Abstract
Autism spectrum disorder (ASD) is a common and severe neuro-developmental disorder in early childhood which is defined by social and communication deficits and repetitive and stereotypic behaviours. The aetiology of ASD remains poorly understood. Susceptibility to development of ASD has significant environmental components, in addition to the profound genetic heritability. Few genes have been associated to the risk for ASD development. There is substantial evidence implicating chronic neurological inflammation and immune dysregulation leading to upregulation of inflammatory cytokines in the ASD brain, probably due to altered blood–brain barrier function. The immune system is characterized by excessive and skewed cytokine responses, modulated T cell reactivity, decreased regulation and production of immunosuppressive cytokines, modified NK function and increased autoantibody production. Conclusion: The perinatal environment generates vulnerability to chronic neuro-inflammation in the brain associated with profound modulation and dysregulation in the immune system leading to the rapid development of ASD in genetically susceptible children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism spectrum disorders (ASD) are a group of neurodevelopmental disabilities characterized by impaired social interaction, qualitative impairments in communication and restrictive, repetitive and stereotyped behaviours [4], as described in the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders. In most cases, ASD is clinically diagnosed during the first 3 years of age and is a lifelong condition for most [70]. Due to the inherent heterogeneity of development, the mean age of diagnosis is 5.7 years, while a substantial number of children remain undiagnosed until the age of 8 years [77].
In December 2012, the American Psychiatric Association approved the fifth DSM, which changes the definition of ASD, in an effort to diagnose more accurately the signs of autism and this edition was published in May 2013. In this new edition, the previously individually diagnosed autistic disorder, pervasive developmental disorder not otherwise specified, Asperger's disorder and childhood disintegrative disorder are all included in one single diagnosis: autism spectrum disorder. Regardless of the controversy surrounding this decision, and whether or not it will make it clearer for clinicians to diagnose patients with autism, it is another indication on how little we know about this disorder since the process of creating proper diagnostic tools and criteria is far from over. What is undeniable is that autism diagnosis has increased dramatically in the past decades. According to the Centre for Disease Control and Prevention Morbidity and Mortality Weekly Report, vol. 61, no. 3 published in March of 2012, 1 in 88 children have been identified with ASD, which marks a 78 % increase since the first report in 2007. Studies in Asia, Europe and North America report an average prevalence of ASD around 1 % [56]. The prevalence of 1/150 to 1/200 is considered a reliable figure, making ASD more prevalent than many other childhood disorders that are considered common.
Although the increase in ASD diagnosis has increased dramatically, it is unclear if the actual prevalence of autism has increased since this increase is partly due to changes in the diagnostic practice and the increasing awareness of parents to the symptoms of these disorders. Overall, ASD are complex neurodevelopment disorders whose theories of causation are still incomplete.
Nonetheless, many aetiologies and causes have been proposed. This review will attempt to revise the most common and strongly supported causes for ASD. It is now clear that autism should not be defined as a disease but more as a syndrome with multiple genetic and environmental causes. Among researchers, there is an increasing support for the theory that ASD does not have a single or main cause but is instead a complex disorder with a set of core aspects that have several different causes [40, 41].
In other words, just like in mental retardation, completely different underlying brain dysfunctions are hypothesized to result in the development of ASD [83]. So far, the consensus between the autism research community is that genetic factors predominate, and additional environmental factors contribute or exacerbate the symptoms of ASD (Fig. 1). Many previously suggested prenatal risk factors for autism, including maternal allergic phenotype and maternal and paternal age, were examined in a meta-analysis, and although none of these factors are implicated in the aetiology of autism, some evidence suggests that pregnancy complications increase the risk [33]. For the purpose of this review, the causal theories of ASD will be divided in three main categories: genetic, prenatal and postnatal causes. For each category, a brief explanation of the main findings and theories will be explained.
Methods
A comprehensive search of PubMed was performed using the terms and MeSH keywords “autism” and “autistic spectrum disorder” associated to the terms “gene”, “genetic”, and “genome”; “pregnancy”, “maternal”, “vitamin D”, “gastrointestinal”, “gut”, “nutrition” and “diet”; and “immunology”, “IgG” and “immune”. The search included all articles in the English, Spanish and Dutch language; additionally, it was limited to articles published from 2000 to March 2013 and only to studies conducted in humans. The search had preference, but was not limited to review articles and meta-analysis.
Genetic-based theories
There is hard evidence to support the theory of a potent genetic cause for ASD development. Monozygotic twin's share 60 to 90 % of autistic traits while fraternal twins only 0 to 23 % [85]. A number of distinct rare genetic disorders related to autism suggest that the genetic tree of this syndrome is very similar to that of mental retardation and epilepsy [64]. Autism is associated with mental retardation in approximately 70 % and seizures in up to 25 % of cases [86].
Rett syndrome represents a clear exception since the majority of cases are caused by de novo mutations or microdeletions of the methyl-CpG-binding protein 2 (MeCP2) gene on Xq28 [31]. MeCP2 is a protein that is encoded by the methyl-CpG-binding gene that is highly expressed in neurons. The exact function of this protein is unclear; it seems to help in the regulation of gene expression by modifying chromatin as CpG binding sites are hotspots for mutations. Usually, it regulates genes involved in brain function, although this protein can be found throughout the body. As the gene is X linked, it is subject to X inactivation. There is no evidence that none of the other subtypes of ASD are linked to any particular genetic disorder. Probably due to this specific aetiology in the latest version of the DSM-5, it has been removed from the group “persuasive developmental disorders”.
Identified genetic disorders associated with ASD
The link between ASD and identified genetic disorders can tilt the scale towards a genetic origin of ASD. It is important to note that the total number of patients diagnosed with ASD who have a clear diagnosis of an additional identified genetic disorder is a small percentage of the whole ASD population, most likely less than 10 % according to population-based studies [30].
A number of specific genetic disorders have been associated with autism including fragile X syndrome, tuberous sclerosis, Smith–Magenis syndrome, Angelman syndrome, Rett syndrome and Prader–Willi syndrome [53]. In general, however, these disorders represent a very small percentage of cases with ASD; as an example, tuberous sclerosis complex (TSC), an autosomal dominant disorder that involves two genes, TSC1 on the chromosome 9q34 and TSC2 on the chromosome 16p13.3 [23]. This syndrome occurs in 1 to 1.7/100,000 persons with approximately 25 % of patients diagnosed with TSC having autism [18]. Additionally, up to 1.3 % of patients diagnosed with ASD have TSC; this percentage may seem low, but when compared to the prevalence of TSC in the general population, it is 30 % higher [42]. Other rare single gene defects have also been associated with ASD in different case studies, which include Williams syndrome, Cowden syndrome and Moebius syndrome, among others [97].
