Abstract
Objective
This study aimed to systematically review clinical trials about the effect of statins as adjunct to mechanical periodontal therapy, on probing pocket depth, clinical attachment level, and intrabony defects, in comparison to mechanical periodontal therapy alone or in association with placebo.
Material and methods
Three databases were searched for controlled clinical trials that used any locally delivered or systemically statin as a sole adjunctive therapy to mechanical periodontal treatment. Weighted mean differences between baseline and 6 months after periodontal treatment for clinical attachment level (CAL), probing pocket depth (PPD), and intrabony defect (IBD) were calculated. A high heterogeneity was detected. Therefore, a meta-regression adjusted for type of statin and year of publication was performed.
Results
Fifteen studies were included in the systematic review, and ten studies were included in the meta-analysis. In the meta-regression, the adjunct use of simvastatin, rosuvastatin, and atorvastatin additionally reduced PPD in comparison to mechanical periodontal therapy and a placebo gel (2.90 ± 0.35, 3.90 ± 0.77, 3.06 ± 0.71 mm, respectively; p < 0.05). Regarding the resolution of IBD, simvastatin and rosuvastatin significantly improved in comparison to control group (0.89 ± 0.35 and 1.93 ± 0.77 mm, respectively; p < 0.05). No statistically significant difference was found between the statins for both PPD and IBD (p < 0.05). Regarding CAL gain, simvastatin provided a statistically significant improvement as compared to the control group (2.02 ± 0.79 mm; p = 0.043).
Conclusions
The use of statins, used as sole adjuncts to mechanical periodontal treatment, improved the periodontal parameters. In the quantitative analyses, simvastatin was the only drug that showed additional benefits in all evaluated parameters.
Clinical relevance
Statins promote significantly clinical periodontal improvements when administered in association with non-surgical scaling and root planning (SRP), when compared to SRP alone or in association with a placebo.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Statins are inhibitors of the 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoA reductase), which is an important enzyme related to the synthesis of cholesterol [1]. Statins are widely used because of their effectiveness on reducing the blood cholesterol levels, excellent tolerability, safety, and low cost [2]. The use of statins is an established therapy for hyperlipidemia and arteriosclerosis, and it is the primary and secondary prevention of coronary artery diseases, mainly due to lowering of low-density lipoprotein cholesterol (LDL-C) [3, 4].
So far, the control of biofilms, along with proper periodontal supportive therapy, is the gold-standard procedures to prevent and treat periodontal diseases [5]. Several treatment modalities are reported in the literature, such as surgical periodontal therapy and scaling and root planing (SRP), both of them alone or with adjunct antimicrobial/several other pharmacological agents [6, 7]. When successful, all those therapies lead to reduction of probing pocket depth (PPD) and gain of clinical attachment. Furthermore, it is known that the healing process after conventional therapy is mainly due to repair, including establishment of a long junctional epithelium [8]. However, as some sites continue to experience periodontal breakdown, despite the conventional treatment [9], recent studies are exploring new strategies to manage periodontal diseases [10, 11].
Additional to its hypolipidemic effects, statins present the pleiotropic mechanisms, which have pharmacologic effects not directly related to the lipid lowering profile, such as antioxidant and antiinflammatory properties, angiogenesis, improvements in the endothelial function, and increased bone formation [12,13,14,15]. The literature also suggests that statins may attenuate periodontal inflammation by decreasing interleukin (IL)-1β and increasing IL-10 levels in gingival crevicular fluid of patients with periodontitis [16]. Positive outcomes of simvastatin in patients with chronic periodontitis were demonstrated in observational studies [17,18,19]. Periodontitis patients treated with statins presented less periodontal pockets in comparison to those that did not use statin [17, 19]. Furthermore, a retrospective cohort study showed that chronic periodontitis patients under statin presented 48% decreased tooth loss rate in comparison to those that did not use the medication [18]. On the other hand, this finding is not consistent in the literature [20].
A previous systematic review about the effects of statins on the treatment of chronic periodontitis included cohort, cross-sectional, and clinical trial studies, but it did not perform a quantitative analysis of the selected clinical trials [11]. Therefore, this study aimed to systematically review clinical trials about the effect of statins in conjunction with mechanical periodontal therapy on probing pocket depth, clinical attachment level, and intrabony defects.
Material and methods
The focused question for this systematic review was “In patients with chronic/aggressive periodontitis, how effective are statins, when used as adjuncts to mechanical periodontal therapy, when compared to mechanical periodontal treatment alone or associated with placebo?” The PICO question comprised patients with chronic or aggressive periodontitis (P), mechanical periodontal treatment with statins (I), compared to mechanical treatment alone or placebo (C), and probing pocket depth, clinical attachment level, and intrabony defect alterations (O).
Search strategy
The search for this systematic review was performed in MEDLINE-Pubmed, Scopus, and EMBASE databases. Search strategy for Pubmed database was developed as follows:
-
(1)
periodontal disease[Title/Abstract] OR periodontal diseases[MeSH Terms]) OR periodontal treatment[Title/Abstract] OR periodontal therapy[Title/Abstract] OR subgingival curettage[MeSH Terms] OR periodontal intervention[Title/Abstract] OR periodontium[MeSH Terms] OR periodontics[MeSH Terms] OR wound healing[MeSH Terms] OR periodontal repair[Title/Abstract] OR periodontal regeneration[Title/Abstract] OR chronic periodontitis [Title/Abstract]
-
(2)
dyslipidemias[MeSH Terms] OR hyperlipidemia[Title/Abstract] OR higher cholesterol[Title/Abstract] OR statin[Title/Abstract] OR hydroxymethylglutaryl-coareductase[MeSH Terms] OR anticholesteremic agents[MeSH Terms] OR cholesterol reductase[MeSH Terms] OR lovastatin[MeSH Terms] OR provastatin[Title/Abstract] OR atorvastatin[Title/Abstract] OR Rosuvastatin [Title/Abstract]
-
(3)
1 and 2
The search strategy for Scopus and EMBASE databases is an adaptation of the above and the literature was searched up to July 2016.
