Abstract
Objectives
This study aimed at evaluating the microtensile bond strength (μTBS) and the resin-dentine ultramorphology (24 h and 10 months ageing) of contemporary universal adhesives applied in self-etch (SE) or etch-and-rinse (ER) mode.
Materials and methods
Sixty-four sound human molars were collected and randomly allocated in 4 main experimental groups (n = 16) according to the adhesive system employed and subsequently divided into two subgroups depending on their application mode SE or ER (n = 8): ZipBond X (ZBX-SE; ZBX-ER), Prime and Bond Active (PBA-SE; PBA-ER), Clearfil Universal Bond Quick (CBQ-SE; CBQ-ER) or Scotchbond Universal (SCH-SE; SCH-ER). The specimens were cut into sticks with a cross-sectional area of approximately 0.9 mm2 and subjected to μTBS testing at 24 h or after 10 months of ageing in artificial saliva (AS). Five representative fractured specimens from each group were analysed using field-emission scanning electron microscopy (FE-SEM). Resin-dentine slabs (Ø 0.9mm2) from each experimental group were immersed in Rhodamine B and subsequently analysed using confocal microscopy analysis (CLSM). The μTBS results were analysed using a two-way ANOVA and Newman–Keuls multiple-comparison test (α = 0.05).
Results
ZBX, PBA and SCH exhibited greater μTBS values than CQB at 24 h in both SE and ER modes (p < 0.05). CQB showed a significant decrease in μTBS values after ageing both when used in SE and ER mode (p < 0.05). ZBX-ER exhibited no significant differences in the μTBS test after ageing (p > 0.05), while a significant drop in μTBS was seen in SCH-ER and APB-ER after 10-month ageing (p < 0.05). Clear signs of degradation were evident in the resin-dentine interface created with CQB regardless of the application mode or the ageing time. In APB-ER and SCH-ER groups, such signs of degradation were evident after ageing in AS. ZBX showed slight dye infiltration both when used in ER and SE mode.
Conclusions
The long-term bonding performance of modern universal adhesives is usually influenced by the adhesive strategy employed; self-etching application should be prioritised during dentine bonding. Moreover, the use of shortened bonding protocols may compromise the quality of the resin-dentine interface and the bonding performance of most modern universal adhesives.
Clinical relevance
The use of etch-and-rinse bonding procedures, as well as “shortened” application protocols should be eluded when using modern universal adhesives in dentine. However, new generation universal adhesives based on innovative chemical formulations may probably allow clinicians to achieve long-term bonding performance with such simplified system also when employed in ER mode.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Modern dental bonding systems, also known as universal adhesives (UA), have been formulated to provide a reliable bonding to tooth substrates (i.e., dentine and enamel) through simplified clinical protocols either when applied in self-etch (SE) and etch-and-rinse (ER) modes [1, 2]. This is possible due to the presence within their compositions of specific functional monomers such as MDP, which can create a strong interaction with calcium ions in hydroxyapatite [3] as well as to their milder pH, which may also allow the use of such adhesives when previous etching is preferred. However, despite their promising results both in vitro [4, 5] and in preliminary clinical studies [6, 7], degradation of the hybrid layer (HL) over time remains a matter of concern, especially when such UAs are used in ER mode. This situation seems to be related to the presence of several hydrophilic compounds within adhesives’ composition, along with incomplete monomer infiltration into the etched collaged fibrils; they are the main responsible factors for the decrease of the bonding performance over time [8, 9].
The consequences of such a scenario may be characterised by the loss of retention over time [10], accompanied by the development of secondary caries in those regions adjacent to the tooth-composite margins [11]. Indeed, this latter aspect represents the major cause for restoration replacement, whose operative procedures result in additional and unnecessary tooth structure loss [12]. In order to overcome such a drawback, new technologies have been adopted by dental companies for the formulation of their latest versions of UAs in order to improve their bonding performance over time [1, 3]. For instance, some of these improvements are based on the use of milder acidic functional monomers, as well as alternative “less-hydrophilic” polymeric compounds [5] and/or optimisation of the mixture of solvents with greater vapour pressure and wettability, which may facilitate monomer diffusion and the evaporation rate of dentine fluids during bonding procedures [12,13,14].
Indeed, it has been demonstrated that the chemical properties of some specific solvents may favour a proper infiltration of resin monomers within the demineralised dentine, so creating a suitable mechanical interlocking (hybrid layer) upon photo-polymerisation [15]. Moreover, it is well known that solvents serve as crucial substances to achieve a homogeneous blending when mixing hydrophilic and hydrophobic compounds in dental adhesives [16]. On the other hand, a greater rate of polymerisation of the adhesive is accomplished only when using specific strategies that favour proper evaporation of the solvents from the dentine [17]; their presence, especially water-based ones, needs to be considerably reduced to attain a high degree of conversion of the adhesive monomers [18] and appropriate physicomechanical properties at the resin-dentine interface [19].
The main solvents used to formulate adhesive systems (i.e. ethanol, acetone and/or water), despite their overall excellent evaporation rate and capacity to form azeotropes, are influenced by the overall composition of adhesives [18, 20]. Indeed, when such systems are characterised by a formulation not properly balanced, or in case clinical application steps are not performed as per manufacturers’ recommendations, solvents may lack in evaporation and lead to an acceleration of degradation processes within the polymer matrix [21]. That is why, in order to improve the evaporation rate of the solvents of the adhesives without compromising their ability to infiltrate into dentine, alternative solvents have been considered. For instance, alternative solvents such as isopropanol or a ternary solvent made of butanone have been incorporated within the composition of some contemporary UA, such as Prime & Bond Active (Dentsply-Sirona, Konstanz, Baden-Württemberg, Germany) and ZipBond X (SDI Ltd. Bayswater, Victoria, Australia), respectively. Moreover, some modern UA systems, such as Kuraray Clearfil Universal Bond Quick (Kuraray Noritake Dental Inc, Osaka, Japan) are claimed to achieve a suitable bonding performance when applied using particular protocols of use based on procedures with reduced application time as short as 5 to 10 s. However, this may be possible not only because of their specific monomers and activator/co-activator composition but also to their specific mixture of solvents [22].
To date, the bonding performance of such modern UA systems applied on dentine in self-etch (SE) and etch-and-rinse (ER) mode has not been thoroughly investigated. Thus, the aim of this in vitro study was to evaluate the effect of prolonged ageing on the bonding performance of modern universal adhesives applied on dentine using different adhesive application strategies (SE; ER mode). This objective was accomplished by evaluating the microtensile bond strength (μTBS) and assessing the resin-dentine ultramorphology by dye-assisted confocal microscopy (CLSM) at 24 h and after 10 months of storage in artificial saliva (AS). The tested hypothesis of this study was that, regardless of their compositions, the tested adhesives would present significant differences in bonding performance both at 24 h and after prolonged ageing in AS when using different application strategies (SE vs. ER).