A number of population-based studies have reported a male-to-female ratio of 3:1 in ASD [101], which could suggest an X-linked disorder in which females would be protected by compensation of the transcription of the X chromosome, but the data to support this theory is until now inconclusive and it is clear that ASD transmission does not follow an X-linked pattern [92]. The formation of non-coding RNA can act as an epigenetic mechanism leading to X chromosome inactivation. Imprinted gene clusters on the X chromosome can use this mechanism to silence adjacent genes, including some rare variants that have been reported in association with ASD, by inducing repressive histone modifications [46]. Therefore, this lack of direct linkage to the X chromosome does not rule out the possibility that X chromosome can modulate the risk of ASD.
Family associations
Children with ASD were far more likely to have a family member with an autoimmune disorder, like type 1 diabetes or coeliac disease, when compared to suitable controls substantiating a connection between ASD and family members [9]. This familial clustering suggested an immune dysfunction to play a role in the pathogenesis of autism [19]. Strong evidence has been found to support the theory that ASD is a heritable disorder: the recurrence risk in siblings of patients diagnosed with ASD is up to 8 % [20], although values up to 18.7 % have also been reported [67]. Moreover, the rate of concordance in monozygotic twins ranges from 36 to 95 % for classic autism, while in dizygotic twins, it ranges from 0 to 23 % [76]. In 2009, a large population study, 277 twin pairs diagnosed with a form of ASD, was conducted in the USA via the Interactive Autism Network reported a concordance rate of 31 % for dizygotic twins and 88 % for monozygotic twins [72]. In 2011, Hallmayer analysed the genetic versus the environmental factors of autism development in twin pairs diagnosed with ASD. This study reported a slightly higher concordance among dizygotic twins (21 % for males and 27 % for females). Additionally, via a fitted model, they analysed the relative importance of genetic versus shared environmental factors for the development of ASD, and they concluded that heritability was estimated at 38 % while the shared environmental component was at 58 % [39]. This study suggests that although there is a strong genetic background in the development of ASD, environmental components play a key role in the development of this syndrome.
Additionally, a genetic background is also supported by the elevated prevalence of obsessive–compulsive disorder, communication disorder and social phobias in family members of ASD patients [47, 80]. Although there are several studies concerning how the family of ASD patients can be affected, no specific gene or genes have been identified to explain this association. Whole genome screenings in families more than one member affected with ASD have shown ten or more genes that could be affected [29]. This would mean that potentially several genetic mutations could be associated or even contributed to the development of autism.
Candidate loci and potential genes
Until now, no definitive genetic mutations have been directly linked to ASD; however, several genes have shown potential involvement in the ASD phenotype [58]. It is now widely accepted that the autism syndrome is part of an underlying genetic syndrome in which several genes interact in order to produce the “autism phenotype”, although each genetic locus identified to date to be associated to ASD represents a very small fraction of the total cases of ASD [74]. Several genes have been consistently linked to cases of ASD, while others have been reported in very few cases; however, all of them have a function directly or indirectly related to brain and neural development. Some of these functions include central nervous system development, neurogenesis and axogenesis and synaptic plasticity and transmission, just to name a few.
In order to understand the complex genetic aetiology of ASD, researchers use different approaches that range from linkage, association, cytogenic and copy number variant (CNV) analysis [59]. A common approach is the use of genome wide association studies (GWA), which compare the frequency of single nucleotide polymorphisms in cases versus control samples. Using the GWA approach, researchers can identify rare de novo and inherited CNVs that can be linked to ASD. Until now, only loci 17q11-17q21 and 7q are considered genome wide significant [1]. The chromosomal translocation breakpoints associated with ASD identified in the 7q22-7q33 [75] are related to the protein reelin localized in this site and potentially involved in neural migration during development, formation on cortical layers and synaptic plasticity encoded by the gene RELN [55]. Reelin is integrally involved in the pathophysiology of ASD. In 2001, the International Molecular Genetic Study of Autism Consortium screened 170 multiplex families and postulated that an autism susceptibility locus, named AUTS1, exited in the location 7q31-q33 for affected family members [50]. This AUTS1 locus contains a number of genes that could potentially be involved in the pathogenesis of ASD. Among them, one example is the candidate gene FOXP2 located in the loci 7q31. This gene encodes the forkhead/winged helix FOX family of proteins, hence the name. The FOXP2 gene is necessary for brain and lung development; knockout mice with only one functional copy of this gene were found to have significantly reduced vocalization as pups [78]. Follow-up studies have narrowed this region on chromosome 7q31 to a 3 cM critical region (located between D7S496 and D7S2418), identifying the candidate gene LAMB1 [48]. This gene encodes the protein laminin, which is the major non-collagenous part of basement membranes and is associated with several biological processes that include cell adhesion, differentiation, migration and neurite outgrowth.
The second region that has been implicated in ASD by a number of studies is the 17q11 where the SLC6A4 candidate gene is located [91]. This gene is involved in the regulation of serotonin and variation in this gene is associated with grey matter volume in the cortex. Additionally, 1 to 4 % of patients diagnosed with ASD have cytogenetic abnormalities at the 15q11-q13 locus [35]. Several population studies and case reports have identified deletions, duplications and inversions at this locus [11, 12, 44]. A “chromosome 15 phenotype” has been described in individuals with chromosome 15 duplications characterized by ataxia, language delay, seizures, mental retardation and facial dysmorphology [96]; since most of these characteristics are the same as found in ASD, there is evidence that an alteration in the chromosome 15 is involved in the pathogenesis of ASD. In the chromosome 15q12, the candidate gene gamma amino butyric acid receptor has been identified, which regulates brain cell migration, differentiation and synapse formation. There are several more locus and candidate genes reported to be associated with ASD and current approach of genetic testing in ASD these finding will continue to grow and evolve. However, no matter how much this field grows, the diagnosis of ASD continues to be clinical based, since until now the pathophysiology of ASD remains too multifactorial [43].
Prenatal-based theories
Biomarkers
ASD develops in susceptible individuals based on genetic and environmental risk factors; the identification of which is still largely incomplete. Analysis of biological samples (increasingly using (epi)genomic, proteomic and metabolomic tools), in combination with neurological analysis (including advanced imaging technologies), could provide potential biomarkers [45]. Such biomarkers are useful for corroborating clinical diagnosis, providing an indicator for the clinical course of the disease, identifying susceptible children based on the predictive value of these markers and underpinning the need for specific therapy and monitoring of the efficacy of that treatment. However, so far, no unequivocal biomarkers for routine clinical use have been defined yet.