Selection criteria and risk of bias assessment
Titles and abstracts resulting from the search as described were screened independently by two reviewers (FWMGM and KT). Any discrepancies with regard to the exclusion/inclusion of the studies of any study were solved by extensive discussion between the two reviewers. When any doubt was still remaining, another investigator (JC) was involved in these processes.
Full text reading and data extraction was performed when the titles or abstracts fulfilled the following criteria:
-
Clinical trials with at least 1-month follow-up;
-
Patients with diagnosis of chronic or aggressive periodontitis;
-
Intervention group should use any statin, as any form of administration, as a solely adjunct to non-surgical mechanical periodontal treatment;
-
The comparison group should comprise non-surgical mechanical periodontal therapy alone or associated with placebo;
-
The outcome should include at least one clinical periodontal measurement, such as probing depth, clinical attachment level, and intrabony defect.
No language or publication date restrictions were applied. However, the studies were excluded, after full text reading, if they presented one of the following characteristics:
-
Observational and experimental animal studies.
-
Case reports, letters, and reviews.
-
Included only patients younger than 18 years old.
-
Those that did not perform any mechanical periodontal therapy.
-
Studies that used statin and any other drug/biomaterial in the same study group.
-
Studies that reported only a secondary analysis of a previously included study.
Studies without abstracts but whose titles suggested that they could be related to the objective of this systematic review were selected, so the full text could be screened for eligibility. All references of related reviews [11, 21] and of the studies included during the electronic search were screened for eligibility. Additionally, after the electronic first screening and selection, all the studies that have cited the included articles, in Scopus database, were also screened for eligibility.
The gray literature was also search through contact with the corresponding author of the included studies and in the following databases: trip database, NYAM gray literature report, Centre for Reviews and Dissemination, and Google scholar. Furthermore, the register of the Clinical Trial website was also screened for eligibility. The above mentioned databases were searched using an adaptation of the search strategy previously described.
The risk of bias of the non-randomized clinical trials was assessed by the ROBINS-I tool, developed by the Cochrane Group [22]. In this tool, different bias are assessed: confounding, selection of participants, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes, selection of the reported result, and overall bias. The risk of bias of the randomized clinical trials was assessed according to the criteria defined by the Cochrane Collaboration [23]. Random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias were evaluated. When sufficient information description was provided, a positive mark was attributed to each item, indicating low risk of bias. In case of missing information, a negative mark was recorded to each of the seven items, indicating high risk of bias. When both low and high risk of bias could not be assessed, the item was classified as unclear. To both tools, each selected study was evaluated independently by two reviewers (FWMGM and JC). Any discrepancies in this regard were solved by extensive discussion between the two reviewers.
Data extraction
The data extraction was performed in a spreadsheet specifically developed for this study. The data extraction included the following variables: authors, date of publication, country of the patients, funding, number of individuals in each group, the statin used, mean or range age, the percentage of male/female, and the results of the periodontal outcomes assessed.
When some of the necessary data were missing in the original studies, a contact with the authors was made by e-mail. Through the same contact, the corresponding authors were asked if they knew any other trial that may fulfill the objectives of this systematic review. All the authors were contacted; however, none of them answered the contact. Studies with missing data were maintained in the systematic review, but not included in the quantitative analysis.
Data synthesis
Due to the larger number of statins locally delivered in periodontal pockets, the quantitative analysis was performed only for these studies, despite the type of statin used. In order to standardize data synthesis, mean alterations (± standard deviation) in probing pocket depth (PPD), intrabony defect assessed radiographically (IBD), and clinical attachment level (CAL), from 6 months to baseline, were included in the meta-analysis. This information was originally available in all included studies. When two different statins were evaluated in the same study, both statin groups were included in the analysis, but the sample size in the group without statin was divided in half.
Statistical analysis: meta-analyses and meta-regression
Meta-analyses were performed using the weighted mean difference (WMD) between baseline and 6 months after periodontal therapy. When difference between 6 months and baseline was not presented in the article, the study was not eligible for meta-analysis. Quantitative analyses were conducted for PPD, IBD, and CAL applying linear meta-analyses. The primary outcome for the meta-analysis was mean alteration in CAL and secondary outcomes were alterations in PPD and IBD. Heterogeneity was assessed by the Q test and quantified with the I2 statistic. Publication bias was assessed using the Egger’s and Begg’s test. Additionally, the overall quality of evidence for each of the main outcomes included in the meta-analyses was rated using the GRADE approach [24].
When high heterogeneity was found (I2 > 40%), sources of effect modification of the pooled WMD were investigated using linear meta-regression [25]. Due to the number of studies (only one/two studies, respectively), we could not test effect modification by diabetes or smoking status. Therefore, only the following study characteristics were included in the meta-regression: year of publication and type of statin.
The heterogeneity parameter (tau2), which denotes the standard deviation of the true between-group variance, was calculated using the method of moment, and p values were estimated with Monte Carlo simulation from 1000 permutations. The analyses were adjusted for the year 2010, as the first study was published in this year. Meta-analyses and meta-regression were conducted using Stata13.1 software [26, 27].
