Abstract
Vitamin D may play an important role in the etiology of Autism Spectrum Disorders (ASD). Vitamin D is regarded as a neuroactive steroid affecting brain development and function. It plays an essential role in myelination, which is important for connectivity in the brain. Studies have shown that decreased vitamin D levels in patients, decreased maternal vitamin D levels during pregnancy, and decreased exposure to solar UVB might increase the risk for ASD. In addition, autism symptoms and global functioning may improve after vitamin D supplementation. Here, we sought to aggregate information from previous publications on vitamin D levels and ASD, in order to achieve a higher statistical power and thereby to determine the validity of vitamin D deficiency as a risk factor for ASD. For this meta-analysis, 11 studies met the inclusion and exclusion criteria, accounting for a total of 870 ASD patients and 782 healthy controls. Levels of serum 25(OH) D in participants with ASD were significantly lower than controls, suggesting that lower vitamin D level might be a risk factor for ASD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism spectrum disorder (ASD) is a brain disorder, characterized by deficits in social interaction, verbal and non-verbal communication, and restricted and repetitive behavior [1, 2]. Over the recent years, the prevalence estimates of ASD have increased for yet unknown reasons [3, 4]. Genetic factors play a prominent role in the etiology of ASD [5], which is confirmed by twin studies [6–9]. A recent study of 2500 ASD simplex families has identified hundreds of gene mutations, including de novo missense mutations (13 %) and likely de novo gene-disrupting (LGD) mutations (43 %). These mutations contribute up to 12 and 9 % of ASD diagnose, respectively [10]. Another study showed that over a hundred of autosomal genes might affect the risk of having ASD [11]. Recent data from a cohort of children born in Sweden point towards a disease etiology including additive genetic and environmental effects [12].
Various environmental events during pregnancy and early life, such as exposure to toxins and medication, have been demonstrated as risk factors for developing ASD [13–16]. Cumulative data suggest that decreased level of Vitamin D may also be an important risk factor for ASD [17–21], although the clinical awareness of this probability is somewhat limited, despite the fact that already in 2008, Cannell hypothesized that vitamin D deficiency during pregnancy could be a risk factor for autism. Cannell further suggested that early childhood vitamin D deficiency could be related to decreased exposure to sunlight, possibly brought on by medical advice to avoid the sun [22].
Vitamin D, structurally part of a group of sterols, plays a crucial role in the calcium and phosphorous metabolism [23]. The main source of vitamin D comes from the conversion of 7-dehydrocholesterol to previtamin D3 in the skin by solar ultraviolet B radiation, while a lesser amount of vitamin D comes from food intake [19, 24]. The active form of vitamin D, 1,25(OH)2-vitamin D, is a steroid with potent endocrine, paracrine, and autocrine effects, induced by binding to its specific ligand—the vitamin D receptor (VDR). The compelling data of vitro, vivo, and animal experiments showed that vitamin D is involved in brain proliferation, differentiation, neurotropism, neuroprotection, neurotransmission, myelination, and neuroplasticity [21, 25]. Vitamin D also stimulates glutathione and can affect the gene expression of a multitude of target genes. Furthermore, Vitamin D is associated with immune response as well [26–28].
Several studies have shown a relationship between (1) the risk for ASD and vitamin D levels in patients, (2) maternal vitamin D levels during pregnancy, and (3) the amount of exposure to solar UVB.
Most data are available with respect to (1) vitamin D levels in patients with ASD. In fact, since 2010, 14 studies have directly investigated serum levels of vitamin D in individuals with autism. Vitamin D levels are expressed in ng/ml or nmol/l (1 ng/ml–2.5 nmol/l) and a short summary of each of the 14 studies is provided.
Meguid and Hashish [29] conducted a study with 70 ASD children and 42 healthy controls in Cairo, Egypt (latitude 30.3 degrees N), aged between 2 and 8 years. Birth season in relation to vitamin D levels and autism was taken into account, but no significant difference was found for the month or season of birth in either group. The ASD group had significantly lower levels of 25(OH) D (28.5 ng/ml) compared with healthy controls (40.1 ng/ml; P < 0.001). Because of the sunny climate in Egypt, 28.5 ng/ml is regarded to be inadequate.
Humble and Gustafsson [30] tested vitamin D levels in adult outpatients with several psychiatric disorders in Sweden (latitude 59.3 degrees N) and found that those with autism or schizophrenia had significantly lower levels of Vitamin D (12.26 ng/ml). This study supports the hypothesis that vitamin D deficiency may be not only a predisposing developmental factor but also related to the psychiatric status of adult patients. However, there was no control group in this study.
Mostafa and Al-Ayadhi [31] measured 25(OH) D and anti-MAG autoantibodies in 50 ASD children (5–12 years old) and 30 age-matched healthy children in Riyadh Saudi Arabia (latitude 24 degrees N). ASD children (18.5 ± 14 ng/ml) had significantly lower serum levels of 25(OH) D than healthy children (33.0 ± 11 ng/ml; P < 0.001). Furthermore, 40 % children with ASD displayed concentrations of 25(OH) D below 10 ng/ml, but none in the control group. Whereas, 48 % ASD children and only 20 % healthy controls had 25(OH) D levels between 10 and 30 ng/ml, suggesting vitamin D deficiency might be a serious risk factor for ASD. This study also demonstrated that serum 25(OH) D levels were significantly negatively correlated with CARS scores (P < 0.001), which implied a possible link between the level of vitamin D deficiency and the severity of autism symptoms. In the same study, serum concentrations of anti-MAG autoantibodies had been found to have significant negative correlations with serum 25(OH) D levels. Thus, Vitamin D deficiency may contribute to the induction of more serum anti-MAG autoantibodies in ASD children.