Maternal autoantibodies
From several studies, it is safe to assume that a dysregulation in the immune system is a crucial part in the development of ASD [7]. A common finding is an elevated number of autoantibodies that react against the brain and central nervous system in children diagnosed with ASD when compared to suitable controls [16, 95]. Recently, a number of studies suggest the presence of maternal autoantibodies that can have a harmful effect in brain development of the foetus during pregnancy [22]. Maternal antibodies, specifically immunoglobulin G (IgG), are able to cross the placenta barrier and provide passive immunity to the foetus during pregnancy. However, along with this beneficial immunoglobulin, pathogenic autoantibodies can also reach the foetus, affecting the foetal brain tissue [100]. Therefore, it is plausible that brain-specific maternal autoantibodies might have an influence in several congenital neurological developmental disorders. In addition, ASD children have a greater family history of autoimmunity like type 1 diabetes and ulcerative colitis [7, 95].
In 2008, Braunschweig examined maternal plasma antibodies against human foetal and adult brain proteins within 61 mothers of a child diagnosed with ASD and 102 suitable controls. This study reported a significantly higher autoreactivity to a protein approximately at 37 and at 73 kDa in mothers of a child with ASD when compared to the respective controls [13]. This previous study was recently replicated with a larger population (n = 560) reporting similar findings, a significant association between maternal IgG reactivity to both proteins and the diagnostic of ASD in the offspring of these mothers [14]. These findings of anti-foetal brain antibodies in the serum of the mothers support the theory of an association between the maternal immune system biomarkers and the diagnosis of ASD [32]. Alterations in the blood–brain barrier result in exposure to neuron-derived antigens that induce a large proportion of specific autoantibodies of different isotypes, including IgM, IgG and IgA. This blood–brain barrier is a highly active and selective interface between the central nervous system and the peripheral immune system. The cytokine production potential of this barrier contributes to the neuro-inflammatory condition in the brain and facilitates the production of autoantibodies and promotes access of inflammatory and immune cells to the brain, creating a vicious circle of the chronic neuro-inflammatory condition in ASD [79].
Maternal levels of vitamin D during pregnancy
Lately, a theory linking the development of ASD to maternal vitamin D deficiency during pregnancy is gaining support. There is strong evidence to suggest that vitamin D has the same role as a neuroactive steroid, which implies that it can affect neuronal differentiation, axonal connectivity and brain structure and function [26]. Moreover, vitamin D deficiency is shown to be common during pregnancy [3, 52] and linked with numerous adverse effects in the foetus, which include intrauterine growth restriction, reduced bone mineral accrual and recurrent wheezing [51, 63].
Vitamin D insufficiency can increase the risk of autism: a theory based on several findings such as children with vitamin D-deficient rickets have autistic characteristics that seem to disappear with high doses of vitamin D [82]; autism is more common in places with impaired UVB penetration; epidemiological studies have found a higher prevalence of ASD when the conception period occurred in the winter months, particularly March, when levels of vitamin D are at their lowest [81]; animal data which found repeatedly that severe vitamin D deficiency during gestation leads to increase brain size and enlarged ventricles; and these anomalies are also found in autistic children [27].
However, the claim that vitamin D deficiency during pregnancy is a risk factor for ASD development still lacks conclusive evidence [57]. Moreover, in 2012, Whitehouse et al. tested if maternal vitamin D deficiency during pregnancy was related to autism phenotype and found little evidence for this association. This study described a significant association between low concentration of maternal 25 OH-vitamin D and offspring with high scores on the attention switching subscale of the AQ, suggesting that low maternal vitamin D levels during pregnancy are not a determinant of ASD [93].
Maternal infections, febrile episodes and antibiotic use during pregnancy
It has been proposed that activation of the maternal immune system during pregnancy could be associated with behaviours of ASD in the offspring of these mothers [69]. Presently, very few studies have investigated the association between common infections, self-reported by the mothers, and ASD in their offspring [10]. Still, viral infections during pregnancy, for example with influenza, rubella, measles, cytomegalovirus and herpes simplex virus, are suggested to increase the risk for ASD development in the offspring [24]. In 2012, a research group in Denmark published a population-based cohort which included 96,736 children born from 1997 to 2003, which found little evidence of an association between a mild common infection or febrile episode during pregnancy and the diagnosis of ASD. However, this study reported a twofold increased risk of ASD after maternal influenza infection during pregnancy and a threefold increase with a prolonged episode of fever during pregnancy and additionally identified the use of antibiotics during pregnancy as a strong risk factor for ASD [8]. Prenatal and early life exposure to acetaminophen was linked to the risk of autism spectrum disorder in susceptible individuals. However, many studies were flawed and have not been independently confirmed.
Postnatal environmental-based theories
It is now widely accepted that environmental factors play an active role in the development of autism, which is why many attempts have been made to identify potential risk factors for ASD. Among these factors, none has been more popular than the link between the gastrointestinal system and ASD.
Gastrointestinal disease
In 2009, Campbell et al. published the first study that demonstrated a probable genetic cause between ASD and gastrointestinal disease by reporting that a variation of the promoter of the gene encoding the mesenchymal epithelial transition factor (MET) receptor tyrosine kinase is related to ASD and that this variation in the MET signalling increases the risk of gastrointestinal dysfunction in ASD [17]. Prior to this study, numerous studies have been published that reported histopathological findings such as ileal lymphoid nodular hyperplasia and colonic lymphoid nodular hyperplasia and the pathological scores to be significantly higher in children with ASD when compared to controls [15]. Additionally, many of the inflammatory transcripts detected in gastrointestinal tract in ASD are similar to those observed in Crohn's disease and ulcerative colitis [89]. Also, difficulties in food consumption are often noticed in ASD as a result of both selectivity in accepting solid foods and gastrointestinal problems due to reflux, constipation or diarrhoea, food allergies or intolerances and coeliac diseases [66].
These findings have been recently questioned since the populations studied were only children who had been referred to a gastroenterologist because they all had previous gastroenterological complaints. Moreover, the so-called significant findings in the ASD children with gastrointestinal symptoms did not differ from the rate usually observed in the general paediatric population [25]. This lack of association was additionally supported by Ibrahim et al. in 2009 when he reported no significant association between cases with ASD and controls in the overall cumulative gastrointestinal symptoms [49]. Recently, a large population study conducted in the UK, comparing the stool patterns of children diagnosed with ASD and normally developed children during the first 3.5 years of life, found no significant differences between the two groups [73]. Thus, there is enough evidence to conclude that the general incidence of gastrointestinal symptoms in the ASD population does not differ from the general paediatric control population [36].
