Abstract
A glycoside hydrolase family 32 exo-inulinase gene was cloned from Sphingomonas sp. JB13 and expressed in Escherichia coli BL21 (DE3). The purified recombinant enzyme (rInuAJB13) showed an apparently optimal activity at pH 5.5 and 55 °C and remained activity at 10–70 °C. The addition of most metal ions and chemical reagents showed little or no effect (retaining more than 76.5 % activity) on the enzyme activity, notably the addition of surfactants SDS, CTAB, Tween 80, and Triton X-100. Most local liquid detergents, including Balin, Walch, Ariel, Tide, Tupperware, and Bluemoon, also showed little or no effect (retaining more than 77.8 % activity) on the enzyme activity. rInuAJB13 exhibited 135.3–163.6 % activity at the NaCl concentration of 1.0–4.5 M. After incubation with up to 57.0 mg mL−1 trypsin and 90.0 mg mL−1 proteinase K at 37 °C for 60 min (pH 7.2), rInuAJB13 retained more than 80 % of its initial activity. The enzyme presents a high proportion (28.0 %) of amino acid residues G, A, and V. This paper is the first to report a detergent-, salt-, and protease-tolerant exo-inulinase.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Inulin, a reserve polysaccharide of plant origin, is composed of α-d-glucopyranosyl-[β-(2,1)-d-fructofuranosyl-d-fructofuranosides] containing 2–140 fructose units (Vijayaraghavan et al. 2009). Most of the plants do not store starch but synthesize and store inulin in their roots, tubers, and bulbs, such as chicory, Jerusalem artichoke, and camas (Vijayaraghavan et al. 2009). Inulin content is as high as up to 22 % of the fresh weight and 80 % of the dry weight of these plants (Kango and Jain 2011). Given the abundance of inulin, inulin is a prominent candidate for being a renewable carbohydrate source and can be produced at the industrial level for various biotechnological uses.
Hydrolysis of inulin for producing fructo-oligosaccharides and fructose, which are important ingredients in food, pharmaceutical, and energy industries, is one of the important biotechnological uses (Kango and Jain 2011; Lima et al. 2011). Compared to the enzymatic hydrolysis, the chemical hydrolysis of inulin is not favourable due to its high operational cost, undesired degradations, and the formation of by-products (Vijayaraghavan et al. 2009). Exo-inulinases (EC 3.2.1.80; fructan β-fructosidases, exo-β-d-fructosidases) are capable of efficiently hydrolysing inulin to produce fructose yields as high as 90–95 % (Lima et al. 2011). In addition, many exo-inulinases from bacteria possess a wide substrate specificity: they hydrolyse levan, sucrose, raffinose, and stachyose as well (Kango and Jain 2011; Vijayaraghavan et al. 2009). Based on their amino acid sequence similarities, exo-inulinases fall into the glycoside hydrolase (GH) family 32 (Cantarel et al. 2009) and carry the consensus pattern H–x(2)-[PV]-x(4)-[LIVMA]-N-D-P–N-[GA] (http://prosite.expasy.org/PS00609).
Specific applications of enzymes usually require one or more significant enzymatic characteristics, such as detergent tolerance for detergent industry (Vijayalaxmi et al. 2013), protease tolerance for food and feed industries (Ghazi et al. 2003), and salt tolerance for processing of sea foods and saline foods (Guo et al. 2009). An exo-inulinase possessing all of the above characteristics has potential in versatile industrial applications and represents a good material for basic research on the structure adaptation to function. However, among the published studies to date, there has not detailed report on an exo-inulinase which is detergent-, salt-, and protease-tolerant.
In this study, a novel exo-inulinase gene from Sphingomonas sp. JB13 was successfully cloned and expressed in Escherichia coli. The purified recombinant enzyme was tolerant to detergents, NaCl, and proteases. Furthermore, the enzyme presents a high proportion (28.0 %) of amino acid residues G, A, and V.
Materials and methods
Bacterial strain, vectors, and reagents
The strain Sphingomonas sp. JB13, a potential novel species isolated from slag of a phosphate rock-stacking site, has been deposited in the China General Microbiological Culture Collection Center under CGMCC 1.10968. The details of strain isolation and identification were described in our previous study (Zhou et al. 2012).
Genomic DNA and plasmid isolation kits were purchased from Tiangen (Beijing, China). DNA polymerases were purchased from TaKaRa (Otsu, Japan). The gene was cloned using a pEASY-T1 vector and E. coli Trans1-T1 and then expressed using a pEASY-E2 vector and E. coli BL21 (DE3) (TransGen, Beijing, China). The Ni2+-NTA agarose used to purify the recombinant protein (His-tagged) was purchased from Qiagen (Valencia, CA, USA). Inulin from dahlia tubers (I3754), d-fructose (F0127), raffinose (R0514), and starch (S9765) were purchased from Sigma (St. Louis, MO, USA). Proteinase K, trypsin (1:250), and isopropyl-β-d-1-thiogalactopyranoside (IPTG) were purchased from Amresco (Solon, OH, USA). Levan from Zymomonas mobilis was purchased from Advanced Technology & Industrial (Hong Kong, China), stachyose from TCI (Shanghai, China), fructo-oligosaccharides set including kestose, nystose, and fructofuranosylnystose from Wako Pure Chemical (Osaka, Japan), and silica gel G plate from Haiyang (Qingdao, China). All other reagents were analytical grade and commercially available.
