Abstract
Meta-analysis of treatment effects of antimicrobial photodynamic therapy (aPDT) adjunct to non-surgical scaling and root planing (SRP) in comparison to SRP alone on patients with chronic periodontitis. The meta-analysis was performed according to PRISMA statement and Cochrane Collaboration guidelines. Electronic search complemented by hand search assured a high yield of randomized controlled trials (RCTs) of aPDT as adjunct modality to SRP. Differences in probing depth (PD) and clinical attachment level (CAL) were calculated with 95% confidence intervals and pooled in a random effects model. Analysis for intra- and inter-study heterogeneity was provided by χ 2 and I 2 tests, and publication bias was checked by funnel plots. Pooled overall effects of 26 RCTs attested significant benefits of aPDT adjunct to SRP with respect to PD reduction (MD 0.37; 95% CI 0.12–0.53; P < 0.0001) and CAL gain (MD 0.33; 95% CI 0.19–0.48; P < 0.00001) after 3 and 6 months. Sensitivity analysis minimized heterogeneity of PD reduction (MD 0.21; 95% CI 0.13–0.30; P < 0.00001) and CAL gain (MD 0.36; 95% CI 0.27–0.46). aPDT adjunct to SRP provides significant PD reduction and CAL gain in treatment of chronic periodontitis. This moderate effect was found after 3 and 6 months which is short from a clinical perspective.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The oral cavity harbors over 700 bacterial species, organized in a complex polymicrobial biofilm that plays a major role in the etiology of periodontitis, periimplantitis, and caries [1, 2]. A “microbial shift” in the biofilm can cause disturbed hemostasis and destructive immunological host responses [3, 4]. This dysbiosis may cause periodontitis, which is characterized by gingival inflammation associated with bleeding, formation of periodontal pockets, periodontal ligament destruction, and tooth loss [1, 4, 5]. Standard therapy is non-surgical removal of the biofilm by SRP [6]. SRP usually leads to clinical improvement and a healthy microbiota [7, 8], but its effectiveness can be compromised by deep pockets, complex root anatomy [6], or bacterial invasion of hard and soft tissues [9]. Treatment can be further compromised by modified adhesion of the polymicrobial biofilm to the root surface and increased resistance against antimicrobial agents [3]. Antibiotic-resistant strains make the adjunct use of antibiotics controversial and should be saved for cases of severe periodontitis [8, 10, 11], and therefore, alternative treatment modalities are needed such as aPDT.
The effects of aPDT are based on three components: light, a photoactive agent (photosensitizer), and the presence of oxygen [12]. Photosensitizers can selectively be incorporated by bacteria, viruses, and/or fungi, whereas host cells remain unaffected. Photosensitizer activation by light of a specific wavelength in the presence of oxygen can produce reactive oxygen species (ROS), such as singlet oxygen, which are cytotoxic to microorganisms [13, 14] in an unspecific manner, as ROS attack various functional systems and pathways, and thus, development of resistance or compromising side effects are unlikely [13, 14]. To evaluate the effectiveness of aPDT as adjunct therapy in chronic periodontitis in an evidence-based manner, four systematic reviews including meta-analysis have already been published in the period of 2009–2013 [15,16,17,18]. The major issue of these reviews was the limited number of studies that were available and methodological bias. After their publication, a number of new studies have been published. The impact of the increased number of clinical studies on the efficiency of aPDT as adjunct therapy of chronic periodontitis is subject of the present systematic review and meta-analysis.
Materials and methods
Inclusion criteria
This systematic review and meta-analysis follows the guidelines of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analysis) Statement [19] and the Cochrane Collaboration (Cochrane Handbook for Systematic Reviews of Interventions 5.1.0., http://www.cochrane-handbook.org ). To address the a priori PICO (Participant, Intervention, Comparison, Outcome) question [20], “Does aPDT adjunct to non-surgical SRP lead to improvements in terms of PD and CAL in patients with chronic periodontitis compared to SRP alone?”, the following inclusion criteria were formulated:
-
Types of studies:
Randomized-controlled clinical trials with ≥ 3-month follow-up in split-mouth or parallel-group design.
-
Types of participants:
Adults over 18 years with chronic periodontitis as defined the “International Workshop for a Classification of Periodontal Diseases and Conditions” [21]. Smokers were included and patients with aggressive periodontitis, systematic diseases, patients taking systemically antibiotics, or other medications that potentially affect periodontal treatment were excluded. Treatments additional to SRP and aPDT, e.g., laser debridement, surgical periodontal therapy, or local antibiotics, entailed exclusion as well.
-
Types of intervention:
aPDT as adjunct treatment to SRP versus SRP alone or SRP in combination with placebo. Single or multiple application of aPDT, irrespective of specific parameters, e.g., concentration or type of photosensitizer, irradiation time, or light source settings.
-
Types of outcome measures:
Primary outcomes: alterations in PD and CAL from baseline measurement to the end of follow-up.