Tostes and Polonini [32] studied serum 25(OH) D levels in children in Juiz de Fora, Brazil (latitude 21.75 degrees S). Results showed a significant decrease of 25(OH) D in the ASD group compared with the control group (26.48 vs 40.52 ng/ml).
Neumeyer and Gates [33] explored bone mineral density (BMD) and 25(OH) D level in 18 boys with ASD and 19 healthy controls, aged from 8 to 14 years in Massachusetts, USA (latitude 41.16 degrees N). BMD in boys with ASD is lower when compared with control group at spine, femoral neck, and total hip. Mean values of serum 25(OH) D were, respectively, 26.7 ng/ml in ASD boys and 31.7 ng/ml in healthy controls (significant difference between the two groups). As known, vitamin D plays an important role in bone growth in human body. This study concluded that BMD is lower in peripubertal boys with ASD and impaired vitamin D status may be associated with lower exercise activity.
Gong and Luo [34] reported the serum levels of 25(OH) D in 48 confirmed ASD patients and 48 healthy controls (matched with age and gender) in Chongqing, China (latitude 29.5 degrees N). The mean serum 25(OH) D level was significantly (P = 0.002) lower in children with ASD as compared with controls (19.9 and 22.6 ng/ml, respectively). In this study, the CARS was used to assess ASD symptoms. Decreased vitamin D levels were significantly correlated with severity of ASD symptoms (P < 0.001). These results indicate that low vitamin D level may be independently associated with the severity of ASD, and lower serum 25(OH) D level could be considered as an independent risk factor for ASD.
Kocovska and Andorsdottir [35] compared serum 25(OH) D levels in ASD young adults (aged 15–24 years) with their normally developing siblings, parents, and healthy controls (matched with age and gender) in Faroe Islands (latitude 61.41 degrees N). The ASD group showed a significantly lower level of serum 25(OH) D (24.8 nmol/l) than the sibling group (46.1 nmol/l, P < 0.001), parent group (46.7 nmol/l, P < 0.001), and healthy control group (37.6 nmol/l, P = 0.002). No associations were found between serum Vitamin D level and IQ, ASD phenotype or scores of Autism Diagnostic Observation Schedule (ADOS).
Bener and Khattab [36] tested serum 25(OH) D levels in 254 ASD children (mean age 5.51 ± 1.58 years) and 254 healthy controls (mean age 5.76 ± 1.56 years) in Qatar (latitude 25.25 degrees N). The mean value of serum vitamin D concentration in children with ASD (18.39 ± 8.2 ng/ml) was significantly lower versus control children (21.59 ± 8.4 ng/ml; P < 0.05). The percentage of children who had vitamin D deficiency was significantly higher in the ASD group than in the control group (14.2 vs 8.3 %; P < 0.01).
Du and Shan [37] showed a possible association between serum vitamin D level and ASD. In this study, the serum 25(OH) D levels have been studied in 117 children with ASD and 109 healthy controls in Changchun, China (latitude 43.88 degrees N). Concentration of serum 25(OH) D in control group was 36 ± 13 ng/ml, which was significantly higher than ASD group (19 ± 9 ng/ml, P < 0.01). The percentage of vitamin D insufficiency plus deficiency in ASD group was significantly higher than that of control group (89.7 vs 52.3 %; P < 0.01).
Saad and Abdel-Rahman [38] measured 25(OH) D in 122 ASD children (3–9 years old) and 100 healthy children (matched with age and gender) in Assiut Egypt (latitude 27.18 degrees N). The ASD group showed a significant lower level of serum 25(OH) D (18.02 ± 8.75 ng/ml) than the control group (42.51 ± 9.48 ng/ml, P < 0.0001). Serum 25(OH)D levels had significantly negative correlations with severity of ASD on CARS scores (P < 0.0001).
Fernell and Bejerot [39] analysed 25(OH) D in 58 Sweden-born sibling pairs, in which 1 child had ASD and the other did not (latitude 36 degrees N). The dried blood spots taken in the neonatal period for metabolic screening had been used to test 25(OH) D level. ASD children had significantly (P = 0.013) lower vitamin D levels (24.0 ± 19.6 ng/ml) compared with their siblings (31.9 ± 27.7 ng/ml).
Although the above-described studies indicated a possible role of vitamin D deficiency in ASD, other studies could not confirm these positive results.
Molloy and Kalkwarf [40] compared the actual serum 25(OH) D levels of 40 Caucasian boys with ASD (4–8 years old) with control group (n = 40) in USA (latitude 23–54 degrees N). No significant differences were observed between the two groups (P = 0.4). In this study, a total of 54 (61 %) children in the entire cohort had a serum 25(OH) D concentration of less than 20 ng/ml (30 ng/ml has been regularly seen as the cutoff value between vitamin D adequate and inadequate). Children from control group were all suffering from acute inflammation, which may potentially affect the plasma vitamin D level.