Results
Study selection
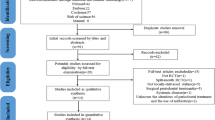
One-thousand three-hundred and sixty-three titles/abstracts were retrieved from the search, of which 15 were selected based on the criteria previously described (Fig. 1) [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42]. One study reported a secondary analysis of an included clinical trial, and was excluded [43]. The additional searches resulted in 393 studies, but did not increase the number of selected studies. All the selected studies were written in English; the demographic sample characteristics and the main results of these studies are shown in Table 1.
Characteristics of included studies
Among the included studies, 3 were non-randomized clinical trials [29, 30, 39], and 12 were randomized clinical trials [28, 31,32,33,34,35,36,37,38, 40,41,42]. All the studies included in the systematic review comprised chronic periodontitis patients. Statins used were atorvastatin, simvastatin, and rosuvastatin. Eleven studies used locally delivered statins with a 1.2% concentration [32,33,34,35,36,37,38,39,40,41,42]. From these, 5 used simvastatin [32,33,34,35, 37], 3 used atorvastatin [36, 40, 42], and 1 used rosuvastatin [38]. Furthermore, one study used rosuvastatin and atorvastatin [41] and another used simvastatin and atorvastatin in different patients [39]. All these studies used statins as adjunct to site-specific mechanical periodontal treatment and compared to a placebo gel plus site-specific mechanical periodontal treatment. One study used 2% atorvastatin in a dentifrice compared to a control dentifrice [31]. Atorvastatin was the only drug used systemically as adjunctive to whole-mouth scaling and root planing in three studies. In one study, it was compared to placebo pills [28], and in the two others, the comparison was solely with mechanical treatment [29, 30].
Two studies treated patients with both periodontitis and hyperlipidemia [29, 30]. Another study treated only patients with type 2 diabetes [35], and Rosenberg et al. (2015) [31] included patients without diabetes and well-controlled diabetics. The others studies included only systemically healthy patients with no use of systemic statin [28, 32,33,34, 36,37,38,39,40,41,42]. Furthermore, only two studies included smokers with periodontitis [31, 37]. Regarding to the side effects of statins, 13 studies reported no adverse effects in any patient [28, 31,32,33,34,35,36,37,38,39,40,41,42]. However, two studies did not report adverse events [29, 30].
Risk of bias assessment
Figure 2 presents the quality analysis of the RCT included in the present systematic review [28, 31,32,33,34,35,36,37,38, 40,41,42]. Only one study fulfilled all criteria with low risk of bias [28]. The majority of the studies did not provide any explanation of how allocation concealment was performed [31,32,33,34,35,36,37,38, 40,41,42]. Despite of that, all RCT had low risk of bias for random sequence generation [28, 31,32,33,34,35,36,37,38, 40,41,42]. Additionally, all RCT had low risk of bias for incomplete outcome data. No study had unclear risk of bias. Overall, this analysis showed that there is a moderate heterogeneity in the risk of bias in the selected studies, ranging from zero negative marks (low risk of bias) to four negative marks (high risk of bias).
Table 2 shows the risk of bias of the non RCT included in the present systematic review. All studies presented an overall bias ranging from moderate [29, 30] to critical [39]. Risks of bias due to confounding were classified as moderate in all non-RCT. One study was classified in the other domains as low risk of bias [30]; meanwhile, another study presented a moderate risk of bias only in measurement of outcomes [29]. The other non-RCT was evaluated as critical/serious in almost all domains [39].
Qualitative results—statins used systemically
Considering the three studies that used systemic administration of atorvastatin, only one demonstrated significant improvements in PPD and dental mobility, favoring the group that used statin [28]. The two remaining studies included hyperlipidemic patients with prescribed atorvastatin and compared with normolipidemic patients [29, 30]. In one study, no statistically significant differences between both groups for all periodontal parameters were shown [30]. Comparisons between groups with and without atorvastatin as adjunct to mechanical periodontal treatment in relation to periodontal parameters are not reported in the other study [29].
Qualitative results—statins used in a dentifrice
One of the selected studies reported 1-month follow-up results of a 2% atorvastatin dentifrice compared with a placebo dentifrice [31]. Both groups showed improvements in periodontal parameters after non-surgical periodontal treatment. Furthermore, when compared to a placebo dentifrice, atorvastatin dentifrice was able to additionally enhance CAL gain, reduce PPD, and bleeding on probing.
Qualitative results—statins used locally
Eleven studies used locally delivered statins adjunctive to periodontal therapy [32,33,34,35,36,37,38,39,40,41,42], of which nine were performed by the same research group [32, 33, 35,36,37,38, 40,41,42]. All studies, but one [39], showed statistically significant reduction in PPD, favoring the statin group. Similarly to PPD, only one study [39] showed no statistically significant improvements in the IBD favoring the statin group. Regarding the CAL gain, only two studies did not demonstrate statistically significant differences between groups with and without statin [34, 39]. All of these studies performed intraoral radiographic analyses.
Meta-analyses and meta-regression for alterations in probing pocket depth
From the 15 selected studies, ten were included in the quantitative analysis of PPD [32,33,34,35,36,37,38, 40,41,42]. For PPD at 6 months, this analysis showed a pooled WMD of 1.93 mm (95% CI 1.44; 2.41), favoring the statin group (Fig. S1). However, this analysis showed a high heterogeneity (93.9%, p < 0.001). No publication bias was shown in both tests for this analysis (p = 0.213 and p = 0.146 for Begg’s and Egg’s tests, respectively) (Fig. S2). In the sensitivity analysis, no major changes were detected for the pooled WMD ranging from 1.81 mm (95% CI 1.44; 2.18) to 2.04 mm (95% CI 1.62; 2.47) (Fig. S3).