Gene cloning
The genomic DNA from the strain JB13 was extracted using a Tiangen genomic DNA isolation kit following the manufacturer’s instructions. Two degenerate primers, eInu32F (CCCACAACTGGATGAACgayccnaaygg) and eInu32R (TCTGCTCGTGCCAGAACAcnttnggrtcnc) (Zhou et al. 2014), were used to clone a partial exo-inulinase gene. A touchdown-PCR was set as described in our previous study (Zhou et al. 2014).
Based on the partial exo-inulinase gene, the full-length gene inuAJB13 was amplified via a GC TAIL-PCR procedure (thermal asymmetric interlaced-PCR specific for GC-rich genes) (Zhou et al. 2010) with four nested insertion-specific primers: inuAJB13uSP1 (AGAACGCCATCACGTCGGTTTCG), inuAJB13uSP2 (CAATGGACCAGATCGCGGCTGA), inuAJB13dSP1 (TGGGACAACACCAGCGGCTTCG), and inuAJB13dSP2 (TCGCAATTCGTCGCCTACAGCAA). PCR products of the expected size, which appeared between the second and third rounds of amplifications, were gel purified and directly sequenced by Beijing Genomics Institute (Guangzhou, China). All of the primers were synthesized by Generay (Shanghai, China).
Sequence analysis
Multiple sequences were assembled and aligned using Vector NTI 10.3 software (InforMax, Gaithersburg, MD, USA). The signal peptide in the amino acid sequence (InuAJB13 deduced from inuAJB13) was predicted using SignalP (http://www.cbs.dtu.dk/services/SignalP/). The identity values of protein sequences were calculated using BLASTP program (http://www.ncbi.nlm.nih.gov/BLAST/). Classification of the glycoside hydrolase family of InuAJB13 was determined with the InterPro online tool (http://www.ebi.ac.uk/interpro/).
Expression of inuAJB13 in E. coli
To express inuAJB13 in E. coli, the coding sequence of the mature peptide (without the predicted signal peptide) was amplified via PCR using Pyrobest DNA polymerase and the primer set rinuAJB13EF (CAGCGGCCGGATATCCGTC) and rinuAJB13ER (AGGCATCAACATGGCCGTGTTC). After adding base A at the 5′ terminal using rTaq DNA polymerase, the resulting PCR product was directly cloned into the pEASY-E2 vector through T-A ligation. Then the recombinant plasmid (pEASY-inuAJB13) was transformed into E. coli BL21 (DE3) competent cells. The positive transformant was identified by PCR analysis, confirmed by DNA sequencing, grown overnight at 37 °C in LB medium with 100 μg mL−1 ampicillin, and then inoculated into 10-L fresh LB medium with ampicillin (1:100 dilution). IPTG was added to a final concentration of 0.7 mM when the A 600 of the culture was approximately 0.7. The culture was then incubated for an additional 8 h at 37 °C, and the cells were finally harvested by centrifugation at 12,000×g for 5 min at room temperature.
Purification and identification of recombinant InuAJB13
The harvested cells were washed and re-suspended in sterilized ice-cold buffer A (20 mM Tris–HCl, 0.5 M NaCl, pH 7.2), and then disrupted by sonication (20–24 kHz) on ice. The precipitation was removed through centrifugation at 12,000×g for 10 min at room temperature. The recombinant InuAJB13 (rInuAJB13; His6-tag at C′ terminal) from the supernatant was then purified with a Ni2+-NTA agarose gel column. The supernatant was loaded onto the column, left undisturbed for 5 min, washed with three column volumes of buffer A, and eluted with one column volume of a linear imidazole gradient of 20–500 mM in buffer A. The purified protein (eluted by 300 mM imidazole) was stored at −20 °C and used for enzyme characterization.
Sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) was performed using 12 % running gel and 5 % stacking gel. The purified protein in the SDS-PAGE gel was identified using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) performed by Tianjin Biochip (Tianjin, China). The Bradford method was employed to determine protein concentration, using bovine serum albumin as the standard (Bradford 1976).
Enzyme assay and substrate specificity
Enzyme activity was determined by measuring the release of reducing sugar from substrate. The standard reaction contained 0.1 mL of appropriately diluted enzyme and 0.9 mL of McIlvaine buffer (pH 5.5) containing 0.5 % (w/v) substrate. The mixture was incubated in a water bath at 37 °C for 5 min and then incubated for an additional 10 min after addition of the enzyme. The reaction was stopped using 1.5 mL of 3,5-dinitrosalicylic acid (DNS) reagent and subsequently boiled in a water bath for 5 min to produce a measurable reddish brown (540 nm) product. One unit (U) of exo-inulinase activity was defined as the amount of enzyme that released 1 μmol of reducing sugar equivalent to d-fructose per minute. The enzyme activity was assayed by following this standard procedure unless otherwise noted.
