Abstract
Objectives
The aim of the present study was to evaluate the percent mean mineral density (MD) change of early caries lesions after the application of silver diamine fluoride (SDF) or glass ionomer cement (GIC).
Materials and methods
This double-blind, crossover study involved two experimental phases of 28 days each. Thirty-two pairs of enamel slabs were created from the proximal surfaces of 16 premolars. Each pair of artificial carious slabs was randomly divided into the control or test group (38 % SDF or GIC). The slabs were attached to orthodontic brackets and bonded to the maxillary first permanent molars of 16 subjects for 28 days. After a 7-day washout period between phases, the subjects received the other material for the second phase. The mean MD of the lesions was measured by microcomputed tomography.
Results
SDF yielded a percent mean MD increase at a depth of 0–84 μm, although increase in the GIC group was observed at a depth of 24–108 μm. The percent mean MD changes of the SDF and GIC groups were similar (p = 0.100) and significantly higher than in control (p < 0.001, p = 0.003, respectively).
Conclusions
The two materials increased the percent mean MD change of early proximal caries lesions to a similar extent, but with different spatial patterns.
Clinical relevance
Due to deeper level of GIC remineralization, the refractive index of the GIC applied enamel might be closer to sound enamel. Hence, GIC is recommended for remineralization of anterior teeth. SDF staining makes it unsuitable for use in anterior teeth; thus, it is reserved for use in posterior teeth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clinically, an early enamel caries lesion appears as a white spot lesion. If the lesion progresses, surface cavitation of the tooth will occur. The current caries management aims to limit the progression of demineralization and to remineralize the lesion at the earliest stage possible. The proximal surface of a tooth is vulnerable to develop caries and progress to a cavitated lesion because of greater porosity at the enamel surface [1]. However, only 0–20.6 % of teeth with a proximal radiolucency in the outer half of enamel in bitewing radiographs were found to be cavitated, with 10.5–47 % of the teeth cavitated when the radiolucency was found in the inner half of enamel [2–7]. These studies indicate that the percentage of non-cavitated lesions in radiographic enamel lesions is high. Thus, the use of a remineralizing agent especially topical fluoride can play an important role in increasing the probability that a lesion will regress or not progress, lessening the chance of cavitation.
Topically applied fluoride therapy is classified into self-applied and professionally applied methods. Fluoride mouthrinses and toothpastes are the main forms of self-applied fluoride. Professionally applied products include fluoride gels, fluoride varnish, silver diamine fluoride, and fluoride-containing restorations and sealants, such as glass ionomer cement.
Glass ionomer cement (GIC) chemically bonds to dental hard tissues, releases fluoride over an extended period, and acts as a fluoride reservoir [8, 9]. Several studies have demonstrated that the application of GIC reduced the area of early proximal caries lesions [10, 11].
Silver diamine fluoride (SDF, Ag(NH3)2F) has antibacterial action, inhibits caries progression via inhibiting biofilm formation, and facilitates enamel remineralization [12]. SDF has been demonstrated to be effective for caries reduction in cavitated primary teeth and first permanent molars in 6-year-old schoolchildren during a 36-month study [13]. However, the evidence of SDF application effect on non-cavitated early proximal caries lesions is unclear. The present study aimed to compare the percent mean mineral density (MD) change of early proximal caries lesions after the application of SDF or GIC in situ.
Materials and methods
Subjects
The study protocols were approved by the Human Research Ethics Committee of the Faculty of Dentistry, Chulalongkorn University, Thailand (HREC-DCU 2013-011). A sample size calculation determined that 16 samples were required to demonstrate an absolute difference of 5 % in mean MD change between the two materials with 80 % power (α = 0.05, β = 0.20). Sixteen healthy post-orthodontic patients, aged 19–23 years old, participated in our study. After they had been given verbal and written explanations of the experimental protocol, informed consent was obtained. The study inclusion criteria consisted of having at least 22 teeth, no clinically active caries, periodontal disease, or other oral pathology, and not using antibiotics. The volunteers were in the moderate to high caries risk group (consuming ≥ 2 between-meal sugar-containing snacks or beverages per day).