Materials and methods
Preparation of dentine specimens
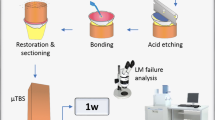
Sixty-four sound human molars were collected according to the guidelines of the local Ethics Committee under the protocol number CEI20/098 and stored in distilled water at 5 °C for no longer than 3 months. The roots were removed 1 mm beneath the cemento–enamel junction using a diamond blade mounted on a low-speed microtome under continuous water cooling (Remet evolution, REMET, Bologna, Italy). A second parallel cut was made to remove the occlusal enamel, and mid-coronal dentine was exposed using 320-grit SiC papers. Four main groups (n = 16 specimens/group) were created based on the adhesive systems employed in this study: CQB: Clearfil Universal Bond Quick; SCH: Scotchbond; PBA: Prime & Bond Active Universal; ZBX: ZipBond X Universal. The information about composition and manufactures are presented in Table 1. The specimens were further divided into two sub-groups (n = 8 specimens/group) based on the application mode: self-etching (SE); etch-and-rinse (ER). The specimens were applied as described in Table 1, followed by a final light-curing procedure of 10 s using a LED light source (> 1000 mW/cm2) (Radii Plus, SDI Ltd., Bayswater VIC, Australia). The specimens were finally restored using a micro-hybrid dental resin composite (Clearfil AP-X, Kuraray Noritake, Tokyo, Japan) in 2-mm increments up to 6 mm and light-cured as per manufacturer’s instructions. A schematic representation of the entire experimental design of this study is presented in Fig. 1.
Micro-tensile bond strength (μTBS), failure modes and FE-SEM fractographic analysis
All the specimens created as previously described were sectioned across the resin-dentine interface using a hard-tissue microtome (Remet evolution, REMET, Bologna, Italy) in both X and Y directions in order to obtain approximately 20 matchstick-shaped specimens from each tooth, with a cross-sectional area of 0.9 mm2. The matchsticks were stored in AS for 24 h (24 h) or 10 months (10 m) at 37 °C in the dark in an incubator and subsequently subjected to μTBS to evaluate their bonding performance. The composition of the artificial saliva (AS) was 0.103 g L−1 of CaCl2, 0.019 g L−1 of MgCl2 × 6H2O, 0.544 g L−1 of KH2PO4, 30 g L−1 of KCl and 4.77 g L−1 HEPES (acid) buffer, pH 7.4. The AS storage media was replaced with a fresh one every 7 days [24].
This latter procedure was performed using a microtensile bond strength device with a stroke length of 50 mm, a peak force of 500 N and a displacement resolution of 0.5 mm. Modes of failure were examined at 30 × magnification using stereoscopic microscopy and classified as a percentage of adhesive (A), mixed (M), or cohesive (C) failures. Five representative fractured specimens from each sub-group were mounted on aluminium stubs with carbon glue after the critical-point drying process. The specimens were gold-sputter coated and analysed with a field-emission scanning electron microscopy (FE-SEM S-4100; Hitachi, Wokingham, UK) at 10 kV and a working distance of 15 mm.
The normality of μTBS data was evaluated using Shapiro–Wilk test (p > 0.05). Homogeneity of variance was calculated using the Brown-Forsythe test. Data were then analysed using a two-way analysis of variance (ANOVA factors: bonding system and ageing protocol) and Newman–Keuls multiple-comparison test (α = 0.05). SPSS V16 for Windows (SPSS Inc., Chicago, IL, USA) was used.
Ultramorphology bonded-dentine interfaces—confocal microscopy evaluation
One bonded composite-dentine slab specimen (Ø 0.9mm2) was selected from each experimental sub-group (n = 8) during the cutting procedures to obtain the matchsticks for μTBS testing. The specimens were aged as previously described in AS and then coated with fast-setting nail varnish, applied 1 mm from the bonded interface and left undisturbed for 30 min. They were subsequently immersed in a Rhodamine B (Sigma Chemicals) water solution (0.1 wt.%) for 24 h. Subsequently, the specimens were ultrasonicated with distilled water for 3 min and then polished for 30 s each side with a 500-grit and subsequently with 2400-grit SiC papers. The specimens were ultrasonicated again in distilled water for 3 min and immediately submitted to confocal microscopy ultramorphology analysis (CLSM-Olympus FV1000, Olympus Corp., Tokyo, Japan), using a 63X/1.4 NA oil immersion lens and 543-nm LED illumination. Reflection and fluorescence images were obtained with a 1-μm z-step to section the specimens optically to a depth of up to 20 μm below the surface [25]. The z-axis scan of the interface surface was pseudo-coloured arbitrarily for improved visualisation and compiled into both single projections using the CLSM image-processing software (Fluoview Viewer, Olympus). The configuration of the system was standardised and used at constant settings for the entire investigation. Each dentine interface was completely investigated and five images were randomly captured; these represented the most common morphological features observed along with the bonded interfaces [26].
Results
Micro-tensile bond strength (μTBS), failure modes
The results of the microtensile bond strength test (mean and ± SD), the number of specimens that pre-failed before testing and the number of specimens that fractured during μTBS in adhesive, mixed and cohesive modes are depicted in Table 2. A significant interaction was identified between the two factors analysed (bonding system and ageing protocol), (p < 0.001). There were no pre-test failures before μTBS assessment in most of the groups, excluding the CQB applied both in ER and SE mode, which had a pre-failure rate of 5% and 8% of their specimens, respectively. Moreover, this latter adhesive (CQB) statistically showed the lowest bond strength (p < 0.05) compared to all the other tested materials, both when used in ER and SE mode. After prolonged storage (10 months in AS), this adhesive showed a significant drop (p < 0.05) in bond strength both when used in SE or ER mode. Regarding the failure mode, CQB applied in ER and SE mode failed prevalently in adhesive mode (62–78%) and mixed mode (38–22%) at 24 h. However, at 10 m of AS storage, the main failure mode observed in both CQB groups was adhesive (> 80%). SCH presented no significant difference (p > 0.05) at 24 h when applied in ER or SE mode, and in both cases, the most prevalent failure mode was mixed. After prolonged ageing in AS, there was a significant drop (p < 0.05) in bond strength only in the group created in ER mode (SCH-ER), but the number of failures in adhesive mode also increased in the group created in SE mode, compared to the same SE group at 24 h. Conversely, at 24 h, PBA presented a significantly greater bond strength (p < 0.05) when applied in ER mode compared to SE mode application. Although PBA-ER presented a greater number of failures in cohesive mode compared to the specimens created in SE mode, in both cases, the most prevalent failure mode was mixed. Likewise, in SCH groups, also PBA after prolonged ageing in AS presented a significant drop (p < 0.05) in bond strength only with the specimens created in ER mode, with an increase in the number of specimens that failed in adhesive and mixed-mode. The adhesive ZBX presented no significant difference (p > 0.05) at 24 h when applied in ER or SE mode, and in both cases, the most prevalent failure mode was mixed. After storage in AS for 10 months, both the specimens in ZBX-ER and ZBX-SE had no significant drop in bond strength, and again the prevalent failure mode in both groups was mixed. ZBX-ER and ZBX-SE also presented a great number of specimens that failed in cohesive mode failure after storage in AS for 10 months.
FE-SEM fractographic analysis
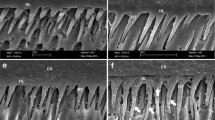
The results of the SEM fractographic analysis performed in this study at 24 h storage are shown in Fig. 2. It was evident that in most of the specimens created with Clearfil Universal Bond Quick (CQB-ER and CQB-ER), which failed in mixed mode, the presence of characteristic honeycomb-like sign of phase separation and/or incomplete evaporation of the solvents and water typically present in dentine during bonding procedures (Fig. 2A and (A1)), and with the presence of demineralised collagen fibrils and dentine tubules completely exposed and poorly infiltrated by the resin adhesive (Fig. 2B). However, the specimens created with CQB in SE mode often presented exposed dentine and dentine tubules partially occluded by thin resin tags (Fig. 2C). The specimens created with Scotchbond Universal in ER mode (SCH-ER) that failed in mixed or adhesive mode (Fig. 2D and E) were characterised by the presence of some demineralised collagen and dentine tubules often filled by loose, fractured resin tags. Conversely, the specimens created with SCH in SE mode (SCH-SE) were regularly covered by resin, and only rarely the presence of exposed dentine tubules was observed (Fig. 2F). The specimens bonded using PBA-ER (Fig. 2G and H) were often characterised by the presence of poorly infiltrated by the resin adhesive and with dentine tubules obliterated by resin tags (Fig. 2I). The specimens in group PBA-SE showed a consistent presence of resin covering the dentine surface and with very few dentine tubules visibly exposed. The specimens bonded with ZBX-ER showed very few exposed collagen fibrils, often localised underneath the hybrid layer (Fig. 2L and M), with most of the dentine tubules clearly occluded by resin tags (Fig. 2N). The specimens in the group ZBX-SE were often characterised by the presence of a layer of resin covering the dentine surface.
