Abstract
Objective
The objective of the study is to evaluate bone repair in rats treated with different alendronate doses.
Matherials and methods
Sixty female rats ovariectomized were randomly divided in three groups: group C (control group), group A1 (ALN/1 mg/kg), and A2 (ALN/ 3 mg/kg). Each animal received subcutaneous applications of sodium alendronate at a dose correspondent to group A1 or A2 three times a week, while the control group received 0.9% saline solution. After 4 weeks of application, a critical defect was created in the calvaria of animals of all groups. The defect was filled by particulate autogenous bone. The applications were maintained until euthanasia, which occurred 15 and 60 days after the surgical procedure. The pieces were sent for histological, histomorphometric and immunohistochemical analysis. The data were submitted to statistical analysis with significance level of 0.05.
Results
The descriptive histological analysis demonstrated an increase in bone neoformation in both groups treated with alendronate when compared to the control group. The histomorphometric analysis showed an increase in the amount of neoformed bone in A1 and A2 groups when compared to group C, both at 15 days (p = 0.0002) and at 60 days (p = 0.001). In the immunohistochemical analysis, it was possible to observe a difference in immunolabeling just for Mmp2 at the time of 60 days in A1 (p = 0.001) and A2 (p = 0.023) when compared to the control group.
Conclusion
Systemic delivery of alendronate, regardless of the dose, increased the amount of bone neoformation.
Clinical relevance
Prescription of sodium alendronate at 1 mg/kg for improvement of bone neoformation in bone graft procedures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bisphosphonates (BPs) are synthetic analogs of inorganic pyrophosphate, which can reduce bone loss and increase bone mineral density. This class of drugs is widely used for the treatment of skeletal disorders such as multiple myeloma, a malignant bone disease, as well as Paget’s disease, and osteoporosis [1]. Some studies have shown that BPs may improve the capacity for physical exercise and bone repair [2,3,4,5], depending on the dose and route of drug administration. BPs are divided into nitrogen-containing and non-nitrogen-containing compounds. Sodium alendronate is the most commonly prescribed BP [6, 7], commonly used to treat osteoporosis, and typically administered orally [8, 9].
The effect of BPs in bone formation may have consequences on the proteins known to be involved in bone metabolism. Hence, studying them may provide new insight on the mechanisms underlying bone regeneration stimulated by BPs.
The Wnt family comprises 19 glycoproteins that activate signaling cascades of embryonic development and tissue repair. These proteins regulate cellular growth, differentiation, function, and apoptosis. A signaling cascade in the canonical pathway (Wnt/β-catenin) is directly related to bone biology, since this pathway contributes to osteoblast proliferation and survival [10]. Wnt3a specifically is involved in odontoblast generation and extracellular matrix (ECM) organization by increasing the proliferation of mesenchymal cells. In addition, it is associated with osteoblastic differentiation by promoting the formation of bone tissue during bone regeneration [11, 12].
Matrix metalloproteinases (MMPs) constitute a group of approximately 28 zinc-dependent enzymes (endopeptidases) responsible for the degradation of the ECM and the basement membrane [13]. Under physiological conditions, MMPs are involved in tissue remodeling, regulation of transcription, activation of zymogens, interaction with specific components of the ECM, and the blockage of natural MMP inhibitors [14, 15]. MMP2 is a gelatinase associated with the lysis of type IV collagen and elastin, and is involved in the differentiation of hematopoietic mesenchymal cells in inflammatory ones, as well as the regulation, migration, and proliferation of epithelial cells [16, 17].
The aim of this study was to evaluate by histology, histomorphometry, and immunostaining, bone repair in rats treated with different doses of alendronate and its influence on Wnt3a and MMP2.