To identify the substrate specificity of purified rInuAJB13, 0.5 % (w/v) inulin, levan, raffinose, stachyose, sucrose, starch, beechwood xylan, birchwood xylan, wheat flour arabinoxylan, or 4-O-methyl-d-glucurono-d-xylan (from beechwood), or 2 mM p-nitrophenyl-β-d-xylopyranoside was added to each reaction solution (determined at pH 5.5 and 55 °C). The liberated p-nitrophenol was determined as described in our previous study (Zhou et al. 2012).
Biochemical characterization
Biochemical characterization of purified rInuAJB13 was determined using inulin as the substrate
The effect of pH on the exo-inulinase activity of purified rInuAJB13 was determined at 37 °C in McIlvaine buffer (pH 3.0–8.0). The enzyme stability at different pH values was estimated by measuring the residual enzyme activity after incubating the enzyme solution in McIlvaine buffer at 37 °C for 60 min, with the untreated enzyme defining 100 % activity.
The temperature-dependent activity of purified rInuAJB13 was determined over 0–70 °C temperature range in McIlvaine buffer (pH 5.5). The thermostability of purified rInuAJB13 was determined under standard assay conditions following pre-incubation of the enzyme in McIlvaine buffer (pH 5.5) at 37, 50, or 60 °C with or without 10 % glycerol (w/v) for various periods, with the untreated enzyme defining 100 % activity.
The effects of various metal ions and chemical reagents on rInuAJB13 activity were analysed under standard assay conditions. Metal ions included 1.0 and 5.0 mM (final concentration) KCl, LiCl, AgNO3, CaCl2, CoCl2, NiSO4, CuSO4, MgSO4, ZnSO4, FeSO4, MnSO4, Pb(CH3COO)2, HgCl2, AlCl3, and FeCl3, and 0.2–4.5 M NaCl. Chemical reagents included 1.0 and 5.0 mM (final concentration) EDTA and β-mercaptoethanol, 1.0–100.0 mM SDS and CTAB, and 1.0–5.0 % (w/v) Tween 80 and Triton X-100. Metal ions and chemical reagents were individually added to the reaction solution. The enzyme stability in 0.2–4.5 M NaCl was estimated by measuring the residual enzyme activity following pre-incubation of the enzyme at 37 °C for 60 min, with the untreated enzyme defining 100 % activity.
To examine resistance to proteases, purified rInuAJB13 (0.05 mg) was incubated at 37 °C for 60 min with 16.7–90.0 trypsin (pH 7.2) or 16.7–97.0 proteinase K (pH 7.2), and the residual enzyme activity was measured under standard assay conditions.
K m, V max, k cat, and k cat/K m of rInuAJB13 were determined using 0.5–20.0 mg mL−1 substrates in McIlvaine buffer (pH 5.5) at 55 °C. Substrates included inulin, levan, sucrose, raffinose, and stachyose. The data were plotted according to the Lineweaver–Burk method (1934).
Effect of local liquid detergents on purified rInuAJB13
Liquid detergents were purchased from a local supermarket, including Balin (Quan Neng), Walch (Brilliant Detergent), Ariel (2 in 1 Oxyblu Detergent), Tide (Total Care 360°), Tupperware (Liquid Laundry Detergent), and Bluemoon (High Performance Laundry Detergent). To simulate the washing conditions, water was used to replace McIlvaine buffer in the standard reaction solution to maintain natural pH of different liquid detergents which were added to the reaction solution at final concentrations of 0.3 and 0.6 % (v/v). The residual activities were measured and compared with that of assay mixture without additive.
Hydrolysis products of inulin, levan, and Jerusalem artichoke tubers
The hydrolysis of 5 mL of 0.5 % (w/v; pH 5.5) inulin, levan, or fructo-oligosaccharides mixture (kestose, nystose, and fructofuranosylnystose) was performed with 0.5 U (0.1 U mL−1 reaction system) of purified rInuAJB13 for 5 min to 6 h at 37 °C. The hydrolysis products were analysed by thin layer chromatography (TLC) as previously described (Zhou et al. 2014). Fructose (1.0 % w/v) and glucose (1.0 % w/v) were used as standards and the substrate with an inactivated rInuAJB13 (90 °C for 5 min) was used as a control.
Jerusalem artichoke tubers (purchased from a local market) were sliced, sun-dried, and then ground into powder form. The hydrolysis reaction contained 30 mL of 10.0 % artichoke powder (w/v; pH 5.5) and 45.0 U (1.5 U mL−1 reaction system) of purified rInuAJB13. The mixture was incubated in a water bath at 37 °C for 10 min to 6 h. Fructose (1.0 % w/v) and glucose (1.0 % w/v) were used as standards, and artichoke powder with an inactivated rInuAJB13 (90 °C for 5 min) was used as a control.