Adams and Audhya [41] studied the nutritional and metabolic status of children (n = 55 aged 5–16 years) with ASD and that of neurotypical children (n = 44) in Arizona, USA (latitude 31–37 degrees N). No significant differences were observed between the two groups concerning 25(OH) D level (P = n.s.)
Uğur and Gürkan [42] examined 25(OH)D levels of children in Ankara, Turkey (latitude 40°02′ degrees N) and found no difference between children with ASD (25.12 ng/ml) and healthy controls (matched with age and gender, 21.11 ng/ml, P = 0.069). Furthermore, they found no correlation between severity of autistic symptoms (ABC and CARS scores) and serum vitamin D levels (P > 0.05). In this study, both ASD children and healthy controls had an average lower level of serum 25(OH) D (less than 30 ng/ml). It could be that the high latitude of Ankara with a relatively short duration of sunshine might contribute to a low level of serum 25(OH) D.
With respect to (2), maternal vitamin D levels during pregnancy and the risk for ASD only a few studies are available, showing inconsistent results. Fernell and Barnevik-Olsson [43] investigated serum 25(OH) D levels in mothers of ASD children in Sweden, who were of Somali origin. Mothers of ASD children had low levels of vitamin D. This might indicate that ASD children could have vitamin D deficit already since prenatal period. A study from India also provided evidence for an association between maternal vitamin D deficit during pregnancy and the risk for ASD [44]. However, a similar Australian study could not support the above conclusion [45].
With respect to (3), the amount of exposure to solar UV-B, which indirectly relates to levels of vitamin D, most results are in line with the hypothesis that lower levels of vitamin D are a risk factor for ASD. Grant and Cannel [46] studied the relationship between UV-B doses and the prevalence of autism in the US for children aged 6–17 years. In that study, an inverse correlation between solar UVB and the prevalence of autism was found. Dark-skinned people require a higher sun exposure to produce the same amount vitamin D as their white counterpart. Therefore, when moving to northern countries, persons with a dark skin may have an increased risk for vitamin D deficiency. This could be an explanation for the phenomenon that the prevalence of ASD is higher in dark-skinned offspring of immigrant mothers to Europe, especially those coming from East Africa to Northern Europe [47]. Hayashi [48] further showed that ASD symptoms might have a seasonal improvement, especially during the summer. It has recently been suggested that seasonal changes of vitamin D levels could be balanced by administering adequate doses of vitamin D in a timely manner [49]. In a recent paper, it was suggested that adequate, perhaps pharmacological, doses of vitamin D may have a treatment effect in the core symptoms of autism [50]. Indeed, this suggestion is actually supported by previous work from our group, Jia and Wang in 2015 first reported a 32-month-old boy with ASD and vitamin D deficiency whose autism symptoms markedly improved after vitamin D supplementation [51]. A very recent trial reported 106 ASD children with low serum 25(OH) D levels (<30 ng/ml) received vitamin D3 supplementation (300 IU/kg/day, not exceed 5000 IU/day) for 3 months. 80.72 % of participants who completed 3 months treatment had significant improvements in scores of the CARS and ABC subscales [38].
In summary, several lines of research suggest that vitamin D may play a role in the etiology of ASD. Some of the evidence for this is still very limited, such as studies of maternal vitamin D levels during pregnancy. However, the number of studies that investigated vitamin D levels in patients with ASD is considerable, allowing a meta-analysis to be performed. A meta-analysis could provide the necessary statistical strength to determine the validity of considering vitamin D as a risk factor for ASD and thereby provide data for future clinical guidelines studies.
Materials and methods
In- and exclusion criteria
All clinical trials that directly investigated serum levels of vitamin D in subjects with ASD and healthy control subjects were considered for inclusion. The inclusion criteria were (1) ASD diagnose according to criteria established in DSM IV(TR); (2) participants aged between 2 and 16 years; (3) measurement of 25-(OH) D concentration in peripheral blood; (4) studies which reported 25-(OH) D concentrations using a mean and standard deviation, with sample size and P value; (5) full text articles that were published in English. The exclusion criteria were studies (1) without a healthy control group; or (2) including children with any disease that could affect serum vitamin D levels.
Search strategy
A computerized search in MEDLINE, EMBASE, PsycINFO, Web of Knowledge, and Scopus from January 1980 to May 2015 has been conducted by using the keywords autism and vitamin D. Detail search terms were 25-OH AND cholecalcifero OR cholecalciferol OR vitamin AND D3 OR vitamin D3 AND autistic disorder OR autism OR ASD. Duplicate articles were deleted. The title and abstract of each article were scanned and assessed independently by two raters (authors Wang and Shan). The full text of each potentially relevant study was then retrieved and assessed for eligibility, according to above-mentioned inclusion and exclusion criteria. The reference lists of these studies were also searched for possible publications that might meet our inclusion criteria. Disagreement between the two initial raters (authors Wang and Shan) during the reviewing process has been regularly solved by discussion. Otherwise, a third independent rater (author Du) would be asked to determine the eligibility.
Data extraction
Data of vitamin D levels for each eligible study were extracted into an Excel spreadsheet (including sample size, mean value, standard deviation, and P value). Two authors (Wang and Shan) extracted the data independently. Any disagreements between them were solved by discussion.