Material and methods
Animals
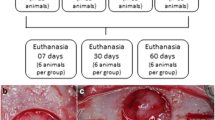
This study was approved by the Ethics and Research Committee (ECUA Protocol 262). Sixty adult Wistar female rats underwent a period of adaptation of 15 days, during which they received commercial pet food (Nuvital, Columbus, PR, Brazil) and water. During the experimental period, the environmental conditions of light, temperature, and humidity were controlled to maintain a 12-h photoperiod, with temperature of 18–22 °C and 65% humidity. All experimental phases of the study are described in Fig. 1. Sixty days after ovariectomy, the application of alendronate/physiological solution was initiated. The calvarial defects were performed 30 days after the beginning of drug administration. The animals were randomly divided into three groups: group C (control group), group A1 (ALN/1 mg/kg), and group A2 (ALN/ 3 mg/kg). Each animal received subcutaneous applications of sodium alendronate at a dose correspondent to group A1 or A2 three times a week, while the control group received 0.9% saline solution. Finally, the groups were subdivided for euthanasia at two different times, 15 days and 60 days after administration of sodium alendronate or saline.
As described by John et al., in 2004 [18], 40-day-old rats were ovariectomized bilaterally using a ventro-dorsal approach with an incision in the midline of all layers (skin, subcutaneous, and linea alba) to access the ovary and with ligature of the ovarian artery and uterine insertion (Fig. 2). The muscular plane and skin were closed using absorbable sutures.
To assess the effectiveness of ovariectomy, approximately 20% of the rats (n = 13) were evaluated for estradiol level. Blood (1 ml) was collected via cardiac puncture at three different time points (15, 30, and 45 days) after ovariectomy and analyzed for estradiol levels (Mouse/Rat Estradiol ELISA, SE120084, Sigma Chemical Co., St. Louis, MO, USA).
Rats of mean age 90 days and weight 230–400 g were randomly divided into three groups: group C, control; group A1, alendronate 1 mg/kg; and group A2, alendroante 3 mg/kg.
Groups A1 and A2 received alendronate sodium subcutaneously three times per week on alternate days, at a dose of 1 mg/kg and 3 mg/kg, respectively. Group C received subcutaneous sodium chloride solution 0.9% during the same period. For all groups, these administrations were started 4 weeks prior to surgery and maintained until euthanasia.
The animals were sedated for 1 min by inhalation with oxygen and isoflurane (Cristália, Itapira, SP, Brazil) and then anesthetized with ketamine 10% (Vetbrands, Paulínia, SP, Brazil) combined with xylazine hydrochloride 2% (Vetbrands, Paulínia, SP, Brazil) by intraperitoneal injection. After anesthesia, trichotomy of the frontoparietal region of the head was carried out with a razor blade, with subsequent vigorous asepsis using povidone iodine. Then, a U incision in the mucoperiosteal anteroposterior area was made with a scalpel (blade no. 15C) in the cranial vault, and with the aid of a Molt elevator, flaps of total thickness were elevated to widely expose the cortical bone region.
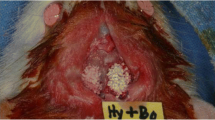
A transosseous critical size defect of 5 mm in diameter, on the median sagittal suture in the parietal bones, was performed with a trephine (Neodent, Curitiba, PR, Brazil) and was well irrigated with a sterile saline solution. The bone block was carefully removed using Molt elevators to avoid rupture of the meninges and exposure of the brain.
In all groups, the defects were filled with an autogenous particulate bone (three beats were standardized to particulate the bone in a pestle-type grinder [Kopp, Curitiba, PR, Brazil]) originating from the bone block removed from the calvaria of the animal. After the defect was filled by the autogenous bone, a collagen membrane was used to cover the defect (Gen Derm, Mogi Mirim, SP, Brazil). The soft tissue was sutured using silk yarn 4-0 (Ethicon, Johnson & Johnson, São José dos Campos, SP, Brazil). In the postoperative period, the animals were kept in cages with access to food and water. For analgesia, morphine sulfate 3 mg/kg (União Química, Jabaquara, SP, Brazil) was administered intramuscularly at the end of surgery. Pain control maintenance was performed by administration of 20 drops of paracetamol (200 mg/kg) diluted in 400 ml of water placed in a water cooler for 3 days.