SEM fractographic analysis of the specimens tested at 24-h storage (baseline). A Representative SEM fractography of a specimen created with Quick Bond (CQB) that failed in mixed mode, where it is possible to see (A1) a clear sign of phase separation and/or incomplete evaporation of the solvents and dentine water (arrows). B At higher magnification in a specimens created with CQB in ER mode, it is possible to observe the presence of demineralised collagen fibrils (arrow) and dentine tubules (dt) completely exposed and not infiltrated by the resin adhesive. C In this specimen created with CQB in SE mode, there is the presence of residual resin as a result of phase separation (arrow) and dentine tubules partially occluded by thin resin tags. D Representative SEM fractography of a specimen created with Scotchbond Universal (SCH) that failed in mixed mode. E In this specimen created with SCH in ER mode and analysed at the zone failed in adhesive mode, it can be seen the presence of some demineralised collagen poorly infiltrated by the resin adhesive (arrow) and patent dentine tubules (dt), that are often filled by loose fractured resin tags (rt). F This specimen created with SCH in SE mode presents a clear resin layer covering the dentine surface (arrow) without any dentine tubules exposed. G Representative SEM fractography of a specimen created with Prime & Bond Active (PBA) that failed in mixed mode. H This specimen created with PBA in ER mode, when analysed at the adhesive failure zone, it showed the presence of few exposed collagen fibrils that were not properly infiltrated by the resin adhesive (arrow) and very few patent dentine tubules (dt), but most of them were occluded by resin tags (rt). I The specimens created with PBA in SE mode show the presence of resin covering the dentine surface and no dentine tubules visible. L Representative SEM fractography of a specimen created with ZipBond X (ZBX) that failed in mixed mode. M This specimen created with ZBX in ER mode shows little exposure of collagen fibrils, which indicates that the fracture occurred underneath the hybrid layer. Moreover, most of the dentine tubules are clearly occluded by resin tags (arrow). N This specimen created with ZBX in SE mode presents a clear layer of resin covering the dentine surface and dentine tubules
The results of the SEM fractographic analysis of the materials tested after prolonged ageing in AS (10 months) are depicted in Fig. 3. Severe degradation of dentine collagen was detected in the specimens created using CQB in ER mode (Fig. 3A). Likewise, the specimens created with CQB in SE mode showed severe signs of dentine degradation (Fig. 3B). The specimens in group SCH-ER showed a clear degradation of dentine collagen and dentine tubules completely exposed (Fig. 3C).
SEM fractographic analysis of the specimens tested after prolonged ageing (10 months in AS). A Representative image of specimens created with CQB in ER mode and failed in adhesive mode. It is possible to observe a severe degradation of dentine collagen (arrow) and dentine tubules (dt) completely exposed and only rarely infiltrated by resin tags (rt). B A severe dentine degradation can be seen in the specimens created with CQB in SE mode, which is also characterised by the presence of residual resin tags (arrow). C Representative image of specimens created with SCH in ER mode failed in adhesive mode, which shows a clear degradation of dentine collagen (arrow) and dentine tubules (dt) completely exposed and only rarely infiltrated by resin tags (rt). D A slight dentine degradation was also observed in the specimens created with SCH in SE mode. It is possible to observe the presence of some partially exposed dentine tubules and residual resin tags (arrow). E Representative image of specimens created with PBA in ER mode failed in adhesive mode, showing clear sign of degradation, but with some dentine collagen fibrils still present (arrow). Moreover, it is possible to see patent dentine tubules (dt) with some fractured resin tags (rt). F In this specimen created with PBA in SE mode, it is possible to note a dentine surface still covered by resin and the presence of some partially exposed dentine tubules and residual resin tags (arrow). G Representative image of specimens created with ZBX in ER mode failed in adhesive mode, showing a slight degradation with several demineralised dentine collagen fibrils still intact (arrow). It is also possible to see patent dentine tubules (dt) with some fractured resin tags (rt). H In this specimen created with ZBX applied in SE mode, it is possible to see a clear presence of resin covering the dentine surface (arrow) and no exposed dentine tubules
Conversely, the specimens bonded with SCH in SE mode presented only slight signs of dentine degradation, but with the presence of partially exposed dentine tubules (Fig. 3D). An evident degradation, along with the presence of some exposed and partially degraded dentine collagen fibrils, was observed in the specimens created with PBA in ER mode (Fig. 3E). Conversely, the specimens created with PBA in SE mode were often characterised by a dentine surface covered by resin, with the presence of residual resin tags (Fig. 3F). The specimens created with ZBX created in ER mode showed only a slight sign of dentine degradation and the presence of intact demineralised dentin collagen fibrils (Fig. 3G). Likewise, the specimens created with ZBX applied in SE mode showed no sign of degradation, and the dentine surface was often covered by the resin (Fig. 3H).
Ultramorphology bonded-dentine interfaces—confocal microscopy evaluation
The results of the ultramorphology confocal microscopy analysis of the bonded-dentine interface at 24-h storage are shown in Fig. 4. The resin-dentine interface created with CQB-ER was devoid of gaps, but with a clear sign of infiltration of the fluorescent dye, in particular within the hybrid layer and adhesive layer (Fig. 4A). Similar intense fluorescent signal, as a consequence of water uptake, was observed in the resin-dentine interface created with CQB-SE (Fig. 4B). Conversely, the resin-dentine interface of the specimens in group SCH-ER was characterised by a hybrid layer totally infiltrated by the fluorescent dye (Fig. 4C). The specimens in group SCH-SE showed a hybrid layer characterised by slight fluorescent infiltration (Fig. 4D). Prime & Bond Active (PBA) applied in ER mode generated a hybrid layer, which was clearly infiltrated by the fluorescent dye (Fig. 4E). The resin-dentine interface of the specimens created using PBA-SE was characterised by fluorescent dye infiltration only within the interdiffusion layer (Fig. 4F). A gap-free resin-dentine interface was observed in the specimens created with ZBX applied in ER mode, and it was often detected a hybrid layer and adhesive layer devoid of infiltration of fluorescent dye (Fig. 4G). The same “dye-free” situation was often observed in the specimens created with ZBX applied in SE mode; only the dentine tubules were filled with the fluorescent dye (Fig. 4F).