The cumulative meta-analysis showed that over the years, the effect size was decreasing significantly across the published studies (p = 0.041). For each year, a mean decrease of 0.21 mm on PPD was observed. On the other hand, the meta-regression showed that all statins, when associated to SRP, reduced significantly PPD in comparison to SRP plus placebo (Table 3). When controlling for the year of publication (at year 2010), rosuvastatin demonstrated numerically the higher coefficient of PPD reduction. However, no statistically significant differences were found between the statins.
Meta-analyses and meta-regression for alterations in intrabony defect
Nine studies were included in the quantitative analysis of alterations in IBD [32, 34,35,36,37,38, 40,41,42]. The resolution of IBD 6 months after periodontal therapy was also included in the meta-analysis. A pooled WMD of 1.54 mm (95% CI 1.24; 1.84) was shown, favoring the statin group. High heterogeneity was also found in this analysis (96.5%, p < 0.001) (Fig. S4). No publication bias was observed in both tests for this analysis (p = 0.721 and p = 0.661 for Begg’s and Egg’s tests, respectively) (Fig. S5). No major changes were detected for the pooled WMD ranging from 1.40 mm (95% CI 1.16; 1.65) to 1.65 mm (95% CI 1.36; 1.94) in the sensitivity analysis (Fig. S6).
The year of publication presented a positive statistically significant effect, with each year increment promoting a decrease of 0.20 mm in the IBD (p = 0.041). In the meta-regression, when the year of publication (at year 2010) and the type of statin were considered, both rosuvastatin and simvastatin showed significantly more resolution of IBD in comparison to the group without statin (p = 0.047 and p = 0.044, respectively). However, no statistically significant differences between the three statin were found (Table 3).
Meta-analyses and meta-regression for alterations in clinical attachment level
Nine studies were included in the quantitative analysis of alterations in CAL [32, 34,35,36,37,38, 40,41,42]. Regarding CAL gain 6 months after the therapy, the pooled WMD found was 1.82 mm (95% CI 1.24; 2.41), also favoring the statin group (Fig. S7). However, a high heterogeneity (96.5%, p < 0.001) was observed. Publication bias was found with the Egger’s test (p = 0.024) (Fig. S8) but not with the Begg’s test (p = 0.592). The sensitivity analysis showed a ranged of pooled WMD of 1.70 mm (95% CI 1.22; 2.19) to 2.01 mm (95% CI 1.44; 2.58) (Fig. S9).
In the meta-regression, this periodontal outcome did not show statistically significant correlation with year of publication (p = 0.951). When statins were compared to the groups without statin, simvastatin was the only statin to promote significantly CAL gain, when adjusted for the year of publication (p = 0.043; Table 3). The comparison among statin groups failed to show statistically significant differences between them. Furthermore, the inclusion of type of statin and year of publication in the meta-regression included more confounders in the model, as the adjusted R2 was − 43.59%.
Quality of evidence at the review level
The GRADE quality of evidence of both primary and secondary outcomes performed in the meta-analyses is presented in Table 4. To all outcomes assessed, the quality of evidence was rated as low.
Discussion
This systematic review aimed to analyze the effect of statins, in any form of administration, as a solely adjuvant to the mechanical periodontal treatment. Generally, most of the included studies showed additional periodontal clinical benefits when statins were used in along with mechanical periodontal treatment. Meta-analyses were performed using locally delivered statins as an adjunct to mechanical periodontal treatment and showed high heterogeneity. Meta-regression showed that simvastatin, atorvastatin, and rosuvastatin significantly reduced PPD in comparison to the group without statin. Simvastatin and rosuvastatin gel significantly decreased IBD when compared to a placebo gel. Regarding CAL gain, the meta-regression showed that only simvastatin was able to significantly improve this periodontal parameter in comparison to a placebo group.
Statins are important drugs used in the treatment of hypercholesterolemia that act through the inhibition of the HMG-CoA reductase. By inhibiting this enzyme, the mevalonate synthesis is reduced, and consequently, other isoprenoid pathways are affected [44]. Therefore, cholesterol is lowered and many cardiovascular diseases may be prevented [1, 44]. Additional to their lipid-lowering effect, statins present the pleiotropic effects, which are dependent of their direct activity in a target site or as consequence to their inhibition on the biosynthesis of cholesterol. These pleiotropic effects include antiinflammatory and antioxidant effects and increase bone formation [15, 45, 46]. In this respect, an interest raised in assessing the possible effects of statins in periodontal treatment outcomes.
The literature showed that statins are able to significantly reduce the expressions of IL-6, IL-8, IL-1β, and tumor necrosis factor-α levels [14, 47]. These drugs also showed an in vitro activity against Gram-positive and Gram-negative bacteria [48]. A decrease on the release of matrix metalloproteinase-1 (MMP-1) and MMP-3 from macrophages and endothelial cells is also reported [14, 49]. Furthermore, simvastatin reduced the levels of inducible nitric oxide synthase (iNOS), receptor activator of nuclear factor κB (RANK), RANK ligand (RANKL), and increased the level of osteoprotegerin (OPG) in periodontal tissues [50].