The groups were divided into two subgroups for sacrifice at 15 and 60 days after the surgical procedure. During the postoperative period, four rats died. For euthanasia, rats were placed in a gas chamber (CO2), for 10 min.
The bone blocks were removed from the cranial defect regions following euthanasia. The margin of safety was approximately 6 mm from the region of the bone defect. Removal was performed using a 702 drill (Beavers Dental, Morrisburg, ON, Canada) mounted on a straight handpiece at 22,000 rpm, and blocks were packed into jars containing formalin 10% (Vetec Química Fina, Duke of Caias, RJ, Brazil).
Histological and histomorphometric analysis
Following decalcification in 7% EDTA solution for 60 days, the samples were hemi-sectioned through the center, parallel to the middle of the defect, processed, and embedded in paraffin. Serial longitudinal sections of 3 μm from the center of the original surgical defect were prepared. Samples were stained by hematoxylin and eosin for light microscopy.
Two histological sections were analyzed from each animal, representing the center of the original surgical defect. Qualitative histological analysis was performed by a single operator, and the images were analyzed with an optical microscope (021/3 Quimis, Diadema, SP, Brazil). The following parameters were evaluated: closure of bone defect, characteristics of the connective tissue, presence of osteoid matrix, and progression of the type of repair present in the surgically created defect.
The stained sections were imaged serially using a digital camera attached to a microscope (Olympus BX41, Melville, NY, USA) with ×40 magnification. Next, images were grouped using Microsoft Powerpoint® (Microsoft Corporation, WA, USA) to form a single continuous image, encompassing the two edges of the defect. ImageJ software (National Institutes of Health, Bethesda, MD, USA) was used to evaluate the following histomorphometric measures:
-
1.
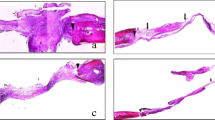
Histological area of the defect (HA): the total area of the surgically created defect. This measurement was made from edge to edge of the original defect, including the variation of thickness in each calvaria (Fig. 3a).
-
2.
Area of osteoid matrix (AO): the grafted bone particles and osteoid matrix areas were measured (Fig. 3b).
Percentage (%): percent of bone, neoformed bone, and osteoid matrix within the histological defect, calculated by a rule of three between the histological area of the defect and the area of osteoid matrix.
Immunohistochemical analysis
For the detection of proteins, immunohistochemistry using streptavidin-biotin immunoperoxidase was performed in 3-μm serial histological sections of the paraffin-embedded specimens (Sigma Chemical Co., St. Louis, MO, USA). The histological sections in the slides were dewaxed in two baths of xylene: (1) submerged for 30 min at 60 °C and (2) immersed for 20 min at room temperature (15 °C). The dewaxed sections were dried using filter paper, rehydrated, and submerged for 10 min in a solution of ammonium hydroxide (10%) diluted in ethanol (95%) to remove formolic pigments.
