Abstract
Objectives
The aim of this study was to evaluate the microhardness of caries-affected dentin and color stability of teeth restored after treatments with silver diamine fluoride (SDF) associated to potassium iodide (KI) and Biosilicate.
Material and methods
Different samples from bovine teeth were obtained. For color readings, 80 cavities (6 mm × 6 mm × 2 mm) were prepared, and for microhardness, teeth were flattened into dentine to obtain 40 samples. All samples were submitted to cariogenic challenge and separated in 4 groups, according to the treatment used: 12% SDF + KI; 38% SDF; Biosilicate and control (no treatment). Cavities were restored with resin-modified glass-ionomer cement (RMGIC, Vitremer, 3 M ESPE) or composite resin (CR, Z350, 3 M ESPE). After restoration, the samples were submitted to thermo-mechanical cycling (TMC) for 1,200,000 cycles. Color readings (EasyShade, Vita) were performed after restorations, after TMC, and 30 days after TMC. Knoop microhardness was evaluated on the planned samples before and after cariogenic challenge, after treatments, and after 30 days. Scanning electron microscopy (SEM) evaluated the dentine surface after treatments. Data were analyzed (ANOVA, Bonferroni, p < .05).
Results
The results showed a higher color alteration for RMGIC than CR. The time of analysis was significant (p < .05) for the 12% SDF + KI and control group. There was no difference (p < .05) in microhardness between groups. However, there was evidence of dentin remineralization after treatments.
Conclusions
It was concluded that the samples treated with Biosilicate resulted in a color alteration similar to control. The treatments presented dentin remineralizing potential for microhardness, below the demineralization level, caused by the cariogenic challenge.
Clinical relevance
Considering the remineralizing potential presented by Biosilicate, this agent is a promising alternative that overcomes the SDF adverse effects such as tooth staining.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dental caries are characterized by demineralization of the dental tissue and progressive degradation of the organic structure [1]. Different treatments such as topical fluoride application, sealants, and silver diamine fluoride (SDF) have been used in caries control [2, 3].
SDF is a transparent and odorless solution with pH close to 10, available in different concentrations, and presents antimicrobial and remineralizing activity [4, 5]. Its chemical composition is based on silver ions, fluoride, and ammonium, the latter the stabilizing agent [5]. The SDF’s antimicrobial activity is mainly attributed to the silver ions [5], and its remineralizing effect occurs through the formation of calcium fluoride and fluorapatite deposits. Also, the collagen degradation is inhibited by fluoride [4,5,6]. Those characteristics make SDF more effective in the control of caries lesions. This agent is considered an accessible, efficient, and safe treatment option, still, its use is following the minimally invasive dentistry approach [7]. Despite its benefits and applicability, SDF adverse effects still compromise patients’ treatment acceptance. SDF causes dark staining on dental tissue, and this adverse effect is considered the most limiting one regarding patient acceptability [2, 8].
Permanent tooth staining with a black color happens due to the precipitation of silver ions over the demineralized dental surface. These silver ions precipitate as Ag2S and react with the organic material, leaving a stain that can be evident depending on the location of the carious lesion [9]. To reduce this drawback, potassium iodide (KI) was proposed after SDF application [8]. KI reacts with the free silver ions in SDF and forms a yellow silver iodide (AgI) precipitate insoluble in water, preventing black staining of teeth [2, 9, 10].
Besides using cariostatic solutions, other therapies with remineralizing agents have been researched. Bioactive glass–ceramics, such as Biosilicate, have shown promising results. Studies demonstrate that this crystallized bioactive glass–ceramic can form hydroxycarbonate apatite (HCA) on mineralized tissues [11]. In contact with dentin, Biosilicate dissolves, releasing calcium and phosphate ions, elevating the pH, and favoring the dental remineralization process [11].
It is important to explore and study the different treatment options available, focusing on minimally invasive dentistry (MID). The MID concept endorses the selective removal of caries tissue, removing only the caries-infected dentin and leaving the caries-affected dentin treated with remineralizing agents. Then the remaining tooth structure is restored with the most appropriate restorative material for each case [12]. The glass-ionomer cement (GIC) is commonly used for its antibacterial activity and adhesiveness to the tooth structure [13].
To improve the esthetics, physical, and mechanical properties of GIC, resin-modified glass-ionomer cement was developed. The incorporation of methacrylate components to the polyacrylic acid allows the initial setting reaction to being initiated by light activation, decreasing the setting time [14]. Nevertheless, another restorative material available with good esthetical characteristics is the composite resin (CR). CR is the most used restorative material and has better color stability and wear resistance than GIC [15].
Within this context, the aim of this study was to evaluate the color stability and microhardness alteration of caries-affected dentin treated with different SDF solutions (12% and 38%) associated with KI and Biosilicate, restored with RMGIC or CR. The first hypothesis tested was that caries-affected dentin treated with SDF would present higher color alteration than those treated with Biosilicate, regardless of the association with KI. The second hypothesis was that there would not be any difference in dentin microhardness, regardless of the treatment used.
Materials and methods
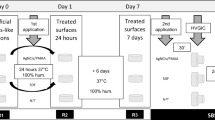
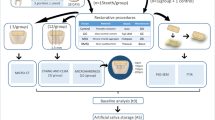
The materials used are presented in Table 1. This study was divided into two analyses: color and microhardness alterations. Figure 1 summarizes the methodology of the study. The sample size was calculated based on a pilot study, comparing means and using www.openepi.com, with a 95% confidence interval and power of 80%.
To perform the microhardness readings, bovine teeth fragments with 6 mm × 6 mm × 2 mm were used, and for the color readings, cavities were prepared and restored on the buccal surface of the bovine teeth selected. For that, 100 bovine incisor teeth were selected and stored in distilled water. For standardization purposes, initial color readings were performed on all selected teeth using a spectrophotometer (EasyShade, VITA Zahnfabrik, BadSckingen, Germany) in a way that the L* values varied between a range of 87 to 93, a* coordinate between 0 and 1, and b* coordinate between 8 and 30. Teeth that presented color values outside this range in any coordinate were discarded.