Quality assessment
To evaluate the quality of potential studies that could be included in our research, a set of predefined criteria of Newcastle-Ottawa Scale (NOS) [52] have been used to make the judgment. The NOS criteria include three aspects: (1) subject selection, 0–4; (2) comparability of subject, 0–2; and (3) clinical outcome, 0–3. Total NOS scores range from 0 (lowest) to 9 (highest). Studies can be classified into two types according to NOS scores: low quality (0–6) and high quality (7–9), respectively. Two raters (Wang and Shan) conducted the NOS assessment independently. Any discrepancies between the two raters on NOS scores of the potential studies were solved by discussion or consultation with a third rater (Du).
Data analysis
The Review Manager Software (Revman 5.2), developed by Cochrane Collaboration and STATA software (version 11.0), were used to pool all data coming from eligible articles to conduct this meta-analysis. We used weighted mean difference (WMD) to describe the overall effect size of the difference in mean level of vitamin D between ASD group and control group in each eligible study. To obtain the WMD with 95 % confidence interval (CI) for both the ASD group and the control group, a random-effects model (DerSimonian and Laird method) was used when heterogeneity was present (P < 0.10) and if the computed I 2 was more than 75 %; otherwise, the fixed-effects model (Mantel–Haenszel method) was used. Significance in CIs is identified by the 95 % CI not encompassing 1; and for mean differences, not encompassing 0, with a P value of less than 0.05. To evaluate the influence of the individual data set on the pooled WMD, a one-way sensitivity analysis was conducted by deleting one study at a time. The funnel plot was constructed to assess publication bias. The symmetry of the funnel plot was further evaluated by Egger’s linear regression test.
Results
Baseline characteristics of included studies
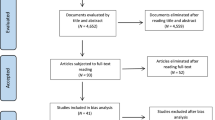
Through search strategy and after full text assessment, 14 potential articles were identified. 3 studies were excluded because of the following reasons: no control group [30] or the control group was not healthy control [40] or participants were over age [35]. The flow chart of this selection process is summarized in Fig. 1. Finally, 11 studies were eligible to take part in this meta-analysis, with a total of 1652 participants (870 ASD patients and 782 healthy controls). Quality scores of all enrolled papers were higher than 7 (high quality). Table 1 summarized the core content and methodological quality of each enrolled study.
Serum levels of 25(OH)D in ASD
A total of 11 case–control studies that evaluated serum 25(OH) D levels in both ASD patients and healthy controls were included. In our meta-analysis, heterogeneity was present (P < 0.00001 I 2 = 98 %). Therefore, we employed a random-effects model. All 11 included trials reported significantly lower concentrations of 25(OH) D in patients with ASD compared to healthy controls. The pooled data showed that there was a significant difference between the ASD group and control group (WMD = −8.63; 95 % CI (−13.17, −4.09), P = 0.0002). These results were presented in a forest plot (see Fig. 2).
Sensitivity analysis and publication bias
Sensitivity analysis showed that the overall statistical significance does not change when any single study was omitted. Therefore, the current result of this meta-analysis is relatively reliable and credible. The graphical funnel plots of the 11 studies are slightly asymmetrical, but Egger’ test showed there was no publication bias in this meta-analysis (P = 0.478) (see Fig. 3).
Discussion
The results from this meta-analysis clearly show that 25(OH) D serum concentrations are significantly reduced in children with ASD compared to healthy control subjects. The observed effects are robust, appear independent of ethnicity, and occur from early childhood into adolescence.
The question rises why vitamin D levels would be reduced in children with ASD children. In general lower vitamin D levels may be caused by reduced intake, reduced production, or increased loss.
Some children with ASD may have unusual preferences or dislikes for certain types of food, and it could cause an inadequate nutritional intake. It could also be the case, that children with ASD may lack sufficient exposure to sunlight, e.g. UV-B. Alternatively, disorders limiting vitamin D absorption, or medical conditions impairing vitamin D conversion into active metabolites may be more prevalent in children with ASD. However, at this point we can only speculate about the mechanism that causes the marked vitamin D shortage in children with ASD.
There are several ways how vitamin D reduction could be related to ASD. First, vitamin D deficiency may affect early brain development [53]. Animal studies have demonstrated that vitamin D is extremely important in brain homeostasis and neurodevelopment, such as neuronal migration and growth, neuronal differentiation, exciting and inhibiting neurotransmission, cell interaction, and synaptic function [54–57]. Not surprising, many neurological and psychiatric conditions such as schizophrenia, MS, and autism, have been linked to developmental (prenatal) vitamin D deficiency [30, 58, 59].