For antigen retrieval and disruption of the cross-linking of proteins caused by formalin, the slides were incubated in a solution of trypsin (1%, pH 6.8) at 37 °C for 45 min. After washing in distilled water, endogenous peroxidase was blocked using a solution of 3% hydrogen peroxide. To prevent significant changes in pH, the slides underwent rapid incubation in phosphate-buffered solution (PBS) (0.05 M, pH 7.4). Next, the sections were incubated overnight with MMP2 (matrix metalloproteinase 2, Santa Cruz Biotechnology Inc., Dallas, TX, USA) and Wnt3a (Santa Cruz Biotechnology Inc., Dallas, TX, USA) primary antibodies at 4 °C in a humidified chamber at the manufacturer-recommended dilution in PBS with 1% bovine serum albumin containing 0.1% sodium azide (Biotest Inc., São Paulo, SP, Brazil). As a negative control, some slides were incubated for 10 min with rabbit IgG polyclonal antibody instead of primary antibody. An LSAB kit (DAKO number KO, 690, Denmark) was used for secondary antibody incubation and tertiary complex formation (streptoavidin-biotin peroxidase) in a humidified chamber for 30 min at room temperature, according to the manufacturer’s instructions. Next, slides were incubated in 300 mg of the chromogen diaminobenzidine (3,3-diaminobenzidine, Sigma Chemical Co., St. Louis, MO, USA) in 100 ml of PBS and activated with 600 μl of H2O2 6% at room temperature for 3 min in a dark chamber. After incubation, the slides were washed in running water and counterstained with Mayer’s hematoxylin for 10 min. Finally, slides were dehydrated using an ascending ethanol gradient (50%, 70%, 90%, and absolute), followed by diaphanized in xylol, and mounted using Permount (Fisher Scientific, Fair Lawn, NJ, USA) for analysis under a light microscope.
Immunohistochemical images were captured using a digital camera (SDC-310, Samsung, South Korea) attached to a light microscope (Zeiss, São Paulo, SP, Brazil) with a ×100 magnification. Each image was saved at 600 dpi resolution, producing a virtual image of 117 × 80 cm.
Qualitative assessment was performed by a single observer, after reviewing all slides within a group. The quantitative evaluation (score for formation/no formation of bone tissue) was given as follows: “−” for 0–5%, “+” for 10–25%, “++” for 25–50%, “+++” for 50–75%, and “++++” for > 75%.
Statistical analysis
Statistical evaluation was performed by analysis of frequency and specific tests using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA), with a confidence interval (CI) of 95%. For comparison of groups’ parametric variables, one-way ANOVA and Tukey’s test were employed. For comparison among groups of ordinal variables, the Mann–Whitney test (two groups) and Kruskall–Wallis test (more than two groups) were used.
Results
Analysis of ovariectomy effectiveness
The estradiol level in a non-ovariectomized rat was measured at 34 pg/ml. Median levels of estradiol were 20.7 pg/ml (20.0–35.4) at 15 days, 20 pg/ml (20.0–62.8) at 30 days, and 20.7 pg/ml (20.0–50.4) at 45 days after ovariectomy. No statistically significant difference was observed among groups C, A1, and A2 (p > 0.05). It might therefore be concluded that, after the initial phase following ovariectomy, there is no difference in estradiol level over time, despite potential hormonal compensation by the adrenal gland. Therefore, 15 days after ovariectomy, the level of estradiol in rats was considered sufficiently low. A detailed description of the results obtained is in Fig. 4.
Histological analysis
In all groups and at all experimental time points, complete bone closure of the defect was observed. In the 15-day control group, highly vascularized dense connective tissue was observed, with chronic inflammation interspersed by viable particulate bone and with osteoblasts within the fragments. In the 60-day control group, dense fibrous connective tissue was interspersed by bone graft particles.
In the 15-day A1 group, dense connective tissue with little inflammatory infiltrate was observed. A greater amount of viable bone tissue was observed in comparison to control, with the presence of osteoblasts. In the 60-day A1 group, dense connective tissue permeated by viable bone particles and mineral osteoid matrix was observed.
In the 15-day A2 group, dense connective tissue with little inflammatory infiltrate was found. A greater amount of viable bone tissue was found compared to that in the control and A1 groups, with a significant number of osteoblasts. In the 60-day A2 group, dense connective tissue permeated by viable bone particles and mineral osteoid matrix was observed.
It is possible to observe all the histological evaluation in Fig. 5.
Histomorphometric analysis
The results of the histomorphometric analysis at 15 and 60 days are shown in Table 1. In the 15-day control group, the percentage of neoformed bone and osteoid matrix in the histological defect was 20.66 ± 7.51 mm2. In contrast, in the 60-day group, the percentage was 15.09 ± 6.51 mm2. In the 15-day and 60-day A1 groups, the percentage was 50.60 ± 12.26 mm2 and 35.02 ± 10.76 mm2, respectively. Finally, in the A2 group, the percentage was 35.69 ± 9.15 mm2 at 15 days and 33.93 ± 9.83 mm2 at 60 days.