Search methods for the identification of studies
The objective of the search was to identify all relevant clinical trials; thus, restrictions with respect to language or publication date were not applied. High sensitivity of the electronic search was achieved by logical connection (Boolean operators) of relevant free-text keywords with database-specific Mesh terms. The individual search algorithms, developed for MEDLINE, EMBASE, Web of Science, LILACS, the Cochrane Oral Health Group Trials Register, and Cochrane Central Register of Controlled Trials (CENTRAL) are summarized in Appendix 1. The electronic search was conducted from 1 January 2000 to 21 September 2016. Additionally, a hand search of major periodontal and laser journals was undertaken. Related review articles and reference lists of all identified articles were searched for further studies. Abstracts of the International Association for Dental Research (IADR), American Academy of Periodontology (AAP), and European Federation of Periodontology (EFP) were screened for unpublished material, and contacts with authors provided information on studies in press or missing data of studies included (Appendix 2).
Data collection and analysis
Study selection and data extraction
Study selection, validation of eligibility, and quality assessment were performed by two blinded, independent reviewers (A.A. and S.D.) to reduce potential reviewer bias. Agreement between the authors was calculated by Kappa statistics [22]. Disagreement was solved on the basis of discussion. Titles and abstracts were screened independently. After examining all identified records for eligibility and removing duplicates, a form was used to extract relevant data from selected full-text publications and their quality was assessed in duplicate by both reviewers (Appendix 3). The following data were retrieved from the publications: author and year of publication, population, gender, age, smokers/non-smokers, follow-up, initial severity of periodontal disease, specific laser parameters, type of photosensitizer, and changes in PD and CAL from baseline to the end of the follow-up period after treatment in test and control groups. Quality assessment was based on modified criteria derived from the Cochrane Collaboration’s tool for assessing risk of bias and the CONSORT Statement [23, 24]. Criteria included masking of randomization, intervention and outcome assessment, completeness of follow-up outcome, comparable test and control groups at baseline of the trial, non-selective reporting, inclusion/exclusion criteria, sample size calculation, and other sources of bias. Each of the potential risks of bias were rated with “met”, “not-met” or “unclear” and were summarized as three final risk classifications with corresponding κ-scores: low risk, when all criteria were met; moderate risk, when one or more criteria were unclear; high risk, when one or more criteria were not met (Appendix 3).
Data analysis
Data of interest for the meta-analysis were continuous mean differences (MDs) and standard deviations (SDs) for PD and CAL (confidence interval (CI) of 95%). When the information was presented in median and interquartile ranges, the means and SDs were estimated [25]. Statistical analysis was performed with RevMan Version 5.1. (Cochrane Collaboration, Copenhagen, Denmark). MDs, CIs, and SEs of studies with split-mouth and parallel-group design were combined in subgroups in the meta-analysis [18, 26]. The pooled overall effect was considered significant when P < 0.05. Individual study weight on patient basis was calculated with generic inverse variance for continuous data. Random-effects model and Z-statistics were selected for analysis because of the possible strong heterogeneity. Variance imputation methods were applied to calculate missing standard deviations of MD in split-mouth studies which did not provide this information [27]. The following formula was the basis of the calculation of the intra-patient correlation coefficient R from studies with complete data [27]:
Heterogeneity was assessed by visual inspection of the forest plots in combination with the χ 2-based Q statistic method to determine intra-study heterogeneity [28] and Higgins I 2 to determine inter-study heterogeneity [29]. Because of the moderate insensitivity of Q statistics, P < 0.1 was considered to indicate significant heterogeneity [28]. I 2 statistics were expressed in a range of 0–100%, with 0% indicating no evidence for heterogeneity and ≥ 75% indicating high level of heterogeneity [30]. A sensitivity analysis was conducted to identify outlier studies which were responsible for heterogeneity. Publication bias was determined by visual analysis of the funnel plots for PD and CAL [31]. Besides the pooled effects at the last follow-up check, subgroups for 3 and 6 months and for activation of the photosensitizer were formed. Subgroups of low- and high-risk studies were analyzed to investigate the influence of study quality on the effect size.
Results
Characteristics of included studies
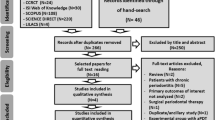
The search strategy yielded 362 references after duplicates were removed. The hand search did not identify further studies. Of the 362 references, 179 met the inclusion criteria on the basis of title screening (κ = 0.72). On the basis of abstracts, 65 references remained eligible for full-text examination (κ = 0.92), which lead to exclusion of 36 studies (κ = 0.90). Frequently occurring reasons for exclusion were study design, systematic diseases, or aggressive periodontitis and treatment modalities. The rejected studies and the reasons for rejection are summarized in Appendix 4. Finally, 29 studies were included for a qualitative systematic review [29, 32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47, 50,51,52,53,54,55,56,57,58,59,60,61]. A summary of their characteristics is shown in Table 1, and PRISMA flow diagram for the study selection process is shown in Appendix 2. Ultimately, three trials had to be excluded from the quantitative meta-analysis because of ambiguities in provided data [47, 59] and heterogeneity in the treatment protocol [52]. Therefore, 26 studies were investigated in the quantitative meta-analysis. All studies included were RCTs published between 2007 and 2016. Twenty of the 26 studies were published after 2012 indicating increasing interest in aPDT for treatment of chronic periodontitis. Twenty studies had a split-mouth design, 11 had two treatment arms, and 2 had four arms (Table 1). Nine studies had a parallel-group design with seven studies with two treatment arms and two with three arms. The 26 included studies comprised 755 patients. Thirteen studies reported a follow-up of 3 months and ten of 6 months whereas three studies evaluated the clinical parameters after 1 year (Table 1).