Confocal microscopy images of the resin-dentine interfaces tested at 24-h storage. A CLSM projection image exemplifying the interfacial characteristics of the resin-dentine interface created by application of quick bond (CQB) in ER mode. It is possible to see a permeable gap-free interface that absorbed the fluorescent solution thought the dentinal tubules (dt) and, in particular within the hybrid layer and adhesive layer (AD) (pointer). B In this CLSM projection image, it is possible to see the resin-dentine interface created by CQB applied in SE mode characterised by important dye uptake within the interdiffusion adhesive layers (pointer). C CLSM projection image of the resin-dentine interface created by application of Scotchbond universal (SCH) in ER mode where it is possible to see a permeable gap-free hybrid layer (pointer) to the fluorescent solution, but the adhesive layer (AD) is devoid of any sign of dye uptake. D This is a CLSM projection image where it is possible to see the resin-dentine interface created by SCH applied in SE mode characterised by slight fluorescent dye uptake within the interdiffusion layer (pointer). E CLSM projection image of the resin-dentine interface created by application of Prime & Bond Active (PBA) in ER mode which shows a hybrid layer (pointer) permeable to the fluorescent dye, but with an adhesive layer (AD) completely devoid of any sign of dye uptake. F In this CLSM projection image, it is possible to observe the resin-dentine interface created by PBA applied in SE mode, which shows fluorescent dye infiltration only within the interdiffusion layer (pointer) and inside the dentine tubules (DT). G CLSM projection image of the resin-dentine interface created by application of ZipBond X (ZBX) in ER mode where it is possible to note gap-free interface with both hybrid layer (pointer) and adhesive layer devoid of infiltration of fluorescent dye. H The same dye-free situation can be observed within the interface created with ZBX applied in SE mode (pointer), with only the dentine tubules (DT) filled with the fluorescent dye
The results of the ultramorphology confocal microscopy analysis of the bonded-dentine interface after 10 months in AS are presented in Fig. 4. An interface highly infiltrated by the fluorescent solution and characterised by clear degradation of the hybrid layer was detected in the specimens created with CQB-ER (Fig. 5A). Such degradation was also observed in the resin-dentine interface created using CQB in SE mode; an important dye infiltration throughout the entire adhesive layer was also observed (Fig. 5B). The specimens created with SCH-ER presented a clear degradation of the hybrid layer and infiltration of the fluorescent dye within the adhesive layer (Fig. 5C). Conversely, the specimens in group SCH-SE were characterised by important fluorescent dye uptake within the interdiffusion layer and adhesive layer (AD) (Fig. 5D). The resin-dentine interface generated with PBA-ER showed a hybrid layer with signs of degradation and adhesive layer (AD) permeable to the fluorescent dye, (Fig. 5E). Conversely, the specimens created with PBA-SE presented no sign of degradation, but only fluorescent dye infiltration within the entire resin-dentine interface (Fig. 5F). No evident gaps or clear signs of degradation were observed in the specimens created with ZBX-ER (Fig. 5G). The same situation was attained in the resin-dentine created with the same adhesive applied in SE mode; very few zones characterised by dye infiltration within the interdiffusion layer were detected (Fig. 5H).
Confocal microscopy images of the resin-dentine interfaces tested after prolonged ageing (10 months in artificial saliva). A CLSM projection image illustrating the interfacial characteristics of the resin-dentine interface created by application of CQB in ER mode and where it is possible to observe a permeable interface that absorbed the fluorescent solution thought the dentinal tubules (dt) and, in particular, it is possible to see a clear degradation (gap) of the hybrid layer (pointer). B In this CLSM projection image, it is possible to see the resin-dentine interface created by CQB applied in SE mode. Please note how this resin-dentine interface is also affected by degradation (pointer) and by important fluorescence within the interdiffusion and adhesive layers (AD). C This is a representative CLSM projection image of the resin-dentine interface created by SCH applied in ER mode. It is possible to note the degradation of hybrid layer (pointer) within a permeable resin-dentine interface to the fluorescent solution. D In this image, the resin-dentine interface created by SCH applied in SE mode is characterised by an important fluorescent dye uptake within the interdiffusion layer and adhesive layer (AD), but without sign of gap (pointer). E This is a representative CLSM projection image of the resin-dentine interface created by application PBA in ER mode, which shows a hybrid and adhesive layer (AD) permeable to the fluorescent dye, with some characteristic signs of degradation within the hybrid layer (pointer). F In this CLSM projection image, it is possible to observe the resin-dentine interface created by PBA applied in SE mode, which shows fluorescent dye infiltration within the entire resin-dentine interface. G A representative CLSM projection image that shows a gap-free resin-dentine interface in specimens created by using ZBX in ER. Note how the hybrid layer (pointer) is characterised by fluorescent dye infiltration, but with no sign of fluorescence at the adhesive layer (AD). H This CLSM single projection image show the resin-dentine interface created by ZBX applied in SE mode. Please note how the resin-dentine interface presents only very few zones of dye infiltration within the interdiffusion layer (pointer), and only the dentine tubules are filled with the fluorescent dye
Discussion
The current study showed important differences in the bonding performance of the tested UA systems employed in ER and SE mode at 24 h and 10 months of ageing. Therefore, the hypothesis that the tested UA systems would present significant differences in bonding performance both at 24 h and after prolonged ageing in AS when using different application strategies (SE vs. ER) must be accepted.
Undeniably, the application strategy of UA systems in ER mode may ensure greater immediate bond strength due to a superior resin-dentine interlocking as a result of the demineralising effect caused by the etching procedure through phosphoric acid application in dentine [2]. Indeed, this latter step permits the exposure of collagen fibrils, which are then infiltrated by the resin monomers of modern UA systems, which upon light-curing can form a hybrid layer and achieve a reliable immediate bond strength; this is usually greater than that obtained by the same UA system applied in SE mode [5, 23, 27]. However, the differences in the chemical composition of UA systems and their adhesive-smear layer interaction, as well as the application protocol employed, may be determinant to accomplish a long-lasting and predictable bonding to dentine [1, 28].
The results of the current study have demonstrated that CBQ applied in “quick” mode had the lowest bonding performance (μTBS), along with the greatest degradation of the HL, regardless the adhesive strategy (SE or ER) or ageing protocol employed. Moreover, the results obtained in this study at 24 h and 10 months during the fractographic and ultramorphological analyses showed clear signs of phase separation (Figs. 2A–B and 3A–B) and degradation (Figs. 4A–B and 5A–B) within the resin-dentine interface.
The manufacturer of this adhesive system (Kuraray, Japan) when launched it for the first time in the market, claimed that a reliable bonding performance could be achieved by using such a shortened “quick” adhesive protocol; that consisted in the “passive” (no brushing time, Table 1) application of the adhesive using short-bonding method and light-curing for 10 s. Despite their commercial marketing strategies, scientific studies have shown that such a “quick and passive” application strategy [29], along with a short-time evaporation period [22, 30] and light-curing procedure [28, 31], could be detrimental when bonding to dentine [28, 32]. In this study, the “quick bonding” protocol suggested by Saikaew et al. [28] was employed and no active brushing application was used. Indeed, the current study is in agreement with those previous studies, and it is possible to affirm that the use of “quick” application protocols when using modern UA systems can increase the risk for a decrease in bonding performance and severe degradation of the resin-dentine interface due to water sorption and hydrolysis of the interface components (i.e., hybrid layer). Indeed, such a passive application of CBQ may not be appropriate to allow adequate infiltration of adhesive resin monomers into the smear layers or the acid-etched dentine [28, 32]. Clearly, it would be necessary to provide adequate time to the bonding procedures in order to perform an “active/scrubbing” application, which will allow acidic monomers to decalcify dentine and create a reliable interlocking and formation of the hybrid layer [29]. Moreover, the passive shortened “quick” application of such a UA system may be responsible for incomplete evaporation of solvents; “short” bonding procedures usually lead to incomplete evaporation of the solvents [17, 29]. This latter issue has been described as the main reason for a lower degree of conversion [31] and phase separation within the resin-dentine interface [33].