Immunohistochemical analysis—Wnt3a
Control group: At 15 days, non-marked bone trabeculae (particles of autogenous bone graft) were observed, and Wnt3a was present only in the matrix of connective tissue. In the 60-day group, Wnt3a was observed only in areas at the edge of the bone, lines of osteoblasts peripheral to the trabecular bone, and in a few areas of connective tissue.
A1 group: At 15 days, Wnt3a-positive staining was observed in osteoblasts peripheral to the trabecular bone and in darker areas of trabeculae, but not in the matrix of connective tissue. At 60 days, staining was similar to the 15-day A1 group, with immunopositivity of osteoblasts found only in the bone trabeculae.
Group A2: At 15 and 60 days, the results were similar to those of the A1 group. However, marked areas of connective tissue and more intense bone trabeculae were observed, with peripheral areas marked by osteoblasts.
The results of immunostaining of Wnt3a at 15 and 60 days are shown in Fig. 6, and the quantitative analysis results are shown in Table 2. No difference in immunostaining was observed between the groups at 15 days (p = 0.3) and at 60 days for Wnt3a (p = 0.11).
Mmp2
Control group: After 15 days, MMP2 was observed only peripherally in the bone trabeculae. In contrast, at 60 days, greater immunostaining was observed more internally in the trabeculae as well as peripherally, with few areas stained in the conjunctive matrix.
A1 group: In the 15-day group, a pattern of immunostaining was observed peripherally in the bone trabeculae (particles of autogenous bone graft). At 60 days, this was maintained, although increased expression was observed in the 15-day group.
A2 group: At both 15 and 60 days, immunostaining for MMP2 was observed peripherally in the bone trabeculae and matrix conjunctiva.
The results of MMP2 immunostaining at 15 and 60 days are shown in Fig. 7. Note the immunostaining peripheral to the autogenous bone, with greater expression in the A1 and A2 groups compared with the control group.
In the quantitative immunohistochemical analysis (Table 3), no difference in immunostaining was observed between the groups at 15 days for MMP2 (p = 0.11).
However, a difference was observed at 60 days (p = 0.002). Greater immunostaining was apparent for groups A1 (p < 0.001) and A2 (p = 0.02) compared with the control group.
Discussion
Sodium alendronate is a nitrogen-containing BP with a high affinity for hydroxyapatite crystals in the bone. It is capable of inhibiting bone resorption, thus directly influencing bone resorption and formation (bone turnover) processes. However, the molecular mechanism by which BP inhibits bone resorption remains unclear [19, 20]. Physicochemical effects such as bone adsorption, cellular effects, cell apoptosis, and mediation of the inhibitory activity of BPs by osteoblasts have been studied to investigate this mechanism. Because of its ability to inhibit osteoclasts, sodium alendronate is widely used to treat patients with bone diseases, including osteoporosis.
With the purpose of developing a model of menopause, ovariectomy in sample rats was carried out to reduce the levels of progesterone and estradiol. According to Riggs and Melton in 1992 [21], an important cause of osteoporosis is the lowering of these hormones, and the ovariectomized adult female rat model, thus reliably, simulates postmenopausal osteoporosis. Following ovariectomy in rats, Sun et al. in 2001 [22] observed an increase in bone turnover caused by bone loss, followed by a deficit in bone mass in some regions of the skeleton. In addition, increased serum alkaline phosphatase and porosity of trabecular bone were observed, as well as changes in the microarchitecture of cancellous bone, characteristic of postmenopausal osteoporosis. Another advantage of this postmenopausal osteoporosis animal model is that, as in humans, rats have a cancellous bone that undergoes remodeling throughout life, even though linear growth has stopped [23]. Following ovariectomy, estradiol levels were found in our study to be sufficiently reduced as soon as 15 days later. This observation allowed for a shorter experimental period without affecting the results, given the effectiveness of ovariectomy 15 days after the procedure.
