Abstract
Objective
This review aimed at evaluating the effects of chronic periodontitis (CP) treatment with local statins as adjuncts to scaling and root planing (SRP), compared with SRP alone or with placebo.
Methods
Electronic and hand searches were conducted in three databases to select randomized controlled trials (RCTs) comparing SRP + statins versus SRP alone. Random effects models were conducted to determine the clinical attachment level (CAL) gain as the primary outcome variable, and probing pocket depth (PPD) reduction, modified sulcus bleeding index (mSBI), and intrabony defect depth (IBD) as the secondary outcomes.
Results
Of the 526 papers identified, 15 articles met the criteria for inclusion in this systematic review, and 13 in the meta-analysis. The meta-analysis showed a statistically significant CAL gain (mean differences [MD] = 1.84 mm, 95% confidence interval [CI] = 1.45 to 2.23; p = 0.000), PPD reduction (MD = 1.69 mm, 95% CI = 1.37 to 2.04; p = 0.000), mSBI change (MD = 0.70, 95% CI = 0.57 to 0.84; p = 0.000), and IBD (MD = 1.48, 95% CI = 1.30 to 1.67; p = 0.000) attributed to SRP + statin treatment (6 months).
Conclusion
Within the limitations of this study, the collective evidence emerging from this systematic review and meta-analysis may support the use of locally applied statins as adjuncts to SRP in CP treatment, based on being an easy, low-cost alternative, with lesser adverse effects on bacterial resistance. These results should be interpreted with caution.
Clinical relevance
Clinicians might consider the use of SRP + statins as an adjunct over other alternative approaches, based on the results of the present review. The informed decision should be taken, considering the patient’s values and preferences, and the intervention to be implemented by the clinician.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Scaling and root planing (SRP) remains an essential part of successful periodontal disease therapy [1]. Evidence from numerous randomized clinical trials (RCTs) reveals a consistency in the clinical responses to chronic periodontitis (CP) treatment by SRP, which is considered the “gold standard.”
The clinical changes after SRP include periodontal pockets depth (PPD) reductions and clinical attachment level (CAL) gains [2]. However, SRP may not sufficiently reduce the periodontal pocket (PPD < 4 mm) in some cases, for example, deeper pockets, tooth type, and tooth location [3]. Adjuvant procedures have been proposed to enhance non-surgical periodontal treatment efficacy, including antibiotics [4], antiseptic agents [5], and photodynamic therapy [6, 7].
Statins are inhibitors of the 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoA reductase), which is an important enzyme related to the synthesis of cholesterol [8]. Statins are the most widely used hypolipidemic agents (as inhibitors of cholesterol biosynthesis), due to their effectiveness in reducing the concentration of blood cholesterol and their excellent tolerability, safety, and low cost [9].
These drugs prevent the synthesis of mevalonate but also of isoprenoid precursors (geranyl and farnesyl pyrophosphates), which are substrates for prenylation (addition of hydrophobic molecule to proteins) of small GTP-binding proteins (Rho, Rac, Rab) [10]. This decrease in prenylation inhibits osteoclast activity because these small proteins cannot anchor to the membrane of osteoclasts by lack of a lipid chain [11]. Mevalonate deprivation suppresses the expression of the receptor for activation of nuclear factor kappa B (NFκB) ligand (RANKL) and activation of NFκB that inhibits osteoclast differentiation and induces osteoclast apoptosis [12],
Both in vitro and in vivo studies demonstrate that statins show convincing anabolic and anti-resorptive bone effects [13, 14], and share these effects on cholesterol pathway downstream to mevalonate with nitrogen-containing bisphosphonates (pamidronate, risedronate, ibandronate, and zoledronate), which inhibit specifically farnesyl pyrophosphate synthase and act as bone anti-resorptive agents [11].
In this sense, statins were considered an almost ideal candidate family of anti-osteoporotic drugs, due to their potential dual anabolic and anti-resorptive effects on bone, and to the extremely large and reassuring experience with these drugs in cardiology (excellent risks to benefits ratio and very low incidence of side effects) [15].
Also, it is reported that use of statins gives rise also to the so-called pleiotropic effects, on the expression of bone morphogenetic protein-2 (BMP-2) gen in bone cells [13], indicating an anabolic effect on bone [8]. Some studies have shown that statins are able to modulate inflammation and alveolar bone loss [16]. Various animal studies reported favorable effects on bone regeneration when statins were applied locally or orally [17,18,19] and a positive effect around dental implants, increasing osteogenesis [20, 21].
Their immunoregulatory effects in human epithelial cells is well-known in vitro studies [22], as well as their antibacterial properties inhibiting oral and perioral microorganisms, in vitro [23]. The pleiotropic effects were observed clinically, where CP patients on statin medication expressed lower IL-1 levels [24]; and a downregulation of IL-1β, myeloperoxidase levels, and higher anti-inflammatory IL-10 levels in gingival crevicular fluid compared to patients without statin treatment [25].
A series of studies in humans have shown that local delivery of statins may result in additional benefits to non-surgical periodontal treatment, when compared to SRP alone [26,27,28,29]. Thereafter, numerous studies were published, investigating the effect of locally applied statins in periodontology. Hence, the aim of the current systematic review is to determine if the adjunctive local use of statins could provide additional benefits for periodontal disease treatment.
Material and methods
The present systematic review was conducted in accordance with the Transparent Reporting of Systematic Reviews and Meta-Analysis—PRISMA Statement [30] and AMSTAR [31] guidelines.
