Abstract
Introduction
This study aimed to observe the effects of long-term alendronate pretreatment on the healing of osteoporotic calvarial defects, and further investigate the effect of alendronate combined with once-weekly parathyroid hormone following 12 weeks of alendronate treatment in ovariectomized rats.
Materials and methods
Thirty 3-month-old female rats were ovariectomized, and 24 rats received alendronate for 12 weeks. Then, a critical defect was created in the calvaria of all animals. Immediately after osteotomy, the animals received one of five treatments for 8 weeks: (1) continuation of vehicle (group E), (2) alendronate followed by vehicle (group A), (3) continuation of alendronate (group B), (4) alendronate followed by once-weekly parathyroid hormone alone (group C), or (5) continuation of alendronate combined with once-weekly parathyroid hormone (group D). Calvarial defect healing was assessed using dual-energy X-ray absorptiometry, micro-computed tomography, histology, and sequential fluorescence labeling.
Results
Group E showed a significantly higher volume of newly formed bone than groups A, B, C, and D. Evidence of new dense bone formation in group E was observed histologically. In addition, the immunohistochemical expression of runt-related transcription factor 2 was increased in group E but inhibited in groups A, B, C, and D. Sequential immunofluorescence also showed inhibited mineral apposition in groups A, B, C, and D compared with group E.
Conclusion
The present study shows that long-term pretreatment with alendronate inhibited calvarial defect healing in osteoporotic rats, and this effect could not be reversed by stopping alendronate, switching to parathyroid hormone, or combining with once-weekly parathyroid hormone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a significant health problem characterized by compromised bone strength that predisposes patients to an increased risk of fractures [1, 2]. The most common medications prescribed for the treatment of osteoporosis are bisphosphonates. Among them, alendronate (ALN) is frequently administered because it increases bone mineral density, reduces the risk of fractures, and is well-tolerated [3]. A previous study suggested that long-term ALN therapy before fracture would favor the formation of a mechanically competent large callus with a slow rate of remodeling in an ovariectomized rat model [4]. In contrast to indirect fracture healing with endochondral callus formation, direct fracture healing occurs via intramembranous ossification without callus formation [5]. Studies on the effects of ALN accumulation in the skeleton on direct healing have shown contradictory results. Previous studies reported that regardless of the dose, ALN enhanced or did not compromise new bone formation in a rat calvarial defect model [6, 7]. However, other studies suggested that bisphosphonates may inhibit osteoblast attachment and spreading, subsequently compromising bone formation in the areas of tooth extraction and delaying bone repair [8,9,10]. Additionally, the influence of the long-term administration of ALN on direct fracture healing in the osteoporotic model remains unknown.
Furthermore, osteoporotic patients undergoing bisphosphonate treatment may also suffer fragility fractures or experience unsatisfactory outcomes, including low bone density that remains in the osteoporotic range [11]. Parathyroid hormone (PTH) has an anabolic effect on bone when administered in a pulsatile fashion [12]. PTH is FDA approved for the treatment of osteoporosis, and preclinical models have shown that PTH enhances fracture healing. PTH has also been used off-label in humans to treat fracture nonunion[13, 14]. Previous studies suggested that systematic PTH therapy accelerated the healing of bone defects in a calvarial window model via its anabolic effects on new bone formation and non-anabolic effects on the inhibition of mast cells in favor of angiogenesis [15, 16]. Studies indicate that combination therapy with bisphosphonates and PTH has additive effects and may potentially be a useful therapy for fracture healing both in normal and osteoporotic conditions [17, 18]. However, the effects of combination therapy with ALN and once-weekly PTH on calvarial bone defects in a direct fracture healing model without callus formation have not been investigated. Importantly, patients previously administered and maintained on long-term bisphosphonate therapy are a clinically essential and large population, and at least 50% of all PTH therapy is initiated based on previous treatment with antiresorptive agents [11]. Therefore, evaluating the effect of switching to or adding PTH on bone defect repair under the condition of the skeletal accumulation of bisphosphonates in osteoporosis is of great significance.