Enamel slab preparation
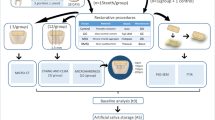
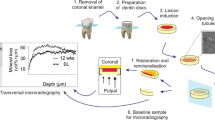
Sixteen extracted human premolar teeth, free of cracks, hypoplasia, and white spot lesions, were used in our study. Each proximal surface was polished using an automatic polishing machine at 100 rpm for 45 s to remove its fluoride-rich layer. Nail varnish (Zoya Professional Lacquer, Cleveland, OH, USA) was applied on all sides of each tooth, except for 1 × 2 mm2 windows on the distal and mesial surfaces where artificial caries lesions would be induced. The artificial lesions were induced on the mesial and distal tooth surfaces by immersion into a 0.2 % polyacrylic acid, 85 % lactic acid, hydroxyapatite, and 6 M sodium hydroxide demineralizing solution at pH 4.8 [14] for 168 h at 37 °C. The depth of the artificial lesions was approximately 100–150 μm.
After demineralization, the teeth were washed in deionized water. Each lesion on the proximal surface was vertically divided into two 1 × 3 × 2 mm3 slabs to obtain 64 slabs (32 pairs) by a low-speed cutting machine (ISOMET 1000, Buehler, Lake Bluff, IL, USA). The surfaces of the slabs were painted with nail varnish, except for a 1 × 1 mm2 window in the middle (Fig. 1). The slabs were sterilized with ethylene oxide for 12 h and stored in deionized water until used.
Experimental protocol
This double-blind, randomized, controlled, crossover study consisted of two 28-day experimental phases with a 7-day washout period in between. The 7-day washout period aimed to regulate the fluoride level in the saliva to the baseline level [15, 16]. Seven days before, and during the experimental period, the subjects brushed for 2 min, 2×/day using a 1000 ppm fluoride-containing dentifrice (Colgate Regular Flavor, Colgate-Palmolive, Chonburi, Thailand), provided by the investigators. The volunteers were not permitted to smoke cigarettes, drink alcohol, use mouthwash, or any xylitol or fluoride-containing products during the study. They were instructed to eat and drink as usual and were required to keep a record of their daily food intake during the first experimental period. During the second experimental period, the volunteers used their daily food records to guide them to eat similar food as during the first experimental period.
Two pairs of slabs from the same tooth were randomly assigned to either the first or second experimental phase for each subject. The subjects were randomly divided into either the 38 % SDF (Saforide, Toyo Seiyaku Kasei Co., Ltd., Osaka, Japan) or GIC (Fuji VII, GC Corporation, Tokyo, Japan) group in the first experimental phase, and they received the other material in the second phase.
Each pair of slabs was allocated to the test or control group by simple randomization. The 1 × 1 mm2 demineralized window in the middle of the test slabs was applied with SDF or GIC according to the manufacturer’s instructions. The test and control slabs were randomly allocated to the left or right side of the oral cavity for each subject equally. The slabs were inserted laterally against the mesial wings of an orthodontic bracket, and flowable composite was placed between the upper and lower mesial wing of orthodontic bracket to simulate proximal contact. The other areas of the slabs except the 1 × 1 mm2 window were bonded to the brackets with flowable composite (Fig. 2). The brackets were bonded to the buccal surface of the maxillary first permanent molars of the subjects with a bonding agent (Transbond XT®, 3 M Unitek, Monrovia, CA, USA) [11, 17, 18].
After each 28-day experimental period, the plaque and flowable composite were removed from the enamel slabs. The SDF or GIC on the surface of the slabs seen from the difference in color from enamel was gently removed using an explorer. The mean MD of the slabs was then measured.
Mineral density measurement
The mean MD of the entire 1 × 1 mm2 window of the intra-oral control and SDF/GIC slabs at baseline and after the experiment were measured in mg HA/cm3 using microcomputed tomography (microCT) (μCT 35 scanner, Scanco, Bruttisellen, Switzerland). The X-ray source was set at 70 kVp and 114 μA with 400-ms integration time and an isometric voxel size of 12 μm. The samples were scanned at a standard resolution (1024 × 1024 pixels). A 0.5-mm aluminum filter was used to reduce beam hardening. The beam hardening correction was calibrated using a 1200 mg HA/cm3 hydroxyapatite phantom. The scanned results were reconstructed and analyzed with SCANCO microCT software (Scanco, Bruttisellen, Switzerland). A notch was marked as a reference point on the upper part of the enamel slabs, and the axis of the polished enamel slabs was set parallel to the base of the holder. The reference point of the depth axis (0 μm) was set at the axial position of the sound enamel surface. For mineral density calibration, a series of mineral reference hydroxyapatite phantoms (0, 100, 200, 400, and 800 mg HA/cm3) were scanned.