Focus question
This systematic review and meta-analysis aimed to answer the following focus question developed in accordance with the recognized Population, Intervention, Comparison, Outcome (PICO) format: “Can the local application of statins improve clinical periodontal parameters, in terms of CAL gain, PPD reduction, modified sulcus bleeding index (mSBI), and IBD reduction, when used as an adjuvant to SRP versus SRP alone (or placebo) in the treatment of patients with CP?”
Population is classified with chronic periodontitis when PPD ≥ 5 mm, CAL ≥ 3 mm, and angular bone loss ≥ 3 mm [32]. Its classification also depends on additional measurements of bleeding on probing (BOP) [33].
Inclusion and exclusion criteria for studies to be considered for inclusion in this review
The studies had to meet the following criteria to be eligible:
-
i.
Randomized controlled trials (RCTs) and split-mouth studies in human individuals (≥ 18 years old).
-
ii.
Studies that assessed the local use of statins as adjuvants to SRP non-surgical treatment (SRP + statins) in CP patients.
-
iii.
Control patients that received the same SRP non-surgical treatment either alone or plus placebo (SRP + placebo).
-
iv.
Studies that quantitatively reported clinical periodontal parameters, such as CAL, PPD, mSBI, and IBD, with at least 6 months of follow-up after randomization.
-
v.
Studies that included individuals with systemic diseases or risk factors (e.g., diabetes or smoking).
Exclusion criteria
The excluded studies comprised prospective, controlled clinical trials without randomization; case-control, cross-sectional case series and case report studies; literature or narrative reviews; animal and in vitro studies; studies that included aggressive periodontitis patients, or in which an adjunct was administered more than 1 week after SRP, or was reapplied to progressively worsening tooth sites.
Search strategy
The electronic search was performed by two authors (J.M.M. and D.S.P.) for articles in English, up to March 2017. The search strategy combined MeSH and EMTREE terms. Other terms not indexed as MeSH were also used. A hand search of relevant primary sources was performed. Finally, the references of the included studies were explored to capture any potential additional records [34].
In addition, the gray literature in the System for Information on Grey Literature in Europe (http://www.opengrey.eu), The New York Academy of Medicine Grey Literature Report (http://www.greylit.org), and Google Scholar databases were screened electronically, as recommended by the high standards for systematic reviews (AMSTAR guideline) [31]. Furthermore, a hand search of relevant primary sources related to the topic was made in Journal of Dental Research, Journal of Clinical Periodontology, Journal of Periodontology, Journal of Periodontal Research, Clinical Oral Investigations, and Archives of Oral Biology. Finally, the references of included studies were explored to capture any potential additional records, as suggested by Greenhalgh and Peacock [34]. The search details tailored for each database are depicted in Appendix-S1.
Data collection, extraction, and management
Screening and selection of papers
Titles and abstracts were screened by two reviewers independently (D.S.P. and D.P.O.). Full-text reports were obtained and reviewed independently for studies that seemed to meet the inclusion criteria (Appendix-S2); kappa scores (Cohen’s ĸ coefficient) were employed during full-text assessment to ensure eligibility and level of agreement between the reviewers. Disagreements were resolved by discussion and consulting a third reviewer (J.M.C.).
Search outcomes and evaluation
Two independent researchers (J.M.M. and D.C.P.) extracted the variables of interest in duplicate, using predefined spreadsheets; disagreements were resolved by discussion with a third reviewer (J.M.C.). In the event of missing data, a request was sent to the authors.
Chronic periodontitis is so classified when PPD ≥ 5 mm, clinical attachment CAL ≥ 3 mm, and angular bone loss ≥ 3 mm [32]. Its classification also depends on additional measurements of bleeding on probing (BOP) [33].
Primary outcome
CAL is used as the main outcome, because PPD fails to capture the effect of non-surgical treatment, and has a predictability of about 50% in probing depths of 7 mm [35,36,37,38].
CAL gain
CAL gain is considered as the mean changes in millimeters between baseline and follow-up. CAL is defined as the extent of the periodontal support that has been destroyed around a tooth. It is estimated by calculating the arithmetic difference between the PPD and the position of the gingival margin (distance from the gingival margin to the cement-enamel junction (CEJ)/recession). If CEJ is not detected, it can be estimated according to the apical margin of restoration.
Secondary outcomes
PPD reduction
PPD reduction is considered as the mean changes in millimeters between baseline and follow-up. PPD is defined as the distance from the free gingival margin to the bottom of the periodontal pocket, measured with a calibrated periodontal probe.
mSBI changes
mSBI changes is considered as the mean changes between baseline and follow-up. The modified sulcus bleeding index (mSBI) quantifies the bleeding on probing (BOP). Individual patients can be monitored for their response to initial therapy and during maintenance by using mSBI with three recommended bleeding scores [39], as well as mean BOP values (with standard deviation) [40].
IBD reduction
IBD reduction is considered as the mean changes in millimeters between baseline and follow-up of the vertical distance from the crest of the alveolar bone to the base of the defect, detected by standardized periapical radiographs. In addition, the Glossary of Periodontal Terms of the American Academy of Periodontology considers an osseous defect as the reduction in or deficiency of the bony architecture around the teeth and implants, caused by disease or trauma; it may be intrabony or interradicular in nature.
Risk of bias in individual studies
Quality assessment was performed by two independent reviewers (J.M.M. and D.S.P.), according to the Cochrane Collaboration tool for assessing risk of bias in randomized trials [41], and using the following assessment criteria: low risk of bias (all domains were met); high risk of bias (when ≥ 1 domain was not met); and unclear (when ≥ 1 domain was partially met). The interexaminer agreement was ascertained by a kappa test; disagreements were resolved by discussion, consulting a third advisor (D.C.P.). A summary of bias appraisal is depicted in Appendix-S3.