Sample preparation
For the color readings, 80 bovine teeth were used. Cavities were prepared on the buccal surfaces (6-mm length, 6-mm width, and 2-mm depth) using no. 1343 diamond burs (KG Sorensen®, Cotia, SP, Brazil) and a high-speed handpiece, guided by a customized device to standardize the depth of the cavity preparations. After that, the cavities were cleaned, and the teeth were embedded in wax in PVC tubes. Then, they were cut 2 mm above and below the cavity’s edges using a low-speed diamond saw under water cooling (Isomet 1000, Isomet, Buehler).
Other samples (without cavity preparation) were prepared for the microhardness analysis. Twenty sound bovine teeth were selected, and the buccal surfaces were cut into 40 fragments (6 mm × 6 mm × 2 mm) using a low-speed diamond saw under water cooling (Isomet 1000, Isomet, Buehler, Lake Bluff, IL, USA). The dentin surface was flattened and polished with SiC abrasive papers (220-, 360-, and 600-grit) under refrigeration to standardize the fragments’ thickness.
Cariogenic challenge
All the specimens (fragments and cavities) were submitted to the cariogenic challenge. For that, all the surfaces of the fragments and cavitated samples, except the dentin, were protected with acid-resistant nail-varnish (Colorama, L’Oréal Brazil, Rio de Janeiro, RJ, Brazil) and adhesive tape (Duct Tape, Shurtape Technologies, LLC, Avon Ohio, USA), respectively. Then, they were fixed in the bottom of a vial and covered with 1.5 mL of 6% carboxymethylcellulose demineralizing gel at a pH 5.0 and stored at 4 °C for 12 h [16]. Subsequently, 1.5 mL of 0.1 M lactic acid (pH = 5.0) adjusted with 10 M NaOH was poured over the specimens, which were incubated for 14 days at 37 °C [16]. At the end of the cycle, they were rinsed with distilled water and dried with absorbent paper.
The fragments were then stored in Eppendorf tubes with distilled water, and the cavitated samples were embedded in self-polymerizing acrylic resin in PVC tubes and then stored in distilled water.
Treatments
After the cariogenic challenge, the infected dentin was manually removed with an excavator (SSWhite Duflex, Juiz de Fora, MG, Brazil). The removal was completed when hard tissue (affected dentin), resistant to the penetration, was detected with the exploratory probe. The specimens (fragments and cavitated samples) were separated into groups according to the treatment received, as described below.
12% SDF + KI group: the specimens were treated with 12% SDF (Cariestop, Biodinâmica, PR, Brazil) followed by 10% KI (Farmácia Liane, Rabeiro Preto, SP, Brazil). One drop of SDF was applied over each specimen with a microbrush for 2 min. Then, a drop of KI solution was applied for 1 min; a second drop was applied; the specimens were rinsed with distilled water and dried with absorbent paper.
38% SDF group: 38% SDF (Riva Star, SDI, Victoria, Australia) was applied according to the manufacturer’s guidelines. The product is a 2-step bottle kit, containing SDF in bottle 1 and KI in bottle 2. So first, a drop of the bottle 1 was applied for 1 min using a microbrush, and then, a drop of bottle 2 was applied, also for 1 min. A second application of bottle 2 was made, and the specimens were rinsed and dried with absorbent paper.
Bio group: Biosilicate was mixed in distilled water, obtaining a 10% suspension applied actively on the dentin surface for 1 min. The specimens were then rinsed and dried with air.
Control group: there was no previous dentin treatment.
Restorations and thermo mechanical cycling
After treatments, only the groups with cavitated samples (prepared for the analysis of the color) were subdivided according to the restorative material used: resin-modified glass-ionomer cement-RMGIC (Vitremer, 3 M ESPE Dental Products, St. Paul, MN, USA) or composite resin-CR (Z350, 3 M ESPE Dental Products, St. Paul, MN, USA).
For the cavitated samples restored with RMGIC, initially, the primer was applied with a microbrush for 30 s, dried with air, and light-cured for 20 s (FLASHlite 1401, Discus Dental, Culver City, CA, USA). After that, the cement was mixed following the manufacturer’s guideline, the cavity was filled in one increment, and the cement was polymerized for 40 s. A final glaze layer was applied and polymerized for 20 s.
For the cavitated samples restored with CR, an adhesive system (Single Bond Universal, 3 M ESPE, St. Paul, MN, USA) was applied in self-etch mode and light-activated for 10 s. The cavities were restored following the incremental technique and polymerized for 20 s. All the samples were stored in artificial saliva at 37 °C for 24 h and then submitted to thermo mechanical cycling (TMC).
The thermo mechanical cycling (ER 37,000, Erios Equipamentos Técnicos e Científicos Ltda, São Paulo, Brazil) was used to simulate the oral environment and the mechanical load applied on the restorations during mastication. The samples were cycled with a load of 133 N, for 1,200,000 cycles (equivalent to 5 years of masticatory force) [17], at a 1.3 Hz of frequency, with varying temperatures of 5 °C, 37 °C, and 55 °C.
Color stability
Color readings were performed on the restored samples, and for that, a spectrophotometer (VITA Zahnfabrik, BadSckingen, Germany) was used. This equipment emits light that is transmitted through optical fibers. Then, the light is reflected and processed as color coordinates according to the CIE L*a*b* system, recommended by CIE (Commission Internationale de l’Éclairage). The CIE L*a*b* system identifies color as cartesian color coordinates, where the L* axis gives the lightness with values from 0 (black) to 100 (white), a* and b* represent the hue axis varying from − 80 to + 80; a* representing the green–red axis and b*, the blue-yellow one.