Gut microbiota
In the past decade, several studies have demonstrated differences between the microbiota composition in ASD cases when compared to healthy suitable controls [2, 28, 94] (see Table 1). However, no clear trend has emerged, and since all the studies used different methodologies, these are difficult to compare. One common finding is that the microbiota profiles from healthy siblings of ASD patients are an intermediate between the findings from the ASD cases and the controls [61].
Additionally, an imbalance in microbiota can have an impact on health by disturbing the fermentation of undigested dietary components and therefore impacting the production of short chain fatty acids and ammonia. It was demonstrated that faecal levels of short chain fatty acid and ammonia are significantly higher in children with ASD when compared to controls [90]. This suggests that fermentation processes or the utilization of the fermentation products is possibly altered in children diagnosed with ASD.
Dysfunctional immune response-based theories
Currently, the scientific community agrees that there is an important genetic component in the development of ASD; as described earlier in this review, there are several genes that have been involved in the development of ASD. Most of these genes are involved in the development of the central nervous system, which potentially affects behavioural, cognitive, learning and memory components, all implicated in the range of symptoms characteristic of ASD. Besides, immune-related genetic variability is also implicated in ASD, since many pro-inflammatory cytokine genes are associated with ASD development [99].
Neurobiological abnormalities
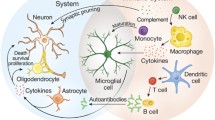
A number of neurological abnormalities have been found in the brains of ASD patients in post-mortem examinations and through magnetic resonance imagining. In 2003, Courchesne described that the brain of children diagnosed with ASD develops normally until the age of 9 months, which is followed by a rapid period of white matter growth during the following 9 to 24 months [21]. Furthermore, Vargas et al. reported that a histologic analysis of the brain tissue of children diagnosed with ASD showed signs of classic inflammation in the areas where excessive growth was registered [60, 87], thus demonstrating an involvement of astroglial and microglial cells with no lymphocyte infiltration or immunoglobulin deposition in the central nervous system (CNS). Microglia are the mononuclear phagocytic cells that participate in the immune surveillance of the CNS. This study also demonstrated an ongoing immune cytokine activation in the post-mortem brain of patients with ASD. Interferon-γ (IFN-γ) is a critical cytokine in adaptive immune response; an abnormality in this cytokine is associated with auto-inflammatory processes and autoimmune diseases. This overproduction of cytokines can lead to a chronic inflammation in the brain tissue, which is consistent with findings reported in white matter tissue in ASD cases.
A particular area of interest is the aberrations found in the limbic system, since this is the centre in the brain that controls behaviour. Until now, increased cell packing and small neuronal size have been reported [68]. Another area of the brain that can influence emotional behaviour is the cerebellum; abnormalities in this area have been reported to be one of the most consistent findings in ASD. These findings include paucity of Purkinje and granular cells [84]. All these findings can explain localized or systemic inflammation, which releases immunomodulatory molecules that could affect neurodevelopment, particularly at critical periods of brain development.
Cytokines
An altered cytokine profile has been described in patients diagnosed with ASD. Cytokines are proteins that control the intensity, duration and type of immune response. Additionally, cytokines are involved in neural development and maintenance and increased cytokine and chemokine production were found in post-mortem ASD brains, including IFN-γ, IL-1β, IL-6, IL-8 and IL-12p40, all of them being pro-inflammatory mediators. Plasma levels of leptin, a cytokine functionally related to IL-6 and IL-12, are increased and able to cross the blood–brain barrier. Also, tumour necrosis factor alpha (TNF-α) and chemokine C–C motif ligand CCL-2 were included [62, 71]. Many of these cytokines are induced by the activation of the NF-κB transcription factor, a critical factor in inflammation and apoptosis, which is found at increased levels in peripheral blood mononuclear cells in ASD. Increased levels of TNF-α and its capacity to block synaptic communication are the most consistent and typical finding in ASD brain, cerebrospinal fluid and blood cells [98]. Blood monocytes of ASD children showed enhanced production of pro-inflammatory cytokines upon TLR2- and TLR4-dependent stimulation, while TLR9 stimulation resulted in decreased cytokine production. Innate immune stimulation of monocytes thus results in an altered cytokine response in ASD. Altered reelin signalling associated with modified cytokine communication in the central nervous system at prenatal (in utero) or early postnatal period is considered to provide an immunological insult. This results in an epigenetic trigger modulating the glucocorticoid receptor gene function with lifelong consequences for the structure and function of the brain and the induction of an inflammatory state in the brain [45].
Several studies have reported decreased plasma levels of transforming growth factor beta (TGF-β), which is related to cell migration, apoptosis and regulation in the immune system and CNS [6, 65]; these results suggest that dysfunction of TGF-β has potentially a role in ASD by not being able to control inflammation, therefore leading to a state of chronic inflammation which has devastating effects in the brain and nervous system. A different cytokine that has also been linked to ASD is macrophage inhibitory factor (MIF), which is a pro-inflammatory immune regulator that influences neural and endocrine systems. Plasma levels of MIF have been reported to be higher in ASD patients when compared to suitable controls. Moreover, the highest levels of plasma MIF correlated to the cases with the most severe behavioural symptoms [38].
Adaptive cellular response
Several immune abnormalities in T and B cells, natural killer (NK) cell activity and differential monocyte responses along with autoantibody production have been described in ASD [54, 88]. A finding that has been common in the immunological studies in patients with ASD is an atypical adaptive T cell response, described as a bias towards T helper 2 (Th2) phenotype and a reduced response of Th1 cells. Circulating antibodies directed toward brain proteins have widely been found in ASD children, along with anti-nuclear antibodies and increased plasma levels of IgG4. After T cell development, naive T cells are spread throughout the body; they express the T cell receptor CD3 complex. This complex is responsible for recognizing antigens bound to the major histocompatibility complex (MHC) molecules, also named the human leukocyte antigen (HLA). The profile activation of circulating T cell is different and CD3+ T cells have higher levels of HLA-DR, which is a marker for late cellular activation [5, 37]. HLA-DR is an MHC class II cell surface receptor and constitutes a ligand for the T cell receptor. HLA-DR is involved in a number of autoimmune diseases. Monocytes are antigen-presenting cells that are actively producing the majority of the pro-inflammatory cytokines due to NF-κB activation. They can differentiate into classical M1 and several populations of M2 macrophages, thereby actively steering the resulting T cell differentiation. A possible impairment of Th1 and perhaps Th17 responses in the ASD children was suggested, making them more vulnerable to certain microbial infection [34, 54]. Additionally, a decreased dendritic cell maturation and resulting decreased frequency of TGF-β+ and/or IL-10 inducible regulatory T (Treg) cells were observed in the ASD children, despite higher increase in TGF-ß production. This decreased level of Treg results in dysregulated Th1 and Th17 functions and an increased Th2 compartment in the ASD children. These findings contribute to the theory that autism could be a state of chronic inflammation; the increased T cell activation could lead to decreased apoptosis, which causes the survival of activated cells that in normal circumstances would be eliminated.
