Abstract
Objectives
This study evaluated the effect of photobiomodulation (PBM) combined with 8% strontium acetate (SA8%) in the treatment of dentin hypersensitivity (DH) in non-carious lesions and analyzed the risk factors with the patient’s quality of life.
Methods
Eighty teeth with DH were randomly allocated into four treatment groups (n = 20): G1, PBM imitation + toothpaste with no active ingredient; G2, PBM imitation + toothpaste with SA8%; G3, PBM + toothpaste without the active ingredient; and G4, PBM + toothpaste with SA8%. Participants were provided with a questionnaire on the experience of dentin hypersensitivity (QEDH) to assess the impact of desensitizing treatment on health-related quality of life (HRQL). Friedman and Kruskal–Wallis tests were used for intra- and intergroup comparisons, and Wilcoxon and Mann–Whitney tests were used to analyze HRQL. All analyses used significance levels of 5%.
Results
Intergroup comparisons revealed a significant difference (p < 0.05); G4 had the best response in terms of HD reduction in G4 compared to the other groups on the 7th day of assessment (T3). Only G4 showed a statistically significant difference (p < 0.05) in the reduction of EDH for intragroup analysis.
Conclusion
The combination of therapies was more effective in reducing DH than the isolated use of these strategies.
Clinical relevance
The combination of therapies is effective in the treatment of DH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dentin hypersensitivity (DH) is a painful, recurrent condition. Its origin is associated with the exposure of dentinal tubules due to the loss of the cementum and dentin structure caused by multiple factors, such as processes of abrasion, erosion, or denudation of the root surface caused by gingival recession [1]. The pain arising from this condition is typically caused by thermal, tactile, and osmotic stimuli [2] characterized by short-term pain that cannot be explained by any other form of dental pathology [3].
Brännström, in 1966 [4], proposed the hydrodynamic theory to explain the mechanism of dentin hypersensitivity. Among all theories suggested at that time, his hypothesis was the most accepted; it stated that thermal and mechanical stimuli, such as spraying with air, exerted pressure changes that caused fluid movement within the dentinal tubules [5]. It is believed that this movement stimulates the A-delta and C fibers [6], triggering the pain perception.
Most treatments that aim to minimize the occurrence of DH are based on chemical or physical occlusion of dentinal tubules and nerve photobiomodulation (PBM) (low-level light therapy) [7]. Moraschini et al. [8] also suggest, in their meta-analysis, that these therapies provide the best results for clinical DH treatment.
Photobiomodulation provides an increase in adenosine triphosphate (ATP) synthesis as it modifies the membrane potential by activating the Na + /K + ATPase pumps. It produces a good analgesic effect [9], a characteristic that makes it an effective and contemporary treatment of DH, according to the systematic review of Machado et al. [10].
Strontium acetate works by depositing a thin layer of insoluble particles that are absorbed at the dentin connective tissue level and the rest of the calcified tissues, in depth. This entire process produces the obliteration of the dentinal tubules, which results in a desensitizing effect [11]. Grünberg et al. [12] point out that dentifrices with formulations containing strontium acetate appear to have a good impact on DH therapy and can be recommended for daily use.
DH is an uncomfortable condition that affects individuals’ quality of life experiencing this symptom [13]. Patel et al. [14] define the oral health-related quality of life (HRQL) as the patient’s perception of how the mouth condition affects the quality of life in daily activities, such as eating, drinking, and social interaction, in addition to a more subtle impact on emotions and identity [15].
The dentin hypersensitivity experience questionnaire (QEDH) has been established to detect these functional limitations, confrontation behaviors, and emotional and social impacts caused by DH. The use of this questionnaire in clinical trials [15,16,17] demonstrates the reliability of the results obtained through its use.
This work aims to evaluate the effect of photobiomodulation combined with strontium acetate in the treatment of DH in non-carious lesions and analyze the risk factors to patients’ quality of life. The null hypotheses tested in this study are as follows: H01, there is no difference in the reduction of HD with PBM associated with the daily use of dentifrice with strontium acetate and other types of treatments, after 1 month of evaluation; H02, PBM combined with daily use of strontium acetate dentifrice does not influence the quality of life of volunteers.
Materials and method
Ethical aspects.