Summary of measures and synthesis of results
Statistical data handling was performed by one author (J.M.C.). Random effects meta-analyses were conducted at 3 and 6 months of follow-up. Pooled outcomes were expressed as mean differences (MD) with a 95% confidence interval (CI). Forest plots were created to illustrate the effects of the meta-analysis results. Studies involving furcation lesions were excluded from the meta-analysis, because the prognosis differs in terms of defect composition and healing.
Subgroup analyses were conducted according to different statin types. If the analysis failed to detect any significant difference, the locally applied statins were considered as a sole group in the meta-analysis. In studies with multiple treatment arms, in which data from the control group were compared with data from more than one other group, the number (n) of subjects in the control group was divided by the number of comparisons. The I2 statistics and corresponding nullity Q test were employed [42]; I2 values of 25, 50, and 75% were interpreted as low, moderate, and high heterogeneity, respectively. The potential for publication bias was determined using Egger’s test [43]. In addition, the classic fail-safe number was used to test data robustness regarding publication bias and average effect size of the intervention. This test estimates the number of additional “negative” studies (studies in which the effect of the intervention was zero) that would be needed to increase the P value of the meta-analysis above 0.05. A sensitivity meta-analysis was planned to determine the main outcome, based on the inclusion or exclusion of trials that include patients with systemic diseases or risk factors that may exert effects, such as confounders or effect size modifiers. The analysis was performed using the Comprehensive Meta-Analysis Ver. 3 software package, Biostat Inc. Englewood, NJ, USA.
Grading the evidence
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach was employed to assess the quality of the body of evidence related to the primary outcome. The GRADEpro Guideline Development Tool https://gradepro.org was used to create a “summary-of-findings” (SoF) table [44, 45], considering that the RCTs begin the appraisal process as high-quality studies “⊕⊕⊕⊕,” and base their final score on the limitations or strengths of the studies.
Results
Study selection
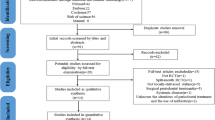
The search strategy identified 526 potentially eligible papers. After removal of duplicates and screening of titles/abstracts, the full texts of 18 titles were selected and assessed; the reviewers showed excellent agreement (K = 0.84). Three studies were excluded because they did not fulfill the eligibility criteria [46,47,48], and two studies were excluded from the meta-analysis, because they involved furcation lesions [49, 50]. At the end, 15 articles met the criteria for inclusion in the systematic review, and 13, in the meta-analysis (Fig. 1). One title [29] was retrieved by gray literature search.
Study characteristics
The included reports were conducted between 2010 and 2017; all were RCT with a placebo control group (Table 1). Data from 864 individuals with CP were collected and analyzed at 3 and 6 months. Five studies involved patients with systemic or local risk factors, smokers [51, 52], diabetics [53, 54], and post-menopausal women [29], called risk populations in this study. Furthermore, only two studies involved sites with furcation lesions, which were included only in the qualitative synthesis and not in the meta-analysis [49, 50]. Critical changes were established from baseline up to 6 months in the parameters assessed. The gel was applied locally by subgingival injection with a blunt cannula; three types of statins were described across the available literature in the present study: simvastatin (SMV) [29, 49, 51, 55,56,57,58], atorvastatin (ATV) [28, 30, 37,38,39], and rosuvastatin (RSV) [38, 40].
The CAL gain changes were measured from the cement-enamel junction to the base of the pocket, except in three studies [49, 50, 54] that evaluated the relative attachment level (RAL). This parameter was calculated by measuring the distance from the stent (a custom-made acrylic guide that serves as a standardized reference point) to the base of the pocket, minus the distance from the stent to the cement-enamel junction. All the studies evaluated the mSBI according to Mombelli et al. [59], except two reports [29, 57], which were excluded from the analysis due to incompatible data. Only two reports offered no data regarding intrabony defects [29, 57], and two studies [49, 50] evaluated the change in furcation lesions, but were excluded from the meta-analysis, as mentioned above. These lesion changes were evaluated radiographically by an image analyzer and a periapical radiograph with a parallel-angle technique for assessment. The radiographs were scanned with a digital scanner, at different resolutions across the studies.
Risk of bias in individual studies
The interexaminer agreement was substantial (K = 0.80), according to the Landis and Koch scale [42]. The allocation concealment is not clearly reported in most studies. The second less reported item was the blinding of participants. Only one study had a “high” risk of bias for the incomplete data item. Finally, four studies were judged as “high” risk for other sources of bias (Fig. 2).
Summary of the risk of bias of the trials included in the systematic review, according to the Cochrane Collaboration’s tool, [41]. Plus sign indicates yes; minus sign indicates no; question mark indicates not specified/unclear
Synthesis of meta-analysis results
At 9 months, there was no clinical effect on the outcomes assessed in the intervention group, compared with the control (data not shown). Interstudy heterogeneity appears significant for parameters of interest at 3 and 6 months; moreover, in regard to IBD reduction, only 6-month data were included in the meta-analysis. Although subgroup and sensitivity analyses were performed, the I2 values remain high. The results of the subgroup analyses according to statin type are depicted in Appendix S5.
Clinical attachment level gain
At 3 months, the random effects meta-analyses showed a CAL gain favoring SRP + statins (MD = 1.09; 95% CI 0.86–1.33) (Fig. 3a), showing high heterogeneity (Q test p value < 0.000; I2 = 98.6%). The same trend and high heterogeneity was maintained at 6 months for SRP + statins (MD = 1.84; 95% CI 1.45–2.23) (Fig. 3c), showing high heterogeneity (Q test p value < 0.000; I2 = 99.4%). The subgroup analysis at 3 and 6 months failed to detect significant differences; all subgroups showed high heterogeneity with I2 values over 90%.