Nucleotide sequence accession number
The nucleotide sequence for the exo-inulinase gene (inuAJB13) was deposited in GenBank under the accession number JF745874.
Results
Gene cloning and sequence analysis
A fragment of inuAJB13 (408 bp) was amplified by PCR using the degenerate primers eInu32F and eInu32R. DNA fragments amplified by GC TAIL-PCR were assembled with the inuAJB13 fragment. The results showed that the full-length sequence of inuAJB13 (JF745874; 1,518 bp) starts with the putative codon ATG, ends with TGA, and encodes a 505-residue polypeptide (InuAJB13) with 55.6 kDa calculated mass. Frequencies of amino acid residues G, A, and V of InuAJB13 are 11.3, 8.9, and 7.7 %, respectively, and the total frequency of G, A, and V of InuAJB13 is 27.9 % (Table 1).
InuAJB13 contains a predicted signal peptide from M1 to A20 and a catalytic domain of GH 32 from H30 to P341. The consensus pattern of GH 32, H–x(2)-[PV]-x(4)-[LIVMA]-N-D-P–N-[GA] (http://prosite.expasy.org/PS00609), was also detected in InuAJB13 (HFAPQRNWMNDPNG in Fig. 1). The two amino acids D40 and E213 from InuAJB13 (Fig. 1), corresponding to D41 and E241 of the exo-inulinase from Aspergillus awamori var. 2250 (CAC44220 or 1Y4 W) (Nagem et al. 2004), were found to be the putative nucleophile and catalytic acid/base, respectively.
Amino acid sequence alignment of InuAJB13 with GH 32 enzymes. Sequence names are shown with accession numbers (except InuAJB13) as follows: the exo-inulinase from G. stearothermophilus KP 1289 (accession no. BAC45010) (Tsujimoto et al. 2003), the exo-inulinase from Bacillus sp. snu-7 (AAK00768) (Kim et al. 2004), the exo-inulinase from A. awamori var. 2250 (CAC44220) (Nagem et al. 2004), and the β-fructosidase from S. yanoikuyae B1 (WP_026109703). Asterisks show the putative catalytic residues. The internal peptides identified by MALDI-TOF MS are underlined with black bars
BLASTP results revealed that the amino acid sequence of InuAJB13 shared the highest identity of 71.5 % with the putative GH 32 β-fructosidase from Sphingobium yanoikuyae B1 (accession no. WP_026109703). Aligned with characterized GH 32 enzymes, the sequence showed less than 50 % identity. For example, the sequence showed 42.1 % identity with the GH 32 exo-inulinase from Geobacillus stearothermophilus KP 1289 (BAC45010) (Tsujimoto et al. 2003) and 43.4 % identity with the GH 32 exo-inulinase from Bacillus sp. snu-7 (AAK00768) (Kim et al. 2004) (Fig. 1).
Expression, purification, and identification of rInuAJB13
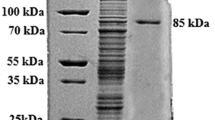
The recombinant enzyme was expressed as a soluble, intracellular His-tagged enzyme in E. coli BL21 (DE3) cells. Following Ni2+-NTA metal chelating affinity chromatography purification, the enzyme was purified to electrophoretic homogeneity as visualized on SDS-PAGE (Fig. 2). The apparent molecular weight of the purified enzyme was approximately 55 kDa, which is close to the value of theoretical prediction of rInuAJB13 (55.9 kDa). The single band in polyacrylamide gel was cut and analysed using MALDI-TOF MS. Results revealed that the MALDI-TOF MS spectrum (data not shown) matched the molecular mass of the known internal peptides of rInuAJB13, confirming that the purified enzyme was rInuAJB13. Five representative internal peptides of rInuAJB13 detected by MALDI-TOF MS analysis are selected and highlighted in (Fig. 1) (peptides: WGHMSWGHAVSR, TGNQSQFVAYSNDHGR, AVIYTSPNLKDWR, LAQAPVKEIEALR, WSASALGGTATMDVTAWPLGPER).
Enzyme characterization
Determined at pH 5.5 and 55 °C, the specific activities of purified rInuAJB13 towards substrates of 0.5 % (w/v) inulin, levan, sucrose, raffinose, stachyose, and starch were 73.7 ± 9.3, 27.6 ± 0.2, 1,655.3 ± 63.5, 244.0 ± 8.9, 152.6 ± 10.1, and 16.1 ± 1.8 U mg−1, respectively. However, no activity of rInuAJB13 was detected towards substrates of beechwood xylan, birchwood xylan, wheat flour arabinoxylan, 4-O-methyl-d-glucurono-d-xylan (from beechwood), or p-nitrophenyl-β-d-xylopyranoside.
Purified rInuAJB13 showed an apparently optimal pH of 5.5 and exhibited over 50 % of its maximal inulinase activity at pH 4.0–7.0 (Fig. 3a). The enzyme was stable in the pH range 4.0–7.0; it retained more than 90 % of its initial activity after 60 min pre-incubation (Fig. 3b).