For the color readings, all the samples were placed over a white background (White Standard Sphere for 45°, 0° Reflectance and Color Gardner Laboratory, Germany) and inside a standardized lightbox (CL6i-45S, T&M INSTRUMENTS, São Paulo, SP, Brazil) with D65 illuminant, simulating the light spectrum of the day. The spectrophotometer’s tip was positioned perpendicular to the surface of the restoration. Three readings were performed for each sample, and the mean was considered as the color coordinates of the sample. Readings were done right after the restoration, after the thermo mechanical cycling, and 30 days after the thermo mechanical cycling.
The color alteration was calculated using the ∆E00 formula as follows:
where ΔL′, ΔC′, and ΔH′ are the differences in lightness, chroma, and hue, respectively, between two measures, and RT (rotation function) is a function that accounts for the interaction between chroma and hue differences in the blue region. SL, SC, and SH are the weighting functions for the lightness, chroma, and hue components, respectively; and KL, KC, and KH, are the parametric factors according to different viewing parameters that were set to 1 [18].
Knoop microhardness
The microhardness alteration was evaluated on the fragments (6 mm × 6 mm × 2 mm), before and after the cariogenic challenge, after the treatments, and 30 days after the treatments. For that, a microhardness tester (Micro Hardness Tester HMV-2, Shimadzu®, Tokyo, Japan) was used, with a pyramid-shaped diamond indenter set to a load of 10 g for 5 s. The largest diagonal of the indention was measured, and the value was applied on the following formula:
Where KHN is Knoop microhardness value, F is the applied load (10 g), and d is the length of the largest indention diagonal.
For each sample, at each time of reading, three measures were performed: a central, 1 mm to the left, and 1 mm to the right. The mean was considered as the Knoop microhardness value. The relative microhardness alteration was calculated using the formula:
where ∆KHN is the relative microhardness alteration value, KHNi is the initial microhardness value, and KHNf, the final one.
Scanning electron microscopy (SEM)
The samples were analyzed under SEM (EVO MA10, Carl Zeiss Microscopy GmbH, Jena, Germany) to characterize the surface morphology. Two restored teeth per group were cut in a mesiodistal direction through the center of each restoration using a low-speed diamond saw under water cooling (Isomet 1000, Isomet, Buehler, Lake Bluff, IL, USA), and the adhesive interfaces were analyzed.
For that, the fragments were dehydrated for 24 h using a desiccator with silica gel, fixed in aluminum stubs (Electron Microscopy Sciences, Washington, USA), sputter-coated with gold–palladium alloy (Bal-Tec, model SCD 050 sputter coater, Balzers, Liechtenstein) and observed at 1000 × , 2000 × , and 3000 × magnifications (20 kV, 30 mm WD and spot size 28 mm) [19].
Statistical analysis
The ∆E00 and relative microhardness values were analyzed with the Shapiro–Wilk test, with a 95% significance level, and considered within a normal distribution. Thus, ∆E00 values were analyzed by 3-way ANOVA with repeated measures (variation factors: restorative material, time of analysis, and treatment) and the relative microhardness values using 2-way ANOVA with repeated measures (variation factors: treatment and time of analysis). Both tests were done with a 95% significance level.
Results
The color alteration (∆E00) values are described in Table 2 and Fig. 2. Table 2 shows the comparison within the same restorative material, and the figure demonstrates the comparison between materials.
Among the samples restored with CR, the control group presented the highest color alteration, regardless of the time of analysis. After TMC, the control group was different (p < 0.05) from 38% SDF group; and 30 days after TMC, was different (p < 0.05) from Bio and 12% SDF + KI groups. Comparing the time of analysis for each treatment, an increase (p < 0.05) in color alteration was found for 38% SDF and control groups 30 days after TMC.
Among the samples restored with RMGIC, after TMC, the highest alteration occurred for 38% SDF group, different from the other groups (p < 0.05). Thirty days after TMC, the control group demonstrated the highest change different (p < 0.05) from Bio group. Comparing the time of analysis, 12% SDF + KI and control groups presented higher values (p < 0.05) 30 days after TMC than immediately after TMC.
The color alteration values were compared among the restorative materials within the same time of analysis (Fig. 2). After TMC, the samples restored with RMGIC presented higher color alteration than the ones restored with CR (p < 0.05) when treated with 38% SDF and Bio. Thirty days after TMC, all the samples restored with both RMGIC and CR presented differences (p < 0.05) between them, except when they were treated with Bio, with higher values for the samples restored with RMGIC.
Figure 3 compares the ∆L*, ∆a*, and ∆b* immediately after and 30 days after TMC. All the groups presented negative alterations in ∆L*, regardless of the time of analysis, indicating the darkening of the samples. All the groups showed high alteration 30 days after TMC, except for 12% SDF + KI + CR, Bio + RMGIC, and 38% SDF + RMGIC groups.
Both control groups (RMGIC and CR) and Bio + RMGIC group presented an increase in ∆a* 30 days after TMC, showing a decrease in red chroma. For all the other groups, ∆a* values were stable. In addition, all the groups revealed an increase in ∆b*, except for 38% SDF + CR and Bio + CR, indicating an increased saturation of the yellow chroma.
The relative microhardness values are shown in Table 3. The microhardness alterations (after the cariogenic challenge, after the treatments, and 30 days after the treatments) are related to the initial microhardness values. The remineralizing potentials (initial and after 30 days) are related to the microhardness values obtained after the cariogenic challenge. There was no difference among the groups at any time of analysis, except in the initial remineralizing potential, where the control group presented a difference from the other groups.
After the cariogenic challenge, there was a decrease in the microhardness for all the groups. Negative values show that the treatments were not able to increase the microhardness to values similar to the sound dentin. The microhardness values were similar (p > 0.05) after the cariogenic challenge, immediately after the treatments, and 30 days after them. However, for the remineralization potential values there was difference (p < 0.05) from all other times of analysis, regardless of the treatment employed. Regarding the remineralizing potential, control group showed difference to all other groups (p < 0.05) for the initial remineralizing potential.