However, it is important to highlight that when simplified adhesives are applied in the etch-and-rinse mode in dentine, they may be more susceptible to degradation of the hybrid layer. The use of ortho-phosphoric acid to etch the dentine causes the removal of the underlying intact mineral and exposes the collagen matrix up to a depth of 8–12 μ [34, 35]. Such a collagen network forms a template for the diffusion of adhesive monomers that, upon polymerisation, generate the hybrid layer. However, it is well known that monomers are often unable to fully infiltrate such a demineralised zone, leaving unprotected, poorly resin-infiltrated dentine collagen [10, 36].
These unprotected collagen fibrils are prone to rapid hydrolysis, which is the main degradation threshold with etch-and-rinse adhesives and contributes to bond-strength reduction over time [37]. It has been demonstrated that metalloproteinases (e.g. MMP-2, MMP-9), intrinsic proteases within the collagen matrix in dentine, may play a crucial role in the degradation of the hybrid layer. However, the presence of unprotected collagen has also been identified at the bottom of the interdiffusion layer created when using simplified adhesives applied in self-etch mode [38, 39].
A further degradation process at the resin-dentine interface created with simplified adhesives applied in self-etching or etch-and-rinse mode also occurs due to water sorption and incomplete evaporation of solvents, especially under simulated pulpal pressure [40]. It is also demonstrated that in the presence of important water sorption, mild acidic adhesives might release protons that can exacerbate the degradation of the silane-filler interface [34, 41]. In this regard, artificial saliva was employed in the current study as an ageing media to simulate an environment as similar as possible to that find in the oral cavity. Several studies have shown the effectiveness of using artificial saliva to promote ageing in the dentine-adhesive interface as confirmed by the results obtained in the present study [42, 43].
The results of the current study also showed that PBA could achieve a greater bond strength when applied to dentine in the ER mode rather than SE mode. As previously mentioned, the use of phosphoric acid etching may have created a morphological scenario that promoted the diffusion of the PBA system to create a reliable interlocking with the dentine (hybrid layer). In particular, such an effective resin monomers infiltration may have been due to the high hydrophilicity of both its solvent (isopropanol) and functional monomers MDP and PENTA (Fig. 2E) and immediate bond strength [44] (Table 2). Conversely, when such adhesive was used in SE mode, the results were significantly lower compared to those obtained in ER mode. We hypothesise that it was probably caused by the presence of two functional monomers in a single-bottle UA system. Indeed, dipentaerythritol penta-acrylate monophosphate (PENTA), a high molecular weight hydrophilic molecule, might have interfered with the interaction of MDP with the dentinal substrate due to some sort of competition for calcium ions in hydroxyapatite [45, 46]. However, PBA applied in ER mode showed a significant drop in bond strength after prolonged storage with a clear sign of degradation at the hybrid layer, along with important fluorescent dye infiltration within the entire resin-dentine interface (Fig. 5E), while PBA applied in SE mode showed no significant drop in bond strength after prolonged storage with no clear sign of degradation but only fluorescent dye infiltration within the entire resin-dentine interface (Fig. 5F).
Such a difference in bonding longevity between the results obtained with PBA applied in SE or ER may be due to the fact that the resin-dentine interface created with mild SE adhesives has greater resistance to ageing degradation. This is a result of a higher amount of residual apatite crystallites left within a very thin partially demineralised collagen matrix upon application of mild-acidic SE systems, which prevented the hydrolytic denaturation of dentine collagen fibrils [47, 48] and promoted the interaction of functional monomers [49] with such inorganic molecules to produce stable salts less prone to degradation over time [3]. The SE approach also reduces water displacement within the HL, and considering that hydrodynamics are essential to produce degradation and plasticisation of the polymers, reduced water presence can preserve HL [5]. This is evident in our fractographic and ultra-morphological analysis, where the SE strategy leads to less fluorescence and collagen degradation after ageing.
Scotchbond Universal (SCH) is probably one of the most tested adhesives in dental research, and it is essentially considered as a sort of gold standard material to use as a control group in such a type of study. The results obtained in the current research are in accordance with previous studies, which showed no differences in the immediate bonding [2, 47, 50]. One of the reasons why this adhesive system can achieve similar immediate results either when used in ER or SE mode may be attributed to its chemical composition. SCH is formulated with an ethanol-based solvent, which is known to be effective in the displacement of the water from the dentine and in favouring a reliable resin monomer infiltration into demineralised collagen fibrils [48]; this allows the formation of an interface characterised by a well-defined HL with evident resin tags penetration into the dentinal tubules, especially when SCH was used in ER mode (Fig. 2E).
Conversely, the bonding performance of a UA system depends on the capability of its functional and/or acidic monomers to interact with the smear layer and the underlying mineralised dentine. Indeed, SCH is one of few simplified adhesives able to form nanolayers within the resin-dentine interface; such a chemical interaction created by MDP seems to allow additional advantages to HL’s interlocking [51]. Furthermore, the use of a patented polyacrylic acid monomer, known as Vitrebond Copolymer, within its composition has been advocated to provide a further chemical interaction with hydroxyapatite [52]; the combination of such self-etch chemical mechanisms may result in stable bonding over time, as it was confirmed in the present study.
In the current study, all tested adhesives presented mild to ultra-mild pHs. The pH of SE adhesives can be considered a primary aspect when predicting the behaviour of the adhesive after ageing [5]. In this sense, excluding the results observed in CBQ applied in quick bonding procedures, the degradation observed after ageing in AS was mainly seen when the ER strategy was used in PBA and SCH. As previously mentioned, acid-etching exposes a great deal of collagen fibrils which are often incompletely infiltrated by the resin monomers adhesive due to the presence of a wet environment. Therefore, in such a situation, the HL formed in the ER technique is more prone to degradation [53] of both collagen and adhesive over time [54]. Moreover, the important presence of hydrophilic molecules and solvents, within the composition of such simplified one-bottle systems, may contribute to an increase of sorption and solubility [8, 55] which may lead to the formation of porosities at the hybrid and adhesive layer. These aspects were confirmed in this study during the ultramorphological analysis (Fig. 5B) and in the fractographic analysis that showed severe collagen degradation after 10 months of storage (Fig. 3B).
In the present study, ZBX obtained the best bonding performance between all the other tested UA systems, especially when applied in ER mode. Moreover, there was no significant difference between the bond strength obtained when it was applied in SE or ER mode. In both application modes, there was no significant drop bond strength after 10-month storage in AS, showing a resin-dentine interface free of gaps and with no evident sign of degradation at the hybrid layer (Fig. 5G and H).
One of the main differences in ZBX when compared to the other tested UA systems can be attributed to its chemical composition, in particular, to the ternary system of solvents based on ethanol, water and a methyl-ethyl-ketone, also known as butanone molecule (Table 1); the viscosity and surface tension of butanone, in combination with water and ethanol, may have allowed better diffusion of the resin monomers into dentine. Indeed, butanone presents intermediate viscosity values (0.41 cp) to that of ethanol (1.10 cp) or acetone (0.316 cp) [48]; this latter is known to be the lowest viscosity solvent used in adhesive formulations. Regarding the surface tension, butanone (24.6 dyn/cm) behaves similarly to ethanol, which has a surface tension of 22.3 dyn/cm. On the other hand, vapour pressure and boiling temperature are essential aspects to determine the time a solvent will need to evaporate from its site of application at a certain temperature. It is established that vapour pressure can influence the applicability of adhesives in either wet or dry dentine. Excessive high vapour pressure, as in the case of acetone, can lead to easy and quick evaporation of the solvent [56]. In spite of the advantages of volatilising vehicles with regard to polymerisation and achieving optimal mechanical properties when applying the adhesive system [30], high vapour pressure may compromise the interaction of some vehicles like acetone to dry dentine [57, 58]. In this sense, butanone presents higher vapour pressure (89 mmHg at 25 °C) compared to that of ethanol (40 mmHg at 19 °C), but still lower than that of acetone (184 mmHg at 20 °C).