The most frequently occurring treatment modality was a single session of aPDT adjunctive to SRP, whereas only two studies applied aPDT twice in a separate treatment arm in comparison to single treatment. Three studies applied three aPDT cycles, two studies 4 cycles, and one study performed 5 cycles (Table 1). Apart from three studies using toluidine blue O as photosensitizer, three used indocyanine green and one study curcumin, whereas all other studies applied methylene blue/phenothiazine chloride (Table 1). Standard light sources were diode lasers with wavelengths in the range of 650–810 nm and irradiation periods of 20–180 s. Three studies used LED lamps for activation [36, 55, 58].
None of the studies reported adverse effects or discomfort during the application of aPDT. Inclusion criteria for chronic periodontitis varied with PD ≥ 3 mm in 2 studies, ≥ 4 mm in 7 studies, ≥ 5 mm in 14 studies, ≥ 6 mm in 1 study, 4–6 mm in 2 studies, 4–7 mm in 1 study, and 6–9 mm in 2 studies (Table 1). Fifteen studies excluded smokers, 8 studies included smokers, 2 studies included exclusively smokers, and 4 studies did not provide any information about on smoking or not (Table 1).
Risk of bias in included studies
Quality assessment of the included studies was performed independently by two blinded clinicians (A.A. and S.D.). Fourteen RCTs were rated to have a high risk for bias, five had a moderate risk for bias, and ten had a low risk for bias (κ = 1; Table 1). All 29 RCTs were free from selective reporting and had precise inclusion/exclusion criteria. Randomization and allocation concealment were not given in one study and was unclear in three studies. Blinding of patient and personnel was not adequate in four studies and unclear in two studies. Completeness of follow-up or reasons for dropout were not described in four studies and unclear in three studies. Four studies had an insufficient description of the sample size calculation, and in nine studies, a calculation was not mentioned at all. Therefore, missing sample size calculation was the dominant methodological flaw. A summary of the quality assessment of the 29 studies included is presented in Appendix 3.
Effects of intervention
Interpolation of missing data
For some split-mouth studies, standard deviations and standard errors were interpolated [27]. Reasonable intra-patient correlation coefficients R for PD were calculated on the basis of data from Dilsiz et al. [46], Berakdar et al. [37], and Queiroz et al. [56]. Only Dilsiz et al. [46] provided the necessary information from the raw data to obtain an R value for CAL. Imputations with an averaged R value of 0.5 for PD and 0.43 for CAL were finally applied in order to pool the primary outcomes. These values are in accordance with Follmann et al. [27], who calculated that 0.5 is a proper assumption for imputation. Additionally, a sensitivity analysis with a conservative R of 0 was conducted and showed nearly the same effect size. A meta-analysis on secondary outcomes was not possible, due to variation in scoring methodology or incomplete data.
Overall treatment effects
Primary outcomes of 26 studies were pooled for a follow-up of 3 or 6 months, respectively. Adjunct aPDT resulted in a significant PD reduction (MD 0.37; 95% CI 0.20–0.53; P < 0.0001) and CAL gain (MD 0.33; 95% CI 0.19–0.48; P < 0.00001) in comparison to SRP alone. χ 2-based Q statistics and I 2 test revealed a significant high grade of heterogeneity in PD reduction (χ 2 = 203.11; P < 0.00001; I 2 = 86%) and CAL gain (χ 2 = 73.81; P < 0.00001; I 2 = 65%). Mean difference of PD reduction was significant in the subgroup analysis of parallel studies (MD 0.53; 95% CI 0.10–0.96; P = 0.01) with significant heterogeneity (χ 2 = 63.38; P < 0.00001; I 2 = 87%) and in split-mouth studies (MD 0.29; 95% CI 0.12–0.45; P = 0.0007) with significant heterogeneity (χ 2 = 112.35; P < 0.00001; I 2 = 83%). CAL gain was in both subgroups significantly higher after adjunct aPDT (MD 0.33 mm; 95% CI 0.16–0.51; P = 0.0002) for parallel studies and MD of 0.33 mm (95% CI 0.13–0.54; P = 0.001) for split-mouth studies. Heterogeneity was not significant in parallel studies (χ 2 = 11.37; P = 0.18; I2 = 30%), but significant in split-mouth studies (χ 2 = 62.44; P < 0.00001; I 2 = 73%) (Fig. 1).
Subgroups
Treatment effects at 3-, 6-, and 12-month follow-up
To investigate the effects of adjunct aPDT treatment, subgroups for a follow-up period of 3, 6, and 12 months were analyzed. Significant PD reduction (MD 0.40; 95% CI 0.23–0.58; P < 0.00001) and strong evidence for heterogeneity (χ 2 = 197.69; P < 0.00001; I 2 = 88%) was obtained by adjunct aPDT treatment after 3 months. After 6 months, PD reduction was significant for adjunct aPDT with a MD of 0.29 mm (95% CI 0.06–0.52; P = 0.01) and strong evidence for heterogeneity (χ 2 = 52.28; P < 0.00001; I 2 = 77%). CAL gain in adjunct aPDT group was 0.32 mm (95% CI 0.20–0.45; P < 0.00001) after 3 months and 0.39 mm (95% CI 0.07–0.71; P = 0.02) after 6 months.