Conclusions
A number of aetiologies for ASD have been proposed, but the scientific community is far from understanding the theory of causation of ASD. Currently, some consensus exists that genetic factors predominate but the genetics of autism is complex, and until now, the information is incomplete. It is clear that a variety of genes are associated with the development of ASD and that most of these genes have a function that impacts directly or indirectly the neural development and the immune system. However, no particular or specific mutation in a candidate gene has a consistent association with ASD. The two more consistent findings identified are at the 15q11-q13 locus and at the 7q22-q33 locus. All of these suggest that there is a genetic predisposition in ASD that involves several genes that have a function relative to neural development.
Another theory that is gaining strength involves the altered immune response in patients with ASD characterized by an atypical cytokine production, altered T cell activation and an impaired apoptotic activity, all of them leading to a chronic state of inflammation in the central nervous system (due to impaired functioning of the blood–brain barrier) and in the peripheral immune system. An immunological implication is also suggested by the observations in the prevalence of autoimmune diseases in family members of patients with ASD. Additionally, the cytokine profile of ASD patients suggests a state of chronic inflammation, which is consistent with the histological findings in the brain tissue samples of ASD patients. Recently, there is increasing evidence that maternal autoantibodies are able to affect the foetal brain development, in particular IgG autoantibodies. Moreover, there are some interesting findings regarding febrile episodes during pregnancy, antibiotic use and prolonged infections, all of them have been more frequently observed in mothers who later received a diagnosis of ASD in their offspring. However, these findings are only observational and have not been undeniably linked as the cause of autism. Another important theory concerns the reduced levels of vitamin D during the first trimester of pregnancy, which coincides with a higher prevalence of ASD. This theory is relatively new, and more population-based studies are necessary for it to gain validity among the scientific community.
Gastrointestinal disorders are one of the most common complaints among ASD patients ranging from chronic constipation, diarrhoea to inflammatory bowel conditions. Nonetheless, when compared to the general pediatric population, there is no significant difference in the prevalence of GI disorders between cases of ASD and healthy controls. A repeated finding is the gut microbiota in ASD patients which differs significantly from healthy controls. An imbalance in microbiota can impact health by disturbing the fermentation of undigested dietary components. For better understanding of this complex syndrome, much scientific research is still necessary.
There is substantial evidence implicating chronic neurological inflammation and immune dysregulation leading to upregulation of inflammatory cytokines in the ASD brain, probably due to altered blood–brain barrier function. The immune system is characterized by excessive and skewed cytokine responses, modulated T cell reactivity, decreased regulation and production of immunosuppressive cytokines, modified NK function and increased autoantibody production. Thus, the perinatal environment generates vulnerability to chronic neuro-inflammation in the brain associated with profound modulation and dysregulation in the immune system leading to the rapid development of ASD in genetically susceptible children.
References
Abrahams BS, Geschwind DH (2008) Advances in autism spectrum disorder: on the threshold of a new neurobiology. Nat Rev Genet 9:341–355
Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA (2011) Gastrointestinal flora and gastrointestinal status in children with autism—comparisons to typical children and correlation with autism severity. BMC Gastroenterol 11:22. doi:10.1186/1471-230X-11-22
Aghajafari F, Nagulesapilai T, Ronksley PE, Tough SC, O’Beirne M, Rabi DM (2013) Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. Br Med J 346:1136–1169
American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders, 4th edn. American Psychiatric Association, Washington, DC
Ashwood P, Corbertt BA, Kantor A, Shulman H, Van de Water J, Amaral DG (2011) In search of cellular immunophenotype in blood of children with autism. PLoS One 6:19299
Ashwood P, Enostrom A, Krakowiak P, Hertz-Picciotto I, Hansen R, Croen LA, Pessah IN, Van de Water J (2008) Decreased transforming growth factor beta1 in autism: a potential link between immune dysregulation and impairment in clinical behaviour outcomes. J Neuroimmunol 204:149–153
Ashwood P, Wills S, van de Water J (2006) The immune response in autism: a new frontier for autism research. J Leukocyte Biol 80:1–15
Atladottir HO, Henriksen TB, Schendel DE, Parner ET (2012) Autism after infection, febrile episodes and antibiotic use during pregnancy: an exploratory study. Pediatrics 130:1447–1454
Atladottir HO, Pedersen MG, Thorsen P, Mortensen PB, Deleuran B, Eaton WW, Parner ET (2009) Association of family history of autoimmune diseases and autism spectrum disorders. Pediatrics 124:687–694
Atladóttir HO, Thorsen P, Østergaard L, Schendel DE, Lemcke S, Abdallah M, Parner ET (2010) Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord 40:1423–1430