Representative SEM images are shown in Fig. 4. In general, the samples treated with RMGIC (Fig. 4B, D, F, H) demonstrated greater interaction with the dentin than those restored with CR, regardless of the treatment performed. The samples restored with CR (Fig. 4A, C, E, and G) presented a thin hybrid layer when treated with Biosilicate and under control conditions. Nonetheless, it was absent when the samples were treated with SDF, regardless of the concentration used. Irrespective of the restorative material, the dentin demonstrated a different appearance when treated with Biosilicate, more similar to the control group, while the dentin treated with SDF seemed more disorganized.
Restorations in dentin treated with SDF were detached from the tooth surface, resembling adhesive fractures and demonstrating poor adhesion to the substrate, regardless of the restorative material employed. The same was seen in the untreated samples restored with RMGIC, however, appearing mixed fractures.
Discussion
This study evaluated the color stability and microhardness of caries-affected dentin treated with SDF and Biosilicate, restored with composite resin or resin-modified glass-ionomer cement. The study had two hypotheses: that the SDF treatments would cause higher color alteration than Biosilicate and that there would be no difference in the microhardness of the dentin, regardless of the treatment employed. The first hypothesis was rejected since there was no difference in the color stability between the groups, irrespective of the treatment used, except for the group treated with 38% SDF and restored with RMGIC that presented higher values than the one treated with Biosilicate. The second hypothesis was accepted because although the microhardness of the dentin decreased after the cariogenic challenge, there was no significant difference between the proposed treatments.
SDF is an agent used for caries prevention and treatment and is found in different concentrations: 12%, 30%, and 38%. SDF at a concentration of 38% has been described as the most effective one [20]. The fluoride present in this product increases the resistance of dental tissue to the acid attack produced by the bacterial metabolism [4], decreasing its solubility. Most studies demonstrate higher efficiency for 38% SDF than for other concentrations. However, Braga et al. [21] reported positive results with 12% SDF. Thus, in the present study, both concentrations were used, mostly because of the increasing concerns about the effect of high fluoride concentrations in children [22].
Despite its efficacy, the greatest disadvantage of SDF is the color alteration caused by the precipitation of silver phosphate and silver sulfate on the substrate [23]. The darkening effect can impair the patient’s and parents’ acceptance of SDF treatment [24]. To reduce this adverse effect, Knight et al. [25] proposed potassium iodide salt as an additional treatment for dental surfaces treated with SDF. Potassium iodide (KI) reacts with the remaining silver ions producing silver iodide, a yellow precipitate, preventing the formation of silver phosphate, and decreasing the dental staining [8, 25]. Considering the results found in the literature regarding color alteration [22, 23], the present study evaluated the two concentrations of SDF associated with KI. Note that in the case of 38% SDF, KI was supplied by the same manufacturer as a second step.
Biosilicate is a crystallized bioactive glass–ceramic that presents in its composition 23.75Na2O–23.75CaO–48.5SiO2–4P2O5 (%weight) [11]. It forms hydroxycarbonate apatite in contact with body fluids, enabling the remineralization of hard tissues [26, 27]. Besides, many studies demonstrate its efficacy in the remineralization of caries-affected substrates [19, 28, 29]. The Biosilicate suspension does not produce any color alteration. Thus, one of the hypotheses of the study was that the substrate treated with Biosilicate would not present any color change, different from SDF, as demonstrated by the results found.
All the samples used for the color analysis were submitted to thermo mechanical cycling (TMC), simulating the effect of the masticatory forces and oral thermal stress on the restorations. Studies describe that the association of KI to SDF does not present a long-term effect, showing color alteration over time [30, 31]. Thus, TMC was used to evaluate this long-term alteration and the color readings performed 30 days after TMC.
The time of analysis was significant for the samples treated with 38% SDF and control group restored with composite resin and for the ones treated with 12% SDF + KI, and control group restored with RMGIC, with higher alteration 30 days after TMC. The different concentrations (12% and 38%) were not different regarding color alteration, similar to the results found by Patel et al. [23], except for the ones restored with RMGIC after TMC.
The TMC used in the present study simulated 5 years of clinical use [17]. Therefore, the proposed treatments can be evaluated as a long-term treatments. After TMC, the results from the 38% SDF group restored with CR were lower than those found for the control group. Thus, the color alteration in these groups could not be due to the precipitation of silver ions. However, 30 days after TMC, there was an increase in color alteration compared to those immediately after TMC.
This can be justified by the formation of silver iodide after applying KI, which is highly photosensitive and can dissociate into silver and iodide by exposure to light [32]. Regarding the 38% SDF group restored with RMGIC, higher color alteration happened immediately after TMC than 30 days after TMC. In that case, the photosensitivity of silver iodide may have caused this alteration since the reaction after exposure to light can be immediately after the treatment [2].
The color readings were performed using a device that emits light and quantifies the reflection of light in L*, a*, and b* color coordinates. The light coming from the spectrophotometer could have caused dissociation of these coordinates, resulting in increased color alteration 30 days after TMC. However, it is important to highlight that the amount of KI applied over SDF can influence this junction [9]. Thus, an insufficient amount of iodide can lead to an excess of silver ions, resulting in higher substrate staining [9].
The color alterations found in the control group (only with the adhesive system) result from the color alteration of the restorative material itself. Composite resin presents water sorption [33], so, the presence of water in TMC could cause color alteration. The water penetrates within the resin polymeric chain, altering the light reflection and diffraction in its interior [34].