In the present study, ovariectomized rats, which are recommended by the FDA as a model of postmenopausal osteoporosis, were used. To assess the effects of long-term ALN on cranial defect healing, the animals received subcutaneous ALN injections before osteotomy to simulate clinical patients who experience bone defects after long-term osteoporosis therapy with ALN. Furthermore, the optimal selection of the type of pharmacological therapy, such as switching to or adding PTH, to accelerate defect healing was investigated. To the best of our knowledge, this is the first study to evaluate the influence of long-term ALN treatment on osteoporotic cranial defect healing and assess the effects of combination therapy with ALN and once-weekly PTH on calvarial defect healing following the use of long-term ALN for osteoporosis therapy.
Materials and methods
Animals and grouping
All experimental procedures and protocols were approved by the Peking University Third Hospital Committee on Ethics for the Care and Use of Laboratory Animals (A2020258). Thirty 12-week-old female Sprague–Dawley rats weighing approximately 250 g each were used in this study. The rats were housed in a standard room with a 12-h light/dark cycle and given free access to food and water. After a 1-week acclimation period, all rats received bilateral ovariectomy (OVX) surgery. The study began when all rats were 6 months old, after a 12-week development of osteoporosis in the OVX rats. Identification of osteoporosis was conducted in the substudy in our previous published research[19]. The treatment plan consisted of two phases over a total of 20 weeks (phase 1: weeks 0–12 and phase 2: weeks 12–20; Fig. 1). In phase 1, 24 rats received subcutaneous ALN injections (alendronate sodium trihydrate, 28 μg/kg; Sigma Aldrich, St. Louis, MO, USA), and 6 rats received subcutaneous saline injections twice per week for three months. In phase 2, all rats underwent surgery to produce a calvarial defect. Based on an established technique, critical-sized full-thickness calvarial bone defects were made using a trephine (Ø 5 mm) under low-speed drilling with continuous cool saline irrigation [20]. Then, all animals were randomly allocated to 5 groups (n = 6 for each group): (1) Vehicle (Veh)-Veh (group A), continued receiving subcutaneous saline injections twice a week; (2) ALN-Veh (group B), received subcutaneous saline injections twice a week after stopping ALN treatment; (3) ALN-ALN (group C), continued receiving subcutaneous ALN injections; (4) ALN-PTH (group D), received subcutaneous injections of PTH (56.5 μg/kg, S20110021, Lilly, Fegersheim, France) once a week; and (5) ALN-ALN + PTH (group E), administered subcutaneous PTH injections once a week together with the continuation of subcutaneous ALN twice a week. The injections were initiated immediately after the experimental osteotomy, the dose of ALN (28 μg/kg twice per week) was based on the minimum dose reported to completely prevent OVX-induced bone loss in rats [21], the body weights were measured weekly, and doses were adjusted accordingly. After 8 weeks, the rats were euthanized with excessive anesthesia, and the calvarium was excised, wrapped in gauze soaked in isotonic saline, and frozen at − 80 °C for subsequent experiments. All calvaria obtained from each group were used for micro-CT and histology.
Schematics of surgery and treatment strategies. Surgeries included bilateral ovariectomy (OVX) and calvarial defect creation. The treatment plan consisted of two phases. All OVX rats received saline or ALN therapy for 12 weeks in phase 1 and then one of four different treatments (ALN-Veh, ALN-ALN, ALN-PTH, or ALN-ALN + PTH) after osteotomy in phase 2. ALN alendronate, Veh vehicle, PTH parathyroid hormone
DXA
A small-animal high-resolution collimator (DiscoveryTM, Hologic, Inc., Boston, MA) was used to capture radiographs of calvarial defects.
Micro-CT
New bone formation at the defect site was evaluated by micro-CT using an Inveon MM system (Siemens, Erlangen, Germany) with a slice thickness of 0.625 mm and a pixel size of 0.215 mm, and three-dimensional (3D) images were reconstructed. The bone volume/total volume (BV/TV) in the defect was calculated by two independent examiners who were blinded to the experimental treatment using multimodal 3D visualization software (Inveon Research Workplace, Siemens, Erlangen, Germany).
Histology and immunohistochemical staining
Specimens were fixed in phosphate-buffered 4% paraformaldehyde, decalcified in 10% ethylenediaminetetraacetic acid (EDTA), and embedded in paraffin. Bone sections of 5-mm thickness were stained with hematoxylin and eosin (H&E) and Masson’s trichrome. In addition, Sects. (5 mm) were treated with 3% H2O2 for 10 min and then incubated in 10% goat serum diluted in PBS for 30 min and a rabbit anti-runt-related transcription factor 2 (RUNX-2) (Cell signaling technology #125,556) antibody overnight at 4 °C. After incubation with a biotinylated anti-rat secondary antibody for 30 min and peroxidase for 10 min, signals were detected using diaminobenzidine and observed under light microscopy (E 800, Nikon, Tokyo, Japan).