Bolton PF, Dennis NR, Browne CE, Thomas NS, Veltman MW, Thompson RJ, Jacobs P (2002) The phenotypic manifestations of interstitial duplications of proximal 15q with special reference to the autistic spectrum disorders. Am J Med Genet 105:675–685
Boyar FZ, Whitney MM, Lossie AC, Gray BA, Keller KL, Stalker HJ, Zori RT, Geffken G, Mutch J, Edge PJ, Voeller KS, Williams CA, Driscoll DJ (2001) A family with grand maternally derived interstitial duplication of proximal 15q. Clin Genet 60:421–230
Braunschweig D, Ashwood P, Krakowiak P, Heetz-Picciotto I, Hansen R, Croen LA, Pessah IN, van de Water J (2008) Autism: maternally derived antibodies specific for fetal brain proteins. Neurotoxicology 29:226–231
Braunschweig D, Duncanson P, Boyce R, Hansen R, Ashwood P, Pessah IN, Hertz-Picciotto I, Van de Water J (2012) Behavioral correlates of maternal antibody status among children with autism. J Autism Dev Disord 42:1435–1445
Buie T, Campbell DB, Fuchs GJ, Futura GT, Levy J, van de Water J, Whitaker AH, Atkins, Bauman ML, Beaudet AL, Carr EG, Gershon MD, Hyman SL, Jirapinvo P, Jyonouchi H, Kooros K, Kushak R, Levitt P, Levy SE, Lewis JD, Murray KF, Naotwocz MR, Sabra A, Wershil BK, Weston SC, Zeltzer L, Winter H (2010) Evaluation, diagnosis and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics 125:S1–S18
Cabanlit M, Wills S, Goines P, Ashwood P, van de Water J (2007) Brain-specific autoantibodies in the plasma of subjects with autistic spectrum disorder. Ann N Y Acad Sci 1107:92–103
Campbell DB, Buie TM, Winter H, Bauman M, Sutcliffe JS, Perrin JM, Levitt P (2009) Distinct genetic risk based on associations of MET in families with co-occurring autism and gastrointestinal conditions. Pediatrics 123:1018–1024
Chakrabarti S, Fombonne E (2001) Pervasive developmental disorders in preschool children. JAMA 285:3093–3099
Comi AM, Zimmerman AW, Frye VH, Law PA, Peeden JN (1999) Familial clustering of autoimmune disorders and evaluation of medical risk factors in autism. J Child Neurol 14:388–394
Cornew L, Dobkins KR, Akshoomoff N, McCleery JP, Carver LJ (2012) Atypical social referencing in infant siblings of children with autism spectrum disorders. J Autism Dev Disord 42:2611–2621
Courchense E, Carper R, Akshoomoff N (2003) Evidence of brain overgrowth in the first year of life in autism. JAMA 290:337–344
Croen LA, Braunschweig D, Haapanen L, Yoshida CK, Fireman B, Grether JK, Kharazi M, Hansen RL, Ashwood P, Van de Water J (2008) Maternal mid-pregnancy autoantibodies to fetal brain protein: the early markers for autism study. Biol Psychiatry 64:583–588
Curatolo P, Bombardieri R, Jozwiak S (2008) Tuberous sclerosis. Lancet 372:657–668
Depino AM (2013) Peripheral and central inflammation in autism spectrum disorders. Mol Cell Neurosci 53:69–76
Erickson CA, Stigler KA, Corkins MR, Posey DJ, Fitzgerald JF, McDougle CJ (2005) Gastrointestinal factors in autistic disorder: a critical review. J Autism Dev Dis 35:713–727
Eyles DW, Burne TH, McGrath JJ (2013) Vitamin D: effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol 34:47–64
Feron F, Burne TH, Brown J, Smith E, McGrath JJ, Mackay-Sim A, Eyles DW (2005) Developmental vitamin D3 deficiency alters the adult rat brain. Brain Res Bull 153:61–68
Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, Youn E, Summanen PH, Granpeesheh D, Dixon D, Liu M, Molitoris DR, Green JA (2010) Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 16:444–453
Folstein SE, Rosen-Sheidley B (2001) Genetics of autism: complex etiology for heterogeneous disorder. Nat Rev Genet 2:943–955
Fombonne E (2002) Epidemiological trends in rates of autism. Mol Psychiatry 7:S4–S6
Fombonne E (2003) Epidemiological surveys of autism and other pervasive developmental disorders: an update. J Autism Dev Disord 33:365–382
Fox E, Amaral D, van de Water J (2012) Maternal and fetal antibrain antibodies in development and disease. Dev Neurobiol 72:1327–1334
Gardener H, Spiegelman D, Buka SL (2009) Prenatal risk factors for autism: comprehensive meta-analysis. Br J Psychiatry 195:7–14
Geschwind DH (2009) Advances in autism. Annu Rev Med 60:367–380
Gesundheit B, Rosenzweig JP, Naor D, Lerer B, Zachor DA, Prochazka V, Melamed M, Kristt DA, Steinberg A, Shulman C, Hwang P, Koren G, Walfisch A, Passweg JR, Snowden JA, Tamouza R, Lebover M, Farge-Bancel D, Ashwood P (2013) Immunological and autoimmune considerations of autism spectrum disorders. J Autoimmun 44:1–7. doi:10.1016/j.jaut.2013.05.005
Gilger MA, Redel CA (2009) Autism and the gut. Pediatrics 124:796–798
Goines P, van de Water J (2010) The immune systems role in the biology of autism. Curr Opin Neurol 23:111–117
Grigorenko EL, Han SS, Yrigollen CM, Leng L, Mizue Y, Anderson GM, Mulder EJ, de Bildt A, Minderaa MB, Volkmar FR, Chang JT, Bucala R (2008) Macrophage migration inhibitory factor and autism spectrum disorders. Pediatrics 122:438–445
Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, Miller J, Fedele A, Collins J, Smith K, Lotspeich L, Croen LA, Ozonoff S, Lajonchere C, Grether JK, Risch N (2011) Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry 68:1095–1102
Happe F, Ronald A (2008) The ‘fractionable autism triad’: a review evidence from behavioural, genetic, cognitive and neural research. Neuropsychol Rev 18:287–304
Happe F, Ronald A, Plomin R (2006) Time to give up on a single explanation for autism. Nat Neurosci 9:1218–1220
Heil KM, Schaaf C (2013) The genetics of autism spectrum disorders—a guide for clinicians. Curr Psychiatry Rep 15:334–343
Herzing LB, Cook EH, Ledbetter DH (2002) Allele-specific expression analysis by RNA-FISH demonstrates preferential maternal expression of UBE3A and imprint maintenance within 15q11-q13 duplications. Hum Mol Genet 11:1707–1718