Periodontal probing depth reduction
The mean differences showed the same trend for estimated significance effect sizes at 3 and 6 months. Thus, a significant PPD reduction favoring SRP + statins could be seen at 3 months (MD = 0.95; 95% CI 0.78–1.12) (Fig. 4a), showing high heterogeneity (Q test p value < 0.000; I2 = 94.1%) and following the same high heterogeneity trend at 6 months (MD = 1.69; 95% CI 1.37–2.04) (Q test p value < 0.000; I2 = 98.4%), in favor of SRP + statins (Fig. 4c). Both subgroup analyses failed to detect significant differences; all subgroups showed high heterogeneity with I2 values over 90%.
Modified sulcus bleeding index
The mean differences at 3 months indicated statistical significance in mSBI reduction in favor of SRP + statins (MD 0.41; 95% CI 0.28–0.54) (Fig. 5a) and showed high heterogeneity (Q test p value < 0.000; I2 = 98.1%). The intervention effect followed the same trend at 6 months for SRP + statins, but showed a slightly greater effect (MD 0.70; 95% CI 0.57–0.84) and heterogeneity that remained high (Q test p value < 0.000; I2 = 99.2%) (Fig. 5c). According to the statin type analysis at both time intervals, the Q test detected a more significant effect for RSV, as compared with ATV and SMV. All subgroups showed high heterogeneity, at I2 values over 90%.
Intrabony defect depth reduction
The random effects meta-analyses were assessed only at 6 months. They showed an IBD reduction in favor of SRP + statins (MD 1.48; 95% CI 1.30–1.67) (Fig. 6a) and showed high heterogeneity (Q test p value < 0.000; I2 = 99.0%) (Fig. 6). The Q test detected a slightly greater effect size for RSV, according to the statin type. The heterogeneity remained at I2 values over 95%.
Publication bias
The funnel plots visually showed slight asymmetry. However, the Egger’s regression intercept p value showed no significant p values over 0.05, thus suggesting a low probability of publication bias. The p values obtained for CAL at 3 and 6 months were 0.464 and 0.288, respectively (Fig. 3b, d). The PPD values at 3 and 6 months were 0.180 and 0.638, respectively (Fig. 4b, d). The mSBI values at 3 and 6 months were 0.128 and 0.086, respectively (Fig. 5b, d). Finally, the IBD value at 6 months was 0.097 (Fig. 6b). The classic fail-safe number suggests high tolerance, indicating a low risk of publication bias for the average effect size estimations among studies included in the meta-analysis (data not shown).
Sensitivity analyses
The sensitivity test failed to detect a study that may introduce bias for estimation (data not shown).
Grading the evidence
The grading process allows us to give a reasonable explanation for several aspects depicted in Table 2. Moreover, our judgment process was thoroughly explained in the online data. We determined that the quality of evidence for the main CAL gain outcome was “moderate” (Table 3), with a substantial effect size (Fig. 7), and that the recommendation derived was “weak” in favor of SRP + statins. The terminology of being “weak” refers to variability; it is related to the quality of evidence and to a lower confidence rate for the balance between desirable intervention and undesirable consequences (Fig. 8).
Level of certainty to arrive at a clinical recommendation according to the GRADE approach [61]; (adapted from Smiley et al. 2015) [62]
Discussion
This systematic review and meta-analysis made a thorough assessment of the effect of local application of statins as adjuvants in non-surgical periodontal treatments up to March 2017. The data focused on at least three types of statins with different chemical properties regarding molecular affinity [43, 44], including hydrophilic/rosuvastatin and lipophilic/simvastatin-atorvastatin types. The risk of bias across the studies was high and unclear. The two most inadequately reported aspects were allocation concealment and personnel blinding. It is also noteworthy to mention that one study [29] was obtained through (gray/unpublished) data searches.
The meta-analysis showed that the SRP + statins group provided a significant improvement in the parameters assessed; the authors observed that greater changes occur at 6 months. The effect size for CAL gain was considered large at 3 and 6 months for the test group. This statistically significant and clinically relevant (MD = 1.84 mm) result was interpreted as substantial, based on the scale described in (Fig. 7). Furthermore, significant differences were noticed for PPD, mSBI, and IBD changes. The subgroup analyses failed to detect significant differences. However, we should point out the greater and slightly greater effects for rosuvastatin, in mSBI and IBD, compared with atorvastatin and simvastatin. However, this result is not conclusive, since the observation was based on two reports; therefore, new studies must be performed to confirm this apparently better effect.
Regarding publication bias, funnel plots demonstrated slight asymmetry; the authors cannot explain the source of heterogeneity despite the subgroup and sensitivity analyses. However, we did observe that the primary potential source is related to the publication year, because trials with positive results are published sooner than other more conservative trials, suggesting a possible time-lapse bias [63]. Additionally, the great majority of reports providing data from the Asia region suggest a kind of location bias or “developed-country bias,” which tends to show more significant results, as suggested by empirical evidence [64]. On the other hand, sensitivity analyses failed to detect a study that may introduce bias for estimation. A quantitative interaction was observed with effect size changes across the studies [65].