Regarding the resin-modified glass-ionomer cement, immediately after TMC, there was higher color alteration for the group treated with 38% SDF than for all the other groups. Thirty days after TMC, Biosilicate presented lower color alteration than control group, but was similar to all the other groups. RMGIC has a polymeric portion, mostly formed by Bis-GMA and TEGDMA, thus, this material is also susceptible to water sorption. However, immediately after TMC, the samples restored with RMGIC presented higher color alteration than the ones restored with composite resin, when treated with 38% SDF and Biosilicate; and in all the groups 30 days after TMC, except for the samples treated with Biosilicate.
Glass-ionomer is hydrophilic and has higher water sorption than composite resin, resulting in higher color alteration [35]. What is more, the presence of filler content in the composite resin contributes to the light dispersion within the restoration [36], better simulating the natural tooth color than glass-ionomer cement. Thus, composite resin masks any color alteration inside the cavity and allows better color dispersion through the filler, resulting in lower color alteration than glass-ionomer cement.
It is important to highlight that, when using Biosilicate, the restorative material employed becomes unimportant for color alteration, as demonstrated in Fig. 2. So, regardless of the material, the samples treated with Biosilicate presented the same esthetic characteristics 30 days after TMC.
Color alteration always results from the alteration of the color coordinates L*, a*, and b* that compose color. Thus, it is possible to verify that the color alterations resulted from darkening, similar to Patel et al. [23], and yellowing of the restorations. For the a* axis, the alterations depended on the restorative material. Samples restored with RMGIC, regardless of the treatment used, showed a decrease in the red chroma immediately after TMC. In contrast, the ones restored with CR showed a small positive alteration, resulting in low reddening of the restorations.
To analyze the SDF’s remineralizing efficacy, the present study used the microhardness test and cariogenic challenge, to simulate the dentin demineralization in the cariogenic process. The cariogenic challenge presented similar effect in all the groups, evidenced by the lack of statistical difference (Table 3).
The SDF can increase the microhardness and reduce mineral loss of dental tissues [37]. In the present study, it was demonstrated that after the treatments, the microhardness values from all the groups did not regress to the initial levels (before the cariogenic challenge). However, when evaluating the relative microhardness between the final readings (after the treatment) and the ones after the cariogenic challenge, it was found that all the treatments presented a positive effect but without difference among them. Thereby demonstrating that the treatments induced some remineralization on the caries-affected dentin.
Remineralizing agents act in different pH levels. The fluoride-releasing system is efficient at low pH [38]. However, both SDF and Biosilicate are alkaline, and their mechanisms of action can be different in an acidic environment. The pH of SDF is around 10, favoring the formation of fluorapatite [4]. The fluoride shields the collagen molecules by adhering to calcium binding sites. It also inhibits MMPs 2, 8, 9, and cathepsins B and K by binding to calcium and zinc ions, which are required to activate these endogenous proteases [6]. Furthermore, the alkaline pH of SDF can prevent the activation of cathepsins and MMPs [39] and induce the nucleation of apatite through the formation of covalent bonds between phosphate ions and the collagen resulting in the binding of calcium ions [6].
When in contact with body fluids, Biosilicate increases the pH to 9 or 10 [11]. Thus, the reactions associated with SDF can also occur for Biosilicate. However, the deposition of hydroxycarbonate apatite on mineralized tissues is lower for Biosilicate because it depends on the material aggregation, followed by the formation of silanol groups and adjacent calcium uptake [40]. Therefore, Biosilicate needs more time to remineralize dental tissue, which can justify the relative microhardness values found 30 days after TMC in relation to the initial remineralizing potential (Table 3).
SDF also increases the surface hardness of demineralized dentin [39]. Silver phosphate and silver iodide contribute to the hardening of the surface [39] through the reaction of silver, which is associated to the remineralization mediated by fluoride [41]. Thus, SDF + KI is efficient to recover the mechanical properties of the demineralized dentin surface [37], as seen in the present study.
SDF penetrates 50–200 µm into dentin, and fluoride can penetrate the dentin more than into enamel [42]. However, when there is silver associated, its synergic effect can be relatively smaller [43]. Thus, in the present study, the dentin microhardness alteration when treated with SDF can be justified by the silver ions deposition.
The 38% SDF has 44,800 fluoride ppm, and 12% SDF has 14,150 fluoride ppm [44]. The higher the concentration of fluoride, the higher the increase in microhardness [45]. Nonetheless, in the present study, there was no difference between 38% SDF and 12% SDF, different from other studies that recommend a higher concentration to achieve more efficiency [22].
The association of SDF and hydroxyapatite forms calcium fluoride and silver phosphate in an alkaline environment [46]. Calcium fluoride acts as a fluoride reservoir, releasing fluoride ions during the cariogenic process and regulating the pH. In addition, hydrogen phosphate ions (HPO4)2 facilitate the conversion of calcium fluoride to fluorapatite. However, silver phosphate is easier to dissolve than hydroxyapatite and fluorapatite. It works as a phosphate ions reservoir making possible the conversion of the calcium fluoride to fluorapatite [47].
SDF also presents an antibacterial effect [5], arresting the caries progression by the biofilm modification. Silver ions inhibit biofilm formation by inactivation and interference with bacterial synthesis of cellular polysaccharides. This mechanism occurs by the inactivation of the glycosyltransferase that is responsible for the synthesis of glucan (the most prevalent component of the biofilm that promotes bacterial adhesion and has high resistance to antimicrobial treatments) [48]. Fluoride ions can also inhibit biofilm formation. It binds to bacterial cell constituents and inhibits bacterial enzymes, reducing the production of glucan.
Materials with remineralizing potential have become popular in dentistry due to the global dissemination of the minimally invasive approach. The present study demonstrated that all the proposed treatments are an option for the management of carious lesions, despite their limitations, such as darkening of the dentin by SDF. Regarding the esthetic outcome, Biosilicate could be a good alternative, but it needs a longer time to promote remineralization.