Second, vitamin D reduction may alter immune responses in individuals with ASD [60]. Vitamin D regulated immune function was first proposed after the identification of vitamin D receptors in lymphocytes. The active form of vitamin D—1,25(OH)2D3, can activate helper T cells, regulatory T cells, activated B cells and dendritic cells [61]. Thus, vitamin D can decrease inflammation and produce immune protective effects [62, 63]. Vitamin D could down regulate pro-inflammatory mediators in monocyte-derived macrophages, IL-1β and IL-6 levels in patients with rheumatoid arthritis [64]. Not surprising, many autoimmune disorders have been associated with low vitamin D level [65]. Hayes and Nashold reviewed a diverse and rapidly growing body of epidemiological, climatological, genetic, nutritional and biological evidence and indicated that the vitamin D endocrine system functions in the establishment and/or maintenance of immunological self-tolerance [66]. CD4(+)CD25(+) regulatory T cells (Tregs) play an important role in the establishment of immunological self-tolerance, thereby preventing autoimmune reactions. Interestingly, in a very recent meta-analysis, nineteen cytokines were assessed, and it was shown that concentrations of interleukin (IL)-1beta, IL-6, IL-8, interferon-gamma, eotaxin and monocyte chemotactic protein-1 were significantly higher in the participants with ASD compared with the healthy controls, while concentrations of transforming growth factor-β1 were significantly lower [67]. Other studies have indicated an altered activation profile for T cells, which might change adaptive cellular immune function in children with ASD [68]. Indeed, autism is considered an autoimmune disease [69, 70]. Many autoimmune markers have been found higher in autism patients, such as anti-nuclear antibodies [71], anti-ganglioside M1 antibodies [72], Osteopontin [73], anti-MBP autoantibodies [74], and antinucleosome-specific antibodies [75]. Besides that, levels of these markers had significant positive correlations with the severity of autism. Thus, autism may be, in part, one of the pediatric autoimmune neuropsychiatric disorders.
Third, accumulating evidence suggests that oxidative stress may be a common feature in autism [76]. The effect of oxidative stress might exert their deleterious effects by further exacerbating the interaction with genetically susceptible alleles [77]. Antioxidants, especially glutathione (GSH), are essential for neural survival during the early critical period [78]. Vitamin D can increase the cellular level of GSH [79] and is important for controlling detoxification processes in the brain [80]. Halicka and Zhao suggested that 1,25-dihydroxyvitamin D3 contributed to enhance DNA repair efficiency through its anti-oxidant activity [81].
Fourth, lower levels of vitamin D may affect the production of serotonin and the oxytocin metabolism. With respect to serotonin, it is known that Vitamin D activates the transcription of the serotonin-synthesizing gene—tryptophan hydroxylase 2 (TPH2) in the brain and inversely affects serotonin production in peripheral tissues [82]. 5-HT plays a critical role in regulating emotions during social decision-making [83]. Individuals with ASD may display lower concentrations of serotonin in their brains [84], and higher serotonin concentrations in their blood [85]. With respect to oxytocin, Vitamin D regulates both the production of the oxytocin and the response to it. Vitamin D also regulates oxytocin-related genes [86]. Several researches have reported oxytocin’s therapeutic effects on deficits in social communication and interaction in autism [87–89]. It has been shown that autistic children have lower plasma concentrations of oxytocin as compared with non-autistic children [60].
Fifth, vitamin D deficiency may increase the risk for genetic mutations through inhibition of DNA repair of de novo mutations early during development. Indeed, mutations contribute up to 12 and 9 % of ASD diagnose, respectively [10].
This article is the first meta-analysis that investigated a possible relationship between low serum vitamin D level and ASD. To appreciate our data, it is important to bear in mind that there are some limitations. First, possible selection bias cannot be ruled out, despite careful assessment concerning methodology and quality of the included studies. Heterogeneity among these articles is still significant, which may attribute to the fact that the serum vitamin D concentrations might not be comparable between studies. Second, possible publication bias may be involved. We only included eligible English studies in this meta-analysis, while studies writing in other languages were excluded based on language criteria. Also, negative publications may not have been published.
Despite these limitations, the findings from our study appear quite robust and the observed vitamin D reduction in children with ASD is likely to have clinical consequences. First, we believe that routine assessment of vitamin D status is indicated in all the children with ASD and also a clear vision on vitamin D supplementation is warranted. Animal evidence indicates vitamin D deficiency-induced brain damage may be malleable, thus vitamin D may partially reverse the brain damage if given in adequate doses [22, 90]. In addition, indications exist that supplementation of vitamin D actually may directly improve autistic symptoms [38, 51].
It remains under debate whether children with autism should be supplemented with higher doses vitamin D3, in order to maintain their 25(OH) D concentrations optimal. Average population 25(OH)D levels are un-known but natural levels, that is, levels found in humans who live or work in the sun, are around 50 ng/ml [91]. To confirm this, long-term controlled clinical trials with large sample sizes will be needed.
References
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders. 5th edn. American Psychiatric Association, Washington DC.