Subgroup analysis of parallel studies showed a higher PD reduction of 0.46 mm (95% CI 0.07–0.85; P = 0.02) than in split-mouth studies (MD 0.36; 95% CI 0.17–0.56; P = 0.0003) after 3 months. After 6 months, PD reduction was significant for parallel studies (MD 0.40; 95% CI 0.02–0.78; P = 0.04), whereas the MD in split-mouth studies was not significant (MD 0.24; 95% CI − 0.04–0.52; P = 0.1).
The MD of CAL gain was lower in parallel studies (MD 0.25; 95% CI 0.09–0.4; P = 0.002) than in split-mouth studies (MD 0.39; 95% CI 0.2–0.58; P < 0.0001) after 3 months, but increased after 6 months (MD 0.39; 95% CI 0.12–0.67; P = 0.005) whereas the MD of CAL gain in split-mouth studies decreased very little but was not significant anymore (MD 0.37; 95% CI − 0.15–0.9; P = 0.16).
The pooled effects after 12 months were based on only two studies [33, 52]. PD reduction (MD 0.52; 95% CI − 0.17–1.21; P = 0.14) and CAL gain (MD 0.68; 95% CI 0.53–1.89; P = 0.27) were both not significant (Appendix Figs. 1, 2, and 3).
Period of incubation and activation time of the photosensitizer
Subgroups on the basis of period of incubation and activation time of the photosensitizer were formed to investigate effects of aPDT-specific parameters on clinical outcomes.
Pooled studies with an incubation time of 30 s showed a significant PD reduction after adjunct aPDT treatment (MD 0.28; 95% CI 0.01–0.55; P = 0.05) and non-significant heterogeneity, whereas an incubation time of 60 s gave a similar PD reduction that was not significant (MD 0.28; 95% CI − 0.01–0.57; P = 0.06) with strong heterogeneity (χ 2 = 51.22; P < 0.00001, I 2 = 84%). PD reduction in subgroups with an incubation period of 90 s or more were both significant (MD90 0.91; 95% CI 0.13–1.69; P = 0.02, and MD>90 0.14; 95% CI − 0.03–0.31; P = 0.0008). CAL gain was significant after 30-s incubation (MD 0.39; 95% CI 0.15–0.62; P = 0.001) with no heterogeneity, whereas 60-s incubation and over 90-s incubation did not result in significant CAL gain (MD60 0.38; 95% CI − 0.02–0.77; P = 0.06, and MD>90 0.17; 95% CI − 0.01–0.36; P = 0.07). MD after 90 s was significant (MD 0.96; 95% CI 0.2–1.72; P = 0.01) on the basis of one study (Appendix Fig. 4).
With respect to activation time, 60 s induced a significant reduction of PD (MD 0.36; 95% CI 0.18–0.54; P = 0.0001) and CAL gain (MD 0.31; 95% CI 0.16–0.47; P < 0.0001), but with strong heterogeneity. An activation time under 60 s or over 90 s did not result in significant PD reduction (MD<60s 0.13; 95% CI − 0.2–0.38; P = 0.3, and MD>90s − 013; 95% CI − 0.56–0.31; P = 0.57) or CAL gain (MD<60s 0.21; 95% CI − 0.35–0.78; P = 0.46, and MD>90s − 015; 95%CI − 0.68–0.38; P = 0.58) and was associated with strong heterogeneity (Appendix Fig. 5).
Low and high bias studies
Aspects of quality assessment on treatment outcomes were taken into account by analyzing subgroups of low bias and high bias studies. When comparing low bias and high bias studies, both groups showed a significant PD reduction and CAL gain by aPDT adjunct therapy. However, low bias studies showed a PD reduction of 0.31 mm (MD 0.31; 95% CI 0.05–0.57; P = 0.02) and a CAL gain of 0.28 mm (MD 0.28; 95% CI 0.07–0.48; P = 0.007) which is a lower effect size than high bias studies had with a PD reduction of 0.38 mm (MD 0.38; 95% CI 0.21–0.55; P < 0.0001) and CAL gain of 0.33 mm (MD 0.33; 95% CI 0.15–0.51; P = 0.0003; Appendix Figs. 6 and 7).
Sensitivity analysis
The occurrence of significant heterogeneity was the reason to conduct a sensitivity analysis by identifying outlier studies. After visual inspection of the Forest plots and exclusion of two parallel studies [38, 50] and eight split-mouth studies [35,34,37, 41, 45, 57, 58, 60], the overall PD reduction diminished from 0.37 mm to a significant overall effect of 0.21 mm (MD 0.21; 95% CI 0.13–0.30; P < 0.00001) after aPDT treatment without signs of heterogeneity (χ 2 = 14.57; P = 0.63; I 2 = 0%). Conversely, CAL gain increased from 0.33 mm to a significant 0.36 mm (95% CI 0.27–0.46; P < 0.00001) after adjusting, with no evidence for heterogeneity (χ 2 = 17.47; P = 0.56; I 2 = 0%). For CAL gain, one parallel group study [50] and six split-mouth studies were excluded [33,33,36, 45, 57, 60] (Fig. 2).