This double-blind randomized clinical trial followed the recommendations of the Consolidated Standards of Reporting Trials (CONSORT) [18]. The study was approved by the ethics and research committee of the Institute of Health Sciences, Federal University of Pará, under number 290.779, and registered in the Clinical Trials Registry (http://www.ClinicalTrials.gov) under the protocol NCT04642001. The research volunteers were properly informed and informed about the risks, methods, and objectives of this study, respecting the norms of the Declaration of Helsinki [19].
Inclusion and exclusion criteria
Participants included in this study were examined and selected based on the following inclusion criteria: individuals aged 18 to 50 years, of both genders, in good general health reporting dental sensitivity with a response ≥ 4 on the 10-cm-long visual analog scale (VAS), and with at least two hypersensitive teeth with up to 1 mm depth (measured with a millimeter probe). Teeth of both the upper and lower arch were included, of all dental groups, including molars, premolars, canines, and incisors, with an exposed root surface caused by abrasion, erosion, or gingival recession. The following were excluded: volunteers using analgesic medication or having teeth with evidence of carious lesions, pulpitis, defective restorations, and moderate or severe periodontal disease, as well as those who had undergone any whitening therapy or professional or homemade desensitizer in the last 6 months and patients with fixed orthodontic appliances. Drug users and pregnant women were also excluded.
Sample size calculation
The sample size calculation was based on a previous pilot trial of this study using the G Power 3.1 software (Heinrich-Heine-Universität, Düsseldorf, Germany). The calculation was performed considering a test power of 80% and a significance level of 5% and 10% loss in sample size, with the estimate of the relevant main difference of 2.26 between the means, resulting in 80 teeth.
Randomization and allocation concealment
A random list was generated in the BioEstat 5.0 software (Civil Society, Mamirauá, Pará, Brazil) by an individual not involved in the intervention or evaluation. Participants were divided into four blocks in the randomization process, and the treatment sequence (placebo or desensitizing protocol) was randomly defined for each block using computer-generated tables. The sequence was inserted in sealed envelopes, numbered 1 to 80, which were opened by the operator only at the intervention time. Patients were numbered according to the registration sequence.
Blinding
In this double-blind clinical trial, the volunteers were unaware of the treatment protocol employed for each group. The evaluators who assessed the degree of sensitivity were also unaware of which group each volunteer belonged to. A single operator performed the entire clinical part of the study.
Study intervention
All participants received prophylaxis 7 days before the start of the study, with fluoride-free prophylactic paste (NuPro Supremo, Dentsply Int., New York, PA, USA) and rubber cup (Microdont, São Paulo, Brazil), to remove stains and external plaque and an oral hygiene kit containing a toothbrush with soft bristles (Soft ORAL-B, São Paulo, Brazil), dental floss (ORAL-B, São Paulo, Brazil), and a placebo or desensitizing toothpaste, with similar coloring and consistencies, for daily use three times a day for 7 days, supplied in identical bottles marked only with codes. A total of three treatment sessions were performed, with a 72-h interval between them. Table 1 describes the materials used in this study.
According to the corresponding desensitizing protocol in this study, 80 teeth belonging to 24 patients (11 men and 13 women) were randomly distributed into 4 groups (n = 20).
To avoid bias in the results, all study volunteers received placebo toothpaste (My First/Colgate®) in the last treatment session for daily use, three times a day, until their return to the clinic for the reassessment of sensitivity after 30 days.
Desensitizing protocol
Groups G2, G3, and G4 received desensitizing treatment according to the protocol of this study (Table 2).
Dental hypersensitivity experience questionnaire (QEDH)
The participants carried out a self-reported assessment. Before the start of treatment and 1 month after the end of treatment, the participants received a questionnaire based on the model described by Boiko et al. [20], with Portuguese adaptation by Douglas-De-Oliveira et al. [17], to assess the impact of desensitizing treatment on the health-related quality of life (HRQL). The short version of the questionnaire consisted of 15 questions (QEDH15) that could assess the impact of DH on HRQL on 5 subscales: functional restrictions, adaptation, social impact, emotional impact, and impact on personal identity. The questions were answered according to a 7-point Likert scale, with a rating from 15 to 105 in the final score. Higher scores indicated a worse HRQL.
Hard tissue depth
The depth of the injuries was measured with the aid of a millimeter-graduated periodontal probe of the Williams type (Trinity, São Paulo, Brazil), which was positioned perpendicularly along the tooth’s long axis in the center of the injury. In a study conducted by Freitas et al. [21], it was observed that lesions greater than 1 mm in depth present a better response to treatment when performing restorative protocols. Because of this, the depth of the lesion was measured in order to standardize the study and obtain a more satisfactory response to the treatments used.