The percent mean MD change was calculated using the following equation:
where ΔZd = difference in area under the densitometric profile of the demineralized lesion and the median sound enamel and ΔZr = difference in area under the densitometric profile of the remineralized lesion and the median sound enamel [19].
The lesion depth was determined as the depth of the region where the mineral content reached 95 % of the maximum MD [20].
Statistical analysis
The mean MD values of the pre- and post-test SDF, GIC, and intra-oral control groups were compared using the dependent t test. The percent mean MD changes of the entire lesion and every 12 μm through the depth of the lesion in the SDF, GIC, and intra-oral control groups were compared using one-way ANOVA, followed by post hoc tests for multiple comparisons. Statistical analysis was performed using SPSS version 20 (SPSS Inc., Chicago, IL, USA) with significance level set at 0.05.
Results
Seven men and nine women with a mean age of 21.6 ± 0.9 years old participated in our study. None withdrew during the present crossover study. Neither adverse events were reported by the participants nor white spot was found at the bonded bracket surface and surrounding. The mean depth of the artificial caries lesions of the enamel slabs was 134.4 ± 28.6 μm.
We used microCT to evaluate MD at baseline and after the experimental periods. The baseline MD of the enamel slab groups ranged from 1500.0 ± 154.4 to 1605.1 ± 149.3 mg HA/cm3; however, these values were not significantly different (p = 0.299; Table 1).
The mean MD values of the SDF and GIC test groups at 1687.4 ± 97.8 and 1687.0 ± 107.6 mg HA/cm3, respectively, were significantly higher than their baseline MD (p < 0.001 and p < 0.001, respectively). In contrast, there was no significant difference between the baseline and post-test MD of the SDF/GIC intra-oral control groups (p = 0.067 and p = 0.944, respectively).
The percent mean MD changes of SDF and GIC groups were significantly higher than those in their intra-oral control groups (p < 0.001 and p = 0.003, respectively). However, there was no significant difference in percent mean MD change between two test groups (p = 0.100; Table 1).
When compared by lesion depth, the percent mean MD change of the SDF group was significantly higher compared to that of its intra-oral control group at a depth of 0–84 μm (Fig. 3). In contrast, the percent mean MD change of the GIC group was significantly higher compared to that of its intra-oral control group at a depth of 24–108 μm (Fig. 4).
Discussion
The present study investigated the effect of silver diamine fluoride (SDF) and glass ionomer cement (GIC) in remineralizing artificially induced caries lesions in situ. Our results indicated that although SDF and GIC generated similar increases in mineral density, they did so differentially throughout the depth of the lesions.
Our study found no significant differences between the baseline and post-test MD of the SDF or GIC intra-oral control groups, indicating that the oral environments of both experimental periods were similar. Therefore, the results of the two periods could be compared. The use of a 1000 ppm fluoride-containing dentifrice 2×/day only maintained the average MD level or did not increase demineralization as well as remineralization. Therefore, it is necessary to use an adjunctive remineralizing agent, if an individual cannot change their dietary habit.
The 38 % SDF solution used in our study had a high concentration of silver (253,870 ppm) and fluoride (44,800 ppm) ions [21]. An in vitro study showed that SDF and hydroxyapatite formed calcium fluoride and silver phosphate in a basic environment [12]. Calcium fluoride acts as a pH-regulated slow-release fluoride reservoir during cariogenic challenge. In addition, hydrogen phosphate ions (HPO4 2−) facilitate calcium fluoride to form fluorapatite. Silver phosphate is more soluble than hydroxyapatite and fluorapatite; thus, it acts as a reservoir of phosphate ions facilitating the formation of fluorapatite from calcium fluoride [22]. This mechanism was shown in other studies using 38 % SDF applied to dentin blocks resulting in higher calcium and phosphorus weight percentages in the outer 25 μm compared to the those of the control group [21, 23] and to a depth of 50 μm in dentin caries lesions [21].
