata). Fauna New Zeal 52:1–400
Fan Q-H, Flechtmann CHW, De Moraes GJ (2016) Annotated catalogue of Stigmaeidae (Acari: Prostigmata), with a pictorial key to genera. Zootaxa 4176:1–199. https://doi.org/10.11646/zootaxa.4176.1.1
Flechtmann CHW (1985) Eustigmaeus bryonemus, sp. n., a moss-feeding mite from Brasil (Acari, Prostigmata: Stigmaeidae). Rev Bras Zool 2:387–391. https://doi.org/10.1590/s0101-81751984000200010
Galvão AS, Melo JWS, Monteiro VB, Lima DB, De Moraes GJ, Gondim MGC Jr (2012) Dispersal strategies of Aceria guerreronis (Acari: Eriophyidae), a coconut pest. Exp Appl Acarol 57:1–13. https://doi.org/10.1007/s10493-012-9527-z
Gerson U (1969) Moss-arthropod associations. Bryologist 72:495–500. https://doi.org/10.2307/3241388
Gerson U (1972) Mites of the genus Ledermuelleria (Prostigmata: Stigmaeidae) associated with mosses in Canada. Acarologia 13:319–343
Gerson U (1982) Bryophytes and invertebrates. In: Smith A (ed) Bryophyte ecology. Springer, Dordrecht, pp 291–332
Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321. https://doi.org/10.1093/sysbio/syq010
Haines WP, Renwick JAA (2009) Bryophytes as food: comparative consumption and utilization of mosses by a generalist insect herbivore. Entomol Exp Appl 133:296–306. https://doi.org/10.1111/j.1570-7458.2009.00929.x
Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS (2018) UFBoot2: improving the ultrafast bootstrap approximation. Mol Biol Evol 35:518–522. https://doi.org/10.1093/molbev/msx281
Imada Y (2021) Moss mimesis par excellence: integrating previous and new data on the life history and larval ecomorphology of long-bodied craneflies (Diptera: Cylindrotomidae: Cylindrotominae). Zool J Linn Soc 193:1156–1204. https://doi.org/10.1093/zoolinnean/zlaa177
Imada Y, Kato M (2016) Bryophyte-feeders in a basal brachyceran lineage (Diptera: Rhagionidae: Spaniinae): adult oviposition behavior and changes in the larval mouthpart morphology accompanied with the diet shifts. PLoS ONE 11:1–20. https://doi.org/10.1371/journal.pone.0165808
Imada Y, Kawakita A, Kato M (2011) Allopatric distribution and diversification without niche shift in a bryophyte-feeding basal moth lineage (Lepidoptera: Micropterigidae). Proc R Soc B Biol Sci 278:3026–3033. https://doi.org/10.1098/rspb.2011.0134
Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods 14:587–589. https://doi.org/10.1038/nmeth.4285
Kato M, Yamamori L, Imada Y (2022) Diversity underfoot of agromyzids (Agromyzidae, Diptera) mining thalli of liverworts and hornworts. Zookeys 1133:1–164. https://doi.org/10.3897/zookeys.1133.94530
Kaźmierski A (2000) Prostigmatic mites (Acari: Actinedida) from the nature reserve słońsk. part I: the families stigmaeidae, raphignathidae. Caligonellidae and Camerobiidae Biol Bull Pozn 37:317–325
Khaustov AA (2019) Contribution to systematics of the genus Eustigmaeus (Acari: Stigmaeidae) of Russia. Acarologia 59:152–173. https://doi.org/10.24349/acarologia/20194320
Khaustov AA (2021a) Contribution to the Stigmaeidae (Acari: Prostigmata) fauna of the Altai Republic, Russia. Acarina 29:43–66. https://doi.org/10.21684/0132-8077-2021-29-1-43-66
Khaustov AA (2021b) A new species and new records of Stigmaeidae (Acari: Prostigmata) from Western Siberia. Acarina 29:169–188. https://doi.org/10.21684/0132-8077-2021-29-2-169-188
Khaustov AA, Kravchenko SV, Kazakov DV (2023) Two new species and a new synonym of Eustigmaeus (Acari: Stigmaeidae) from Russia with COI barcode. Acarina 31:77–99. https://doi.org/10.21684/0132-8077-2023-31-1-77-99
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120. https://doi.org/10.1007/BF01731581
Klimov PB et al (2018) Comprehensive phylogeny of acariform mites (Acariformes) provides insights on the origin of the four-legged mites (Eriophyoidea), a long branch. Mol Phylogenet Evol 119:105–117. https://doi.org/10.1016/j.ympev.2017.10.017
Krantz GW, Lindquist EE (1979) Evolution of phytophagous mites (Acari). Annu Rev Entomol 24:121–158. https://doi.org/10.1146/annurev.en.24.010179.001005