Sequential fluorescence labeling
To monitor bone mineral apposition, double-fluorochrome labels were administered as follows. Calcein green (20 mg/kg, Sigma-Aldrich, USA) and Alizarin red (30 mg/kg, Sigma-Aldrich, USA) were injected via the tail vein 14 and 7 days before euthanasia, respectively. Specimens were randomly selected from each group, fixed in 10% neutral buffered formalin, dehydrated in increasing gradients of alcohol, and embedded in methyl methacrylate resin. Next, the undecalcified sections were ground and polished to 500 μm for imaging (EXAKT Cutting & Grinding System; EXAKT Advanced Technologies, Norderstedt, Germany).
Statistical analysis
All data are expressed as the mean ± standard deviation (SD). Statistical analyses were performed using SPSS 20.0 (SPSS Inc., Chicago, IL, USA). Multiple comparisons between groups were performed using one-way ANOVA and Tukey’s post hoc test. P-values < 0.05 were considered statistically significant.
Results
DXA
Radiographs of calvarial defects showed that new bone formation was inhibited in the long-term ALN pretreatment groups (ALN-Veh, ALN-ALN, ALN-PTH, and ALN-ALN + PTH) compared with the saline treatment group (Veh-Veh) (Fig. 2).
Representative radiographs of the five different treatment groups after calvarial defect surgery. New bone formation was observed in the control group, whereas almost no bone regeneration was observed in the four long-term ALN administration groups (ALN-Veh, ALN-ALN, ALN-PTH, and ALN-ALN + PTH). ALN alendronate, Veh vehicle; PTH parathyroid hormone
Micro-CT
The 3D reconstructions and quantity of bone formation within the calvarial defects were analyzed by micro-CT at 8 weeks after osteotomy. The long-term ALN pretreatment groups showed significantly limited bone formation compared with the saline-treated group. Furthermore, no significant differences in new bone formation were found among the ALN-Veh, ALN-ALN, ALN-PTH, and ALN-ALN + PTH groups (Fig. 3).
Representative three-dimensional reconstruction images of calvarial defects. a Long-term ALN treatment (ALN-Veh, ALN-ALN, ALN-PTH, and ALN-ALN + PTH groups) inhibited bone formation, whereas new bone formation was observed in the control group. b Quantitative analysis of bone formation in the long-term ALN treatment and control groups. Data are expressed as the mean ± SD; error bars indicate SD. *P < 0.01 (compared with the control group). ALN alendronate, Veh vehicle, PTH parathyroid hormone
H&E
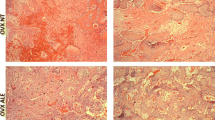
We also performed H&E staining to measure bone formation in the calvarial defects. The smallest amount of bone formation was observed in the long-term ALN pretreatment group, and the calvarial defects were mostly covered with fibrous-like tissues. The saline-treated control group (Veh-Veh) showed an increase in the new bone formation surrounding the edge of the defect compared with the long-term ALN pretreatment groups (ALN-Veh, ALN-ALN, ALN-PTH, and ALN-ALN + PTH). A similar extent of inhibited bone formation and fibrous-like tissues was observed among different long-term pretreatment groups (ALN-Veh, ALN-ALN, ALN-PTH, and ALN-ALN + PTH) (Fig. 4).
H&E staining of calvarial defects. New bone formation was found in the control group (E), whereas almost no bone formation was observed in the long-term ALN groups (a ALN-Veh, b ALN-ALN, c ALN-PTH, and d ALN-ALN + PTH). The scale bar represents 2.5 mm. Black arrows indicate the edge of the calvarial defect, and the squares show the new bone formation. ALN alendronate, Veh vehicle, PTH parathyroid hormone
Masson’s trichrome
Masson’s trichrome was used to observe the calcification of new bones, and calcific bones in the defect appeared blue. Compared with the saline alone group, the long-term ALN pretreatment group exhibited reduced calcific bone formation, and the defects were covered with mostly fibrous-like tissues. No difference of calcific bone formation was found in the different long-term ALN pretreatment groups (ALN-Veh, ALN-ALN, ALN-PTH, and ALN-ALN + PTH) (Fig. 5).