The main mechanism of GIC in remineralization of caries lesion has been shown under the lesion without actual exposure to the oral environment [24]. The carboxyl group (COO–) of the polycarboxylic acid in GIC forms ionic bonds to the calcium in enamel and dentin [25, 26]. When the GIC powder and liquid are mixed, metals (e.g., strontium, calcium, and aluminum) and fluoride ions are released [26], resulting in a hypermineralized zone between the material and tooth structure [17, 24, 27, 28]. However, our results differ from those of previous studies. In our study, the percentage mean MD change after GIC application was not significantly different compared to that of the control group at depths less than 24 μm; thus, we did not find a hypermineralized zone at these depths. This finding implies that the acid–base reaction of the powder–liquid type of GIC used in the present study did not produce a surface hypermineralized zone.
In contrast to our results, a preliminary study by Thepyou et al. found that the percent mean MD change of the GIC group compared to its intra-oral control group was 42.3 %, higher than that in our study [17]. This may be because the subjects in Thepyou et al. were in the high caries risk group, who would have had a more frequently acidic oral environment. The amount of fluoride released from GIC at low pH is greater than that at higher pH [29]. SEM analysis revealed the presence of a large number of voids, cracks, and microporosities of material surface at low pH causing more release fluoride. Moreover, GIC can take-up and re-release fluoride, acting as a fluoride reservoir [29, 30]. The higher amount of fluoride released from GIC at low pH in the Thepyou et al. study may have resulted in a significantly higher MD compared to its intra-oral control at a lesion depth of 0–68 μm [17], as opposed to our study that found a higher MD from 24–108 μm. This difference may be because the GIC used in our study was the powder–liquid type in contrast to the capsule type used in their study. We chose the powder–liquid type because it is widely used in our country due to its lower cost. Although the handling of the GIC was performed according to the manufacturer’s instruction, the capsule type is resistant to contamination by moisture and, due to a more consistent powder-to-liquid ratio, may release more fluoride, generating a surface hypermineralized zone which may inhibit the fluoride ion penetration through deeper level of the lesion. This could explain why the remineralization in our study occurred in a deeper area than that of Thepyou et al.
Compared to each material’s intra-oral control group, the percent mean MD change of the GIC group was significant at a lesion depth of 24–108 μm, whereas this change was significant at a depth of 0–84 μm in the SDF group. This could be because the free fluoride ions released from applied GIC penetrate into the demineralizing lesion. This contrasts with SDF, which forms calcium fluoride, a slow-release fluoride reservoir during cariogenic challenge. Due to deeper level of GIC remineralization, the refractive index of the GIC applied enamel might be closer to sound enamel.
The results of the present study are consistent with a 24-month clinical study that compared the effectiveness of SDF and GIC in 3–4-year-old children. Zhi et al. demonstrated that annual application of either SDF or GIC arrested active dentin caries. Increasing the frequency of SDF application to every 6 months increased the caries arrest rate [31].
Both SDF and GIC have antibacterial effects; however, they exert their effects by different mechanisms. The silver of SDF interacts with the sulfhydryl groups (−SH) of proteins and DNA, altering hydrogen bonding, DNA unwinding, cell wall synthesis, cell division, and inhibiting biofilm formation [12]. Alternatively, the aluminum and fluoride from GIC inhibit ATPase in Streptococcus mutans that plays an important role in the maintenance of intracellular pH. Inhibition of this enzyme disrupts bacterial metabolism and the aciduric capability of S. mutans [32]. In addition, fluoride alters bacterial adhesion, leading to less acidogenic plaque flora. However, no correlation was found between the zinc released from GIC and antibacterial activity [33].
Although fluoride varnish is used for caries management at proximal surfaces, GIC is more effective than fluoride varnish in remineralization of proximal caries lesions. Trairatvorakul et al. found that the average caries lesion area under GIC is significantly less than that under fluoride varnish [11].
An advantage of our crossover study was that the two materials were evaluated in similar oral environments. In addition, all the subjects were in the same caries risk group, had similar dietary habits during the experimental periods, and received the same amount and frequency of fluoride exposure, further normalizing the study conditions between subjects.
MicroCT was used in the present study to evaluate the mineral density of the samples because microCT provides non-destructive, three-dimensional images of an object. This makes it possible to generate localized 3D imaging of the degree of remineralization of the lesions without sectioning the tooth, and these images can be assessed after each cycle of demineralization or remineralization [34].
The present study shows that both of SDF and GIC increased the percent mean MD change of early proximal caries lesions. In early proximal lesion, the teeth can be separated with elastic rings and then applied with either materials. Although SDF is easier to apply, it is recommended to only be used on the proximal caries of posterior teeth due to its potential to stain the enamel. GIC takes more time to apply; however, it is esthetically more acceptable to use in treating proximal caries on anterior teeth.