Hewitson L (2013) Scientific challenges in developing biological markers for autism. OA Autism 1(1):7
Hochberg Z, Feil R, Constancia M, Fraga M, Junien C, Carel JC, Boileau P, Le Bouc Y, Deal CL, Lillycrop K, Scharfmann R, Sheppard A, Skinner M, Szyf M, Waterland RA, Waxman DJ, Whitelaw E, Ong K, Albertsson-Wikland K (2011) Child health, developmental plasticity, and epigenetic programming. Endocr Rev 32:159–224
Hollander E, King A, Delaney K, Smith CJ, Silverman JM (2003) Obsessive compulsive behaviors in parents of multiplex autism families. Psychiatry Res 117:11–16
Hutcheson HB, Olson LM, Bradford Y, Folstein SE, Santangelo SL, Sutcliffe JS, Haines JL (2004) Examination of NRCAM, LRRN3, KIAA0716 and LAMB1 as autism candidate genes. BMC Med Genet 5(12):1–14
Ibrahim SH, Voigt RG, Katusic AK, Weaver AL, Barbaresi WJ (2009) Incidence of gastrointestinal symptoms in children with autism in Olmsted County, Minnesota, 1976–1997: a population based study. Pediatrics 124:680–686
IMGSAC (2001) Further characterization of the autism susceptibility locus AUTS1 in chromosome 7q. Hum Mol Genet 10:973–982
James WH (2012) A potential explanation of some established major risk factors for autism. Dev Med Child Neurol 54:301–305
Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, Boucher BJ, Arden NK, Godfrey KM, Cooper C, Princess Anne Hospital Study Group (2006) Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet 367:36–43
Johnson DD, Wagner CL, Husley TC, McNeil RB, Ebeling M, Hollis BW (2011) Vitamin D deficiency and insufficiency is common during pregnancy. Am J Perinat 28:007–012
Johnson NL, Giarelli E, Lewis C, Rice CE (2012) Genomics and autism spectrum disorder. J Nurs Scholarsh 45:69–78
Jyonouchi H, Geng L, Streck DL, Totuner A (2012) Immunological characterization and transcription profiling of peripheral blood (PB) monocytes in children with autism spectrum disorders (ASD) and specific polysaccharide antibody deficiency (SPAD): a case study. J Neuroinflammation 9(4):1–12
Kemper TL, Bauman ML (2002) Neuropathology of infantile autism. Mol Psychiatry 7:12–13
Kim YS, Leventhal BL, Koh YJ, Fombonne E, Laska E, Lim EC, Cheon KA, Kim SJ, Kim YK, Lee H, Song DH, Grinker RR (2011) Prevalence of autism spectrum disorders in a total population sample. Am J Psychiatry 168:904–912
Kocovska E, Fernell E, Billstedt E, Minnis H, Gillberg C (2012) Vitamin D and autism: clinical review. Res Dev Disabil 33:1541–1550
Korvatska E, van de Water J, Anders TF, Gershwin ME (2002) Genetic and immunologic considerations in autism. Neurobiol Dis 9:107–125
Kumar RA, Christian SL (2009) Genetics of autism spectrum disorder. Curr Neurol Neurosci Rep 9:188–197
Li X, Chauhan A, Sheikh AM, Patil S, Chauhan V, Li XM, Ji L, Brown T, Malik M (2009) Elevated immune response in the brain of autistic patients. J Neuroimmunol 207:111–116
Louis P (2012) Does the human gut microbiota contribute to the etiology of autism spectrum disorder? Dig Dis Sci 57:1987–1989
Monnet-Tschudi F, Deafaux A, Braissant O, Cagnon L, Zurich MG (2011) Methods to assess neuroinflammation. Curr Protoc Toxicol. 11; Chapter 12: Unit 12.19. DOI: 10.1002/0471140856.tx1219s50
Morley R, Carlin JB, Pasco JA, Wark JD (2006) Maternal 25-hydroxyvitamin D and parathyroid hormone concentrations and offspring birth size. J Clin Endocrinol Meth 91:906–912
Muhle R, Trentacoste SV, Rapin I (2004) The genetics of autism. Pediatrics 113:472–486
Okada K, Hashimoto K, Iwata Y et al (2007) Decreased serum levels of transforming growth factor beta-1 in patients with autism. Prog Neuropsychopharmacol Biol Psychiatry 31:187–190
Olivié H (2012) Clinical practice. The medical care of children with autism. Eur J Pediatr 271:741–749
Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, Bryson S, Carver LJ, Constantino JN, Dobkins K, Hutman T, Iverson JM, Landa R, Rogers SJ, Sigman M, Stone WL (2011) Recurrence risks for autism spectrum disorders: a baby siblings research consortium study. Pediatrics 128:e1. doi:10.1542/peds.2010-2825
Palmen SJ, van Engeland H, Hof PR, Schmitz C (2004) Neuropathological findings in autism. Brain 127:2572–2583
Patterson P (2011) Maternal infection and immune involvement in autism. Trends Mol Med 17:389–394
Ratacczak HV (2011) Theoretical aspects of autism: causes—a review. J Immunotoxicol 8:68–79
Ricci S, Businaro R, Ippolliti F, Lo Vasco VR, Massoni F, Onofri E, Trolli GM, Pontecorvi V, Morelli M, Rapp Ricciardi M, Archer T (2013) Altered cytokine and BDNF levels in autism spectrum disorder. Neurotoxicol Res. doi:10.1007/s12640-013-9393-4
Rosenberg RE, Law JK, Yenokyan G, McGready J, Kaufamann WE, Law PA (2009) Characteristics and concordance of autism spectrum disorders among 277 twin pairs. Arch Pediatr Adolesc Med 163:907–914
Sandhu B, Steer C, Golding J, Emond A (2009) The early stool patterns of young children with autistic spectrum disorder. Arch Dis Child 94:497–500
Schaaf CP, Sabo A, Sakai Y, Crosby J, Muzny D, Hawes A, Lewis L, Akbar H, Varghese R, Boerwinkle E, Gibbs RA, Zoghbi HY (2011) Oligogenic heterozygosity in individuals with high functioning autism spectrum disorder. Hum Mol Genet 20:3366–3375