Characterization of purified rInuAJB13. a Effect of pH on enzyme activity. The enzyme activity was determined at 37 °C from pH 3.0–8.0. b pH stability of rInuAJB13 at 37 °C for 60 min at pH 3.0–7.0. The residual activity was measured in McIlvaine buffer (pH 5.5) at 37 °C. c Effect of temperature on enzyme activity measured in McIlvaine buffer (pH 5.5). d Thermostability assay. The enzyme was determined at 37 °C after pre-incubation of the enzyme in McIlvaine buffer (pH 5.5) at 37, 50, or 60 °C with or without 10 % glycerol (w/v). e Effect of NaCl on enzyme activity and stability determined at pH 5.5 and 37 °C. The enzyme stability in NaCl was estimated by measuring the residual enzyme activity following pre-incubation of the enzyme at 37 °C for 60 min. f Stability of rInuAJB13 in various concentrations of trypsin and proteinase K at 37 °C for 60 min at pH 7.2. The residual enzyme activity was determined in McIlvaine buffer (pH 5.5) at 37 °C. Error bars represent the mean ± SD (n = 3)
When assayed at pH 5.5, purified rInuAJB13 was apparently optimal at 55 °C. The enzyme was active over a broad temperature range (10–70 °C), showing more than 35 % of the maximal activity at 30–70 °C and 20.0 % activity at 20 °C (Fig. 3c). rInuAJB13 was stable at 37 °C for more than 60 min. Half-lives of the enzyme were approximately 15 and 2 min at 50 and 60 °C, respectively (Fig. 3d). The thermostability of this enzyme can be enhanced by glycerol at the temperature of 50 °C. In the presence of 10 % glycerol (w/v), rInuAJB13 remained 134.4 % of the initial activity after pre-incubation for 60 min at 50 °C (Fig. 3d).
The addition of most metal ions and chemical reagents showed little or no effect (retaining more than 76.5 % activity) on the enzyme activity of purified rInuAJB13, notably the addition of surfactants SDS, CTAB, Tween 80, and Triton X-100 (Tables 2, 3). Purified rInuAJB13 also exhibited good NaCl tolerance, retaining 143.9 % activity even in the presence of 4.5 M NaCl (Fig. 3e) and 101.5–131.4 % of its initial activity following incubation with 0.2–4.5 M NaCl at 37 °C for 60 min (Fig. 3e). Furthermore, the enzyme activity was completely inhibited by HgCl2 and 5.0 mM AgNO3 and partially inhibited (retaining 64.7–68.8 % activities) by 1 mM AgNO3, 5.0 mM MnSO4, and 100.0 mM CTAB, but it was enhanced by approximately 0.4-fold in the presence of 1.0 mM β-mercaptoethanol.
rInuAJB13 could be tolerant to up to 57.0 mg mL−1 trypsin and 90.0 mg mL−1 proteinase K, retaining more than 80 % of its initial activity (Fig. 3f). However, almost all inulinase activity was lost after incubation with 90.0 mg mL−1 trypsin or 97.0 mg mL−1 proteinase K at 37 °C for 60 min (pH 7.2) (Fig. 3f).
The apparent K m, V max, and k cat values of purified rInuAJB13 towards different substrates (Table 4) were calculated based on the Lineweaver–Burk plots. The results are as follows: K m values of 10.2, 11.1, 6.5, 5.9, and 7.3 mg mL−1 towards inulin, levan, sucrose, raffinose, and stachyose, respectively; V max values of 294.1, 22.3, 1666.7, 212.8, and 163.9 µmol min−1 mg−1 towards inulin, levan, sucrose, raffinose, and stachyose, respectively; k cat values of 273.8, 20.7, 1551.7, 198.1, and 152.6 s−1 towards inulin, levan, sucrose, raffinose, and stachyose, respectively; and k cat /K m values of 26.8, 1.9, 238.7, 33.6, and 20.9 mL mg−1 s−1 towards inulin, levan, sucrose, raffinose, and stachyose, respectively.
Effect of local liquid detergents on purified rInuAJB13
Like surfactants, the addition of most local liquid detergents showed little or no effect (retaining more than 77.8 % activity) on the enzyme activity of purified rInuAJB13 (Table 3). Furthermore, the enzyme was partially inhibited by 0.6 % (v/v) Bluemoon (retaining 71.3 % activity).
Hydrolysis products
The products formed upon hydrolysis of inulin, levan, fructo-oligosaccharides mixture, and artichoke powder were analysed by TLC (Fig. 4). These results revealed that fructose was the primary product released from fructans and fructo-oligosaccharides in these substrates and showed that the fructose content increased during long-term incubation.