The effect of SRP + statins in risk populations provides interesting results, compared with other local adjuvants in well-controlled diabetics or in smokers [66,67,68]. Locally applied antibiotics as adjuncts to non-surgical periodontal treatment in smokers with PPD ≥ 5 mm result in significant reductions in PPD and CAL (0.81/0.91 mm, respectively) at 6 months [66]. Strikingly, the SRP + statins results seem to be slightly higher, even considering the less plausible value attributable from the lower boundaries of the 95% confidence intervals at 6 months, for both parameters (Figs. 3 and 4). Moreover, only one report involves post-menopausal women [29] and observes lesser effect size values, showing 0.70 mm for CAL gain at 6 months. This finding corroborates that of a previous study reporting greater CAL loss in patients with this condition [69]. Our results corroborate those of another systematic review [70] focused on evaluating the effect of statins on periodontal intrabony defects. Nevertheless, some methodological differences in design and execution allowed us to include more articles.
Finally, the side effects from taking statins include myopathy, myalgia, rhabdomyolysis, and elevated liver function that could possibly lead to liver damage [71]. However, these adverse events are rare when given at standard doses [72], and the potential events may be prevented using local delivery systems.
This review has several strengths and some limitations, such as the comprehensive literature search, the effort to use the methodology tools for the qualitative and quantitative synthesis of data, and the subgroup and sensitivity analyses to test the robustness of results. The assessment of confidence of results determined the main outcome by the GRADE approach. Furthermore, we included mostly small randomized trials and observed methodological flaws that may influence the meta-analysis results across studies.
Conclusion
Within the limitations of this study, the collective evidence may support the use of locally applied statins as adjuncts to SRP in CP treatment, based on being easy, low-cost alternatives, with lesser adverse effects on bacterial resistance. Even though the confidence in the estimates for CAL gain is moderate, the strengths and recommendations emerging from this review are “weak” in regard to the application of statins, owing to study limitations, inconsistencies, and unreported, unknown potential adverse effects. These results should be interpreted with caution. An informed decision should be taken considering the patient’s values and preferences, and the intervention to be performed by the clinician.
References
van der Sluijs M, van der Sluijs E, van der Weijden F, Slot DE. "The effect on clinical parameters of periodontal inflammation following non-surgical periodontal therapy with ultrasonics and chemotherapeutic cooling solutions" a systematic review =. J Clin Periodontol. 2016;
Goodson JM, Haffajee AD, Socransky SS, Kent R, Teles R, Hasturk H, Bogren A, van Dyke T, Wennstrom J, Lindhe J Control of periodontal infections: a randomized controlled trial I. The primary outcome attachment gain and pocket depth reduction at treated sites. J Clin Periodontol [Internet]. 2012 [cited 2016 Apr 11];39:526–36. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22512461, 536
Tomasi C, Leyland AH, Wennström JL. Factors influencing the outcome of non-surgical periodontal treatment: a multilevel approach. J Clin Periodontol [Internet]. 2007 [cited 2016 Apr 11];34:682–90. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17635246, 690
Cosgarea R, Juncar R, Heumann C, Tristiu R, Lascu L, Arweiler N, et al. Non-surgical periodontal treatment in conjunction with 3 or 7 days systemic administration of amoxicillin and metronidazole in severe chronic periodontitis patients. A placebo controlled randomized clinical study. J Clin Periodontol [Internet]. 2016;n/a-n/a. Available from: http://doi.wiley.com/10.1111/jcpe.12559
Decker E-M, Bartha V, Kopunic A, von Ohle C. Antimicrobial efficiency of mouthrinses versus and in combination with different photodynamic therapies on periodontal pathogens in an experimental study. J Periodontal Res [Internet]. 2016 [cited 2016 Apr 13]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/27038101
Betsy J, Prasanth CS, Baiju K V, Prasanthila J, Subhash N. Efficacy of antimicrobial photodynamic therapy in the management of chronic periodontitis: a randomized controlled clinical trial. J Clin Periodontol [Internet]. 2014 [cited 2016 Apr 13];41:573–81. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24617449, 581
Kolbe MF, Ribeiro F V, Luchesi VH, Casarin RC, Sallum EA, Nociti FH, et al. Photodynamic therapy during supportive periodontal care: clinical, microbiologic, immunoinflammatory, and patient-centered performance in a split-mouth randomized clinical trial. J Periodontol [Internet]. 2014 [cited 2016 Apr 13];85:e277–86. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24555751
Horiuchi N, Maeda T. Statins and bone metabolism. Oral Dis [Internet]. 2006 [cited 2016 Apr 4];12:85–101. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16476028
Maron DJ, Fazio S, Linton MF. Current perspectives on statins. Circulation [Internet]. 2000 [cited 2016 Apr 13];101:207–13. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10637210, 213
Coxon FP, Ebetino FH, Mules EH, Seabra MC, McKenna CE, Rogers MJ. Phosphonocarboxylate inhibitors of Rab geranylgeranyl transferase disrupt the prenylation and membrane localization of Rab proteins in osteoclasts in vitro and in vivo. Bone [Internet]. 2005 [cited 2016 Nov 8];37:349–58. Available from: http://linkinghub.elsevier.com/retrieve/pii/S875632820500181X, 358