Assessment of pain sensitivity
A triple syringe connected to an air compressor at room temperature applied for 1 s at 1 cm from the tooth surface was used to assess the degree of sensitivity [22]. Neighboring teeth were isolated during the test using the operator’s fingers and cotton rolls. For the tactile test, an exploratory probe no. 5 (Golgran, São Paulo, Brazil) was used with a cross-shaped probe contact on the dental surfaces: from apical to incisal and another from mesial to distal [15]. The degree of sensitivity of each dental element was quantified by the patient using the visual analog pain scale (VAS) immediately after the stimuli. The scale consists of a dashed horizontal ruler, numbered from 0 to 10, representing the following pain scores: 0, no pain; 1 to 3, mild pain; 4 to 5, moderate pain; 6 to 8 severe pain; and 9 to 10, unbearable pain [23]. The data recorded in the first session (before therapy was applied) determined the baseline representing the initial pain level of each sensitive tooth. Evaluations were carried out in four stages: baseline, after the first treatment application (T1), after 72 h (T2), 7 days (T3), and 30 days (T4) after the end of the treatment.
Data analysis
The values corresponding to the DH reported by the participants were tabulated in an Excel spreadsheet (Microsoft Windows, 2010) and analyzed using the Bioestat 5.4 program. Data were initially submitted to the Kolmogorov–Smirnov normality test. This analysis showed a non-normal distribution of data, so the Friedman and Kruskal–Wallis nonparametric tests were used for the comparative analysis, the Friedman nonparametric test was performed for the intragroup analysis, to compare the G1 as well as G2 groups, G3 and G4, in different periods of time, so multiple comparisons were made; the intergroup analysis was performed with the Kruskal–Wallis test comparing different groups in pairs in the same time interval. Wilcoxon and Mann–Whitney tests were used to analyze the QEDH before and after 1 month of desensitizing treatments taken. All analyses considered significance levels of 5%.
Results
Participants
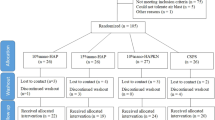
A total of 46 participants were evaluated, 24 of which were randomized, treated, and followed up according to Fig. 1. Eighty teeth were analyzed.
Demographic characteristics
The demographic characteristics of the 24 participants completing the study are described in Table 3.
There was a higher proportion of female participants (N = 13; 54.2%) in the final sample when compared to male participants (N = 11; 45.8%). The participants’ average age was 22 years (SD = 20.6; range = 18–26). When comparing different treatment groups, no significant difference was found in any of these characteristics (p > 0.05).
Depth of injuries
The depth of the lesions was measured on eighty teeth. Table 4 shows the mean and standard deviation of depth in the different groups, with group G3 having a higher mean depth compared to the other groups.
Sensitivity assessment
Tactile stimulus
As shown in the box graph (Fig. 2), groups G1 and G2 showed similar behavior, with no reduction in the level of pain in any evaluation period. G3 had decreased pain level only in the third treatment session (T3) and increased pain sensitivity after 1 month. G4 (combination of PBM and dentifrice containing strontium acetate) showed decreased sensitivity to pain at T1 and T3, which represents the first and third treatment sessions, respectively, with pain stabilization after 1 month.
Boxplots comparing HD, by the visual analog scale (VAS), of the groups in the different evaluation periods with the tactile stimulus. *Different capital letters represent statistically significant intergroup difference (p ≤ 0.05); **different lowercase letters represent statistically significant intragroup difference (p ≤ 0.05); †p values calculated according to the Kruskal–Wallis test and Friedman test. Kruskal–Wallis test and Friedman test, minimum, first quartile, median, third quartile, and maximum values in centimeters
Comparisons between groups at the same time interval revealed a reduction in HD (p < 0.05) in group G4 (combination of PBM and dentifrice containing strontium acetate) at T3 and after 1 month compared to the other groups (G1, G2, and G3). It was also observed that G3 had decreased pain when compared to G1 at T3 and after 1 month.