Kanner L (1943) Autistic disturbances of affective contact. Nerv Child 2:217–250
Elsabbagh M, Divan G, Koh YJ, Kim YS, Kauchali S, Marcín C, Montiel-Nava C, Patel V, Paula CS, Wang C, Yasamy MT, Fombonne E (2012) Global prevalence of autism and other pervasive developmental disorders. Autism Res 5:160–179
Atladottir HO, Gyllenberg D, Langridge A et al (2015) The increasing prevalence of reported diagnoses of childhood psychiatric disorders: a descriptive multinational comparison. Eur Child Adolesc Psychiatry 24:173–183
Heil KM, Schaaf CP (2013) The genetics of autism spectrum disorders—a guide for clinicians. Curr Psychiatry Rep 15:334
Smalley SL, Asarnow RF, Spence MA (1988) Autism and genetics. A decade of research. Arch Gen Psychiatry 45:953–961
Schaefer GB, Mendelsohn NJ (2013) Clinical genetics evaluation in identifying the etiology of autism spectrum disorders: 2013 guideline revisions. Genet Med Off J Am Coll Med Genet 15:399–407
Mendelsohn NJ, Schaefer GB (2008) Genetic evaluation of autism. Semin Pediatr Neurol 15:27–31
Posthuma D, Polderman TJ (2013) What have we learned from recent twin studies about the etiology of neurodevelopmental disorders? Curr Opin Neurol 26:111–121
Iossifov I, O’Roak BJ, Sanders SJ et al (2014) The contribution of de novo coding mutations to autism spectrum disorder. Nature 515:216–221
De Rubeis S, He X, Goldberg AP et al (2014) Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 515:209–215
Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, Reichenberg A (2014) The familial risk of autism. Jama 311:1770–1777
Kolevzon A, Gross R, Reichenberg A (2007) Prenatal and perinatal risk factors for autism: a review and integration of findings. Arch Pediatr Adolesc Med 161:326–333
Gardener H, Spiegelman D, Buka SL (2009) Prenatal risk factors for autism: comprehensive meta-analysis. Br J Psychiatry J Ment Sci 195:7–14
Munger KL, Levin LI, Massa J, Horst R, Orban T, Ascherio A (2013) Preclinical serum 25-hydroxyvitamin D levels and risk of type 1 diabetes in a cohort of US military personnel. Am J Epidemiol 177:411–419
Ronald A, Hoekstra RA (2011) Autism spectrum disorders and autistic traits: a decade of new twin studies. Am J Med Genet B Neuropsychiatr Genet Off Publ Int Soc Psychiatr Genet 156b:255–274
Freitag CM, Staal W, Klauck SM, Duketis E, Waltes R (2010) Genetics of autistic disorders: review and clinical implications. Eur Child Adolesc Psychiatry 19:169–178
Uher R (2014) Gene-environment interactions in severe mental illness. Frontiers in psychiatry 5:48
Neggers YH (2014) Increasing prevalence, changes in diagnostic criteria, and nutritional risk factors for autism spectrum disorders. ISRN Nutr 2014:514026
Bakare MO, Munir KM (2011) Autism spectrum disorders (ASD) in Africa: a perspective. Afr J Psychiatry 14:208–210
DeLuca GC, Kimball SM, Kolasinski J, Ramagopalan SV, Ebers GC (2013) Review: the role of vitamin D in nervous system health and disease. Neuropathol Appl Neurobiol 39:458–484
Cannell JJ (2008) Autism and vitamin D. Med Hypotheses 70:750–759
Grant WB, Soles CM (2009) Epidemiologic evidence supporting the role of maternal vitamin D deficiency as a risk factor for the development of infantile autism. Dermato-endocrinology 1:223–228
Cannell JJ, Grant WB (2013) What is the role of vitamin D in autism? Dermato-endocrinology 5:199–204
Kocovska E, Fernell E, Billstedt E, Minnis H, Gillberg C (2012) Vitamin D and autism: clinical review. Res Dev Disabil 33:1541–1550
Becker KG (2011) Autism, immune dysfunction and Vitamin D. Acta psychiatrica Scandinavica 124:74–75
Wang TT, Du L, Shan L, Jia FY (2014) Research advances in immunological dysfunction in children with autism spectrum disorders. Chinese journal of contemporary pediatrics (Zhongguo dang dai er ke za zhi) 16:1289–1293
Noriega DB, Savelkoul HF (2014) Immune dysregulation in autism spectrum disorder. Eur J Pediatr 173:33–43
Meguid NA, Hashish AF, Anwar M, Sidhom G (2010) Reduced serum levels of 25-hydroxy and 1,25-dihydroxy vitamin D in Egyptian children with autism. J Altern Complement Med (New York, NY) 16:641–645
Humble MB, Gustafsson S, Bejerot S (2010) Low serum levels of 25-hydroxyvitamin D (25-OHD) among psychiatric out-patients in Sweden: relations with season, age, ethnic origin and psychiatric diagnosis. J Steroid Biochem Mol Biol 121:467–470
Mostafa GA, Al-Ayadhi LY (2012) Reduced serum concentrations of 25-hydroxy vitamin D in children with autism: relation to autoimmunity. J Neuroinflammation 9:201
Tostes MH, Polonini HC, Gattaz WF (2012) Low serum levels of 25-hydroxyvitamin D (25-OHD) in children with autism. Trends in Psychiatry and Psychotherapy 3:161–163
Neumeyer AM, Gates A, Ferrone C, Lee H, Misra M (2013) Bone density in peripubertal boys with autism spectrum disorders. J Autism Dev Disord 43:1623–1629
Gong ZL, Luo CM, Wang L et al (2014) Serum 25-hydroxyvitamin D levels in Chinese children with autism spectrum disorders. NeuroReport 25:23–27