These important pleiotropic effects may be the main reason why statin showed additional improvements in PPD reduction, CAL gain, and IBD decrease in most of the included studies in this systematic review. The beneficial effects of statins on the periodontal tissues were also reported on others systematic reviews [11, 21]. However, to the best of the authors’ knowledge, this is the first study to show a quantitative analysis of these studies. Additionally, this is the first systematic review to report the risk of bias of the included studies.
Regarding the studies that used systemically statin, only one study showed additional improvements in PPD in normolipidemic patients, favoring the group that used atorvastatin [28]. The atorvastatin antiinflammatory effect may be responsible for this additional improvement [51]. Two other studies used systemically atorvastatin in patients with hyperlipidemia [29, 30]. The literature shows a significant association between altered lipid profile and periodontal disease [52, 53]. Therefore, it may be hypothesized that the use of statins, in patients with altered lipid profile, may not promote additionally reduction of PPD when compared to normolipidemics without statins use.
After an oral administration, statins are quickly absorbed because of their higher liver specificity, making their bioavailability very low (approximately 5–30% of the administered dose) [44]. Therefore, it may be important to have a higher drug concentration in the target site. Locally delivered drug is an approach that presents the advantage of achieving high intrasucular drug concentration directly in the target site with a reduced dosage, better patient compliance, and reduced side effects in comparison to its systemic administration [54, 55]. Due to the higher number of studies using locally delivered statins, a meta-analysis was performed only on those studies.
Three different meta-analyses were performed, and demonstrate that statins, when used as adjunct to mechanical treatment, promote additional improvements in clinical parameters. The pooled WMD (95% CI) for PPD, IBD, and CAL were, respectively, 1.93 mm (1.44; 2.41), 1.54 mm (1.24; 1.84), and 1.82 mm (1.24; 2.41) (Figs. S1, S4, and S7). Despite these interesting findings, a higher heterogeneity was detected in all the analyses. Therefore, due to the lack of consistency, the clinical relevance of these results should be interpreted with caution, as it may not be directly translated into the clinical practice. Similar results are also reported in a recent published systematic review [56]. This study showed a pooled WMD (95% CI) for PPD and CAL of, respectively, 1.51 mm (1.04; 1.97) and 1.84 mm (1.37; 2.31). High heterogeneity was also demonstrated in this systematic review. Moreover, this study showed that rosuvastatin and a longer follow-up period were associated with improved periodontal outcomes [56]. However, no meta-regression was detailed to address the heterogeneity, which is one of the main strength of the present systematic review.
Additionally, the GRADE evaluation showed that evidence provided for the studies included in the meta-analyses was ranked as low quality. Another point to be raised is statistical versus clinical significance. This fact needs to be individually interpreted. However, a statistically significant difference in such a pool of studies at least points out for a strong tendency of better results. The clinical extrapolation of them needs to consider other points of evidence-based approaches, such as the skills of the professional and the preferences and beliefs of the patients.
Overall, in the meta-regression, all the tested statins (simvastatin, atorvastatin, and rosuvastatin) showed additional benefits in reducing PPD. However, simvastatin was the only drug able to additionally improve CAL gain when compared to a placebo gel. The higher number of studies using this statin may explain this result.
The use of other drugs as an adjunct to mechanical periodontal treatment is also reported in the literature. Local and systemic antibiotics are largely studied. Overall, despite of their statistically significant improvements in periodontal parameters, this additional benefit is small and may not represent a clinical benefit. For example, a systemic review with meta-analysis showed that the use of systemic amoxicillin/metronidazole, as adjunct to mechanical periodontal treatment, promote significantly CAL gain (WMD = 0.21; 95% CI = 0.02 to 0.4) and PPD reduction (WMD = 0.43; 95% CI = 0.24 to 0.63) [57]. Additionally, the subgingival application of antimicrobials, adjunctive to periodontal treatment, also showed a significantly improvement in PPD reduction and CAL gain, with a WMD of 0.407 and 0.310, respectively [58]. Comparing with these results of the present meta-analyses, the magnitude of CAL gain and PPD reduction was higher with the adjunct use of statins than with the adjunct use of antimicrobials.
All kinds of statin administration were included to give a broader view of this drug when associated with mechanical periodontal therapy. Of the 15 studies included in this systematic review, 11 used locally delivered statin. From these, 9 were performed by the same research group in India. All those studies were included in at least one of the performed meta-analyses/meta-regression. Additionally, these 11 studies performed periodontal treatment and the gel application in only one/two sites of each participant. These facts should be put in perspective when analyzing the quantitative data presented in this study, as a research center effect may be expected and one/two sites treatment may not represent the periodontal treatment as a whole.
The study of Surve et al. (2015) [39] was the only study included in this systematic review that did not show an additional benefit of locally delivered statin to any periodontal parameter evaluated. This study was not included in the quantitative analysis, as the data extraction could not be performed in a standardized manner. Despite its methodology similarities to the included ones, this study did not present a sample size calculation, and used only 15 sites on each group. Additionally, it should be highlighted that this study presented a critical/serious risk of bias in several domains of the ROBINS-I tool (Table 2). Those observations may explain the discrepancies between the studies.
The most common side effects related to statin are muscle toxicity with myopathy and rhabdomyolysis, which occur in patients using higher statin doses or drugs that interact with the hepatic metabolism [59]. Almost of all the included studies in the systematic review showed no adverse events related to use of statin either locally delivered or systemically. This is in agreement with the literature that states that statins are well tolerated and the adverse events are rare [60].