Publication bias
The funnel plots for overall PD reduction and CAL gain showed only slight asymmetries. Therefore, the risk of publication bias was estimated to be low (Appendix Fig. 8).
Discussion
Our systematic review and meta-analysis was aimed at the quantitative analysis of effectiveness of aPDT adjunct to SRP in the treatment of chronic periodontitis. For that purpose, an extended search strategy without language restriction with rigorous exclusion and inclusion criteria and quality assessment was performed. The primary outcomes calculated on the basis of 26 RCTs were pooled in a meta-analysis that showed significant PD reduction and CAL gain after SRP and adjunct aPDT. These findings showed significant heterogeneity, possibly due to heterogeneous demographic factors, such as severity of chronic periodontitis, as the initial PD varied widely, sample size, inclusion of smokers or not, and different follow-up procedures. Differences in aPDT protocols, such as number of cycles of aPDT, laser settings, and the photosensitizer applied (type, concentration, incubation time) may contribute to heterogeneity.
A confounder was the use of indocyanine as photosensitizer, because its antimicrobial effects are based on photothermal destruction of bacterial membranes rather than ROS induction [61]. Another confounder was the number of a PDT cycles, which was only considered in two studies with more than one treatment arms, both showed that repeated aPDT applications did not result in further clinical improvement [48, 51]. A carry-across effect by leakage of photosensitizer in split-mouth studies was considered by comparing split-mouth and parallel-group design in individual subgroups [26]. Some differences in effect size and heterogeneity between studies were found but these differences disappeared after removing outliers. Therefore, a carry-across effect seems unlikely.
The strong heterogeneity in subgroups urged the identification of outlier studies by visual inspection of Forest plots. After removing outlier studies, two parallel group studies [38, 50] and eight split-mouth studies [35,34,37, 41, 45, 57, 58, 60], heterogeneity was eliminated completely. After adjustment, PD reduction was 0.21 mm (originally 0.37 mm) and CAL 0.36 mm (originally 0.33 mm). Heterogeneity was for both outcomes not significant (I 2 = 0%).
Comparison of low bias and high bias studies showed quantitative differences in PD reduction and CAL gain. Both subgroups showed significant PD reduction and CAL gain, with a moderate difference of 0.07 mm for PD reduction and 0.05 mm for CAL gain in favor of high bias studies. After exclusion of outlier studies, the treatment effect on PD reduction had disappeared in low bias studies, whereas CAL gain was 0.07 mm higher than in high bias studies. Apparently, effects of study quality on PD reduction and CAL gain values cannot be ruled out when comparing effects of the 3- and 6-month studies.
Most studies reported a short follow-up period of 3 months, whereas ten studies had a follow-up of 6 months and only two studies had a follow-up period of 12 months. Therefore, subgroup analysis should be interpreted with caution, but PD reduction was stronger at 3-month follow-up than at 6-month follow-up. Particularly, the subgroup of split-mouth studies showed no significant difference after 6-month follow-up. In contrast, CAL gain increased between 3- and 6-month follow-up from 0.32 to 0.39 mm, whereas the subgroup of split-mouth studies again did not show differences. The two studies with 12-month follow-up showed contrary results [33, 52]. While Alwaeli et al. [33] found a significant PD reduction of 0.91 mm and CAL gain of 1.35 mm, PD and CAL did not change in the study of Lulic et al. [49]. These data suggest that long-term effectiveness of aPDT adjunct therapy to SRP is doubtful.
Subgroups on the basis of variations in effects of irradiation time and incubation time were also heterogeneous. Two studies reported photosensitizer activation during < 60 s and two studies during > 90 s. Both subgroups showed no significant PD reduction or CAL gain. All other studies used an irradiation time of 60 s with significant PD reduction and CAL gain.
The subgroup of studies with incubation time > 90 s also showed no PD reduction and CAL gain. Therefore, we conclude that irradiation time and incubation time of the photosensitizer of 60 s induce optimal effects on clinical parameters. Due to incomplete and unclear information on concentrations of photosensitizer, any further analysis was not possible.
In agreement with previous systematic reviews [15,16,17,18], none of the included studies reported side effects or discomfort for the patients. Therefore, application of aPDT adjunct to SRP of chronic periodontitis can be considered as save. A meta-analysis on secondary outcomes, e.g., gingiva recession, bleeding, and plaque indices, was not possible because of incomplete data or incomparable indices.
Four meta-analyses on the effects of aPDT adjunct to SRP of chronic periodontitis were identified [15,16,17,18]. Two of these included reviews [15, 16] were based on a low number of studies that were partially of low quality. These reviews showed strong heterogeneity and dealt with different types of periodontitis which lead to inconsistent estimates of treatment effects. Sgolastra et al. [17] included exclusively studies with parallel-group design to reduce heterogeneity. The positive effect of aPDT adjunct to SRP over a time span of 3–6 months correlated rather well with the findings of the present meta-analysis. The only difference was a non-significant PD reduction after 6 months, which was significant in the present analysis. The latest meta-analysis on this topic [18] was the first meta-analysis that combined split-mouth and parallel group studies of 360 patients. The findings of our meta-analysis are completely in agreement with the outcome of that meta-analysis [18], despite the fact that 12 additional RCTs were included and comprised over 700 patients. These findings show that adjunct aPDT has short-term effects only. It has to be investigated whether aPDT treatment repeats after, for example, 6 and 12 months the initial treatment can provide additional long-term benefits for the patients.