Kocovska E, Andorsdottir G, Weihe P et al (2014) Vitamin d in the general population of young adults with autism in the faroe islands. J Autism Dev Disord 44:2996–3005
Bener A, Khattab AO, Al-Dabbagh MM (2014) Is high prevalence of Vitamin D deficiency evidence for autism disorder?: In a highly endogamous population. J Pediatr Neurosci 9:227–233
Du L, Shan L, Wang B, Feng JY, Xu ZD, Jia FY (2015) Serum levels of 25-hydroxyvitamin D in children with autism spectrum disorders. Chinese journal of contemporary pediatrics (Zhongguo dang dai er ke za zhi) 17:68–71
Saad K, Abdel-Rahman AA, Elserogy YM et al. (2015) Vitamin D status in autism spectrum disorders and the efficacy of vitamin D supplementation in autistic children. Nutritional neuroscience. (PMID:25876214)
Fernell E, Bejerot S, Westerlund J et al (2015) Autism spectrum disorder and low vitamin D at birth: a sibling control study. Mol Autism 6:3
Molloy CA, Kalkwarf HJ, Manning-Courtney P, Mills JL, Hediger ML (2010) Plasma 25(OH)D concentration in children with autism spectrum disorder. Dev Med Child Neurol 52:969–971
Adams JB, Audhya T, McDonough-Means S et al (2011) Nutritional and metabolic status of children with autism vs. neurotypical children, and the association with autism severity. Nutr Metab 8:32
Uğur Ç, Gürkan CK (2014) Serum vitamin D and folate levels in children with autism spectrum disorders. Res Autism Spectr Disord 8:1641–1647
Fernell E, Barnevik-Olsson M, Bagenholm G, Gillberg C, Gustafsson S, Saaf M (2010) Serum levels of 25-hydroxyvitamin D in mothers of Swedish and of Somali origin who have children with and without autism. Acta Paediatrica (Oslo, Norway: 1992) 99:743–747
Vadeyar S, Shetye S, Somani S, Shah P (2014) Maternal vitamin D deficiency correlation with neonatal vitamin D deficiency. BJOG Int J Obstet Gynaecol 121:165–166
Whitehouse AJ, Holt BJ, Serralha M, Holt PG, Hart PH, Kusel MM (2013) Maternal vitamin D levels and the autism phenotype among offspring. J Autism Dev Disord 43:1495–1504
Grant WB, Cannell JJ (2013) Autism prevalence in the United States with respect to solar UV-B doses: an ecological study. Dermato-endocrinology 5:159–164
Dealberto MJ (2011) Prevalence of autism according to maternal immigrant status and ethnic origin. Acta Psychiatr Scand 123:339–348
Hayashi E (2001) Seasonal changes in sleep and behavioral problems in a pubescent case with autism. Psychiatry Clin Neurosci 55:223–224
Adams JB, Audhya T, McDonough-Means S et al (2011) Effect of a vitamin/mineral supplement on children and adults with autism. BMC Pediatr 11:111
Cannell JJ (2013) Autism, will vitamin D treat core symptoms? Med Hypotheses 81(2):195–198
Jia F, Wang B, Shan L, Xu Z, Staal WG, Du L (2015) Core symptoms of autism improved after vitamin d supplementation. Pediatrics 135:e196–e198
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25:603–605
Duan XY, Jia FY, Jiang HY (2013) Relationship between vitamin D and autism spectrum disorder. Chinese journal of contemporary pediatrics (Zhongguo dang dai er ke za zhi) 15:698–702
Almeras L, Eyles D, Benech P et al (2007) Developmental vitamin D deficiency alters brain protein expression in the adult rat: implications for neuropsychiatric disorders. Proteomics 7:769–780
Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ (2005) Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat 29:21–30
Eyles DW, Burne TH, McGrath JJ (2013) Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol 34:47–64
Harms LR, Burne TH, Eyles DW, McGrath JJ (2011) Vitamin D and the brain. Best Pract Res Clin Endocrinol Metab 25:657–669
Al-Daghri NM, Al-Attas OS, Alokail MS et al (2014) Lower vitamin D status is more common among Saudi adults with diabetes mellitus type 1 than in non-diabetics. BMC Public Health 14:153
Brance ML, Brun LR, Lioi S, Sanchez A, Abdala M, Oliveri B (2015) Vitamin D levels and bone mass in rheumatoid arthritis. Rheumatol Int 35:499–505
Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J (2011) Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun 25:40–45
Jones AP, Tulic MK, Rueter K, Prescott SL (2012) Vitamin D and allergic disease: sunlight at the end of the tunnel? Nutrients 4:13–28
Guillot X, Semerano L, Saidenberg-Kermanac’h N, Falgarone G, Boissier MC (2010) Vitamin D and inflammation. Jt Bone Spine Rev du Rhum 77:552–557
Mao L, Ji F, Liu Y, Zhang W, Ma X (2014) Calcitriol plays a protective role in diabetic nephropathy through anti-inflammatory effects. Int J Clin Exp Med 7:5437–5444
Neve A, Corrado A, Cantatore FP (2014) Immunomodulatory effects of vitamin D in peripheral blood monocyte-derived macrophages from patients with rheumatoid arthritis. Clin Exp Med 14:275–283
Munoz LE, Schiller M, Zhao Y, Voll RE, Schett G, Herrmann M (2012) Do low vitamin D levels cause problems of waste removal in patients with SLE? Rheumatology (Oxford, England) 51:585–587