Furthermore, solvents are necessary to allow the UA to form an azeotrope between the solvent in its composition with water in wet-dentine. Once the azeotrope is formed, the physical properties of water change, approaching its physical properties to those of the solvent, and thus the remaining water from dentine can be easily removed to create a complementary system to improve the adhesive wettability and change dentine hydrodynamics when the HL is formed [56]. This can be considered a further advantage of having butanone as part of the solvents mixture in adhesives. Therefore, we can hypothesise that the homogeneous results obtained by ZBX in all modes and ageing times can be mainly attributed to the capacity of its solvents to allow better monomer diffusion into dentine, promoting optimal polymerisation of ZBX due to evaporation of water from dentine. However, improved potential interaction between MDP present in the composition of ZBX and dentinal hydroxyapatite should not be excluded as a mechanism of stabilisation of the hybrid layer in the long-term performance. Indeed, the formation of stable MDP-Ca salts may depend on the overall composition of UAs, in particular on the concentration of MDP and water; the efficacy of smear layer removal and bonding performance may be influenced by the water concentration in adhesive systems [59, 60]. Moreover, a high concentration of ethanol may limit the ionic dissociation of phosphate groups of the MDP [61]. It has also been proposed that MDP-based adhesives formulated without HEMA can present a better bonding performance since HEMA can bring solvents back into solution and create and complex aggregation with MDP, which can compromise its interaction with the dentine [62,63,64]. However, this type of solvent formulation in modern UA is quite new, so further studies analysing the degree of conversion, volatilisation and the interaction within dentine of butanone-based adhesives are required to support the findings of the current study.
Conclusions
Within the limitations of the present study, it is possible to conclude that the adhesive quick bond universal applied by using simplified “quick and passive” application protocols both in SE and ER mode may have its immediate and long-term bonding performance jeopardised. The adhesive strategy (ER or SE), as well as the overall formulation of the bonding systems, can influence the long-term performance of resin-bonded dentine interface. Indeed, self-etch application mode should be prioritised when using modern universal adhesive systems in dentine. On the other hand, new generation universal adhesives based on innovative chemical formulations may probably allow clinicians to achieve stable bonding performance over time. Indeed, the simplified butanone-based adhesive system ZipBond X might represent a suitable choice to create a hybrid layer with reduced degradation over time even when applied in ER mode.
References
Hardan L, Bourgi R, Kharouf N, Mancino D, Zarow M, Jakubowicz N, Haikel Y, Cuevas-Suarez CE (2021) Bond strength of universal adhesives to dentin: a systematic review and meta-analysis. Polymers (Basel) 13.https://doi.org/10.3390/polym13050814
Rosa WL, Piva E, Silva AF (2015) Bond strength of universal adhesives: a systematic review and meta-analysis. J Dent 43:765–776. https://doi.org/10.1016/j.jdent.2015.04.003
Van Meerbeek B, Yoshihara K, Van Landuyt K, Yoshida Y, Peumans M (2020) From Buonocore’s Pioneering Acid-Etch Technique to self-adhering restoratives. A status perspective of rapidly advancing dental adhesive technology. J Adhes Dent 22:7–34. https://doi.org/10.3290/j.jad.a43994
Munoz MA, Luque-Martinez I, Malaquias P, Hass V, Reis A, Campanha NH, Loguercio AD (2015) In vitro longevity of bonding properties of universal adhesives to dentin. Oper Dent 40:282–292. https://doi.org/10.2341/14-055-L
Cuevas-Suarez CE, da Rosa WLO, Lund RG, da Silva AF, Piva E (2019) Bonding performance of universal adhesives: an updated systematic review and meta-analysis. J Adhes Dent 21:7–26. https://doi.org/10.3290/j.jad.a41975
Zanatta RF, Silva TM, Esper M, Bresciani E, Goncalves S, Caneppele T (2019) Bonding performance of simplified adhesive systems in noncarious cervical lesions at 2-year follow-up: a double-blind randomized clinical trial. Oper Dent 44:476–487. https://doi.org/10.2341/18-049-C
de Paris MT, Perdigao J, de Paula E, Coppla F, Hass V, Scheffer RF, Reis A, Loguercio AD (2020) Five-year clinical evaluation of a universal adhesive: a randomized double-blind trial. Dent Mater. https://doi.org/10.1016/j.dental.2020.08.007
Zhang ZY, Tian FC, Niu LN, Ochala K, Chen C, Fu BP, Wang XY, Pashley DH, Tay FR (2016) Defying ageing: an expectation for dentine bonding with universal adhesives? J Dent 45:43–52. https://doi.org/10.1016/j.jdent.2015.11.008
Sauro S, Makeeva I, Faus-Matoses V, Foschi F, Giovarruscio M, Maciel Pires P, Martins Moura ME, Almeida Neves A, Faus-Llacer V (2019) Effects of ions-releasing restorative materials on the dentine bonding longevity of modern universal adhesives after load-cycle and prolonged artificial saliva aging. Materials (Basel) 12.https://doi.org/10.3390/ma12050722
Spencer P, Ye Q, Park J, Topp EM, Misra A, Marangos O, Wang Y, Bohaty BS, Singh V, Sene F, Eslick J, Camarda K, Katz JL (2010) Adhesive/dentin interface: the weak link in the composite restoration. Ann Biomed Eng 38:1989–2003. https://doi.org/10.1007/s10439-010-9969-6
Astvaldsdottir A, Dagerhamn J, van Dijken JW, Naimi-Akbar A, Sandborgh-Englund G, Tranaeus S, Nilsson M (2015) Longevity of posterior resin composite restorations in adults - a systematic review. J Dent 43:934–954. https://doi.org/10.1016/j.jdent.2015.05.001
Demarco FF, Correa MB, Cenci MS, Moraes RR, Opdam NJ (2012) Longevity of posterior composite restorations: not only a matter of materials. Dent Mater 28:87–101. https://doi.org/10.1016/j.dental.2011.09.003
Szesz AL, Pereira GMA, Siqueira FSF, Cardenas AFM, Bandeca MC, Armas-Vega A, Reis A, Loguercio AD (2021) Effect of addition of dimethyl sulfoxide to simplified adhesives on dentin bond durability after three years of water storage. J Adhes Dent 23:159–165. https://doi.org/10.3290/j.jad.b1079585
Pashley DH, Tay FR, Carvalho RM, Rueggeberg FA, Agee KA, Carrilho M, Donnelly A, Garcia-Godoy F (2007) From dry bonding to water-wet bonding to ethanol-wet bonding. A review of the interactions between dentin matrix and solvated resins using a macromodel of the hybrid layer. Am J Dent 20:7–20
Eick JD, Gwinnett AJ, Pashley DH, Robinson SJ (1997) Current concepts on adhesion to dentin. Crit Rev Oral Biol Med 8:306–335. https://doi.org/10.1177/10454411970080030501