Conclusion
On the basis of our meta-analysis and that of Sgolastra et al. [18], it can be concluded that aPDT as adjunct treatment to SRP of chronic periodontitis has a modest but significant effect of 0.21 mm PD reduction and 0.36 mm CAL gain at 3-month follow-up than at 6-month follow-up. These data suggest that long-term effectiveness of aPDT adjunct therapy to SRP is doubtful and further investigations regarding laser settings, type of photosensitizer, number of applications, and various other aspects are necessary.
References
Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE (2005) Defining the normal bacterial flora of the oral cavity. J Clin Microbiol 43(11):5721–5732
Beikler T, Flemmig TF (2011) Oral biofilm-associated diseases: trends and implications for quality of life, systemic health and expenditures. Periodontol 55(1):87–103
Marsh PD, Moter A, Devine DA (2011) Dental plaque biofilms: communities, conflict and control. Periodontol 55(1):16–35
Berezow AB, Darveau RP (2011) Microbial shift and periodontitis. Periodontol 55(1):36–47
Page RC, Offenbacher S, Schroeder HE, Seymour GJ, Kornman KS (1997) Advances in the pathogenesis of periodontitis: summary of developments, clinical implications and future directions. Periodontol 14:216–248
Heitz-Mayfield LJA, Lang NP (2013) Surgical and nonsurgical periodontal therapy. Learned and unlearned concepts. Periodontol 62:218–231
Hung HC, Douglass CW (2002) Meta-analysis of the effect of scaling and root planing, surgical treatment and antibiotic therapies on periodontal probing depth and attachment loss. J Clin Periodontol 29(11):975–986
Flemmig TF, Beikler T. (2011) Control of oral biofilms. Periodontology 2000. 55(1):9–15.
How KY, Song KP, Chan KG (2016) Porphyromonas gingivalis: an overview of periodontopathic pathogen below the gum line. Front Microbiol 7:53
Ardila CM, Lopez MA, Guzman IC (2010) High resistance against clindamycin, metronidazole and amoxicillin in Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans isolates of periodontal disease. Med Oral Patol Oral Cir Bucal 15(6):E947–EE51
van Winkelhoff AJ, Winkel EG (2009) Antibiotics in periodontics: right or wrong? J Periodontol 80(10):1555–1558
Meyer-Betz F. (1913) Untersuchung über die biologische (photodynamische) Wirkung des Hämatoporphyrins und anderer Derivate des Blut-und Gallenfarbstoffes. Dtsch Arch Klin Med 112:476–503
Jori G, Fabris C, Soncin M, Ferro S, Coppellotti O, Dei D et al (2006) Photodynamic therapy in the treatment of microbial infections: basic principles and perspective applications. Lasers Surg Med 38(5):468–481
Wainwright M, Crossley KB (2004) Photosensitising agents—circumventing resistance and breaking down biofilms: a review. Int Biodeterior Biodegrad 53(2):119–126
Atieh MA (2010) Photodynamic therapy as an adjunctive treatment for chronic periodontitis: a meta-analysis. Lasers Med Sci 25(4):605–613
Azarpazhooh A, Shah PS, Tenenbaum HC, Goldberg MB (2009) The effect of photodynamic therapy for periodontitis: a systematic review and meta-analysis. J Periodontol 81(1):4–14
Sgolastra F, Petrucci A, Gatto R, Marzo G, Monaco A (2013) Photodynamic therapy in the treatment of chronic periodontitis: a systematic review and meta-analysis. Lasers Med Sci 28(2):669–682
Sgolastra F, Petrucci A, Severino M, Graziani F, Gatto R, Monaco A (2013) Adjunctive photodynamic therapy to non-surgical treatment of chronic periodontitis: a systematic review and meta-analysis. J Clin Periodontol 40(5):514–526
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical research ed) 339:b2700
Forrest JL, Miller SA. (2001) Enhancing your practice through evidence-based decision making: finding the best clinical evidence. J Evid Based Dent Pract 1(3):227–36
Armitage GC (1999) Development of a classification system for periodontal diseases and conditions. Ann Periodontol / Am Acad Periodontol 4(1):1–6
Landis JR, Koch GG (1977) An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics 33(2):363–374
Higgins JPT, Green S (2011). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from http://handbook.cochrane.org
Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D et al (2001) The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med 134(8):663–694
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5:13
Lesaffre E, Philstrom B, Needleman I, Worthington H (2009) The design and analysis of split-mouth studies: what statisticians and clinicians should know. Stat Med 28(28):3470–3482
Follmann D, Elliott P, Suh I, Cutler J (1992) Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol 45(7):769–773
Lau J, Ioannidis JP, Schmid CH (1997) Quantitative synthesis in systematic reviews. Ann Intern Med 127(9):820–826