Hayes CE, Nashold FE, Spach KM, Pedersen LB (2003) The immunological functions of the vitamin D endocrine system. Cell Mol Biol (Noisy-le-Grand, France) 49:277–300
Masi A, Quintana DS (2015) Cytokine aberrations in autism spectrum disorder: a systematic review and meta-analysis. Mol Psychiatry 20:440–446
Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J (2011) Altered T cell responses in children with autism. Brain Behav Immun 25:840–849
Sweeten TL, Bowyer SL, Posey DJ, Halberstadt GM, McDougle CJ (2003) Increased prevalence of familial autoimmunity in probands with pervasive developmental disorders. Pediatrics 112:e420
Croen LA, Grether JK, Yoshida CK, Odouli R, Van de Water J (2005) Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: a case-control study. Arch Pediatr Adolesc Med 159:151–157
Mostafa GA, Kitchener N (2009) Serum anti-nuclear antibodies as a marker of autoimmunity in Egyptian autistic children. Pediatr Neurol 40:107–112
Mostafa GA, Al-Ayadhi LY (2011) Increased serum levels of anti-ganglioside M1 auto-antibodies in autistic children: relation to the disease severity. J Neuroinflammation 8:39
Al-ayadhi LY, Mostafa GA (2011) Increased serum osteopontin levels in autistic children: relation to the disease severity. Brain Behav Immun 25:1393–1398
Mostafa GA, Al-Ayadhi LY (2011) A lack of association between hyperserotonemia and the increased frequency of serum anti-myelin basic protein auto-antibodies in autistic children. J Neuroinflammation 8:71
Al-A LY, Mostafa GA (2014) Serum antinucleosome-specific antibody as a marker of autoimmunity in children with autism. J Neuroinflammation 11:69
Rossignol DA, Frye RE (2012) A review of research trends in physiological abnormalities in autism spectrum disorders: immune dysregulation, inflammation, oxidative stress, mitochondrial dysfunction and environmental toxicant exposures. Mol Psychiatry 17:389–401
Hegazy HG, Ali EH, Elgoly AH (2015) Interplay between pro-inflammatory cytokines and brain oxidative stress biomarkers: evidence of parallels between butyl paraben intoxication and the valproic acid brain physiopathology in autism rat model. Cytokine 71:173–180
Dringen R (2000) Metabolism and functions of glutathione in brain. Prog Neurobiol 62:649–671
Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D (2002) New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab TEM 13:100–105
Garcion E, Thanh XD, Bled F et al (1996) 1,25-Dihydroxyvitamin D3 regulates gamma 1 transpeptidase activity in rat brain. Neurosci Lett 216:183–186
Halicka HD, Zhao H, Li J, Traganos F, Studzinski GP, Darzynkiewicz Z (2012) Attenuation of constitutive DNA damage signaling by 1,25-dihydroxyvitamin D3. Aging 4:270–278
Patrick RP, Ames BN (2014) Vitamin D hormone regulates serotonin synthesis. Part 1: relevance for autism. FASEB J Off Publ Fed Am Soc Exp Biol 28:2398–2413
Crockett MJ, Clark L, Tabibnia G, Lieberman MD, Robbins TW (2008) Serotonin modulates behavioral reactions to unfairness. Science (New York, NY) 320:1739
Chugani DC, Muzik O, Behen M et al (1999) Developmental changes in brain serotonin synthesis capacity in autistic and nonautistic children. Ann Neurol 45:287–295
McBride PA, Anderson GM, Hertzig ME et al (1989) Serotonergic responsivity in male young adults with autistic disorder. Results of a pilot study. Arch Gen Psychiatry 46:213–221
Prufer KJG (1997) 1.25-Dihydroxyvitamin D3 receptor is partly colocalized with oxytocin immunoreac-tivity in neurons of the male rat hypothalamus. Cellular and molecular biology (Noisy-le-Grand, France) 43:543–548
Aoki Y, Watanabe T, Abe O et al. (2015) Oxytocin’s neurochemical effects in the medial prefrontal cortex underlie recovery of task-specific brain activity in autism: a randomized controlled trial. Mol Psychiatry 20:447–453
Dadds MR, MacDonald E, Cauchi A, Williams K, Levy F, Brennan J (2014) Nasal oxytocin for social deficits in childhood autism: a randomized controlled trial. J Autism Dev Disord 44:521–531
Guastella AJ, Gray KM, Rinehart NJ et al. (2015) The effects of a course of intranasal oxytocin on social behaviors in youth diagnosed with autism spectrum disorders: a randomized controlled trial. J Child Psychol Psychiatry 56:444–452
Burne TH, Feron F, Brown J, Eyles DW, McGrath JJ, Mackay-Sim A (2004) Combined prenatal and chronic postnatal vitamin D deficiency in rats impairs prepulse inhibition of acoustic startle. Physiol Behav 81:651–655
Vieth R (2006) What is the optimal vitamin D status for health? Prog Biophys Mol Biol 92:26–32
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Wang, T., Shan, L., Du, L. et al. Serum concentration of 25-hydroxyvitamin D in autism spectrum disorder: a systematic review and meta-analysis. Eur Child Adolesc Psychiatry 25, 341–350 (2016). https://doi.org/10.1007/s00787-015-0786-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00787-015-0786-1