Jadhav SB, Jain GK. Statins and osteoporosis: new role for old drugs. J Pharm Pharmacol [Internet]. Blackwell Publishing Ltd; 2006 [cited 2016 Nov 8];58:3–18. Available from: http://doi.wiley.com/10.1211/jpp.58.1.0002
Mo H, Yeganehjoo H, Shah A, Mo WK, Soelaiman IN, Shen C-L. Mevalonate-suppressive dietary isoprenoids for bone health. J Nutr Biochem [Internet]. 2012 [cited 2016 Nov 8];23:1543–51. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0955286312002008
Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, Boyce B, Zhao M, Gutierrez G Stimulation of bone formation in vitro and in rodents by statins. Science [Internet]. UNITED STATES; 1999 [cited 2016 Apr 13];286:1946–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10583956, 1949
Liu S, Bertl K, Sun H, Liu Z-H, Andrukhov O, Rausch-Fan X. Effect of simvastatin on the osteogenetic behavior of alveolar osteoblasts and periodontal ligament cells. Hum Cell [Internet]. 2012 [cited 2016 Nov 9];25:29–35. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/s13577-011-0028-x
Baigent, C., Keech, A., Kearney PM. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. Lancet [Internet]. 2005 [cited 2016 Nov 8];366:1267–78. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0140673605673941
Poston CCJ, Pierce TCT, Li Y, Brinson CWC, Lu Z, Lauer AAW, Leite RS, Huang Y Statin intake is associated with MMP-1 level in gingival crevicular fluid of patients with periodontitis. Oral Dis [Internet]. 2016 [cited 2016 Apr 13];22:438–44. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26988924, 444
Stein D, Lee Y, Schmid MJ, Killpack B, Genrich MA, Narayana N, et al. Local simvastatin effects on mandibular bone growth and inflammation. J Periodontol [Internet]. 2005 [cited 2018 Apr 21];76:1861–70. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16274305
Nyan M, Sato D, Oda M, Machida T, Kobayashi H, Nakamura T, Kasugai S Bone formation with the combination of simvastatin and calcium sulfate in critical-sized rat calvarial defect. J Pharmacol Sci [Internet]. 2007 [cited 2018 Apr 21];104:384–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17721043, 386
Goes P, Lima NA, Rodrigues JAG, Benevides NMB, Brito GAC, Lima V (2016) Anti-inflammatory and anti-resorptive effects of atorvastatin on alveolar bone loss in Wistar rats. Braz Dent J Brazil 27:267–272
Moraschini V, Almeida DCF, Calasans-Maia JA, Diuana Calasans-Maia M. The ability of topical and systemic statins to increase osteogenesis around dental implants: a systematic review of histomorphometric outcomes in animal studies. Int J Oral Maxillofac Surg [Internet]. 2018 [cited 2018 Apr 21]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/29352637
Kellesarian S, Al Amri M, Al-Kheraif A, Ghanem A, Malmstrom H, Javed F. Efficacy of local and systemic statin delivery on the osseointegration of implants: a systematic review. Int J Oral Maxillofac Implants [Internet]. 2017 [cited 2018 Apr 21];32:497–506. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28494034
Sakoda K, Yamamoto M, Negishi Y, Liao JK, Node K, Izumi Y. Simvastatin decreases IL-6 and IL-8 production in epithelial cells. J Dent Res [Internet]. 2006 [cited 2016 Nov 9];85:520–3. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16723648
Ting M, Whitaker EJ, Albandar JM. Systematic review of the in vitro effects of statins on oral and perioral microorganisms. Eur J Oral Sci [Internet]. 2016;124:4–10. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26718458
Suresh S, Narayana S, Jayakumar P, Sudhakar U, Pramod V. Evaluation of anti-inflammatory effect of statins in chronic periodontitis. Indian J Pharmacol [Internet]. Medknow Publications and Media Pvt. Ltd.; 2013 [cited 2016 Oct 18];45:391–4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24014917
Cicek Ari V, Ilarslan YD, Erman B, Sarkarati B, Tezcan I, Karabulut E, Oz SG, Tanriover MD, Sengun D, Berker E Statins and IL-1β, IL-10, and MPO levels in gingival crevicular fluid: preliminary results. Inflammation [Internet]. 2016 [cited 2016 Nov 12];39:1547–57. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/s10753-016-0390-7, 1557
Pradeep AR, Thorat MS. Clinical effect of subgingivally delivered simvastatin in the treatment of patients with chronic periodontitis: a randomized clinical trial. J Periodontol [Internet]. 2010 [cited 2016 Apr 12];81:214–22. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20151799
Rath A, Mahenra J, Thomas L, Sandhu M, Namasi A, Ramakrishna T. A clinical radiological and IL-6 evaluation of subgingivally delivered simvastatin in the treatment of chronic periodontitis. Int J Drug Deliv A. Rath, Department of Periodontology and Implantology, Meenakshi Ammal Dental College and Hospital, Chennai −600 095, Tamil Nadu, India; 2012;4:70–81
Pradeep AR, Karvekar S, Nagpal K, Patnaik K, Guruprasad CN, Kumaraswamy KM. Efficacy of locally delivered 1.2% rosuvastatin gel to non-surgical treatment of patients with chronic periodontitis: a randomized, placebo-controlled clinical trial. J Periodontol [Internet]. 2015 [cited 2016 Apr 12];86:738–45. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25786565
Gaekwad SS, Gujjari SK, Kumar TMP, Devishree S. Effect of subgingivally delivered simvastatin in postmenopausal women with chronic periodontitis: a randomized controlled trial. J Adv Clin Res Insights [Internet]. 2015;2:253–8. Available from: http://jcri.net/eJournals/ShowText.aspx?ID=89&Type=FREE&TYP=TOP&IN=_eJournals/images/JPLOGO.gif&IID=9&Value=1&isPDF=YES
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol [Internet]. 2009 [cited 2014 Jul 16];62:1006–12. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19631508
Shea BJ, Hamel C, Wells GA, Bouter LM, Kristjansson E, Grimshaw J, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol [Internet]. 2009 [cited 2016 Feb 9];62:1013–20. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19230606
Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol [Internet]. 1999 [cited 2018 Jan 26];4:1–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10863370
Tonetti MS, Claffey N, European Workshop in Periodontology group C. Advances in the progression of periodontitis and proposal of definitions of a periodontitis case and disease progression for use in risk factor research. Group C consensus report of the 5th European Workshop in Periodontology. J Clin Periodontol [Internet]. 2005 [cited 2018 26];32 Suppl 6:210–3. Available from: http://doi.wiley.com/10.1111/j.1600-051X.2005.00822.x
Greenhalgh T, Peacock R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: audit of primary sources [Internet]. Br. Med. J. 2005 [cited 2017 Apr 21]. p. 1064–5. Available from: http://www.bmj.com/content/331/7524/1064
Smiley CJ, Tracy SL, Abt E, Michalowicz BS, John MT, Gunsolley J, et al. Systematic review and meta-analysis on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J Am Dent Assoc [Internet]. 2015 [cited 2017 Apr 12];146:508–524.e5. Available from: http://www.sciencedirect.com/science/article/pii/S0002817715003463
Michalowicz BS, Hodges JS, Pihlstrom BL. Is change in probing depth a reliable predictor of change in clinical attachment loss? J Am Dent Assoc [Internet]. 2013 [cited 2017 Apr 23];144:171–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23372133
Claffey N, Egelberg J. Clinical characteristics of periodontal sites with probing attachment loss following initial periodontal treatment. J Clin Periodontol [Internet]. 1994 [cited 2017 Apr 23];21:670–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7852611, 679
Badersten A, Nilvéus R, Egelberg J. Scores of plaque, bleeding, suppuration and probing depth to predict probing attachment loss. 5 years of observation following nonsurgical periodontal therapy. J Clin Periodontol [Internet]. 1990 [cited 2017 Apr 23];17:102–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2406291, 107
Newbrun E. Indices to measure gingival bleeding. J Periodontol [Internet]. 1996 [cited 2018 Jan 27];67:555–61. Available from: http://doi.wiley.com/10.1902/jop.1996.67.6.555, 561
Holtfreter B, Albandar JM, Dietrich T, Dye BA, Eaton KA, Eke PI, et al. Standards for reporting chronic periodontitis prevalence and severity in epidemiologic studies: proposed standards from the Joint EU/USA Periodontal Epidemiology Working Group J Clin Periodontol [Internet]. 2015 [cited 2018 Jan 27];42:407–12. Available from: http://doi.wiley.com/10.1111/jcpe.12392
Higgins A, Gotzsche J, Moher O et al (2011) The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928–d5928
Higgins JPT, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ [Internet]. 1997 [cited 2017 Jun 12];315:629–34. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9310563, 634
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck Y, Alonso-coello P et al (2008) Rating quality of evidence and strength of recommendations : GRADE : an emerging consensus on rating quality of evidence and strength of recommendations rating quality of evidence of recommendations GRADE : of evidence an emerging and consensus of on rati. Br Med J 336:924–926
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, Jaeschke R, Rind D, Meerpohl J, Dahm P, Schünemann HJ (2011) GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 64:383–394
Grover H, Kapoor S, Singh A. Effect of topical simvastatin (1.2 mg) on gingival crevicular fluid interleukin-6, interleukin-8 and interleukin-10 levels in chronic periodontitis—a clinicobiochemical study. J oral Biol craniofacial [Internet]. 2016 [cited 2017 Apr 20]; Available from: http://www.sciencedirect.com/science/article/pii/S221242681500127X
Suresh S, Narayana S, Jayakumar P, Sudhakar U, Pramod V (2013) Evaluation of anti-inflammatory effect of statins in chronic periodontitis. Indian J Pharmacol India 45:391–394
Surve SM, Acharya AB, Thakur SL (2015) Efficacy of subgingivally delivered atorvastatin and simvastatin as an adjunct to scaling and root planing. Drug Metab Pers Ther Germany 30:263–269
Pradeep AR, Priyanka N, Kalra N, Naik SB, Singh SP, Martande S. Clinical efficacy of subgingivally delivered 1.2-mg simvastatin in the treatment of individuals with Class II furcation defects: a randomized controlled clinical trial. J Periodontol [Internet]. 2012 [cited 2016 Apr 12];83:1472–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22348696
Garg S, Pradeep A. 1.2% rosuvastatin and 1.2% atorvastatin gel local drug delivery and redelivery in the treatment of class II furcation defects: a randomized controlled clinical. J Periodontol [Internet]. 2017 [cited 2017 Apr 20]; Available from: http://www.joponline.org/doi/abs/10.1902/jop.2016.160399
Rao NS, Pradeep AR, Bajaj P, Kumari M, Naik SB. Simvastatin local drug delivery in smokers with chronic periodontitis: a randomized controlled clinical trial. Aust Dent J [Internet]. 2013 [cited 2016 Apr 12];58:156–62. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23713634, 162
Kumari M, Martande SS, Pradeep AR. Subgingivally delivered 1.2% atorvastatin in the treatment of chronic periodontitis among smokers: a randomized, controlled clinical trial. J Investig Clin Dent. Department of Periodontics, Vydehi Institute of Dental Sciences and Research Centre, Bangalore, India.; Department of Periodontics, Dr D.Y. Patil Dental College and Hospital, Pimpri, Pune, India.; Department of Periodontics, Government Dental College and : John Wiley & Sons Australia, Ltd; 2016;
Pradeep AR, Rao NS, Bajaj P, Kumari M. Efficacy of subgingivally delivered simvastatin in the treatment of patients with type 2 diabetes and chronic periodontitis: a randomized double-masked controlled clinical trial. J Periodontol [Internet]. United States; 2013 [cited 2016 Apr 12];84:24–31. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22420871