TLC chromatograms. The hydrolysis of 5 mL of 0.5 % (w/v; pH 5.5) inulin, levan, or fructo-oligosaccharides mixture (kestose, nystose, and fructofuranosylnystose) was performed with 0.5 U (0.1 U mL−1 reaction system) of purified rInuAJB13 for 5 min to 6 h at 37 °C. The hydrolysis of 30 mL of 10.0 % artichoke powder (w/v pH 5.5) was performed with 45.0 U (1.5 U mL−1 reaction system) of purified rInuAJB13 for 10 min to 6 h at 37 °C. Lanes: G 1.0 % (w/v) glucose; F 1.0 % (w/v) fructose; CK, substrates with the inactivated rInuAJB13
Discussion
Sphingomonas strains have been found in various environments owing to their ability to utilize a wide range of organic compounds as well as some environmental contaminants (Farias et al. 2011; Miller et al. 2010; White et al. 1996). Inulin is abundant in nature (Kango and Jain 2011). The ability of hydrolysing inulin in various conditions help some Sphingomonas strains survive under various environments, especially survive under harsh environments, suggesting that exo-inulinase genes can be cloned from some Sphingomonas strains and these enzymes can be active in harsh conditions. However, to the best of our knowledge, only two hypothetical GH 32 proteins (accession no. AHE53215, AHE53217) from the genus Sphingomonas have been revealed in available literatures and databases. The activity ratio of inulinase/sucrase (0.045) and TLC analysis reveal that rInuAJB13 is an exo-inulinase (Kango and Jain 2011; Moriyama et al. 2002). Thus, the current study is the first to report the identification and characterization of a GH 32 exo-inulinase from a Sphingomonas strain, and it is the first to report that the purified exo-inulinase is detergent-, salt-, and protease-tolerant.
Salt-tolerant enzymes have the potential for use in harsh industrial processes, such as food processing, biosynthetic processes, and washing (Margesin and Schinner 2001). Fermentation and material processing under high NaCl concentration can also reduce the total cost by eliminating the sterilization process (Margesin and Schinner 2001). Salt-tolerant xylanases have been given considerable research interests (Guo et al. 2009; Hung et al. 2011; Prakash et al. 2012). However, significant inulinase activity of over 135 % at the high NaCl concentration of 1.0–4.5 M is not a usual feature of inulinases reported to date. For example, the inulinase from Aspergillus fumigatus MTCC 3009 remains 77 % activity in the presence of 1 mM NaCl (Gill et al. 2004); the inulinase from Kluyveromyces marxianus var. bulgaricus remains 50 % activity in the presence of 50 mM NaCl (Kushi et al. 2000); and the inulinase from Sphingobacterium sp. GN25 remains 91–14 % activity when NaCl was added to the reaction solution at the concentrations from 1.0 to 4.5 M (data not published).
Among the published studies to date, inulinases are sensitive to SDS, such as the inulinases from Cryptococcus aureus G7a, Streptomyces sp. ALKC 4, Sphingobacterium sp. GN25, and A. fumigatus MTCC 3009 (Gill et al. 2006; Sharma and Gill 2007; Sheng et al. 2008; Zhou et al. 2014), because SDS is an anionic detergent and causes strong denaturation of proteins when the concentration is above 7 mM in water (Manning and Colon 2004). Thus, the application of inulinases in detergent industry has been extremely limited. Unlike SDS-sensitive inulinases, purified rInuAJB13 showed 77.9–94.2 % activity in the presence of 1.0–100.0 mM SDS and remained more than 77.8 % activity in the presence of most local liquid detergents. Furthermore, the enzyme was active over a broad temperature range (10–70 °C) and was active towards substrates of levan and starch. Many microorganisms produce levan as an extracellular polysaccharide for the attachment to a surface and for the formation of a biofilm (Han 1990; Takahashi et al. 1983). Dirt comes in many forms, such as proteins and starches. Therefore, rInuAJB13 shows the potential for use in liquid detergent industry.
Proteases are often supplemented in food, feed, and detergent (Kuddus and Ramteke 2012). Endogenous proteases are widely found in prokaryotes, fungi, plants and animals (Kuddus and Ramteke 2012). Thus, protease-tolerant xylanases and α-galactosidases have attracted much attention (Cao et al. 2009; Fontes et al. 1995; Ghazi et al. 2003; Morgavi et al. 2001). However, protease-tolerant inulinases have not been reported. The current study revealed that rInuAJB13 was resistant to digestion by up to 57.0 mg mL−1 trypsin and 90.0 mg mL−1 proteinase K.
Salt-tolerant enzymes present a higher proportion of amino acid residues G, A, and V than their homologues (Madern et al. 2000). SDS and protease tolerances have been proposed as common properties of kinetically stable proteins which are characterized by a high occurrence of hydrophobic residues, such as A and V (Jaswal et al. 2002; Manning and Colon 2004). Compared with most inulinases shown in Table 1, such as the SDS-sensitive inulinases from C. aureus G7a and Sphingobacterium sp. GN25, the total frequency of amino acid residues G, A, and V of InuAJB13 is much higher. The result suggests that amino acid residues G, A, and V may contribute to the salt, SDS, and protease tolerances of InuAJB13. Given the low identity (less than 40 %) and QMEAN4 (less than −5.95) of rInuAJB13 with the known templates of crystal structures, the homology modelling and structural analysis are ambiguous for clarifying these assumptions.