The results herein presented are challenging in terms of interpretation. However, the possible benefits of the adjunct use of statins in periodontal therapy should be considered, with further clinical studies exploring this hypothesis, especially due to some of the limitations of the included studies, with a high degree of heterogeneity and the possible strong research center effect.
Conclusion
It was concluded that statins, used as sole adjuncts to mechanical periodontal treatment, additionally improved at least one of the following periodontal parameters: probing pocket depth, clinical attachment level, and intrabony defect. The use of locally delivered statins in periodontal pockets is largely studied. Within the limits of this review, in the meta-regression analyses, simvastatin was the only drug that showed additional benefits in probing depth, clinical attachment level, and intrabony defect, when compared to the groups without statin.
References
Baigent C, Keech A, Kearney PM et al (2005) Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 366:1267–1278
Maron DJ, Fazio S, Linton MF (2000) Current perspectives on statins. Circulation 101:207–213
Todd PA, Goa KL (1990) Simvastatin. A review of its pharmacological properties and therapeutic potential in hypercholesterolaemia. Drugs 40(4):583–607
Zhou Q, Liao JK (2009) Statins and cardiovascular diseases: from cholesterol lowering to pleiotropy. Curr Pharm Des 15(5):467–478. https://doi.org/10.2174/138161209787315684
Ramfjord SP, Knowles JW, Nissle RR, Burgett FG, Shick RA (1975) Results following three modalities of periodontal therapy. J Periodontol 46(9):522–526. https://doi.org/10.1902/jop.1975.46.9.522
Deas DE, Moritz AJ, Sagun RS, Gruwell SF, Powell CA (2016) Scaling and root planing vs. conservative surgery in the treatment of chronic periodontitis. Periodontol 2000 71:128–139
Muniz FW, de Oliveira CC, de Sousa Carvalho R, Moreira MM, de Moraes ME, Martins RS (2013) Azithromycin: a new concept in adjuvant treatment of periodontitis. Eur J Pharmacol 705(1-3):135–139. https://doi.org/10.1016/j.ejphar.2013.02.044
Caton JG, Greenstein G (1993) Factors related to periodontal regeneration. Periodontol 2000 1:9–15
Dye BA (2012) Global periodontal disease epidemiology. Periodontol 58(1):10–25. https://doi.org/10.1111/j.1600-0757.2011.00413.x
Chen J, Chen Q, Hu B, Wang Y, Song J (2016) Effectiveness of alendronate as an adjunct to scaling and root planing in the treatment of periodontitis: a meta-analysis of randomized controlled clinical trials. J Periodontal Implant Sci 46(6):382–395. https://doi.org/10.5051/jpis.2016.46.6.382
Estanislau IM, Terceiro IR, Lisboa MR, Teles PB, Carvalho RS, Martins RS, Moreira MM (2015) Pleiotropic effects of statins on the treatment of chronic periodontitis—a systematic review. Br J Clin Pharmacol 79(6):877–885. https://doi.org/10.1111/bcp.12564
Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, Boyce B, Zhao M, Gutierrez G (1999) Stimulation of bone formation in vitro and in rodents by statins. Science 286(5446):1946–1949. https://doi.org/10.1126/science.286.5446.1946
Mennickent CS, Bravo DM, Calvo MC, Avello LM (2008) Pleiotropic effects of statins. Rev Med Chil 136:775–782
Kavalipati N, Shah J, Ramakrishan A, Vasnawala H (2015) Pleiotropic effects of statins. Indian J Endocrinol Metab 19(5):554–562. https://doi.org/10.4103/2230-8210.163106
Adam O, Laufs U (2008) Antioxidative effects of statins. Arch Toxicol 82(12):885–892. https://doi.org/10.1007/s00204-008-0344-4
Cicek Ari V, Ilarslan YD, Erman B, Sarkarati B, Tezcan I, Karabulut E, Oz SG, Tanriover MD, Sengun D, Berker E (2016) Statins and IL-1β, IL-10, and MPO levels in gingival crevicular fluid: preliminary results. Inflammation 39(4):1547–1557. https://doi.org/10.1007/s10753-016-0390-7
Lindy O, Suomalainen K, Mäkelä M, Lindy S (2008) Statin use is associated with fewer periodontal lesions: a retrospective study. BMC Oral Health 8(1):16. https://doi.org/10.1186/1472-6831-8-16
Cunha-Cruz J, Saver B, Maupome G, Hujoel PP (2006) Statin use and tooth loss in chronic periodontitis patients. J Periodontol 77(6):1061–1066. https://doi.org/10.1902/jop.2006.050280
Saxlin T, Suominen-Taipale L, Knuuttila M, Alha P, Ylöstalo P (2009) Dual effect of statin medication on the periodontium. J Clin Periodontol 36:997–1003
Saver BG, Hujoel PP, Cunha-Cruz J, Maupomé G (2007) Are statins associated with decreased tooth loss in chronic periodontitis? J Clin Periodontol 34(3):214–219. https://doi.org/10.1111/j.1600-051X.2006.01046.x
de Mones E, Schlaubitz S, Catros S, Fricain JC (2015) Statins and alveolar bone resorption: a narrative review of preclinical and clinical studies. Oral Surg Oral Med Oral Pathol Oral Radiol 119(1):65–73. https://doi.org/10.1016/j.oooo.2014.09.030
Sterne JA, Hernán MA, Reeves BC (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919. https://doi.org/10.1136/bmj.i4919