Another limitation of the SDF is that the silver precipitate on the dentin surface could occlude the dentinal tubules and form an insoluble layer that interferes [49] with the ability of the bonding agent to impregnate the peritubular and intratubular dentin and infiltrate within the collagen matrix [50], thus, no hybrid layer is formed (Fig. 4). As a stable bond is not achieved, the restoration could dislocate from the tooth structure, as seen by SEM in the samples treated with SDF, regardless of the restorative material employed; and as also reported by Quock et al. [49] and Van Duker et al. [51].
It has also been observed by SEM that the samples restored with the composite resin presented a thin hybrid layer when treated with Biosilicate and under control conditions; however, when SDF was applied, there was no formation of this layer. Again, the silver deposition might have impaired the penetration of the bonding agent into the dentinal tubules and the formation of a meshwork with the underlying collagen matrix [50]. What is more, its alkaline pH would have hampered the etching efficacy of the acidic monomers present in the adhesive system applied in self-etch mode. Thus, no hybrid layer is formed [50].
Meanwhile, the Biosilicate particles employed in the present study have a diameter of 4 µm. Most of them can be dissolved in distilled water at the time of its preparation, but some remain on the dentin surface [19], as noted by SEM. However, apparently, this did not interfere with the formation of the hybrid layer, and a more organized dentin structure was observed.
On the other hand, it was noticed a good interaction between the RMGIC and the dentin substrate. Glass ionomer cements present chemical and micromechanical adhesion to dentin, and the incorporation of a polymerizable monomer 2-hydroxyethyl methacrylate (HEMA) in RMGIC improves the bond [52].
As stated in the study, the treatments present pros and cons. Further studies should be carried out to evaluate the effect of these conservative treatments on the bond strength over time, using different restorative materials and concentrations.
It is important to reassure that caries is the most common oral disease worldwide, especially in developing countries, where the population has less access to dentists [10]. In addition, the management of carious lesions can be very traumatic for the patients, which makes their dental treatment difficult. Thereby, the search for less invasive treatments using materials that are accessible, easy to use, and effective, are the key to make dentistry more inclusive.
One of the limitations of this study is the artificial method of dentin caries induction used, which could not reflect the exact in vivo color change. Caries-affected dentin obtained by in vitro protocols attempt to reproduce caries-like lesions in vivo protocols that are valid for the development of research [53]. However, several factors may influence caries progression, providing variability in the results [53]. Therefore, caution should be exercised in interpreting the results. The results cannot be extrapolated directly to in vivo conditions. In vivo studies should be performed.
Conclusion
Based on the results found, it is concluded that the SDF and Biosilicate can alter the color of composite resin and resin-modified glass-ionomer cement restorations, according to the material used and aging. For microhardness, there was no difference of the treated teeth nor the remineralizing potential of the remineralizing agents tested.
References
Pitts NB, Zero DT, Marsh PD, Ekstrand K, Weintraub JA, Ramos-Gomez F, Tagami J, Twetman S, Tsakos G, Ismail A (2017) Dental caries. Nat Rev Dis Prim 3:1–16. https://doi.org/10.1038/nrdp.2017.30
Nguyen V, Neill C, Dds JF, Primus C (2017) Potassium iodide: the solution to silver diamine fluoride discoloration? Adv Dent Oral Health 5:1–6. https://doi.org/10.19080/ADOH.2017.05.5555655
Nelson T (2016) Silver diamine fluoride in pediatric dentistry training programs: survey of graduate program directors. Pediatr Dent 38:212–217
Mei ML, Nudelman F, Marzec B, Walker JM, Lo ECM, Walls AW, Chu CH (2017) Formation of fluorohydroxyapatite with silver diamine fluoride. J Dent Res 96:1122–1128. https://doi.org/10.1177/0022034517709738
Seifo N, Robertson M, Maclean J, Blain K, Grosse S, Milne R, Seeballuck C, Innes N (2020) The use of silver diamine fluoride (SDF) in dental practice. Br Dent J 228:75–81. https://doi.org/10.1038/s41415-020-1203-9
Firouzmandi M, Shafiei F, Jowkar Z, Nazemi F (2019) Effect of silver diamine fluoride and proanthocyanidin on mechanical properties of caries-affected dentin. Eur J Dent 13:255–260. https://doi.org/10.1055/s-0039-1693237
Huang WT, Shahid S, Anderson P (2019) Applications of silver diamine fluoride in management of dental caries. Elsevier Ltd. https://doi.org/10.1016/b978-0-08-102476-8.00023-2
Haiat A, Ngo HC, Samaranayake LP, Fakhruddin KS (2021) The effect of the combined use of silver diamine fluoride and potassium iodide in disrupting the plaque biofilm microbiome and alleviating tooth discoloration: a systematic review. PLoS ONE 16:1–18. https://doi.org/10.1371/journal.pone.0252734
Sorkhdini P, Gregory RL, Crystal YO, Tang Q, Lippert F (2020) Effectiveness of in vitro primary coronal caries prevention with silver diamine fluoride—chemical vs biofilm models. J Dent 99:103418. https://doi.org/10.1016/j.jdent.2020.103418