Masson’s trichome staining of calvarial defects. New calcific bone formation was found in the control group (E), whereas almost no calcific bone formation was observed in the long-term ALN groups (a ALN-Veh, b ALN-ALN, c ALN-PTH, and d ALN-ALN + PTH). The scale bar represents 2.5 mm. Black arrows indicate the edge of the calvarial defect, and the squares show the new bone formation. ALN alendronate, Veh vehicle, PTH parathyroid hormone
Immunohistochemical staining (IHC)
IHC staining for the bone formation marker RUNX-2 showed that positive brown staining was observed in the saline-treated group. However, reduced positive RUNX-2 expression was found in the different long-term ALN pretreatment groups (ALN-Veh, ALN-ALN, ALN-PTH, and ALN-ALN + PTH) (Fig. 6).
Immunohistochemical staining of the bone formation marker RUNX-2 in calvarial defects. Higher RUNX-2 expression was found in the control group (E) compared with the long-term ALN groups (a ALN-Veh, b ALN-ALN, c ALN-PTH, and d ALN-ALN + PTH). The scale bar represents 500 μm. Runt-related transcription factor 2, RUNX-2; ALN alendronate, Veh vehicle, PTH parathyroid hormone
Sequential fluorescence label
More bone mineralization detected by Alizarin red and Calcein was observed in the calvarial defect in the saline-treated group compared with the different ALN pretreatment groups. No difference was found among the different ALN pretreatment groups (ALN-Veh, ALN-ALN, ALN-PTH, and ALN-ALN + PTH) (Fig. 7).
Representative fluorescence images of calvarial defects. New bone mineralization was found in the control group (E), whereas inhibited bone mineralization was observed in the long-term ALN groups (a ALN-Veh, b ALN-ALN, c ALN-PTH, and d ALN-ALN + PTH). ALN alendronate, Veh vehicle, PTH parathyroid hormone
Discussion
This study shows that the long-term use of ALN for osteoporosis treatment may inhibit calvarial defect healing, which is a type of direct fracture healing without callus formation. Additionally, the negative effect of ALN on defect healing could not be reversed by stopping treatment, continuing ALN, switching to once-weekly PTH, or adding once-weekly PTH. Eight weeks after calvarial defect surgery, new bone formation was inhibited, and more fibrous-like tissues in the defect region were observed in the long-term ALN pretreatment groups compared with the saline-treated group. In addition, the immunohistochemical expression of RUNX-2 and bone mineralization labeled by Alizarin red and Calcein were both inhibited.
To enhance bone regeneration, various physical and chemical strategies have been developed to functionalize bone tissue scaffolds with ALN and achieve controlled release profiles [22, 23], suggesting that systematic ALN pretreatment before the development of calvarial defects may provide benefits in osteogenesis. In our study, the inhibition of new bone formation was substantially different from the previously reported increase in bone regeneration following local ALN application [20, 24] and distinct to the results obtained by systematic ALN application [25]. The exact reasons for these contrasting results are unknown but may be associated with the dosage of ALN, route of administration, animal bone defect model, or other factors.
However, it was reported that bisphosphonate injection immediately after surgery does not enhance bone volume and may actually negatively affect bone graft incorporation, whereas delaying bisphosphonate administration following cleft bone graft surgery significantly increases bone volume and integration compared with saline controls [26]. Although the inhibition of bone defect healing caused by ALN treatment was also observed in our study, stopping ALN administration after calvarial defect surgery did not lead to improvements in new bone formation. This may be due to the ALN that accumulates in the bone after its long-term use. Specifically, ALN may still exert its effects after the generation of calvarial defects, even though it is not being administered. Additionally, it was reported that zoledronic acid use potentially suppressed bone remodeling, which led to impaired healing at the extracted socket but full regeneration of the tibia defect [27, 28]. Therefore, it was speculated that bisphosphonates impair bone defect healing by decreasing bone turnover in direct fracture healing without callus formation, such as during calvarial or socket healing, whereas the opposite response occurs during indirect fracture healing with callus formation. The inhibitory effect on direct fracture healing found in a tibial osteotomy model that was rigidly fixed further supported our assumption [29].