Evaporative stimulus
In the intragroup analysis, the box graph (Fig. 3) demonstrates that groups G1 and G2 did not show a decrease in the level of pain in any evaluation period. G3 showed a reduction in pain level only in the third treatment session (T3) and an increase in pain sensitivity after 1 month. G4 (combination of PBM and dentifrice containing strontium acetate) showed decreased sensitivity to pain at T1 and T3, which represents the first and third treatment sessions, respectively, with pain stabilization after 1 month. In the intergroup analysis, G4 showed a reduction in HD (p < 0.05) compared to the other groups in periods T3 and after 1 month, and G3 had decreased pain compared to G1 in periods T3 and after 1 month.
Boxplots comparing HD, by the visual analog scale (VAS), of the groups in the different evaluation periods with the evaporative stimulus. *Different capital letters represent statistically significant intergroup difference (p ≤ 0.05); **different lowercase letters represent statistically significant intragroup difference (p ≤ 0.05); †p values calculated according to the Kruskal–Wallis test and Friedman test. Kruskal–Wallis test and Friedman test, minimum, first quartile, median, third quartile, and maximum values in centimeters
Health-related quality of life (HRQL): dental hypersensitivity experience questionnaire (QEDH)
Table 5 represents the mean and standard deviation of the questionnaire of dentin hypersensitivity experiences (QEDH) used to assess the volunteers’ HRQL before and after the desensitizing treatments. Only the G4 group (combination of PBM and strontium acetate containing toothpaste) showed a statistically significant difference (p < 0.05) in QEDH reduction in the intragroup analysis. In the intergroup analysis, G4 differed statistically from the other groups 1 month after the end of the desensitizing treatment.
Discussion
Dental hypersensitivity is a chronic problem [23] characterized by episodes of discomfort or acute pain, stimulated by activities or substances found in daily life, such as toothbrushing, consumption of cold, sugary, or acidic liquids, and cold air [24]. Patients describe this pain as problematic enough to affect diet and work [25], directly interfering with their quality of life [26].
The Sensodyne® Rápido Alívio desensitizing dentifrice used in this study has 8% strontium acetate in its formulation. This agent’s action mechanism occurs through organic precipitation and odontoblastic denaturation, forming a sealing film that prevents the circulation of liquids inside the dentinal tubules, reducing permeability [27] and promoting an obliterating action [28]. Although this agent can occlude dentinal canaliculi from the deposition of strontium salts on the dentin surface [29], in this study, G2 (the group treated with strontium acetate) did not cause a reduction in the pain level, with similar results to G1 (placebo group), which corroborates with the results obtained by West et al. [30]. Therefore, its ineffectiveness can be explained by the limited use of desensitizing toothpaste (about 8 days), suggesting that it is not sufficient to form the sealing film. Increasing the number of applications and time of use may be necessary to obtain DH relief [31].
The G3 (group treated with photobiomodulation therapy) during the third treatment session showed a significant reduction in the level of pain, as observed in the study by Lopes et al. [32]. A percentage increase in pain sensitivity was observed after 1 month, representing a non-lasting effect for this therapy. Photobiomodulation induces an analgesic effect by changing the depolarization of the afferent C fiber and preventing nerve transmission of the pain stimulus to the central nervous system, which causes pain relief [9]. The LASER analgesic mechanism prevents pain transmission [33]; however, the analgesic effect tends to decrease over time if the treatment does not continue, suggesting a possible explanation for the results of this clinical study.
PBM therapy combined with strontium acetate containing desensitizing toothpaste (G4) obtained the best results for both intragroup and intergroup comparisons, showing to be statistically significant (p < 0.05) in relation to the other treatments, rejecting H01. In scientific literature, several clinical trials have studied the efficacy of strontium acetate in controlling DH [33,34,35,36,37]; however, there are no findings that address its combination with photobiomodulation. Strontium is naturally present in enamel and dentin. Since it acts in a similar way to calcium, replacing it in the formation of hydroxyapatite [calcium strontium apatite, Ca6Sr4(PO4)6(OH)2], strontium also has a remineralizing activity, maintaining its effectiveness in strontium deposition [38] and dentin tubule obliteration [39]. PBM also acts in biomodulatory therapy in dental pulp, resulting in the obliteration of dentinal tubules by increasing the cellular metabolic activity of odontoblasts, redoubling the production of tertiary dentin [40]; this may explain the stabilization of pain, in this group, 1 month after treatment completion. PBM increases the metabolic activity of cells and the production of tertiary dentin [41]. PBM may accelerate the formation of strontium apatite, increasing its effect on dentin. To the best of the authors’ knowledge, no previous clinical trial has evaluated the potential of these protocols and their combinations in reducing DH.