Roberts A, Bradley J, Merkley S, Pachal T, Gopal JV, Sharma D (2020) Does potassium iodide application following silver diamine fluoride reduce staining of tooth? A systematic review. Aust Dent J 65:109–117. https://doi.org/10.1111/adj.12743
Crovace MC, Souza MT, Chinaglia CR, Peitl O, Zanotto ED (2016) Biosilicate® - a multipurpose, highly bioactive glass-ceramic. In vitro, in vivo and clinical trials. J Non Cryst Solids 432:90–110. https://doi.org/10.1016/j.jnoncrysol.2015.03.022
Banerjee A (2013) Minimal intervention dentistry: Part 7. Minimally invasive operative caries management: rationale and techniques. Br Dent J 214:107–111. https://doi.org/10.1038/sj.bdj.2013.106
Yoshida Y, VanMeerbeek B, Nakayama Y, Snauwaert J, Hellemans L, Lambrechts P, Vannerle G, Wakasa K (2000) Evidence of chemical bonding at biomaterial-hard tissue interfaces. J Dent Res 79:709–714. https://doi.org/10.1177/00220345000790020301
Wiegand A, Buchalla W, Attin T (2007) Review on fluoride-releasing restorative materials-fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent Mater 23:343–362. https://doi.org/10.1016/j.dental.2006.01.022
Ferracane JL (2011) Resin composite—state of the art. Dent Mater 27:29–38. https://doi.org/10.1016/j.dental.2010.10.020
Moron BM, Comar LP, Wiegand A, Buchalla W, Yu H, Buzalaf MAR, Magalhães AC (2013) Different protocols to produce artificial dentine carious lesions in vitro and in situ: hardness and mineral content correlation. Caries Res 47:162–170. https://doi.org/10.1159/000345362
Kohal RJ, Wolkewitz M, Tsakona A (2011) The effects of cyclic loading and preparation on the fracture strength of zirconium-dioxide implants : an in vitro investigation. Clin Oral Implants Res 22:808–814. https://doi.org/10.1111/j.1600-0501.2010.02067.x
Sharma G, Wu W, Dalal EN (2005) The CIEDE2000 color-difference formula: implementation notes, supplementary test data, and mathematical observations. Color Res Appl 30:21–30. https://doi.org/10.1002/col.20070
GengVivanco R, Tonani-Torrieri R, Souza ABS, Marquele-Oliveira F, de Carvalho Panzeri Pires-de-Souza F (2021) Effect of natural primer associated to bioactive glass-ceramic on adhesive/dentin interface. J Dent 106:103585. https://doi.org/10.1016/j.jdent.2021.103585
Gao SS, Zhao IS, Hiraishi N, Duangthip D, Mei ML, Lo ECM, Chu CH (2016) Clinical trials of silver diamine fluoride in arresting caries among children : a systematic review. JDR Clin Transl Res 1:201–210. https://doi.org/10.1177/2380084416661474
Braga MM, Mendes FM, De Benedetto MS, Imparato JCP (2009) Effect of silver diammine fluoride on incipient caries lesions in erupting permanent first molars: a pilot study. J Dent Child 76:5–9
Fung MHT, Duangthip D, Wong MCM, Lo ECM, Chu CH (2018) Randomized clinical trial of 12 % and 38 % silver diamine fluoride treatment. J Dent Res 97:171–178. https://doi.org/10.1177/0022034517728496
Patel J, Anthonappa RP, King NM (2018) Evaluation of the staining potential of silver diamine fluoride: in vitro. Int J Paediatr Dent 28:514–522. https://doi.org/10.1111/ipd.12401
Crystal YO, Kreider B, Raveis VH (2019) Parental expressed concerns about silver diamine fluoride (sdf) treatment. J Clin Pediatr Dent 43:155–160. https://doi.org/10.17796/1053-4625-43.3.2
Knight GM, Mcintyre JM (2006) The effect of silver fluoride and potassium iodide on the bond strength of auto cure glass ionomer cement to dentine. Aust Dent J 51:42–45. https://doi.org/10.1111/j.1834-7819.2006.tb00399.x
Granito RN, Rennõ AC, Ravagnani C, Bossini PS, Mochiuti D, Jorgetti V, Driusso P, Peitl O, Zanotto ED, Parizotto NA, Oishi J (2011) In vivo biological performance of a novel highly bioactive glass-ceramic (Biosilicate®): a biomechanical and histomorphometric study in rat tibial defects. J Biomed Mater Res - Part B Appl Biomater 97 B:139–147. https://doi.org/10.1002/jbm.b.31795
Roriz VM, Rosa AL, Peitl O, Zanotto ED, Panzeri H, De Oliveira PT (2010) Efficacy of a bioactive glass-ceramic (Biosilicate®) in the maintenance of alveolar ridges and in osseointegration of titanium implants. Clin Oral Implants Res 21:148–155. https://doi.org/10.1111/j.1600-0501.2009.01812.x
de Morais RC, Silveira RE, Chinelatti MA, de Carvalho Panzeri Pires-de-Souza F (2016) Biosilicate as a dentin pretreatment for total-etch and self-etch adhesives: In vitro study. Int J Adhes Adhes 70:271–276. https://doi.org/10.1016/j.ijadhadh.2016.07.007
de Morais RC, Silveira RE, Chinelatti M, Geraldeli S, de Carvalho Panzeri Pires-de-Souza F (2018) Bond strength of adhesive systems to sound and demineralized dentin treated with bioactive glass ceramic suspension. Clin Oral Investig 22:1923–1931. https://doi.org/10.1007/s00784-017-2283-z
Vennela E, Sharada J, Hasanuddin S, Suhasini K, Hemachandrika I, Singh PT (2021) Comparison of staining potential of silver diamine fluoride versus silver diamine fluoride and potassium iodide under tooth-colored restorations: an in vitro study. J Indian Soc Pedod Prev Dent 39:47–52. https://doi.org/10.4103/jisppd.jisppd
Li R, Lo ECM, Liu BY, Wong MCM, Chu CH (2016) Randomized clinical trial on arresting dental root caries through silver diammine fluoride applications in community-dwelling elders. J Dent 51:15–20. https://doi.org/10.1016/j.jdent.2016.05.005
Zhao IS, Chu S, Yu OY, Mei ML, Chu CH, Lo ECM (2019) Effect of silver diamine fluoride and potassium iodide on shear bond strength of glass ionomer cements to caries-affected dentine. Int Dent J 69:341–347. https://doi.org/10.1111/idj.12478