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, C.B.M. Group, C.S.M. Group (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343(oct18 2):d5928. https://doi.org/10.1136/bmj.d5928
Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A (2011) GRADE guidelines: a new series of articles in the journal of clinical epidemiology. J Clin Epidemiol 64(4):380–382. https://doi.org/10.1016/j.jclinepi.2010.09.011
Higgins JPT, Green S (editors) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org. Chichester, UK: John Wiley & Sons, Ltd. Acessed 22th Nov 2016
Harbord R, Higgins J (2008) Meta-regression in Stata. Stata J 8:493–519
Sterne J, Bradburn M, Egger M (2008) Meta-analysis in StataTM. In: Egger M, Smith G, Altman D (eds) Systematic review in health care, 2nd edn. BMJ Publishing Group, London, pp. 347–369
Fajardo ME, Rocha ML, Sanchez-Marin FJ, Espinosa-Chavez EJ (2010) Effect of atorvastatin on chronic periodontitis: a randomized pilot study. J Clin Periodontol 37(11):1016–1022. https://doi.org/10.1111/j.1600-051X.2010.01619.x
Fentoglu O, Kirzioglu FY, Ozdem M, Kocak H, Sutcu R, Sert T (2012) Proinflammatory cytokine levels in hyperlipidemic patients with periodontitis after periodontal treatment. Oral Dis 18(3):299–306. https://doi.org/10.1111/j.1601-0825.2011.01880.x
Sangwan A, Tewari S, Singh H, Sharma RK, Narula SC (2016) Effect of hyperlipidemia on response to nonsurgical periodontal therapy: statin users versus nonusers. Eur J Dent 10(1):69–76. https://doi.org/10.4103/1305-7456.175685
Rosenberg DR, Andrade CX, Chaparro AP, Inostroza CM, Ramirez V, Violant D, Nart J (2015) Short-term effects of 2% atorvastatin dentifrice as an adjunct to periodontal therapy: a randomized double blind clinical trial. J Periodontol 86(5):623–630. https://doi.org/10.1902/jop.2015.140503
Pradeep AR, Thorat MS (2010) Clinical effect of subgingivally delivered simvastatin in the treatment of patients with chronic periodontitis: a randomized clinical trial. J Periodontol 81(2):214–222. https://doi.org/10.1902/jop.2009.090429
Pradeep AR, Priyanka N, Kalra N, Naik SB, Singh SP, Martande S (2012) Clinical efficacy of subgingivally delivered 1.2-mg simvastatin in the treatment of individuals with class II furcation defects: a randomized controlled clinical trial. J Periodontol 83(12):1472–1479. https://doi.org/10.1902/jop.2012.110716
Rath A, Mahenra J, Thomas L, Sandhu M, Namasi A, Ramakrishna T (2012) A clinical, radiological and IL-6 evaluation of subgingivally delivered simvastatin in the treatment of chronic periodontitis. Int J Drug Deliv 4:70–81
Pradeep AR, Rao NS, Bajaj P, Kumari M (2013) Efficacy of subgingivally delivered simvastatin in the treatment of patients with type 2 diabetes and chronic periodontitis: a randomized double-masked controlled clinical trial. J Periodontol 84(1):24–31. https://doi.org/10.1902/jop.2012.110721
Pradeep AR, Kumari M, Rao NS, Martande SS, Naik SB (2013) Clinical efficacy of subgingivally delivered 1.2% atorvastatin in chronic periodontitis: a randomized controlled clinical trial. J Periodontol 84(7):871–879. https://doi.org/10.1902/jop.2012.120393
Rao NS, Pradeep AR, Bajaj P, Kumari M, Naik SB (2013) Simvastatin local drug delivery in smokers with chronic periodontitis: a randomized controlled clinical trial. Aust Dent J 58(2):156–162. https://doi.org/10.1111/adj.12042
Pradeep AR, Karvekar S, Nagpal K, Patnaik K, Guruprasad CN, Kumaraswamy KM (2015) Efficacy of locally delivered 1.2% rosuvastatin gel to non-surgical treatment of patients with chronic periodontitis: a randomized, placebo-controlled clinical trial. J Periodontol 86(6):738–745. https://doi.org/10.1902/jop.2015.140631
Surve SM, Acharya AB, Thakur SL (2015) Efficacy of subgingivally delivered atorvastatin and simvastatin as an adjunct to scaling and root planing. Drug Metabol Personal Ther 30(4):263–269. https://doi.org/10.1515/dmpt-2015-0024
Kumari M, Martande SS, Pradeep AR (2016) Subgingivally delivered 1.2% atorvastatin in the treatment of chronic periodontitis among smokers: a randomized, controlled clinical trial. J Investig Clin Dent 8(2):e12213. https://doi.org/10.1111/jicd.12213
Pradeep AR, Garg V, Kanoriya D, Singhal S (2016) 1.2% Rosuvastatin versus 1.2% atorvastatin gel local drug delivery and re-delivery in treatment of intrabony defects in chronic periodontitis: a randomized placebo controlled clinical trial. J Periodontol 87(7):756–762. https://doi.org/10.1902/jop.2016.150706
Pradeep AR, Kanoriya D, Singhal S, Garg V, Manohar B, Chatterjee A (2016) Comparative evaluation of subgingivally delivered 1% alendronate versus 1.2% atorvastatin gel in treatment of chronic periodontitis: a randomized placebo-controlled clinical trial. J Investig Clin Dent 8(3):e12215. https://doi.org/10.1111/jicd.12215