Scherer SW, Cheung J, MacDonald JR, Osborne LR, Nakabayashi K, Herbrick JA, Carson AR, Parker-Katiraee L, Skaug J, Khaja R, Zhang J, Hudek AK, Li M, Haddad M, Duggan GE, Fernandez BA, Kanematsu E, Gentles S, Christopoulos CC, Choufani S, Kwasnicka D, Zheng XH, Lai Z, Nusskern D, Zhang Q, Gu Z, Lu F, Zeesman S, Nowaczyk MJ, Teshima I, Chitayat D, Shuman C, Weksberg R, Zackai EH, Grebe TA, Cox SR, Kirkpatrick SJ, Rahman N, Friedman JM, Heng HQ, Pelicci PG, Lo-Coco F, Belloni E, Shaffer LG, Pober B, Morton CC, Gusella JF, Bruns GAP, Korf BR, Quade BJ, Ligon AH, Ferguson H, Higgins AW, Leach NT, Herrick SR, Lemyre E, Farra CG, Kim HG, Summers AM, Gripp KW, Roberts W, Szatmari P, Winsor EJT, Grzeschik KH, Teebi A, Minassian BA, Kere J, Armengol L, Pujana MA, Estivill X, Wilson MD, Koop BF, Tosi A, Moore GE, Boright AP, Zlotorynski E, Kerem B, Kroisel PM, Petek E, Oscier DG, Mould SJ, Döhner H, Döhner K, Rommens JM, Vincent JB, Venter JC, Li PW, Mural RJ, Adams MD, Tsui LC (2003) Human chromosome 7: DNA sequence and biology. Science 300:767–772
Shao Y, Wolpert CM, Raiford KL, Menold MM, Donnelly SL, Ravan SA, Bass MP, McClain C, von Wendt L, Vance JM, Abramson RH, Wright HH, Ashley-Koch A, Gilbert JL, DeLong RG, Cuccaro ML, Pericak-Vance MA (2002) Genomic screen and follow up analysis for autistic disorder. Am J Med Genet 69:327–340
Shattuck PT, Durkin M, Maenner M, Newschaffer C, Mandell DS, Wiggins L, Lee LC, Rice C, Giarelli E, Kirby R, Baio J, Pinto-Martin J, Cuniff C (2009) Timing of identification among children with an autism spectrum disorder: findings from a population-based surveillance study. J Am Acad Child Adolesc Psychiatry 48:474–483
Shu W, Cho JY, Jiang Y, Zhang M, Weisz D, Elder GA, Schmeidler J, De Gasperi R, Sosa MA, Rabidou D, Santucci AC, Perl D, Morrisey E, Buxbaum JD (2005) Altered ultrasonic vocalization in mice with a disruption in the Foxp2 gene. Proc Natl Acad Sci 102:9643–9648
Siniscalo D, Bradstreet JJ, Antonucci N (2013) Therapeutic role of hematopoietic stem cells in autism spectrum disorder-related inflammation. Front Immunol 4:140. DOI 10.3389/fimmu.2013.00140
Spiker D, Lotspeich LJ, Dimiceli, Szatmari P, Myers RM, Risch N (2001) Birth order defects on nonverbal IQ scores in autism multiplex families. J Autism Dev Disord 31:449–460
Stevens MC, Feith DH, Waterhouse LH (2000) Season of birth effects in autism. J Clin Exp Neuropsychol 22:399–407
Stewart C, Latif A (2008) Symptomatic nutritional rickets in a teenager with autistic spectrum disorder. Child Care Health Dev 34:276–278
Steyaert JG, De La Marche W (2008) What's new in autism? Eur J Pediatr 167:1091–1101
Sudarov A (2013) Defining the role of cerebellar Purkinje cells in autism spectrum disorders. Cerebellum 12:950–955. doi:10.1007/s12311-013-0490-y
Tchaconas A, Adesman A (2013) Autism spectrum disorder: a pediatric overview and update. Curr Opin Pediatr 25:130–144
van Eeghen AM, Pulsifer MB, Merker VL, Neumeyer AM, van Eeghen EE, Thibert RL, Cole AJ, Leigh FA, Plotkin SR, Thiele EA (2013) Understanding relationships between autism, intelligence and epilepsy: a cross-disorder approach. Dev Med Child Neurol 55:146–153
Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA (2005) Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol 57:67–81
Vojdani A, Campbell AW, Anyanwu E, Kashanian A, Bock K, Vojdani E (2002) Antibodies to neuron-specific antigens in children with autism: possible cross-reaction with encephalitogenic proteins from milk, Chlamydia pneumoniae and Streptococcus group A. J Neuroimmunol 129:168–177
Walker SJ, Fortunato J, Gonzalez LG, Krigsman A (2013) Identification of unique gene expression profile in children with regressive autism spectrum disorder (ASD) and ileocolitis. PLoS ONE 8:e58058. doi:10.1371/journal.pone.0058058
Wang LV, Claus C, Sorich MJ, Gerber JP, Angley MT, Conion MA (2010) Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig Dis Sci 57:2096–2102
Wassink TH, Hazlett HC, Epping EA, Arndt S, Dager SR, Schellenberg GD, Dawson G, Piven J (2007) Cerebral cortical gray matter overgrowth and functional variation of the serotonin transporter gene in autism. Arch Gen Psychiatry 64:709–717
Werling DM, Geschwind DH (2013) Sex differences in autism spectrum disorder. Curr Opin Neurol 26:146–153
Whitehouse AJ, Holt BJ, Serralha M, Holt PG, Hart PH, Kusel MM (2012) Maternal vitamin D levels and the autism phenotype among offspring. J Autism Dev Disord 43:1495–1504
Williams BL, Horning M, Buie T, Bauman ML, Cho Paik M, Wick I, Bennett JA, Jabado O, Hirschberg DL, Lipkin WI (2011) Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS One 6:987–991
Wills S, Cabanlit M, Bennett J, Ashwood P, Amaral D, van de Water J (2007) Autoantibodies in autism spectrum disorders. Ann N Y Acad Sci 1107:79–91
Wolpert CM, Menold MM, Bass MP, Qumsiyeh MB, Donnelly SL, Ravan SA, Vance JM, Gilbert JR, Abranson RK, Wright HH, Cuccaro ML, Pericak-Vance MA (2000) Three probands with autistic disorder and isodicentric chromosome 15. Am J Med Genet 96:365–372
Yeargin-Allsopp M, Rice C, Karapurkar T, Doernberg N, Boyle C, Murphy C (2003) Prevalence of autism in a US metropolitan area. JAMA 289:49–55
Zhang H, Dougherty PM (2011) Acute inhibition of signaling phenotype of spinal GABAergic neurons by tumor necrosis-alpha. J Physiol Lond 589:4511–4526
Ziats MN, Rennert OM (2013) Aberrant expression of long noncoding RNAs in autistic brain. J Mol Neurosci 49:589–593. doi:10.1007/s12031-012-9880-8
Zimmerman AW, Connors SL, Matteson KJ, Lee LC, Singer HS, Castaneda JA, Pearce DA (2007) Maternal antibrain antibodies in autism. Brain Behav Immun 21:351–357
Zwaigenbaum L, Bryson SE, Szatmari P, Brian J, Smith IM, Roberts W, Vaillancourt T, Roncadin C (2012) Sex differences in children with autism spectrum disorder identified within a high risk infant cohort. J Autism Dev Disord 42:22585–22596
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Noriega, D.B., Savelkoul, H.F.J. Immune dysregulation in autism spectrum disorder. Eur J Pediatr 173, 33–43 (2014). https://doi.org/10.1007/s00431-013-2183-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-013-2183-4