Albuquerque PPAC, Moreira ADL, Moraes RR, Cavalcante LM, Schneider LFJ (2013) Color stability, conversion, water sorption and solubility of dental composites formulated with different photoinitiator systems. J Dent 41:e67–e72. https://doi.org/10.1016/j.jdent.2012.11.020
Fonseca ASQS, Labruna Moreira AD, de Albuquerque PPAC, de Menezes LR, Pfeifer CS, Schneider LFJ (2017) Effect of monomer type on the C=C degree of conversion, water sorption and solubility, and color stability of model dental composites. Dent Mater 33:394–401. https://doi.org/10.1016/j.dental.2017.01.010
Sidhu S, Nicholson J (2016) A review of glass-ionomer cements for clinical dentistry. J Funct Biomater 7:16. https://doi.org/10.3390/jfb7030016
Muhittin U, Burak TU, Kam HO (2019) Color stability of microhybrid and nanofilled composite resins: effect of surface sealant agents containing different filler content. J Contemp Dent Pract 20:1045–1050. https://doi.org/10.5005/jp-journals-10024-2636
Cai J, Burrow MF, Manton DJ, Tsuda Y, Sobh EG, Palamara JEA (2019) Effects of silver diamine fluoride/potassium iodide on artificial root caries lesions with adjunctive application of proanthocyanidin. Acta Biomater 88:491–502. https://doi.org/10.1016/j.actbio.2019.02.020
Ten Gate JM (1997) Review on fluoride, with special emphasis on calcium fluoride mechanisms in caries prevention. Eur J Oral Sci 105:461–465. https://doi.org/10.1111/j.1600-0722.1997.tb00231.x
Mei ML, Lo ECM, Chu CH (2018) Arresting dentine caries with silver diamine fluoride: what’s behind it ? J Dent R 97:751–758. https://doi.org/10.1177/0022034518774783
Renno ACM, Bossini PS, Crovace MC, Rodrigues ACM, Zanotto ED, Parizotto NA (2013) Characterization and in vivo biological performance of Biosilicate. Biomed Res Int. https://doi.org/10.1155/2013/141427
Seto J, Horst JA, Parkinson DY, Frachella JC, DeRisi JL (2017) Silver microwires from treating tooth decay with silver diamine fluoride. BioRxiv. https://doi.org/10.1101/152199
Horst JA, Ellenikiotis H, UCSF Silver Caries Arrest Committee, Milgrom PM (2016) USCF protocol for silver diamine fluoride: rationale, indications, and consent. J Calif Dent Assoc 44:16–28
Zhi QH, Lo ECM, Kwok ACY (2013) An in vitro study of silver and fluoride ions on remineralization of demineralized enamel and dentine. Aust Dent J 58:50–56. https://doi.org/10.1111/adj.12033
Gao SS, Zhang S, Mei ML, Lo EC, Chu C (2016) Caries remineralisation and arresting effect in children by professionally applied fluoride treatment—a systematic review. BMC Oral Health 16:1–9. https://doi.org/10.1186/s12903-016-0171-6
Wiegand A, Krieger C, Attin R, Hellwig E, Attin T (2005) Fluoride uptake and resistance to further demineralisation of demineralised enamel after application of differently concentrated acidulated sodium fluoride gels. Clin Oral Investig 9:52–57. https://doi.org/10.1007/s00784-005-0306-7
Rosenblatt A, Stamford TCM, Niederman R (2009) Silver diamine fluoride: a caries “Silver-Fluoride Bullet.” J Dent Res 88:116–125. https://doi.org/10.1177/0022034508329406
Akyildiz M, Sönmez IS (2019) Comparison of remineralising potential of nano silver fluoride, silver diamine fluoride and sodium fluoride varnish on artificial caries: an in vitro study. Oral Heal Prev Dent 17:469–477. https://doi.org/10.3290/j.ohpd.a42739
Mei ML, Li Q, Chu C, Lo EC, Samaranayake LP (2013) Antibacterial effects of silver diamine fluoride on multi-species cariogenic biofilm on caries. Ann Clin Microbiol Antimicrob 12:1–7. https://doi.org/10.1186/1476-0711-12-4
Quock RL, Barros JA, Yang SW, Patel SA (2012) Effect of silver diamine fluoride on microtensile bond strength to dentin. Oper Dent 37:610–616. https://doi.org/10.2341/11-344-L
Lutgen P, Chan D, Sadr A (2018) Effects of silver diammine fluoride on bond strength of adhesives to sound dentin. Dent Mater J 37:1003–1009. https://doi.org/10.4012/dmj.2017-401
Van Duker M, Hayashi J, Chan DC, Tagami J, Sadr A (2019) Effect of silver diamine fluoride and potassium iodide on bonding to demineralized dentin. Am J Dent 32:143–146
Nicholson JW (2016) Adhesion of glass-ionomer cements to teeth: a review. Int J Adhes Adhes 69:33–38. https://doi.org/10.1016/j.ijadhadh.2016.03.012
Pacheco LF, Banzi ECDF, Rodrigues E, Soares LES, Pascon FM, Correr-Sobrinho L, Puppin-Rontani RM (2013) Molecular and structural evaluation of dentin caries-like lesions produced by different artificial models. Braz Dent J 24:610–618. https://doi.org/10.1590/0103-6440201302357
Funding
This study was performed by Adriana Cavalcanti Ferreira as partial fulfillment of her MSc degree at the School of Dentistry of Ribeirao Preto–University of Sao Paulo. This study was partially supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES)-Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Considering the institutional guidelines, ethical approval is not required for this type of study.
Informed consent
Informed consent is not required for this type of study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ferreira, A.C., de Lima Oliveira, R.F., Amorim, A.A. et al. Remineralization of caries-affected dentin and color stability of teeth restored after treatment with silver diamine fluoride and bioactive glass–ceramic. Clin Oral Invest 26, 4805–4816 (2022). https://doi.org/10.1007/s00784-022-04445-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-022-04445-6