Postmenopausal osteoporosis is a disease characterized by increased bone resorption compared with bone neoformation, thus increasing the rate of bone turnover. This disease leads to an increased susceptibility to fractures due to a reduced bone mineral density. Thus, pharmacological treatment in women with postmenopausal osteoporosis aims to reduce the risk of fracture by increasing the bone mass [22]. The majority of patients with osteoporosis receive sodium alendronate. Kim et al. [4] showed that higher doses of this medication, when applied systemically, might contribute to decreased bone resorption, thus ensuring greater control over the process of bone remodeling. Bone et al. in 2004 [2], in a clinical study of women diagnosed with osteoporosis, found that treatment with 10 mg of sodium alendronate per day resulted in an increase in mineral bone density among various bone types. An important aspect of treatment with sodium alendronate is the determination of the minimum dose required to accelerate bone repair without causing systemic toxicity. For the treatment of osteoporosis, the standard dose of the drug is 70 mg per week (1 mg/kg). Given the more accelerated metabolism of rats, we used the human dose (1 mg/kg) but administered it three times per week in one of the groups. In the other group, the dose was tripled (3 mg/kg), and was maintained at three times per week until euthanasia [23, 24]. We have shown that alendronate accelerated the process of bone repair when applied systemically, which could be detected both at 15 and 60 days, and regardless of the dose. When the minor dose was used, greater initial bone formation was observed compared with the 3 mg/kg dose. Nevertheless, after 60 days, the amount of newly formed bone was similar between A1 and A2 groups. Hence, the dose of sodium alendronate does not need to be increased in order to obtain increased bone repair in long term.
In order to understand the mechanisms related to bone formation and the use of sodium alendronate, immunostaining for Wnt3a and Mmp2 was performed. Wnt3a is a member of the Wnt protein family, responsible for some functions in bone formation and osteoblastogenesis. In an in vitro study, Wnt3a was shown to stimulate osteoblastogenesis and adipogenesis [11, 25, 26]. Furthermore, Wnt3a promotes cell proliferation and regulates the generation of connective tissue in the initial stage of osteogenic differentiation [27]. Although Wnt3a is related to bone formation and resorption, there was no difference in expression observed by immunostaining among groups C, A1, and A2. However, when the groups were assessed qualitatively, differences among control and case groups were detected, with greater immunostaining for Wnt3a in osteoblasts of bone trabeculae observed in the latter. MMP2, also known as gelatinase, is involved in various processes in the ECM, both physiological and pathological. Nyman et al. in 2011 [28] showed that MMP2 contributes to bone density and trabecular bone architecture, thus potentially affecting biomechanical bone properties. At 15 days after administration of alendronate, no difference in immunoexpression of MMP2 was detected. At 60 days, however, increased expression of MMP2 was observed in the A1 and A2 groups compared with the control group.
Proteolytic processing is required for the activation of MMP2; in a previous study by De Simone et al. in 2013 [29], the expression of MMP2 was shown to be increased in a rat model of induced arthritis. At an initial time point of 7 days, a statistically significant decrease in expression of MMP2 was observed. However, after 60 days, increased immunopositivity for MMP2 was detected. Interestingly, the same effect was not observed for IL-4, IL-6, or TNF-α. This evidence is consistent with the findings of this study, especially with respect to the samples examined after 60 days of treatment. This late increase in the MMP2 expression suggests proteolytic activity after the initial period of bone formation, possibly mediated by treatment with sodium alendronate. In a study by Hashimoto et al. in 2007 [30], alendronate was found to inhibit ovarian cancer cell migration. In another study by the same group in 2005 [31], immunodeficient animals with ovarian cancer treated with sodium alendronate showed decreased invasion of the tumor stroma by inhibiting MMP2 activity. This antitumor effect may have resulted from the inhibition of cancer cell migration and proteolytic activity. Taken together, this evidence suggests that the increased expression of MMP2 at 60 days is the result of increased proteolytic activity, which occurs after initial exposure to alendronate.