Lawson DS, Nyrop JP, Dennehy TJ (1996) Aerial dispersal of European red mites (Acari: Tetranychidae) in commercial apple orchards. Exp Appl Acarol 20:193–202. https://doi.org/10.1007/BF00054511
Li J, Margolies DC (1993) Effects of mite age, mite density, and host quality on aerial dispersal behavior in the twospotted spider mite. Entomol Exp Appl 68:79–86. https://doi.org/10.1111/j.1570-7458.1993.tb01691.x
Lindquist EE (1998) Evolution of phytophagy in trombidiform mites. Exp Appl Acarol 22:81–100. https://doi.org/10.1023/A:1006041609774
Lindquist EE, Oldfield GN (1996) Evolution of eriophyoid mites in relation to their host plants. In: Lindquist EE, Sabelis MW, Bruin J (eds) Eriophyoid mites—their biology, natural enemies and control. World crop pests, vol 6. Elsevier Science Publishing, Amsterdam, pp 277–300
Liu Y, Johnson MG, Cox CJ, Medina R, Devos N, Vanderpoorten A, Hedenäs L, Bell NE, Shevock JR, Aguero B, Quandt D, Wickett NJ, Shaw AJ, Goffinet B (2019) Resolution of the ordinal phylogeny of mosses using targeted exons from organellar and nuclear genomes. Nat Commun 10:1485. https://doi.org/10.1038/s41467-019-09454-w
López-Olmos S, Ferragut F (2023) The newcomer takes it all: the invader Texas citrus mite, Eutetranychus banksi (Acari: Tetranychidae), displaces the resident relatives in citrus agrosystems. Biol Invasions 25:3171–3192. https://doi.org/10.1007/s10530-023-03099-z
Majer A, Laska A, Hein G, Kuczyński L, Skoracka A (2021) Hitchhiking or hang gliding? Dispersal strategies of two cereal-feeding eriophyoid mite species. Exp Appl Acarol 85:131–146. https://doi.org/10.1007/s10493-021-00661-z
Majidi M, Hajiqanbar H, Saboori A (2019) Parasitic stigmaeid mites (Acari: Stigmaeidae) of phlebotomine sandflies (Diptera: Psychodidae) in Fars Province, Southern Iran. Int J Acarol 45:41–47. https://doi.org/10.1080/01647954.2018.1549098
Markham K, Chalk T, Stewart CN Jr (2006) Evaluation of fern and moss protein-based defenses against phytophagous insects. Int J Plant Sci 167:111–117. https://doi.org/10.1086/497651
Michalska K, Skoracka A, Navia D, Amrine JW (2010) Behavioural studies on eriophyoid mites: an overview. Exp Appl Acarol 51:31–59. https://doi.org/10.1007/s10493-009-9319-2
Minh BQ, Lanfear R, Ly-Trong N, Trifinopoulos J, Schrempf D, Schmidt HA (2022) IQ-TREE version 2.2.0: Tutorials and manual. Phylogenomic software by maximum likelihood. http://www.iqtree.org. Accessed 22 November 2023
Nguyen LT, Schmidt HA, Von Haeseler A, Minh BQ (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32:268–274. https://doi.org/10.1093/molbev/msu300
Osakabe M, Isobe H, Kasai A, Masuda R, Kubota S, Umeda M (2008) Aerodynamic advantages of upside down take-off for aerial dispersal in Tetranychus spider mites. Exp Appl Acarol 44:165–183. https://doi.org/10.1007/s10493-008-9141-2
Parker JD, Burkepile DE, Collins DO, Kubanek J, Hay ME (2007) Stream mosses as chemically-defended refugia for freshwater macroinvertebrates. Oikos 116:302–312. https://doi.org/10.1111/j.2007.0030-1299.15289.x
Proctor MCF (2008) Physiological ecology. In: Goffinet B, Shaw AJ (eds) Bryophyte Biology, 2nd edn. Cambridge University Press, Cambridge, pp 237–268
Puillandre N, Lambert A, Brouillet S, Achaz G (2012) ABGD, automatic barcode gap discovery for primary species delimitation. Mol Ecol 21:1864–1877. https://doi.org/10.1111/j.1365-294X.2011.05239.x
Puillandre N, Brouillet S, Achaz G (2021) ASAP: assemble species by automatic partitioning. Mol Ecol Resour 21:609–620. https://doi.org/10.1111/1755-0998.13281
Pyszko P, Plášek V, Drozd P (2020) Don’t eat where you sleep: unexpected diversity of food web for beetles feeding on mosses. Insect Conserv Divers 14:325–334. https://doi.org/10.1111/icad.12453
Rambaut A (2018) FigTree v1.4.4. http://tree.bio.ed.ac.uk/software/figtree/. Accessed 23 April 2023
Reeves WK, Kato CY, Gilchriest T (2008) Pathogen screening and bionomics of lutzomyia apache (Diptera: Psychodidae) in Wyoming, USA. J Am Mosq Control Assoc 24:444–447. https://doi.org/10.2987/5745.1