Wang Y, Spencer P, Yao X, Brenda B (2007) Effect of solvent content on resin hybridization in wet dentin bonding. J Biomed Mater Res A 82:975–983. https://doi.org/10.1002/jbm.a.31232
Reis A, Carrilho M, Breschi L, Loguercio AD (2013) Overview of clinical alternatives to minimize the degradation of the resin-dentin bonds. Oper Dent 38:E1–E25. https://doi.org/10.2341/12-258-LIT
Loguercio AD, Loeblein F, Cherobin T, Ogliari F, Piva E, Reis A (2009) Effect of solvent removal on adhesive properties of simplified etch-and-rinse systems and on bond strengths to dry and wet dentin. J Adhes Dent 11:213–219
Sauro S, Osorio R, Watson TF, Toledano M (2012) Assessment of the quality of resin-dentin bonded interfaces: an AFM nano-indentation, muTBS and confocal ultramorphology study. Dent Mater 28:622–631. https://doi.org/10.1016/j.dental.2012.02.005
Yiu CK, Pashley EL, Hiraishi N, King NM, Goracci C, Ferrari M, Carvalho RM, Pashley DH, Tay FR (2005) Solvent and water retention in dental adhesive blends after evaporation. Biomaterials 26:6863–6872. https://doi.org/10.1016/j.biomaterials.2005.05.011
Carvalho RM, Mendonca JS, Santiago SL, Silveira RR, Garcia FC, Tay FR, Pashley DH (2003) Effects of HEMA/solvent combinations on bond strength to dentin. J Dent Res 82:597–601. https://doi.org/10.1177/154405910308200805
Luque-Martinez IV, Perdigao J, Munoz MA, Sezinando A, Reis A, Loguercio AD (2014) Effects of solvent evaporation time on immediate adhesive properties of universal adhesives to dentin. Dent Mater 30:1126–1135. https://doi.org/10.1016/j.dental.2014.07.002
Breschi L, Mazzoni A, Ruggeri A, Cadenaro M, Di Lenarda R, De Stefano DE (2008) Dental adhesion review: aging and stability of the bonded interface. Dent Mater 24:90–101. https://doi.org/10.1016/j.dental.2007.02.009
Spagnuolo G, Pires PM, Calarco A, Peluso G, Banerjee A, Rengo S, Elias Boneta AR, Sauro S (2021) An in-vitro study investigating the effect of air-abrasion bioactive glasses on dental adhesion, cytotoxicity and odontogenic gene expression. Dent Mater 37:1734–1750. https://doi.org/10.1016/j.dental.2021.09.004
Feitosa VP, Sauro S, Ogliari FA, Stansbury JW, Carpenter GH, Watson TF, Sinhoreti MA, Correr AB (2014) The role of spacer carbon chain in acidic functional monomers on the physicochemical properties of self-etch dental adhesives. J Dent 42:565–574. https://doi.org/10.1016/j.jdent.2014.02.009
Tezvergil-Mutluay A, Seseogullari-Dirihan R, Feitosa VP, Cama G, Brauer DS, Sauro S (2017) Effects of composites containing bioactive glasses on demineralized dentin. J Dent Res 96:999–1005. https://doi.org/10.1177/0022034517709464
Pashley DH, Tay FR, Breschi L, Tjaderhane L, Carvalho RM, Carrilho M, Tezvergil-Mutluay A (2011) State of the art etch-and-rinse adhesives. Dent Mater 27:1–16. https://doi.org/10.1016/j.dental.2010.10.016
Saikaew P, Matsumoto M, Chowdhury A, Carvalho RM, Sano H (2018) Does shortened application time affect long-term bond strength of universal adhesives to dentin? Oper Dent 43:549–558. https://doi.org/10.2341/17-205-L
Thanatvarakorn O, Prasansuttiporn T, Takahashi M, Thittaweerat S, Foxton RM, Ichinose S, Tagami J, Nakajima M (2016) Effect of scrubbing technique with mild self-etching adhesives on dentin bond strengths and nanoleakage expression. J Adhes Dent 18:197–204. https://doi.org/10.3290/j.jad.a36033
Borges BC, Souza-Junior EJ, Brandt WC, Loguercio AD, Montes MA, Puppin-Rontani RM, Sinhoreti MA (2012) Degree of conversion of simplified contemporary adhesive systems as influenced by extended air-activated or passive solvent volatilization modes. Oper Dent 37:246–252. https://doi.org/10.2341/11-248-L
Cadenaro M, Maravic T, Comba A, Mazzoni A, Fanfoni L, Hilton T, Ferracane J, Breschi L (2019) The role of polymerization in adhesive dentistry. Dent Mater 35:e1–e22. https://doi.org/10.1016/j.dental.2018.11.012
Saikaew P, Chowdhury AF, Fukuyama M, Kakuda S, Carvalho RM, Sano H (2016) The effect of dentine surface preparation and reduced application time of adhesive on bonding strength. J Dent 47:63–70. https://doi.org/10.1016/j.jdent.2016.02.001
Spencer P, Wang Y (2002) Adhesive phase separation at the dentin interface under wet bonding conditions. J Biomed Mater Res 62:447–456. https://doi.org/10.1002/jbm.10364
Feitosa VP, Sauro S, Zenobi W, Silva JC, Abuna G, Van Meerbeek B, Sinhoreti MAC, Correr AB, Yoshihara K (2019) Degradation of adhesive-dentin interfaces created using different bonding strategies after five-year simulated pulpal pressure. J Adhes Dent 21:199–207. https://doi.org/10.3290/j.jad.a42510
Sauro S, Pashley DH, Mannocci F, Tay FR, Pilecki P, Sherriff M, Watson TF (2008) Micropermeability of current self-etching and etch-and-rinse adhesives bonded to deep dentine: a comparison study using a double-staining/confocal microscopy technique. Eur J Oral Sci 116:184–193. https://doi.org/10.1111/j.1600-0722.2007.00518.x
Van Meerbeek B, De Munck J, Mattar D, Van Landuyt K, Lambrechts P (2003) Microtensile bond strengths of an etch & rinse and self-etch adhesive to enamel and dentin as a function of surface treatment. Oper Dent 28:647–660
Hashimoto M, Ohno H, Sano H, Kaga M, Oguchi H (2003) In vitro degradation of resin-dentin bonds analyzed by microtensile bond test, scanning and transmission electron microscopy. Biomaterials 24:3795–3803. https://doi.org/10.1016/s0142-9612(03)00262-x
Tay FR, Pashley DH, Yoshiyama M (2002) Two modes of nanoleakage expression in single-step adhesives. J Dent Res 81:472–476. https://doi.org/10.1177/154405910208100708
Nishitani Y, Yoshiyama M, Wadgaonkar B, Breschi L, Mannello F, Mazzoni A, Carvalho RM, Tjaderhane L, Tay FR, Pashley DH (2006) Activation of gelatinolytic/collagenolytic activity in dentin by self-etching adhesives. Eur J Oral Sci 114:160–166. https://doi.org/10.1111/j.1600-0722.2006.00342.x
Bacelar-Sa R, Sauro S, Abuna G, Vitti R, Nikaido T, Tagami J, Ambrosano GMB, Giannini M (2017) Adhesion evaluation of dentin sealing, micropermeability, and bond strength of current hema-free adhesives to dentin. J Adhes Dent 19:357–364. https://doi.org/10.3290/j.jad.a38866
Van Landuyt KL, De Munck J, Mine A, Cardoso MV, Peumans M, Van Meerbeek B (2010) Filler debonding & subhybrid-layer failures in self-etch adhesives. J Dent Res 89:1045–1050. https://doi.org/10.1177/0022034510375285
De Munck J, Mine A, Poitevin A, Van Ende A, Cardoso MV, Van Landuyt KL, Peumans M, Van Meerbeek B (2012) Meta-analytical review of parameters involved in dentin bonding. J Dent Res 91:351–357. https://doi.org/10.1177/0022034511431251