In conclusion, animals treated with sodium alendronate showed greater bone neoformation when compared to the control group. Although both 1 mg/kg and 3 mg/kg doses increased long-term bone repair, the use of higher doses for this purpose was shown to be unnecessary and could lead to systemic implications. Finally, MMP2 appears to be associated with increased bone neoformation when sodium alendronate was administered.
References
Chaudhry AN, Ruggiero SL (2007) Osteonecrosis and bisphosphonates in oral and maxillofacial surgery. Oral Maxillofac Surg Clin N Am 19:199–206
Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Richard P et al (2004) Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med 350:1189–1199
Migliorati CA, Casiglia J, Epstein J, Jacobsen PL, Siegel MA, Woo SB. Managing the care of patients with bisphosphonate-associated osteonecrosis: an American Academy of Oral Medicine position paper. J Am Dent Assoc. 2005 Dec;136(12):1658–68. Review. Erratum in: J Am Dent Assoc. 2006 Jan;137(1):26
Kim JH, Park YB, Li Z, Shim JS, Moon HS, Jung HS, Chung MK (2011) Effect of alendronate on healing of extraction sockets and healing around implants. Oral Dis 17:705–711
Toker H, Ozdemir H, Ozer H, Eren K (2012) A comparative evaluation of the systemic and local alendronate treatment in synthetic bone graft: a histologic and histomorphometric study in a rat calvarial defect model. Oral Surg Oral Med Oral Pathol Oral Radiol 114(5 Suppl):S146–S152
Cao Y, Mori S, Mashiba T, Westmore MS, Ma L, Sato M, Akiyama T, Shi L, Komatsubara S, Miyamoto K, Norimatsu H (2002) Raloxifene, estrogen, and alendronate affect the processes of fracture repair differently in ovariectomized rats. J Bone Miner Res 17:2237–2246
Burch J, Rice S, Yang H, Neilson A, Stirk L, Francis R et al. Systematic review of the use of bone turnover markers for monitoring the response to osteoporosis treatment: the secondary prevention of fractures, and primary prevention of fractures in high-risk groups. Health Technology Assessment 2014; 18 (11)
Scully C, Madrid C, Bagan J (2006) Dental endosseous implants in patients on bisphosphonate therapy. Implant Dent 15:212–215
Migliorati CA, Siegel MA, Elting LS (2006) Bisphosphonate-associated osteonecrosis: a long-term complication of bisphosphonate treatment. Lancet Oncol 7(6):508–514
Krishnan V, Bryant HU, Macdougald OA (2006) Regulation of bone mass by Wnt signaling. J Clin Invest 116(5):1202–1209
De Boer J, Wang HJ, Van Blitterswijk C. Effects of Wnt signaling on proliferation and differentiation of human mesenchymal stem cells. Tissue Eng 2004; 10(3–4):393–401
Moschouris P, Retzepi M, Petrie A, Donos N (2016) Effect of Wnt3a delivery on early healing events during guided bone regeneration. Clin Oral Implants Res 28:283–290. https://doi.org/10.1111/clr.12796
Jabłońska-Trypuć A, Matejczyk M, Rosochacki S (2016) Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J Enzyme Inhib Med Chem 31(1):177–183
Nagase H, Woessner JF (1999) Matrix metalloproteinases. J Oral Chem 274:21491–21494
Sternlicht MD, Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17:463–516
Zhao H, Bernardo MM, Osenkowski P, Sohail A, Pei D, Nagase H, Kashiwagi M, Soloway PD, DeClerck YA, Fridman R (2004) Differential inhibition of membrane type 3 (MT3)-matrix metalloproteinase (MMP) and MT1-MMP by tissue inhibitor of metalloproteinase (TIMP)-2 and TIMP-3 rgulates pro-MMP-2 activation. J Biol Chem 279(10):8592–8601 Epub 2003 Dec 16