Bisphosphonates may compromise bone defect healing processes by inhibiting osteoblast attachment and spreading [9]. Prolonged treatment with ALN was reported to alter cranial repair because of the simultaneous occurrence of low estrogen and suppression of bone morphogenetic protein receptor type 1B and RUNX-2 expression [30]. Consistent with the previous study, the expression of the osteogenesis-related factor RUNX-2 was inhibited in the long-term ALN pretreatment groups in our study. Bone mineralization was also inhibited in the long-term ALN treatment groups in our study, which supports the phenomenon of the inhibition of new bone formation.
Patients previously administered and maintained on long-term bisphosphonate therapy, which are a clinically essential and large population, may still suffer from bone defects. The treatment of osteoporotic bone defects in the non-weight-bearing skeleton after long-term ALN use is unclear. There are two classes of osteoporosis therapies: anabolic agents that stimulate bone formation, such as PTH, and antiresorptive drugs that prevent bone resorption, such as bisphosphonates[31]. Using a combination of both pro-anabolic and anti-catabolic agents to minimize adverse effects and optimize the outcomes of bone grafting for alveolar clefts may be effective, and some recent studies have shown favorable results [32, 33].
Although scientific reports on combination therapies to promote bone repair are particularly limited in the literature, several clinical trials have evaluated combinations of PTH and a variety of antiresorptive agents. The results suggest that using once-weekly PTH to enhance bone formation and bisphosphonates to decrease bone resorption may be a superior treatment method to achieve better outcomes in calvarial defect healing. As a result, combination therapy was proposed to stimulate bone formation and inhibit bone resorption.
However, in contrast to our initial assumption, switching to PTH or adding once-weekly PTH to ALN did not show further benefits in osteoporotic defect healing after long-term ALN pretreatment, but the underlying mechanism remains unknown. Calvarial defect healing in the absence of mechanical loading may restrict the function of PTH [34]. Furthermore, the effects of the route of PTH administration (56.5 μg/kg once weekly) on calvarial defect healing could not be assessed because of the significant inhibition of bone formation induced by long-term ALN treatment.
Strengths and limitations
These findings suggest that the negative influence of long-term ALN treatment on direct fracture healing should be noted in clinical settings, and optimal treatments should be further explored considering the lack of effects observed in this study. However, our study has several limitations. First, the underlying mechanism of the different response to ALN between fracture and calvarial defect healing remains to be explored. Second, the reason why the combination of PTH also failed to alleviate the inhibition of osteogenesis by ALN was unknown. Methods such as using different doses of PTH and comparing with fracture models for further study are needed. Third, because histological studies of both decalcified and undecalcified tissues were conducted in our study, the sample size was insufficient for quantitative histological analysis. Larger sample size was required for further verification.
In conclusion the long-term use of ALN may impair calvarial defect healing and switching to or adding once-weekly PTH to ALN may not be an alternative option in the treatment of calvarial defects.