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ (Clinical research ed) 327(7414):557–560
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558
Sterne JA, Egger M (2001) Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol 54(10):1046–1055
Al-Zahrani MS, Austah ON (2011) Photodynamic therapy as an adjunctive to scaling and root planing in treatment of chronic periodontitis in smokers. Saudi Medical Journal [Internet] 11:1183–1188
Alwaeli HA, Al-Khateeb SN, Al-Sadi A. (2015) Long-term clinical effect of adjunctive antimicrobial photodynamic therapy in periodontal treatment: a randomized clinical trial. Lasers Med Sci. 30(2):801–7
Andersen R, Loebel N, Hammond D, Wilson M (2007) Treatment of periodontal disease by photodisinfection compared to scaling and root planing. J Clin Dent [Internet] 2:34–38
Balata ML, de Andrade LP, Santos DB, Cavalcanti AN, Tunes Uda R, Ribeiro Edel P et al (2013) Photodynamic therapy associated with full-mouth ultrasonic debridement in the treatment of severe chronic periodontitis: a randomized-controlled clinical trial. J Appl Oral Sci Revista FOB 21(2):208–214
Bassir SH, Moslemi N, Jamali R, Mashmouly S, Fekrazad R, Chiniforush N et al (2013) Photoactivated disinfection using light-emitting diode as an adjunct in the management of chronic periodontitis: a pilot double-blind split-mouth randomized clinical trial. J Clin Periodontol 40(1):65–72
Berakdar M, Callaway A, Fakhr Eddin M, Ross A, Willershausen B (2012) Comparison between scaling-root-planing (SRP) and SRP/photodynamic therapy: six-month study. Head Face Med 8:12
Betsy J, Prasanth CS, Baiju KV, Prasanthila J, Subhash N (2014) Efficacy of antimicrobial photodynamic therapy in the management of chronic periodontitis: a randomized controlled clinical trial. J Clin Periodontol 41(6):573–581
Birang R, Shahaboui M, Kiani S, Shadmehr E, Naghsh N (2015) Effect of Nonsurgical Periodontal Treatment Combined With Diode Laser or Photodynamic Therapy on Chronic Periodontitis: A Randomized Controlled Split-Mouth Clinical Trial. Journal of lasers in medical sciences 6(3):112–119
Braun A, Dehn C, Krause F, Jepsen S (2008) Short-term clinical effects of adjunctive antimicrobial photodynamic therapy in periodontal treatment: a randomized clinical trial. Journal of Clinical Periodontology 35(10):877–884
Campos G, Pimentel S, Ribeiro F, Casarin RV, Cirano F, Saraceni CC et al (2013) The adjunctive effect of photodynamic therapy for residual pockets in single-rooted teeth: a randomized controlled clinical trial. Lasers Med Sci 28(1):317–324
Cappuyns I, Cionca N, Wick P, Giannopoulou C, Mombelli A (2012) Treatment of residual pockets with photodynamic therapy, diode laser, or deep scaling. A randomized, split-mouth controlled clinical trial. Lasers in Medical Science 27(5):979–986
Chondros P, Nikolidakis D, Christodoulides N, Rössler R, Gutknecht N, Sculean A (2009) Photodynamic therapy as adjunct to non-surgical periodontal treatment in patients on periodontal maintenance: a randomized controlled clinical trial. Lasers in Medical Science 24(5):681–688
Christodoulides N, Nikolidakis D, Chondros P, Becker J, Schwarz F, Rössler R, Sculean A (2008) Photodynamic Therapy as an Adjunct to Non-Surgical Periodontal Treatment: A Randomized, Controlled Clinical Trial. Journal of Periodontology 79(9):1638–1644
Correa MG, Oliveira DH, Saraceni CH, Ribeiro FV, Pimentel SP, Cirano FR, et al (2015) Short-term microbiological effects of photodynamic therapy in non-surgical periodontal treatment of residual pockets: a split-mouth RCT. Lasers Surg Med 48(10):944–950
Dilsiz A, Canakci V, Aydin T (2013) Clinical effects of potassium-titanyl-phosphate laser and photodynamic therapy on outcomes of treatment of chronic periodontitis: a randomized controlled clinical trial. J Periodontol. 84(3):278–286
Franco EJ, Pogue RE, Sakamoto LHT, Cavalcante LLM, Carvalho DR, de Andrade RV (2014) Increased expression of genes after periodontal treatment with photodynamic therapy. Photodiagn Photodyn Ther 11(1):41–47
Ge L, Shu R, Li Y, Li C, Luo L, Song Z et al (2010) Adjunctive effect of photodynamic therapy to scaling and root Planing in the treatment of chronic periodontitis. Photomed Laser Surg 29(1):33–37
Lulic M, Gorog IL, Salvi GE, Ramseier CA, Mattheos N, Lang NP (2009) One-year outcomes of repeated adjunctive photodynamic therapy during periodontal maintenance: a proof-of-principle randomized-controlled clinical trial. J Clin Periodontol. 36(8):661–666
Monzavi A, Chinipardaz Z, Mousavi M, Fekrazad R, Moslemi N, Azaripour A et al (2016) Antimicrobial photodynamic therapy using diode laser activated indocyanine green as an adjunct in the treatment of chronic periodontitis: a randomized clinical trial. Photodiagn Photodyn Ther 14:93–97