The QEDH applied before and after the treatment disclosed an improvement in the quality of life of the volunteers allocated in the group with the combination of PBM and strontium acetate containing toothpaste, showing a statistically significant difference (p < 0.05) in relation to the other treatments, and a decrease in the Likert scale after 1 month for intragroup comparisons, rejecting H02. QEDH offers a tool to quantify the effects of DH on daily life, with the potential to measure the effectiveness of interventions and treatments associated with the disease [16]. The results of the QEDH confirmed the effect of desensitizing therapies on the quality of life of the participants in this clinical trial. Many current studies have performed this analysis [15, 25, 42,43,44,45]. Baker et al. [46] point out that the QEDH is a valid and responsive measure capable of discriminating treatments of different effectiveness. Assessing the effectiveness of these desensitizing therapies considering the quality of life is essential. It is necessary to perform future research on sensitivity, always using the parameter of quality of life.
Monitoring patients over the time is necessary to treat the pain sensitivity of non-carious lesions, which is a limitation of this study. Evaluation of the long-term effects of the treatment is suggested.
Conclusion
The combination of PBM and SA 8% therapies effectively reduced dentin sensitivity compared to the other combinations for a limited period of 7 days and had a positive impact on the EDH.
References
Suchetha A, Keshava Prasad BS, Apoorva SM, Lakshmi P (2013) Dentinal hypersensitivity- a review. Indian J Dent Sci 5(2):112–116
Patil SA, Naik BD, Suma R (2015) Evaluation of three different agents for in-office treatment of dentinal hypersensitivity: a controlled clinical study. Indian J Dent Res 26(1):38–42
Addy M (1992) Aspectos clínicos da hipersensibilidade à dentina. Proc Finn Dent Soc 88(1):407–412
Brännström M (1966) Sensitivity of dentine. Oral Surg Oral Med Oral Pathol 21(4):517–526
Shibukawa Y, Sato M, Kimura M, Sobhan U, Shimada M, Nishiyama A, Katakura A, Ichinohe T, Tazaki M, Shibukawa Y (2015) Odontoblasts as sensory receptors: transient receptor potential channels, pannexin-1, and ionotropic ATP receptors mediate intercellular odontoblast-neuron signal transduction. Pflugers Arch Eur J Physio 467:843–863. https://doi.org/10.1007/s00424-014-1551-x
Won J, Vang H, Kim JH, Lee PR, Kang Y, Oh SB (2018) TRPM7 mediates mechanosensitivity in adult rat odontoblasts. J Dent Res 97:1039–1046. https://doi.org/10.1177/0022034518759947
Kopycka-Kedzierawski DT, Meyerowitz C, Litaker MS, Heft MW, Tasgaonkar N, Day MR, Porter-Williams A, Gordan VV, Yardic RL, Lawhorn TM, Gilbert GH, National Dental PBRN Collaborative Group (2017) Management of dentin hypersensitivity by practitioners in the National Dental Practice-Based Research Network. J Am Dent Assoc 148:728–736
Moraschini V, Costa LS, Santos GO (2018) Effectiveness for dentin hypersensitivity treatment of non-carious cervical lesions: a meta-analysis. Clin Oral Invest 22:617–631. https://doi.org/10.1007/s00784-017-2330-
Lopes AO, Paula Eduardo C, Aranha ACC (2017) Evaluation of different treatment protocols for dentin hypersensitivity: an 18-month randomized clinical trial. Lasers Med Sci 32(5):1023–1030
Machado AC, Viana ÍEL, Farias-Neto AM, Braga MM, Paula Eduardo C, Freitas PM, Aranha AC (2018) CIs photobiomodulation (PBM) effective for the treatment of dentin hypersensitivity? A systematic review. Lasers Med Sci 33:745–753. https://doi.org/10.1007/s10103-017-2403-7
Parkinson CR, Willson RJ (2010) A comparative in vitro study investigating the occlusion and mineralization properties of commercial toothpastes in a four-day dentin disc model. J Clin Dent 22(3):74–81
Grünberg C, Bauer F, Crispin A, Jakob M, Hickel R, Draenert ME (2017) Effectiveness of dentifrices with new formulations for the treatment of dentin hypersensitivity - a meta-analysis. Am J Dent 30(4):221–226