References
Giannoudis P, Tzioupis C, Almalki T, Buckley R (2007) Fracture healing in osteoporotic fractures: is it really different? A basic science perspective. Injury 38:S90-99
Geusens P (2009) Bisphosphonates for postmenopausal osteoporosis: determining duration of treatment. Curr Osteoporos Rep 7:12–17
Duque G (2013) Osteoporosis in older persons: current pharmacotherapy and future directions. Expert Opin Pharmacother 14:1949–1958
Fu LJ, Tang TT, Hao YQ, Dai KR (2013) Long-term effects of alendronate on fracture healing and bone remodeling of femoral shaft in ovariectomized rats. Acta Pharmacol Sin 34:387–392
McKibbin B (1978) The biology of fracture healing in long bones. J Bone Joint Surg Br. https://doi.org/10.1302/0301-620X.60B2.350882
de Oliveira N, Oliveira J, de Souza ML, Weiss SG, Chaves LH, Casagrande TC, Deliberador TM, Giovanini AF, Zielak JC, Scariot R (2019) Bone repair in craniofacial defects treated with different doses of alendronate: a histological, histomorphometric, and immunohistochemical study. Clin Oral Investig 23:2355–2364
Vieira JS, Giovanini A, Görhinger I, Gonzaga CC, Costa-Casagrande TA, Deliberador TM (2017) Use of low-dose alendronate improves cranial bone repair and is associated with an increase of osteocalcin: an experimental study. J Oral Maxillofac Surg 75:1873–1881
Aguirre JI, Altman MK, Vanegas SM, Franz SE, Bassit AC, Wronski TJ (2010) Effects of alendronate on bone healing after tooth extraction in rats. Oral Dis 16:674–685
Koyama C, Hirota M, Okamoto Y, Iwai T, Ogawa T, Hayakawa T, Mitsudo K (2020) A nitrogen-containing bisphosphonate inhibits osteoblast attachment and impairs bone healing in bone-compatible scaffold. J Mech Behav Biomed Mater 104:103635
Idris AI, Rojas J, Greig IR, Van’t Hof RJ, Ralston SH (2008) Aminobisphosphonates cause osteoblast apoptosis and inhibit bone nodule formation in vitro. Calcif Tissue Int 82:191–201
Bilezikian JP (2008) Combination anabolic and antiresorptive therapy for osteoporosis: opening the anabolic window. Curr Osteoporos Rep 6:24–30
Meng XW, Liang XG, Birchman R, Wu DD, Dempster DW, Lindsay R, Shen V (1996) Temporal expression of the anabolic action of PTH in cancellous bone of ovariectomized rats. J Bone Miner Res 11:421–429
Oteo-Alvaro A, Moreno E (2010) Atrophic humeral shaft nonunion treated with teriparatide (rh PTH 1–34): a case report. J Shoulder Elbow Surg 19:e22-28
Corrigan RA, Miller A, McNally MA, Javaid MK (2013) Treatment of fracture non-union in a young adult with combination anabolic and anti-resorptive bone therapy. Rheumatology (Oxford) 52:1147–1149
Li H, Zhou Q, Bai BL, Weng SJ, Wu ZY, Xie ZJ, Feng ZH, Cheng L, Boodhun V, Yang L (2018) Effects of combined human parathyroid hormone (1–34) and menaquinone-4 treatment on the interface of hydroxyapatite-coated titanium implants in the femur of osteoporotic rats. J Bone Miner Metab 36:691–699
Zhang L, Wang T, Chang M, Kaiser C, Kim JD, Wu T, Cao X, Zhang X, Schwarz EM (2017) Teriparatide treatment improves bone defect healing via anabolic effects on new bone formation and non-anabolic effects on inhibition of mast cells in a murine cranial window model. J Bone Miner Res 32:1870–1883
Casanova M, Herelle J, Thomas M, Softley R, Schindeler A, Little D, Schneider P, Muller R (2016) Effect of combined treatment with zoledronic acid and parathyroid hormone on mouse bone callus structure and composition. Bone 92:70–78
Li YF, Zhou CC, Li JH, Luo E, Zhu SS, Feng G, Hu J (2012) The effects of combined human parathyroid hormone (1–34) and zoledronic acid treatment on fracture healing in osteoporotic rats. Osteoporos Int 23:1463–1474
Zhang C, Zhu J, Jia J, Guan Z, Sun T, Zhang W, Yuan W, Wang H, Leng H, Song C (2020) Once-weekly parathyroid hormone combined with ongoing long-term alendronate treatment promotes osteoporotic fracture healing in ovariectomized rats. J Orthop Res. https://doi.org/10.1002/jor.24953
Wang X, Zeng D, Weng W, Huang Q, Zhang X, Wen J, Wu J, Jiang X (2018) Alendronate delivery on amino modified mesoporous bioactive glass scaffolds to enhance bone regeneration in osteoporosis rats. Artif Cells Nanomed Biotechnol 46:171–181
Seedor JG, Quartuccio HA, Thompson DD (1991) The bisphosphonate alendronate (MK-217) inhibits bone loss due to ovariectomy in rats. J Bone Miner Res 6:339–346