Muller Campanile VS, Giannopoulou C, Campanile G, Cancela JA, Mombelli A (2013) Single or repeated antimicrobial photodynamic therapy as adjunct to ultrasonic debridement in residual periodontal pockets: clinical, microbiological, and local biological effects. Lasers Med Sci 30(1):27–34
Petelin M, Perkič K, Seme K, Gašpirc B (2014) Effect of repeated adjunctive antimicrobial photodynamic therapy on subgingival periodontal pathogens in the treatment of chronic periodontitis. Lasers Med Sci 30(6):1647–56
Polansky R, Haas M, Heschl A, Wimmer G (2009) Clinical effectiveness of photodynamic therapy in the treatment of periodontitis. Journal of Clinical Periodontology 36(7):575–580
Pourabbas R, Kashefimehr A, Rahmanpour N, Babaloo Z, Kishen A, Tenenbaum HC, Azarpazhooh A (2014) Effects of Photodynamic Therapy on Clinical and Gingival Crevicular Fluid Inflammatory Biomarkers in Chronic Periodontitis: A Split-Mouth Randomized Clinical Trial. Journal of Periodontology 85(9):1222–1229
Pulikkotil SJ, Toh CG, Mohandas K, Leong KV (2016) Effect of photodynamic therapy on Aggregatibacter actinomycetemcomitans in periodontitis patients. A randomized split mouth clinical trial. Aust Dent J 61(4):440–445
Queiroz AC, Suaid FA, de Andrade PF, Oliveira FS, Novaes AB, Taba M, Palioto DB, Grisi MFM, Souza SLS (2015) Adjunctive effect of antimicrobial photodynamic therapy to nonsurgical periodontal treatment in smokers: a randomized clinical trial. Lasers in Medical Science 30(2):617–625
Srikanth K, Chandra RV, Reddy AA, Reddy BH, Reddy C, Naveen A (2015) Effect of a single session of antimicrobial photodynamic therapy using indocyanine green in the treatment of chronic periodontitis: a randomized controlled pilot trial. Quintessence international 46(5):391–400
Sreedhar A, Sarkar I, Rajan P, Pai J, Malagi S, Kamath V et al (2015) Comparative evaluation of the efficacy of curcumin gel with and without photo activation as an adjunct to scaling and root planing in the treatment of chronic periodontitis: a split mouth clinical and microbiological study. J Nat Sci Biol Med 6(Suppl 1):S102–S109
Sigusch BW, Engelbrecht M, Volpel A, Holletschke A, Pfister W, Schutze J (2010) Full-mouth antimicrobial photodynamic therapy in Fusobacterium nucleatum-infected periodontitis patients. J Periodontol 81(7):975–981
Theodoro LH, Silva SP, Pires JR, Soares GHG, Pontes AEF, Zuza EP et al (2012) Clinical and microbiological effects of photodynamic therapy associated with nonsurgical periodontal treatment. A 6-month follow-up. Lasers Med Sci 27(4):687–693
Kranz S, Huebsch M, Guellmar A, Voelpel A, Tonndorf-Martini S, Sigusch BW (2015) Antibacterial photodynamic treatment of periodontopathogenic bacteria with indocyanine green and near-infrared laser light enhanced by Trolox(TM). Lasers Surg Med 47(4):350–360
Funding
No funding from sources other than the Department of Operative Dentistry and Periodontology of the University Medical Center Mainz has to be reported.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Appendix Fig. 1
Forest plot of PD reduction and CAL gain after 3 month (JPEG 2366 kb)
Appendix Fig. 2
Forest plot of PD reduction and CAL gain after 6 month (JPEG 1857 kb)
Appendix Fig. 3
Forest plot of PD reduction and CAL gain after 12 month (JPEG 1817 kb)
Appendix Fig. 4
Forest plot of PD reduction and CAL gain after various incubation periods with photosensitizer (JPEG 2356 kb)
Appendix Fig. 5
Forest plot of PD reduction and CAL gain after various periods of activation time of photosensitizer (JPEG 2377 kb)
Appendix Fig. 6
Forest plot of PD reduction and CAL gain in studies with low bias risk (JPEG 1932 kb)
Appendix Fig. 7
Forest plot of PD reduction and CAL gain in studies with high bias risk (JPEG 2187 kb)
Appendix Fig. 8
Funnel plot of overall PD reduction (JPEG 1415 kb)
Appendix 1
Search strategy (DOCX 15 kb)
Appendix 2
Flow diagram according to the PRISMA Statement (JPEG 2187 kb)
Appendix 3
Modified quality assessment according to the Cochrane Handbook for Systematic Reviews of Interventions (DOCX 43 kb)
Appendix 4
Excluded studies from systematic review with reasons for exclusion (DOCX 57 kb)
ESM 1
Appendix list of references (DOCX 20 kb)
Rights and permissions
About this article
Cite this article
Azaripour, A., Dittrich, S., Van Noorden, C.J.F. et al. Efficacy of photodynamic therapy as adjunct treatment of chronic periodontitis: a systematic review and meta-analysis. Lasers Med Sci 33, 407–423 (2018). https://doi.org/10.1007/s10103-017-2383-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-017-2383-7