John M, Jaworski C, Chen Z, Subramanian S, Ma W, Sun F, Li D, Spector A, Carper D (2004) Matrix metalloproteinases are down-regulated in rat lenses exposed to oxidative stress. Exp Eye Res 79(6):839–846
Fernandes C, Leite RS, Lanças FM (2005) Bisfosfonatos: síntese, análises químicas e aplicações farmacológicas. Quím Nova 28(2):274–280
Buzza JA III, Einhorn T (2016) Bone healing in 2016. Clin Cases Miner Bone Metab 13(2):101–105
Riggs BL, Melton LJ III (1992) The prevention and treatment of osteoporosis. N EngI J Med 327:620–627
Sun JS, Huang YC, Tsuang YH, Chen LT, Lin FH (2001) Sintered dicalcium pyrophosphate increases bone mass in ovariectomized rats. J Biomed Mater Res 59(2):246–253
WHO technical report series, No. 843: (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO study group. Geneva: World Health Organization
Toker H, Ozdemir H, Ozer H, Eren K (2012) Alendronate enhances osseous healing in a rat calvarial defect model. Elsevier 57:1545–1550
Siebelt M, Waarsing JH, Groen HC, Müller C, Koelewijn SJ, de Blois E, Verhaar JAN, de Jong M, Weinans H (2014) Inhibited osteoclastic bone resorption through alendronate treatment in rats reduces severe osteoarthritis progression. Bone 66:163–170. https://doi.org/10.1016/j.bone.2014.06.009
Boland GM, Perkins G, Hall DJ, Tuan RS (2004) Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J Cell Biochem 93(6):1210–1230
Bennett CN, Longo KA, Wright WS, Suva LJ, Lane TF, Hankenson KD, MacDougald OA (2005) Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci U S A 102:3324–3329
Luo Q, Kang Q, Si W, Jiang W, Park JK, Peng Y et al (2004) Connective tissue growth factor (CTGF) is regulated by Wnt and bone morphogenetic proteins signaling in osteoblast differentiation of mesenchymal stem cells. J Biol Chem 279:55958–55968
Nyman JS, Lynch CC, Perrien DS, Thiolloy S, O’Quinn EC, Patil CA, Bi X, Pharr GM, Mahadevan-Jansen A, Mundy GR (2011) Differential effects between the loss of MMP-2 and MMP-9 on structural and tissue-level properties of bone. J Bone Miner Res 26(6):1252–1260
De Simone E, Caggiano N, Polli M, Rolando J, Lastra Y, Gullace F et al (2013) Efecto del alendronato sobre el perfil de citoquinas y metaloproteinasas 2 y 9 en un modelo múrido de artritis experimental. Rev Colomb Reumatol 20(4):202–210
Hashimoto K, Morishige K, Sawada K, Tahara M, Shimizu S, Ogata S, Sakata M, Tasaka K, Kimura T (2007) Alendronate suppresses tumor angiogenesis by inhibiting Rho activation of endothelial cells. Biochem Biophys Res Commun 354(2):478–484
Hashimoto K, Morishige K, Sawada K, Tahara M, Kawagishi R, Ikebuchi Y et al (2005 Jan 15) Alendronate inhibits intraperitoneal dissemination in vivo ovarian cancer model. Cancer Res 65(2):540–545
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Informed consent
Not applicable.
Rights and permissions
About this article
Cite this article
de Oliveira, N., Oliveira, J., de Souza Moraes, L. et al. Bone repair in craniofacial defects treated with different doses of alendronate: a histological, histomorphometric, and immunohistochemical study. Clin Oral Invest 23, 2355–2364 (2019). https://doi.org/10.1007/s00784-018-2670-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-018-2670-0