Ruan Y, Konstantinov AS, Damaška AF (2020) The biology and immature stages of the moss-eating flea beetle Cangshanaltica fuanensis sp. nov. (Coleoptera, Chrysomelidae, Galerucinae, Alticini), with description of a fan-driven high-power Berlese funnel. Insects 11:1–27. https://doi.org/10.3390/insects11090571
Stathakis TI, Kapaxidi EV, Papadoulis GTh (2016) The genus Eustigmaeus Berlese (Acari: Stigmaeidae) from Greece. Zootaxa 4191:1–102. https://doi.org/10.11646/zootaxa.4191.1.1
Summers FM (1966) Genera of the mite family Stigmaeidae Oudemans (Acarina). Acarologia 8:230–250
Summers FM, Price DW (1961) New and redescribed species of Ledermuelleria from North America (Acarina: Stigmaeidae). Hilgardia 31:369–382
Swift SF (1994) The superfamily Raphignathoidea (Acari: Acariformes) of the Hawaiian Islands: taxonomy, ecology and distribution. Dissertation, University of Hawaii
Takafuji A, Kuno E, Fujimoto H (1997) Reproductive interference and its consequences for the competitive interactions between two closely related Panonychus spider mites. Exp Appl Acarol 21:379–391. https://doi.org/10.1023/a:1018423711166
Tamura K, Stecher G, Kumar S (2021) MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol 38:3022–3027. https://doi.org/10.1093/molbev/msab120
Ueno T, Osono T, Kanda H (2009) Inter- and intraspecific variations of the chemical properties of high-Arctic mosses along water-regime gradients. Polar Sci 3:134–138. https://doi.org/10.1016/j.polar.2009.04.001
Wood TG (1972) New and redescribed species of Ledermuelleria Oudms. and Villersia Oudms. (Acari: Stigmaeidae) from Canada. Acarologia 13:301–318
Zhang Z-Q, Gerson U (1995) Eustigmaeus johnstoni, new species (Acari: Stigmaeidae), parasitic on phlebotomine sandflies (Diptera: Psychodidae). Tijdschr Voor Entomol 138:297–301
Zhang Z-Q, Fan Q-H, Pesic V, Smit H, Bochkov AV, Khaustov AA, Baker A, Wohltmann A, Wen T, Amrine JW, Beron P, Lin J, Gabrys G, Husband R (2011) Order Trombidiformes Reuter, 1909. In: Zhang, Z-Q (ed) Animal biodiversity: an outline of higher-level classification and survey of taxonomic richness. Zootaxa 3148:129–138. https://doi.org/10.11646/zootaxa.3148.1.24
Acknowledgements
We would like to thank Hiroki Hata, Tomoyuki Katagiri, Takafumi Nakano, and Taku Okamoto for their fruitful discussions. We are particularly thankful to Takafumi Nakano for providing insightful comments on a previous version of the manuscript. Special thanks go to Makiko Fukui for lending us a microscope at Ehime University and to Ryoei Miyoshi for assisting with sampling. We are also grateful to Satoshi Shimano, Minoru Shiba, and Mohamed W. Negm for sharing their knowledge on acarology, and to Alexander A. Khaustov and Denis V. Kazakov for their guidance on DNA amplification methods. We would like to acknowledge Taro Jonishi for his advice on molecular analysis.
Funding
This research received partial support from the Sasakawa Scientific Research Grant from the Japan Science Society under grant number 2023–5007 to Satsuki Ikeda, as well as the Grant-in-Aid for Scientific Research (KAKENHI) grant numbers 18H06077, JP20K15852, JP22H02684 from the Japan Society for the Promotion of Science (JSPS) to Yume Imada.
Author information
Authors and Affiliations
Contributions
Satsuki Ikeda and Yume Imada designed this study, conducted field sampling, and acquired funds. Satsuki Ikeda prepared the specimens, recorded the specimen data, conducted molecular analysis, and wrote the main manuscripts. Yuya Inoue identified moss specimens and edited the manuscript. Yume Imada supervised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Ethical approval
No approval of research ethics committees was required to accomplish this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ikeda, S., Inoue, Y. & Imada, Y. Unveiled species diversity of moss-feeding mites (Stigmaeidae: Eustigmaeus): a research on their distribution, habitat, and host plant use in Japan. Exp Appl Acarol (2024). https://doi.org/10.1007/s10493-024-00954-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10493-024-00954-z