Fentoğlu Ö, Kirzioğlu FY, Tözüm Bulut M et al (2015) Serum Lp-PLA2: as a novel viewpoint in periodontal treatment of hyperlipidaemics. Turk J Med Sci 45(3):619–626. https://doi.org/10.3906/sag-1406-75
Sirtori CR (2014) The pharmacology of statins. Pharmacol Res 88:3–11. https://doi.org/10.1016/j.phrs.2014.03.002
Viereck V, Gründker C, Blaschke S et al (2005) Atorvastatin stimulates the production of osteoprotegerin by human osteoblasts. J Cell Biochem 96:1244–1253
Blanco-Colio LM, Tuñón J, Martín-Ventura JL, Egido J (2003) Anti-inflammatory and immunomodulatory effects of statins. Kidney Int 63(1):12–23. https://doi.org/10.1046/j.1523-1755.2003.00744.x
Sakoda K, Yamamoto M, Negishi Y, Liao JK, Node K, Izumi Y (2006) Simvastatin decreases IL-6 and IL-8 production in epithelial cells. J Dent Res 85(6):520–523. https://doi.org/10.1177/154405910608500608
Jerwood S, Cohen J (2008) Unexpected antimicrobial effect of statins. J Antimicrob Chemother 61(2):362–364. https://doi.org/10.1093/jac/dkm496
Kamio K, Liu XD, Sugiura H, Togo S, Kawasaki S, Wang X, Ahn Y, Hogaboam C, Rennard SI (2010) Statins inhibit matrix metalloproteinase release from human lung fibroblasts. Eur Respir J 35(3):637–646. https://doi.org/10.1183/09031936.00134707
Dalcico R, de Menezes AM, Deocleciano OB et al (2013) Protective mechanisms of simvastatin in experimental periodontal disease. J Periodontol 84(8):1145–1157. https://doi.org/10.1902/jop.2012.120114
Goes P, Lima NA, Rodrigues JA, Benevides NM, Brito GA, Lima V (2016) Anti-inflammatory and anti-resorptive effects of atorvastatin on alveolar bone loss in Wistar rats. Braz Dent J 27(3):267–272. https://doi.org/10.1590/0103-6440201600600
Thapa S, Wei F (2016) The association between high serum Total cholesterol and periodontitis—an NHANES 2011–2012 study of American adults. J Periodontol 87(11):1286–1294. https://doi.org/10.1902/jop.2016.150648
Zhou X, Zhang W, Liu X, Li Y (2015) Interrelationship between diabetes and periodontitis: role of hyperlipidemia. Arch Oral Biol 60(4):667–674. https://doi.org/10.1016/j.archoralbio.2014.11.008
Pragati S, Ashok S, Kuldeep S (2011) Recent advances in periodontal drug delivery systems. Int. J Drug Deliv 1:1–14
Needleman IG, Pandya NV, Smith SR, Foyle DM (1995) The role of antibiotics in the treatment of periodontitis (part 2—controlled drug delivery). Eur J Prosthodont Restor Dent 3(3):111–117
Bertl K, Parllaku A, Pandis N, Buhlin K, Klinge B, Stavropoulos A (2017) The effect of local and systemic statin use as an adjunct to non-surgical and surgical periodontal therapy—a systematic review and meta-analysis. J Dent 67:18–28. https://doi.org/10.1016/j.jdent.2017.08.011
Sgolastra F, Gatto R, Petrucci A, Monaco A (2012) Effectiveness of systemic amoxicillin/metronidazole as adjunctive therapy to scaling and root planing in the treatment of chronic periodontitis: a systematic review and meta-analysis. J Periodontol 83(10):1257–1269. https://doi.org/10.1902/jop.2012.110625
Matesanz-Pérez P, García-Gargallo M, Figuero E, Bascones-Martínez A, Sanz M, Herrera D (2013) A systematic review on the effects of local antimicrobials as adjuncts to subgingival debridement, compared with subgingival debridement alone, in the treatment of chronic periodontitis. J Clin Periodontol 40(3):227–241. https://doi.org/10.1111/jcpe.12026
Jeger R, Dieterle T (2012) Statins: have we found the holy grail? Swiss Med Wkly 142:w13515
Schachter M (2005) Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol 19(1):117–125. https://doi.org/10.1111/j.1472-8206.2004.00299.x
Funding
This study was self-funded by the authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical approval does not apply to systematic reviews.
Informed consent
Informed consent does not apply to systematic reviews.
Electronic supplementary material
Figure S1
Forest plot of PPD reduction. (GIF 25 kb)
Figure S2
Funnel plot of the risk of bias analysis of pocket depth at 6-months follow-up. (GIF 10 kb)
Figure S3
Cumulative meta-analysis of pocket depth at 6-months follow-up. (GIF 17 kb)
Figure S4
Forest plot of IBD decrease. (GIF 18 kb)
Figure S5
Funnel plot of the risk of bias analysis of intrabony defect at 6-months follow-up. (GIF 7 kb)
Figure S6
Cumulative meta-analysis of intrabony defect at 6-months follow-up. (GIF 2 kb)
Figure S7
Forest plot of CAL gain. (GIF 18 kb)
Figure S8
Funnel plot of the risk of bias analysis of clinical attachment level at 6-months follow-up. (GIF 9 kb)
Figure S9
Cumulative meta-analysis of clinical attachment level at 6-months follow-up. (GIF 17 kb)
Rights and permissions
About this article
Cite this article
Muniz, F.W.M.G., Taminski, K., Cavagni, J. et al. The effect of statins on periodontal treatment—a systematic review with meta-analyses and meta-regression. Clin Oral Invest 22, 671–687 (2018). https://doi.org/10.1007/s00784-018-2354-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-018-2354-9