Due to the limitation of the in situ study, longer clinical trial could show better remineralizing effect of SDF and GIC, the best frequency of use, and cost-effectiveness to select the appropriate material to limit caries progression and remineralize early proximal lesions.
In conclusion, SDF and GIC similarly increased the percent mean MD change of early proximal caries lesions but with different spatial patterns.
References
Zero DT (1999) Dental caries process. Dent Clin N Am 43(4):635–664
Akpata ES, Farid MR, Al-Saif K, Roberts EA (1996) Cavitation at radiolucent areas on proximal surfaces of posterior teeth. Caries Res 30(5):313–316
Bille J, Thylstrup A (1982) Radiographic diagnosis and clinical tissue changes in relation to treatment of approximal carious lesions. Caries Res 16(1):1–6
De Araujo FB, Rosito DB, Toigo E, dos Santos CK (1992) Diagnosis of approximal caries: radiographic versus clinical examination using tooth separation. Am J Dent 5(5):245–248
Pitts NB, Rimmer PA (1992) An in vivo comparison of radiographic and directly assessed clinical caries status of posterior approximal surfaces in primary and permanent teeth. Caries Res 26(2):146–152
Rugg-Gunn AJ (1972) Approximal carious lesions. A comparison of the radiological and clinical appearances. Br Dent J 133(11):481–484
Thylstrup A, Bille J, Qvist V (1986) Radiographic and observed tissue changes in approximal carious lesions at the time of operative treatment. Caries Res 20(1):75–84
Mejare I, Mjor IA (1990) Glass ionomer and resin-based fissure sealants: a clinical study. Scand J Dent Res 98(4):345–350
Forsten L (1990) Short- and long-term fluoride release from glass ionomers and other fluoride-containing filling materials in vitro. Scand J Dent Res 98(2):179–185
Hatibovic-Kofman S, Suljak JP, Koch G (1997) Remineralization of natural carious lesions with a glass ionomer cement. Swed Dent J 21(1–2):11–17
Trairatvorakul C, Techalertpaisarn P, Siwawut S, Ingprapankorn A (2009) Effect of glass ionomer cement and fluoride varnish on the remineralization of artificial proximal caries in situ. J Clin Pediatr Dent 34(2):131–134
Rosenblatt A, Stamford TC, Niederman R (2009) Silver diamine fluoride: a caries “silver-fluoride bullet”. J Dent Res 88(2):116–125. doi:10.1177/0022034508329406
Llodra JC, Rodriguez A, Ferrer B, Menardia V, Ramos T, Morato M (2005) Efficacy of silver diamine fluoride for caries reduction in primary teeth and first permanent molars of schoolchildren: 36-month clinical trial. J Dent Res 84(8):721–724
White DJ (1987) Use of synthetic polymer gels for artificial carious lesion preparation. Caries Res 21(3):228–242
Manarelli MM, Delbem AC, Binhardi TD, Pessan JP (2015) In situ remineralizing effect of fluoride varnishes containing sodium trimetaphosphate. Clin Oral Investig. doi:10.1007/s00784-015-1492-6
Shen P, Manton DJ, Cochrane NJ, Walker GD, Yuan Y, Reynolds C, Reynolds EC (2011) Effect of added calcium phosphate on enamel remineralization by fluoride in a randomized controlled in situ trial. J Dent 39(7):518–525. doi:10.1016/j.jdent.2011.05.002
Thepyou R, Chanmitkul W, Thanatvarakorn O, Hamba H, Chob-Isara W, Trairatvorakul C, Tagami J (2013) Casein phosphopeptide-amorphous calcium phosphate and glass ionomer show distinct effects in the remineralization of proximal artificial caries lesion in situ. Dent Mater J 32(4):648–653
Songsiripradubboon S, Hamba H, Trairatvorakul C, Tagami J (2014) Sodium fluoride mouthrinse used twice daily increased incipient caries lesion remineralization in an in situ model. J Dent 42(3):271–278. doi:10.1016/j.jdent.2013.12.012
Walker GD, Cai F, Shen P, Bailey DL, Yuan Y, Cochrane NJ, Reynolds C, Reynolds EC (2009) Consumption of milk with added casein phosphopeptide-amorphous calcium phosphate remineralizes enamel subsurface lesions in situ. Aust Dent J 54(3):245–249. doi:10.1111/j.1834-7819.2009.01127.x