In the present study, we identified and characterized a novel exo-inulinase from Sphingomonas sp. JB13. The purified recombinant enzyme showed detergent, salt, and protease tolerances. To our knowledge, it is the first report on the identification and characterization of a detergent-, salt-, and protease-tolerant exo-inulinase.
References
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B (2009) The Carbohydrate-Active EnZymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res 37:D233–D238
Cao Y, Wang Y, Meng K, Bai Y, Shi P, Luo H, Yang P, Zhou Z, Zhang Z, Yao B (2009) A novel protease-resistant α-galactosidase with high hydrolytic activity from Gibberella sp. F75: gene cloning, expression, and enzymatic characterization. Appl Microbiol Biotechnol 83:875–884
Farias ME, Revale S, Mancini E, Ordonez O, Turjanski A, Cortez N, Vazquez MP (2011) Genome sequence of Sphingomonas sp. S17, isolated from an alkaline, hyperarsenic, and hypersaline volcano-associated lake at high altitude in the Argentinean Puna. J Bacteriol 193:3686–3687
Fontes C, Hall J, Hirst BH, Hazlewood GP, Gilbert HJ (1995) The resistance of cellulases and xylanases to proteolytic inactivation. Appl Microbiol Biotechnol 43:52–57
Ghazi S, Rooke JA, Galbraith H (2003) Improvement of the nutritive value of soybean meal by protease and α-galactosidase treatment in broiler cockerels and broiler chicks. Br Poult Sci 44:410–418
Gill PK, Manhas RK, Singh J, Singh P (2004) Purification and characterization of an exoinulinase from Aspergillus fumigatus. Appl Biochem Biotechnol 117:19–32
Gill PK, Manhas RK, Singh P (2006) Purification and properties of a heat-stable exoinulinase isoform from Aspergillus fumigatus. Bioresour Technol 97:894–902
Guo B, Chen XL, Sun CY, Zhou BC, Zhang YZ (2009) Gene cloning, expression and characterization of a new cold-active and salt-tolerant endo-β-1,4-xylanase from marine Glaciecola mesophila KMM 241. Appl Microbiol Biotechnol 84:1107–1115
Han YW (1990) Microbial Levan. Adv Appl Microbiol 35:171–194
Hung KS, Liu SM, Tzou WS, Lin FP, Pan CL, Fang TY, Sun KH, Tang SJ (2011) Characterization of a novel GH10 thermostable, halophilic xylanase from the marine bacterium Thermoanaerobacterium saccharolyticum NTOU1. Process Biochem 46:1257–1263
Jaswal SS, Sohl JL, Davis JH, Agard DA (2002) Energetic landscape of α-lytic protease optimizes longevity through kinetic stability. Nature 415:343–346
Kango N, Jain SC (2011) Production and properties of microbial inulinases: recent advances. Food Biotechnol 25:165–212
Kim KY, Koo BS, Jo D, Kim SI (2004) Cloning, expression, and purification of exoinulinase from Bacillus sp. snu-7. J Microbiol Biotechnol 14:344–349
Kobayashi T, Uchimura K, Deguchi S, Horikoshi K (2012) Cloning and sequencing of inulinase and β-fructofuranosidase genes of a deep-sea microbulbifer species and properties of recombinant enzymes. Appl Environ Microbiol 78:2493–2495
Kuddus M, Ramteke PW (2012) Recent developments in production and biotechnological applications of cold-active microbial proteases. Crit Rev Microbiol 38:330–338
Kushi RT, Monti R, Contiero J (2000) Production, purification and characterization of an extracellular inulinase from Kluyveromyces marxianus var. bulgaricus. J Ind Microbiol Biotechnol 25:63–69
Kwon YM, Kim HY, Choi YJ (2000) Cloning and characterization of Pseudomonas mucidolens exoinulinase. J Microbiol Biotechnol 10:238–243
Liebl W, Brem D, Gotschlich A (1998) Analysis of the gene for β-fructosidase (invertase, inulinase) of the hyperthermophilic bacterium Thermotoga maritima, and characterisation of the enzyme expressed in Escherichia coli. Appl Microbiol Biotechnol 50:55–64
Lima DM, Fernandes P, Nascimento DS, Ribeiro R, de Assis SA (2011) Fructose syrup: a biotechnology asset. Food Technol Biotechnol 49:424–434
Lineweaver H, Burk D (1934) The determination of enzyme dissociation constants. J Am Chem Soc 56:658–666
Madern D, Ebel C, Zaccai G (2000) Halophilic adaptation of enzymes. Extremophiles 4:91–98
Manning M, Colon W (2004) Structural basis of protein kinetic stability: resistance to sodium dodecyl sulfate suggests a central role for rigidity and a bias toward β-sheet structure. Biochemistry 43:11248–11254