Mehta P, Vimala N, Mandke L (2013) An insight into dentin desensitizing agents–in vivo study. Indian J Dent Res 24(5):571–574. https://doi.org/10.4103/0970-9290.123369
Patel RR, Richards PS, Inglehart MR (2008) Periodontal health, quality of life, and smiling patterns–an exploration. J Periodontol 79(2):224–231. https://doi.org/10.1902/jop.2008.070344
Baker SR, Gibson BJ, Sufi F, Barlow A, Robinson PG (2014) The dentine hypersensitivity experience questionnaire: a longitudinal validation study. J Clin Periodontol 41(1):52–59. https://doi.org/10.1111/jcpe.12181
Ortiz MIG, Melo Alencar C, Paula BLF, Alves EB, Araújo JLN, Silva CM (2019) Effect of the casein phosphopeptide-amorphous calcium phosphate fluoride (CPP-ACPF) and photobiomodulation (PBM) on dental hypersensitivity: a randomized controlled clinical trial. PloS one 14(12):e0225501
Douglas-De-Oliveira DW, Lages FS, Paiva SM, Cromley JG, Robinson PG, Cota LOM (2018) Cross-cultural adaptation of the Brazilian version of the dentine hypersensitivity experience questionnaire (DHEQ-15). Braz Oral Res 32:e37. https://doi.org/10.1590/1807-3107bor-2018.vol32.0037
Pandis N, Chung B, Scherer RW, Elbourne D, Altman DG (2019) CONSORT 2010 statement: extension checklist for reporting within person randomised trials. Bri J Dermatol 180(3):534–552
World Medical Association (2013) World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Med Assoc 310(20):2191
Boiko OV, Baker SR, Gibson BJ, Locker D, Sufi F, Barlow APS, Robinson PG (2010) Construction and validation of the quality of life measure for dentine hypersensitivity (DHEQ). J Clin Periodontol 37(11):973–980. https://doi.org/10.1111/j.1600-051X.2010.01618.x
Freitas SDS, Sousa LLA, Moita Neto JM, Mendes RF, Prado Junior RR (2015) Dentin hypersensitivity treatment of non-carious cervical lesions–a single-blind, split-mouth study. Braz oral res 29(1):1–6
Rees JS, Jin LJ, Lam S, Kudanowska I, Vowles R (2003) The prevalence of dentine hypersensitivity in a hospital clinic population in Hong Kong. J Dent 31(7):453–461
Chiarotto A, Maxwell LJ, Ostelo RW, Boers M, Tugwell P, Terwee CB (2019) Measurement properties of visual analogue scale, numeric rating scale, and pain severity subscale of the brief pain inventory in patients with low back pain: a systematic review. J of Pain 20(3):245–263
Pourshahidi S, Ebrahimi H, Mansourian A, Mousavi Y, Kharazifard M (2019) Comparison of Er, Cr:YSGG and diode laser effects on dentin hypersensitivity: a split-mouth randomized clinical trial. Clin Oral Investig 23(11):4051–4058. https://doi.org/10.1007/s00784-019-02841-z
Idon PI, Sotunde OA, Ogundare TO (2019) Beyond the relief of pain: dentin hypersensitivity and oral health-related quality of life. Front Dent 16(5):325–334. https://doi.org/10.18502/fid.v16i5.2272
Bartold PM (2006) Dentinal hypersensitivity: a review. Aust Dent J 51(3):212–218
Porto IC, Andrade AK, Montes MA (2009) Diagnosis and treatment of dentinal hypersensitivity. J Oral Sci 51(3):323–332
Markowitz K (2009) The original desensitizers: strontium and potassium salts. J Clin Dent 20(5):145–51
Mason S, Hughes N, Layer T (2009) Considerations for the development of over-the-counter dentifrices for the treatment and relief of dentin sensitivity. J clin dente 20(5):167–173
West NX, Addy M, Jackson RJ, Ridge D (1997) Dentine hypersensitivity and the placebo response. A comparison of the effect of strontium acetate, potassium nitrate and fluoride toothpastes. J Clin Periodontol 24(4):209–15
Yu X, Liang B, Jin M, Fu X, Hannig B (2010) Comparative in vivo study on the desensitizing efficacy of dentin desensitizers and one-bottle self-etching adhesives. Operat Dent 35(3):279–286
Lopes AO, Paula Eduardo C, Aranha ACC (2015) Clinical evaluation of low-power laser and a desensitizing agent on dentin hypersensitivity. Lasers Med Sci 30:823–829. https://doi.org/10.1007/s10103-013-1441-z
Karu T (1998) The science of low-power laser therapy, 1st edn. Gordon and Breach Science Publishers, New Delhi