Arends J, Dijkman T, Christoffersen J (1987) Average mineral loss in dental enamel during demineralization. Caries Res 21(3):249–254
Mei ML, Li QL, Chu CH, Lo EC, Samaranayake LP (2013) Antibacterial effects of silver diamine fluoride on multi-species cariogenic biofilm on caries. Ann Clin Microbiol Antimicrob 12:4. doi:10.1186/1476-0711-12-4
Peng JJ, Botelho MG, Matinlinna JP (2012) Silver compounds used in dentistry for caries management: a review. J Dent 40(7):531–541. doi:10.1016/j.jdent.2012.03.009
Chu CH, Mei L, Seneviratne CJ, Lo EC (2012) Effects of silver diamine fluoride on dentine carious lesions induced by Streptococcus mutans and Actinomyces naeslundii biofilms. Int J Paediatr Dent / Br Paedodontic Soc Int Assoc Dent Child 22(1):2–10. doi:10.1111/j.1365-263X.2011.01149.x
Ngo HC, Mount G, McIntyre J, Do L (2011) An in vitro model for the study of chemical exchange between glass ionomer restorations and partially demineralized dentin using a minimally invasive restorative technique. J Dent 39(Suppl 2):S20–S26. doi:10.1016/j.jdent.2011.10.016
Mickenautsch S, Mount G, Yengopal V (2011) Therapeutic effect of glass-ionomers: an overview of evidence. Aust Dent J 56(1):10–15. doi:10.1111/j.1834-7819.2010.01304.x, quiz 103
Tyas MJ (2003) Milestones in adhesion: glass-ionomer cements. J Adhes Dent 5(4):259–266
Ngo HC, Mount G, Mc Intyre J, Tuisuva J, Von Doussa RJ (2006) Chemical exchange between glass-ionomer restorations and residual carious dentine in permanent molars: an in vivo study. J Dent 34(8):608–613. doi:10.1016/j.jdent.2005.12.012
Cenci MS, Tenuta LM, Pereira-Cenci T, Del Bel Cury AA, ten Cate JM, Cury JA (2008) Effect of microleakage and fluoride on enamel-dentine demineralization around restorations. Caries Res 42(5):369–379. doi:10.1159/000151663
Gandolfi MG, Chersoni S, Acquaviva GL, Piana G, Prati C, Mongiorgi R (2006) Fluoride release and absorption at different pH from glass-ionomer cements. Dent Mater: Off Publ Acad Dent Mater 22(5):441–449. doi:10.1016/j.dental.2005.04.036
Markovic D, Petrovic BB, Peric TO (2008) Fluoride content and recharge ability of five glassionomer dental materials. BMC Oral Health 8:21. doi:10.1186/1472-6831-8-21
Zhi QH, Lo EC, Lin HC (2012) Randomized clinical trial on effectiveness of silver diamine fluoride and glass ionomer in arresting dentine caries in preschool children. J Dent 40(11):962–967. doi:10.1016/j.jdent.2012.08.002
Hayacibara MF, Rosa OP, Koo H, Torres SA, Costa B, Cury JA (2003) Effects of fluoride and aluminum from ionomeric materials on S. mutans biofilm. J Dent Res 82(4):267–271
Shashibhushan KK, Basappa N, Subba Reddy VV (2008) Comparison of antibacterial activity of three fluorides- and zinc-releasing commercial glass ionomer cements on strains of mutans streptococci: an in vitro study. J Ind Soc Pedodontics Prev Dent 26(Suppl 2):S56–S61
Zou W, Hunter N, Swain MV (2011) Application of polychromatic µCT for mineral density determination. J Dent Res 90(1):18–30. doi:10.1177/0022034510378429
Acknowledgments
The authors acknowledge Dr. Tewarit Somkotra for his assistance with the statistical analysis. We thank Dr. Kevin Tompkins for aiding in manuscript revision. This study was supported by a Chulalongkorn University Graduate School Thesis Grant.
Conflict of interest
The authors declare no potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nantanee, R., Santiwong, B., Trairatvorakul, C. et al. Silver diamine fluoride and glass ionomer differentially remineralize early caries lesions, in situ. Clin Oral Invest 20, 1151–1157 (2016). https://doi.org/10.1007/s00784-015-1603-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-015-1603-4