De Munck J, Van Landuyt K, Peumans M, Poitevin A, Lambrechts P, Braem M, Van Meerbeek B (2005) A critical review of the durability of adhesion to tooth tissue: methods and results. J Dent Res 84:118–132. https://doi.org/10.1177/154405910508400204
Follak AC, Miotti LL, Lenzi TL, Rocha RO, Soares FZM (2021) Self-etch approach of universal adhesives as an alternative to minimize bond degradation on sound dentin vs caries-affected dentin over time. J Adhes Dent 23:243–252. https://doi.org/10.3290/j.jad.b1367889
Navarra CO, Breschi L, Turco G, Diolosa M, Fontanive L, Manzoli L, Di Lenarda R, Cadenaro M (2012) Degree of conversion of two-step etch-and-rinse adhesives: in situ micro-Raman analysis. J Dent 40:711–717. https://doi.org/10.1016/j.jdent.2012.05.001
Wang R, Shi Y, Li T, Pan Y, Cui Y, Xia W (2017) Adhesive interfacial characteristics and the related bonding performance of four self-etching adhesives with different functional monomers applied to dentin. J Dent 62:72–80. https://doi.org/10.1016/j.jdent.2017.05.010
Hanabusa M, Mine A, Kuboki T, Momoi Y, Van Ende A, Van Meerbeek B, De Munck J (2012) Bonding effectiveness of a new ‘multi-mode’ adhesive to enamel and dentine. J Dent 40:475–484. https://doi.org/10.1016/j.jdent.2012.02.012
Breschi L, Maravic T, Cunha SR, Comba A, Cadenaro M, Tjaderhane L, Pashley DH, Tay FR, Mazzoni A (2018) Dentin bonding systems: from dentin collagen structure to bond preservation and clinical applications. Dent Mater 34:78–96. https://doi.org/10.1016/j.dental.2017.11.005
Ikeda M, Tsubota K, Takamizawa T, Yoshida T, Miyazaki M, Platt JA (2008) Bonding durability of single-step adhesives to previously acid-etched dentin. Oper Dent 33:702–709. https://doi.org/10.2341/08-26
Munoz MA, Luque I, Hass V, Reis A, Loguercio AD, Bombarda NH (2013) Immediate bonding properties of universal adhesives to dentine. J Dent 41:404–411. https://doi.org/10.1016/j.jdent.2013.03.001
Yoshida Y, Yoshihara K, Nagaoka N, Hayakawa S, Torii Y, Ogawa T, Osaka A, Meerbeek BV (2012) Self-assembled nano-layering at the adhesive interface. J Dent Res 91:376–381. https://doi.org/10.1177/0022034512437375
Yoshida Y, Van Meerbeek B, Nakayama Y, Snauwaert J, Hellemans L, Lambrechts P, Vanherle G, Wakasa K (2000) Evidence of chemical bonding at biomaterial-hard tissue interfaces. J Dent Res 79:709–714. https://doi.org/10.1177/00220345000790020301
Marchesi G, Frassetto A, Mazzoni A, Apolonio F, Diolosa M, Cadenaro M, Di Lenarda R, Pashley DH, Tay F, Breschi L (2014) Adhesive performance of a multi-mode adhesive system: 1-year in vitro study. J Dent 42:603–612. https://doi.org/10.1016/j.jdent.2013.12.008
Frassetto A, Breschi L, Turco G, Marchesi G, Di Lenarda R, Tay FR, Pashley DH, Cadenaro M (2016) Mechanisms of degradation of the hybrid layer in adhesive dentistry and therapeutic agents to improve bond durability—a literature review. Dent Mater 32:e41-53. https://doi.org/10.1016/j.dental.2015.11.007
Gutierrez MF, Alegria-Acevedo LF, Mendez-Bauer L, Bermudez J, Davila-Sanchez A, Buvinic S, Hernandez-Moya N, Reis A, Loguercio AD, Farago PV, Martin J, Fernandez E (2019) Biological, mechanical and adhesive properties of universal adhesives containing zinc and copper nanoparticles. J Dent 82:45–55. https://doi.org/10.1016/j.jdent.2019.01.012
Ekambaram MYC, Matinlinna JP (2015) An overview of solvents in resin–dentin bonding. Int J Adhes Adhes 57:22–33. https://doi.org/10.1016/j.ijadhadh.2014.09.007
Reis A, Loguercio AD, Azevedo CL, de Carvalho RM, da Julio SM, Grande RH (2003) Moisture spectrum of demineralized dentin for adhesive systems with different solvent bases. J Adhes Dent 5:183–192
Jacobsen T, Soderholm KJ (1998) Effect of primer solvent, primer agitation, and dentin dryness on shear bond strength to dentin. Am J Dent 11:225–228
Fujita Nakajima K, Nikaido T, Arita A, Hirayama S, Nishiyama N (2018) Demineralization capacity of commercial 10-methacryloyloxydecyl dihydrogen phosphate-based all-in-one adhesive. Dent Mater 34:1555–1565. https://doi.org/10.1016/j.dental.2018.06.027
Teshima M (2018) Effect of the concentration of water in an MDP-based all-in-one adhesive on the efficacy of smear layer removal and on dentin bonding performance. Dent Mater J 37:685–692. https://doi.org/10.4012/dmj.2017-291
Carrilho E, Cardoso M, Marques Ferreira M, Marto CM, Paula A, Coelho AS (2019) 10-MDP based dental adhesives: adhesive interface characterization and adhesive stability—a systematic review. Materials (Basel) 12.https://doi.org/10.3390/ma12050790
Kirihara M, Inoue G, Nikaido T, Ikeda M, Sadr A, Tagami J (2013) Effect of fluoride concentration in adhesives on morphology of acid-base resistant zones. Dent Mater J 32:578–584. https://doi.org/10.4012/dmj.2013-041
Yoshida Y, Yoshihara K, Hayakawa S, Nagaoka N, Okihara T, Matsumoto T, Minagi S, Osaka A, Van Landuyt K, Van Meerbeek B (2012) HEMA inhibits interfacial nano-layering of the functional monomer MDP. J Dent Res 91:1060–1065. https://doi.org/10.1177/0022034512460396
Zhou J, Wurihan SY, Tanaka R, Zhang Z, Zheng K, Li Q, Ikeda S, Gao P, Miyazaki T (2019) Quantitative/qualitative analysis of adhesive-dentin interface in the presence of 10-methacryloyloxydecyl dihydrogen phosphate. J Mech Behav Biomed Mater 92:71–78. https://doi.org/10.1016/j.jmbbm.2018.12.038
Acknowledgements
All the materials used in this study were regularly purchased from local distributors. The authors also gratefully acknowledge SDI Ltd. Bayswater, Victoria, Australia, for a generous donation of the adhesive ZipBond X used in this study. Paula Maciel Pires was undertaking a PhD exchange program at Cardenal Herrera University during a part of the experimental assay and was supported by a CAPES grant from Brazil (grant numbers 88882.424807/2018-01 and 88881.188518/2018-01).
Funding
The research facilities of this study were supported by grants “Ministerio de Ciencia, Innovación y Universidades (PID2020-120346 GB-I00) and Universidad CEU-Cardenal Herrera (Programa FUSP CEU-Santander 2019–2020)” (PI: SS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required. Human molars used in this study were collected according to the guidelines of the local Ethics Committee (CEI20/098).
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Maciel Pires, P., Dávila-Sánchez, A., Faus-Matoses, V. et al. Bonding performance and ultramorphology of the resin-dentine interface of contemporary universal adhesives. Clin Oral Invest 26, 4391–4405 (2022). https://doi.org/10.1007/s00784-022-04402-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-022-04402-3