Zang P, Shaw D (2016) A randomized clinical study to evaluate the efficacy of an 8% (w/w) strontium acetate dentifrice in providing relief from dentinal hypersensitivity. J Clin Dent 27(4):91–96
Schiff T, Mateo LR, Delgado E, Cummins D, Zhang YP, DeVizio W (2011) Clinical efficacy in reducing dentin hypersensitivity of a dentifrice containing 8.0% arginine, calcium carbonate, and 1450 ppm fluoride compared to a dentifrice containing 8% strontium acetate and 1040 ppm fluoride under consumer usage conditions before and after switch-over. J Clin Dent 22(4):128–138
Seong J, Davies M, Macdonald EL, Claydon NC, West NX (2018) Randomized clinical trial to determine if changes in dentin tubule occlusion visualized by SEM of replica impressions correlate with pain scores. Am J Dent 31(4):189–194
West N, Newcombe RG, Hughes N, Mason SC, Maggio B, Sufi F, Claydon N (2013) A 3-day randomised clinical study investigating the efficacy of two toothpastes, designed to occlude dentine tubules, for the treatment of dentine hypersensitivity. J Dent 41(2):187–194. https://doi.org/10.1016/j.jdent.2012.11.007
Docimo R, Perugia C, Bartolino M, Maturo P, Montesani L, Zhang YP, Devizio W, Mateo LR, Dibart S (2011) Comparative evaluation of the efficacy of three commercially available toothpastes on dentin hypersensitivity reduction: an eight-week clinical study. J Clin Dent 22(4):121–127
Parkison CR, Wilson RJ (2011) A comparative in vitro study investigating the occlusion and mineralization properties of commercial dentifrices in a four-day dentin disc model, in vitro. J Clin Dent 22:68–73
Saeki K, Marshall GW, Gansky SA, Parkison CR, Marshall SJ (2016) Strontium effects on root dentin tubule occlusion and nanomechanical properties. Acad Dent Mater 32(2):240–251
Hashim NT, Gasmalla BG, Sabahelkheir AH, Awooda AM (2014) Effect of the clinical application of the diode laser (810 nm) in the treatment of dentine hypersensitivity. BMC Res Notes 7(1):31. https://doi.org/10.1186/1756-0500-7-31
Orhan K, Aksoy U, Can-Karabulut DC, Kalender A (2011) Low level laser therapy of dentin hypersensitivity: a short-term clinical trial. Laser Med Sci 26:591–598
Exarchou C, Betsani I, Sakellari D, Chatzopoulou D, Gillam D (2019) A survey of dentists in the management of dentine hypersensitivity: a questionnaire-based study. Eur J Dent 13(3):383–390. https://doi.org/10.1055/s-0039-1694306
Mason S, Burnett GR, Patel N, Patil A, Maclure R (2019) Impacto do creme dental na qualidade de vida relacionada à saúde bucal em pessoas com hipersensibilidade dentinária. BMC Oral Health 19:226. https://doi.org/10.1186/s12903-019-0919-x
Rocha MOC, Cruz AACF, Santos DO, Oliveira DWD, Flexa OD, Gonçalves PF (2020) Sensitivity and specificity of assessment scales of dentin hypersensitivity – an accuracy study. Braz oral res 34(1807):3107
Baker SR, Gibson BJ, Sufi F, Barlow AP, Robinson PG (2015) The dentin hypersensitivity Experience Questionnaire (DHEQ): um estudo de validação longitudinal. In Dentin Hypersensitivity. Academic Press 18:141–154. https://doi.org/10.1016/B978-0-12-801631-2.00009-9
Acknowledgements
We would like to thank the National Council for Scientific and Technological Development (CNPq/UFPA) for the subsidy provided that contributed to the purchase of the materials used in this study.
Funding
The work was supported by the National Council for Scientific and Technological Development (CNPq/UFPA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and the Helsinki Declaration of 1964 and its subsequent amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Barros, A.P.O., de Melo Alencar, C., de Melo Pingarilho Carneiro, A. et al. Combination of two desensitizing protocols to control dentin hypersensitivity in non-carious lesions: a randomized, double-blind clinical trial. Clin Oral Invest 26, 1299–1307 (2022). https://doi.org/10.1007/s00784-021-04104-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-021-04104-2