Margesin R, Schinner F (2001) Potential of halotolerant and halophilic microorganisms for biotechnology. Extremophiles 5:73–83
Miller TR, Delcher AL, Salzberg SL, Saunders E, Detter JC, Halden RU (2010) Genome sequence of the dioxin-mineralizing bacterium Sphingomonas wittichii RW1. J Bacteriol 192:6101–6102
Morgavi DP, Beauchemin KA, Nsereko VL, Rode LM, McAllister TA, Iwaasa AD, Wang Y, Yang WZ (2001) Resistance of feed enzymes to proteolytic inactivation by rumen microorganisms and gastrointestinal proteases. J Anim Sci 79:1621–1630
Moriyama S, Akimoto H, Suetsugu N, Kawasaki S, Nakamura T, Ohta K (2002) Purification and properties of an extracellular exoinulinase from Penicillium sp. strain TN-88 and sequence analysis of the encoding gene. Biosci Biotechnol Biochem 66:1887–1896
Nagem RAP, Rojas AL, Golubev AM, Korneeva OS, Eneyskaya EV, Kulminskaya AA, Neustroev KN, Polikarpov I (2004) Crystal structure of exo-inulinase from Aspergillus awamori: the enzyme fold and structural determinants of substrate recognition. J Mol Biol 344:471–480
Prakash B, Vidyasagar M, Jayalakshmi SK, Sreeramulu K (2012) Purification and some properties of low-molecular-weight extreme halophilic xylanase from Chromohalobacter sp. TPSV 101. J Mol Catal B-Enzym 74:192–198
Sharma AD, Gill PK (2007) Purification and characterization of heat-stable exo-inulinase from Streptomyces sp. J Food Eng 79:1172–1178
Sheng J, Chi ZM, Gong F, Li J (2008) Purification and characterization of extracellular inulinase from a marine yeast Cryptococcus aureus G7a and inulin hydrolysis by the purified inulinase. Appl Biochem Biotechnol 144:111–121
Takahashi N, Mizuno F, Takamori K (1983) Isolation and properties of levanase from Streptococcus salivarius KTA-19. Infect Immun 42:231–236
Tsujimoto Y, Watanabe A, Nakano K, Watanabe K, Matsui H, Tsuji K, Tsukihara T, Suzuki Y (2003) Gene cloning, expression, and crystallization of a thermostable exo-inulinase from Geobacillus stearothermophilus KP1289. Appl Microbiol Biotechnol 62:180–185
Vijayalaxmi S, Prakash P, Jayalakshmi SK, Mulimani VH, Sreeramulu K (2013) Production of extremely alkaliphilic, halotolerent, detergent, and thermostable mannanase by the free and immobilized cells of Bacillus halodurans PPKS-2. Purification and characterization. Appl Biochem Biotechnol 171:382–395
Vijayaraghavan K, Yamini D, Ambika V, Sowdamini NS (2009) Trends in inulinase production-a review. Crit Rev Biotechnol 29:67–77
White DC, Sutton SD, Ringelberg DB (1996) The genus Sphingomonas: physiology and ecology. Curr Opin Biotechnol 7:301–306
Zhang LH, Zhao CX, Ohta WY, Wang YJ (2005) Inhibition of glucose on an exoinulinase from Kluyveromyces marxianus expressed in Pichia pastoris. Process Biochem 40:1541–1545
Zhou JP, Huang HQ, Meng K, Shi PJ, Wang YR, Luo HY, Yang PL, Bai YG, Yao B (2010) Cloning of a new xylanase gene from Streptomyces sp. TN119 using a modified thermal asymmetric interlaced-PCR specific for GC-rich genes and biochemical characterization. Appl Biochem Biotechnol 160:1277–1292
Zhou JP, Dong YY, Li JJ, Zhang R, Tang XH, Mu YL, Xu B, Wu Q, Huang ZX (2012) Cloning, heterologous expression, and characterization of novel protease-resistant α-galactosidase from new Sphingomonas strain. J Microbiol Biotechnol 22:1532–1539
Zhou JP, Gao YJ, Zhang R, Mo MH, Tang XH, Li JJ, Xu B, Ding JM, Huang ZX (2014) A novel low-temperature-active exo-inulinase identified based on Molecular-Activity strategy from Sphingobacterium sp. GN25 isolated from feces of Grus nigricollis. Process Biochem. doi:10.1016/j.procbio.2014.06.013
Acknowledgments
This work was supported by the Key Technologies Research and Development Program of China (No. 2013BAD10B01), National Natural Science Foundation of China (31260215), and Science Research Foundation of Yunnan Provincial Education Committee (2013Y432).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Oren.
J. Zhou and M. Peng contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, J., Peng, M., Zhang, R. et al. Characterization of Sphingomonas sp. JB13 exo-inulinase: a novel detergent-, salt-, and protease-tolerant exo-inulinase. Extremophiles 19, 383–393 (2015). https://doi.org/10.1007/s00792-014-0724-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-014-0724-z