Kumari M, Martande SS, Pradeep AR, Naik SB (2016) Efficacy of subgingivally delivered 1.2% atorvastatin in the treatment of chronic periodontitis in patients with type 2 diabetes mellitus: a randomized controlled clinical trial. J Periodontol United States 87:1278–1285
Pradeep AR, Thorat MS. Clinical effect of subgingivally delivered simvastatin in the treatment of patients with chronic periodontitis: a randomized clinical trial. J Periodontol A R Pradeep, Department of Periodontics, Government Dental College and Research Institute, Fort Bangalore-560002, Karnataka, India; 2010;81:214–22
Pradeep AR, Rao NS, Naik SB, Kumari M. Efficacy of varying concentrations of subgingivally delivered metformin in the treatment of chronic periodontitis: a randomized controlled clinical trials J Periodontol [Internet]. 2013 [cited 2017 Apr 7];84:212–20. Available from: http://www.joponline.org/doi/10.1902/jop.2012.120025
Agarwal S, Chaubey KK, Chaubey A, Agarwal V, Madan E, Agarwal MC (2016) Clinical efficacy of subgingivally delivered simvastatin gel in chronic periodontitis patients. J Indian Soc Periodontol India 20:409–416
S Martande S, Kumari M, Pradeep AR, Pal Singh S, Kumar Suke D (2017) Comparative evaluation of efficacy of subgingivally delivered 1.2% atorvastatin and 1.2% simvastatin in the treatment of intrabony defects in chronic periodontitis: a randomized controlled trial. J Dent Res Dent Clin Dent Prospects Iran 11:18–25
Mombelli A, van Oosten MA, Schurch E, Land NP. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol Immunol [Internet]. 1987 [cited 2016 Feb 11];2:145–51. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3507627, 151
Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, Guyatt GH (2011) GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 64:401–406
Andrews J, Guyatt G, Oxman AD, Alderson P, Dahm P, Falck-Ytter Y, Nasser M, Meerpohl J, Post PN, Kunz R, Brozek J, Vist G, Rind D, Akl EA, Schünemann HJ (2013) GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol 66:719–725
Smiley CJ, Tracy SL, Abt E, Michalowicz BS, John MT, Gunsolley J, et al. Evidence-based clinical practice guideline on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J Am Dent Assoc [Internet]. Elsevier Inc; 2015;146:525–35. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0002817715003347
Hopewell S, Clarke MJ, Stewart L, Tierney J. Time to publication for results of clinical trials. In: Hopewell S, editor. Cochrane Database Syst Rev [Internet]. Chichester: John Wiley & Sons, Ltd; 2007 [cited 2017 Jul 11]. p. MR000011. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17443632
Vickers A, Goyal N, Harland R, Rees R. Do certain countries produce only positive results? A systematic review of controlled trials. Control Clin Trials [Internet]. 1998 [cited 2017 Jul 9];19:159–66. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9551280
Yusuf S, Wittes J, Probstfield J, Tyroler HA. Analysis and interpretation of treatment effects in subgroups of patients in randomized clinical trials. JAMA [Internet]. 1991 [cited 2017 Jul 8];266:93–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2046134, 98
Chambrone L, Vargas M, Arboleda S, Serna M, Guerrero M, de Sousa J, et al. Efficacy of local and systemic antimicrobials in the non-surgical treatment of smokers with chronic periodontitis: a systematic review. J Periodontol [Internet]. 2016 [cited 2016 Oct 26];87:1320–32. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27498712
Bajaj P, Pradeep AR, Agarwal E, Kumari M, Naik SB (2012) Locally delivered 0.5% clarithromycin, as an adjunct to nonsurgical treatment in chronic periodontitis with well-controlled type 2 diabetes: a randomized controlled clinical trial. J Investig Clin Dent 3:276–283
Rovai ES, Souto MLS, Ganhito JA, Holzhausen M, Chambrone L, Pannuti CM. Efficacy of local antimicrobials in the non-surgical treatment of patients with periodontitis and diabetes: a systematic review. J Periodontol [Internet]. 2016 [cited 2017 Sep 9];87:1406–17. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27468792
Penoni DC, Fidalgo TKS, Torres SR, Varela VM, Masterson D, Leão ATT, et al. Bone density and clinical periodontal attachment in postmenopausal women. J Dent Res [Internet]. 2017;96:261–9. Available from: http://journals.sagepub.com/doi/10.1177/0022034516682017
Sinjab K, Zimmo N, Lin G-H, Chung M-P, Shaikh L, Wang H-L. The effect of locally-delivered statins on treating periodontal intrabony defects: a systematic review and meta-analysis. J Periodontol [Internet]. 2016;1–18. Available from: http://www.joponline.org/doi/10.1902/jop.2016.160384, 2017
Silva MA, Swanson AC, Gandhi PJ, Tataronis GR. Statin-related adverse events: a meta-analysis. Clin Ther [Internet]. 2006 [cited 2017 Jul 10];28:26–35. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0149291806000191
Armitage J. The safety of statins in clinical practice. Lancet (London, England) [Internet] 2007 [cited 2017 Jul 10];370:1781–90. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0140673607607168, 1790
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
No ethical approval was required for this study, since no human participants or animals were used in this study.
Informed consent
Formal consent is not required for a systematic review and meta-analysis.
Electronic supplementary material
ESM 1
(DOCX 230 kb)
Rights and permissions
About this article
Cite this article
Meza-Mauricio, J., Soto-Peñaloza, D., Peñarrocha-Oltra, D. et al. Locally applied statins as adjuvants to non-surgical periodontal treatment for chronic periodontitis: a systematic review and meta-analysis. Clin Oral Invest 22, 2413–2430 (2018). https://doi.org/10.1007/s00784-018-2507-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-018-2507-x