Tarafder S, Bose S (2014) Polycaprolactone-coated 3D printed tricalcium phosphate scaffolds for bone tissue engineering: in vitro alendronate release behavior and local delivery effect on in vivo osteogenesis. ACS Appl Mater Interfaces 6:9955–9965
Zeng Y, Zhou M, Mou S, Yang J, Yuan Q, Guo L, Zhong A, Wang J, Sun J, Wang Z (2020) Sustained delivery of alendronate by engineered collagen scaffold for the repair of osteoporotic bone defects and resistance to bone loss. J Biomed Mater Res A. https://doi.org/10.1002/jbm.a.36997
Kim SE, Yun YP, Shim KS, Kim HJ, Park K, Song HR (2016) 3D printed alendronate-releasing poly(caprolactone) porous scaffolds enhance osteogenic differentiation and bone formation in rat tibial defects. Biomed Mater 11:055005
Toker H, Ozdemir H, Ozer H, Eren K (2012) Alendronate enhances osseous healing in a rat calvarial defect model. Arch Oral Biol 57:1545–1550
Cheng N, Park J, Olson J, Kwon T, Lee D, Lim R, Ha S, Kim R, Zhang X, Ting K, Tetradis S, Hong C (2017) Effects of bisphosphonate administration on cleft bone graft in a rat model. Cleft Palate Craniofac J 54:687–698
Lim SS, Lee B, Kim IS, Hwang SJ (2017) Differential modulation of zoledronate and etidronate in osseous healing of an extracted socket and tibia defect. Oral Surg Oral Med Oral Pathol Oral Radiol 123:8–19
Amanat N, McDonald M, Godfrey C, Bilston L, Little D (2007) Optimal timing of a single dose of zoledronic acid to increase strength in rat fracture repair. J Bone Miner Res 22:867–876
Savaridas T, Wallace RJ, Salter DM, Simpson AH (2013) Do bisphosphonates inhibit direct fracture healing? A laboratory investigation using an animal model. Bone Joint J. https://doi.org/10.1302/0301-620X.95B9.31562
Giovanini AF, de Sousa Passoni GN, Göhringer I, Deliberador TM, Zielak JC, Storrer CLM, Costa-Casagrande TA, Scariot R (2018) Prolonged use of alendronate alters the biology of cranial repair in estrogen-deficient rats’ associated simultaneous immunohistochemical expression of TGF-β1+, α-ER+, and BMPR1B. Clin Oral Investig 22:1959–1971
Camacho P (2016) 2016 American Association of Clinical Endocrinologists/American College of Endocrinology Clinical Practice guidelines on postmenopausal osteoporosis. US Endocrinol 12:74
Belfrage O, Flivik G, Sundberg M, Kesteris U, Tagil M (2011) Local treatment of cancellous bone grafts with BMP-7 and zoledronate increases both the bone formation rate and bone density: a bone chamber study in rats. Acta Orthop 82:228–233
Harding AK, Aspenberg P, Kataoka M, Bylski D, Tagil M (2008) Manipulating the anabolic and catabolic response in bone graft remodeling: synergism by a combination of local BMP-7 and a single systemic dosis of zoledronate. J Orthop Res 26:1245–1249
Kim CH, Takai E, Zhou H, von Stechow D, Müller R, Dempster DW, Guo XE (2003) Trabecular bone response to mechanical and parathyroid hormone stimulation: the role of mechanical microenvironment. J Bone Miner Res 18:2116–2125
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (Project Nos. 81672133 and 81874010). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. We acknowledge the technical support provided by the Peking University Third Hospital Central Laboratory. We thank Melissa Crawford, PhD, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors participated in the design, interpretation of the studies, acquisition, analysis of the data and review of the manuscript. CGZ and CLS designed the experiments. CGZ and JXZ conducted the experiments and performed analysis. CGZ, JXZ, JLJ, ZYG, TTS, WZ, WQY, and HW participated in acquiring the data. CGZ and CLS wrote the manuscript. All authors have read and approved the final version of the submitted manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Chenggui Zhang, Junxiong Zhu, Jialin Jia, Zhiyuan Guan, Tiantong Sun, Wang Zhang, Wanqiong Yuan, Hong Wang and Chunli Song declare that they have no conflict of interest.
Ethical approval
All experimental procedures and protocols were approved by the Peking University Third Hospital Committee on Ethics for the Care and Use of Laboratory Animals (A2020258).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Zhang, C., Zhu, J., Jia, J. et al. Long-term pretreatment with alendronate inhibits calvarial defect healing in an osteoporotic rat model. J Bone Miner Metab 39, 925–933 (2021). https://doi.org/10.1007/s00774-021-01235-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-021-01235-0