Abstract
Extracellular vesicles (EVs) are phospholipid membrane-enclosed entities containing specific proteins, RNA, miRNA, and lncRNA. EVs are released by various cells and play a vital role in cell communication by transferring their contents from the host cells to the recipient cells. The role of EVs has been characterized in a wide range of physiological and pathophysiological processes. In this context, we highlight recent advances in our understanding of the regulatory effects of EVs, with a focus on bone metabolism and the bone microenvironment. The roles of EVs in cell communication among bone-related cells, stem cells, tumor cells, and other cells under physiological or pathological conditions are also discussed. In addition, promising applications for EVs in treating bone-related diseases are proposed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
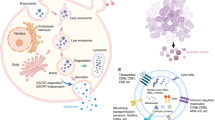
In multicellular organisms, there exist complex communication networks that are involved in regulating biological activity. Cells exchange information and interact with one another through the secretion of factors (cytokines, chemokines, hormones, nucleic acids, etc.) either directly or via vehicles known as extracellular vesicles (EVs) [1]. EVs are a series of small phospholipid membrane-enclosed entities released by a wide spectrum of cell types and present in various body fluids [2]. Based on their size, EVs are generally categorized into three classes: small vesicles (10–100 nm) [3, 4], larger vesicles (100–1000 nm) [3, 5], and large vesicles (1–4 μm) [3, 6]. The small vesicles, also known as exosomes, are released through exocytosis and contain specific proteins and nucleic acid. Larger vesicles are formed by budding from the plasma membrane and include microvesicles, shed vesicles, and matrix vesicles that play a role in bone formation. Large vesicles are released by dying cells and are called apoptotic bodies [3].
Among the EVs, exosomes and exosome-like vesicles may act on target cells directly via receptor-mediated interactions and transfer their content (cargo) from the host cells to the recipient cells [7]. Exosomes are characterized by the presence of proteins, including the tumor susceptibility gene (TSG) 101 protein, flotillin (Flot)1, heat shock proteins (HSPs), integrins, CD63, CD81, and CD82 [8]. An increasing number of studies have indicated that exosome cargos are also full of nucleic acids, such as messenger RNA (mRNA), microRNA (miRNA), circular RNA (circRNA), and long non-coding RNA (lncRNA) [9, 10]. In some cases, the exosomes carry more specific miRNA than the host cells from which they derive. It is still not clear how exosomes sort and carry nucleic acids, but research has suggested that Y-box protein 1 is required in the process of exosome miRNA sorting [11]. MicroRNAs are small endogenous non-coding RNAs, containing approximately 22 nucleotides. They are well-known as post-transcriptional regulators of mRNA expression [12]. LncRNAs are newly recognized regulators that can affect transcription patterns by targeting proteins to specific genomic loci, modulating the activity of protein-binding partners, or playing a role as precursors for small RNAs [13]. As non-coding RNA-enriched spherical bilayered proteolipids, exosomes play a vital role in cell communication, and of all the EVs, they have attracted the most attention from researchers.
Bone forms from a complex microenvironment that hosts a great diversity of tissues and cells, including osteocytes, osteoblasts (OBs), osteoclasts (OCs), hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs), fat cells, endothelial cells, cartilage, and nerves. These bone-related cells and tissues communicate with others through the secretion of regulatory factors [14] or via direct physical interactions [15]. A recent study has indicated that these members of the bone microenvironment all secrete exosomes [16]. It is conceivable that EVs play paracrine/endocrine roles within the bone microenvironment. The role of EVs (exosomes) has been studied comprehensively in many important fields, including tumorigenesis in the tumor microenvironment [17,18,19], crosstalk among cells [20,21,22,23], tissue repair and regeneration [24, 25], regulation of chronic inflammatory and immune processes [26] and angiogenesis [27], early diagnosis of disease [28, 29], and drug delivery [30]. Until recently, however, studies on EVs (exosomes) in bone metabolism and the bone microenvironment have been limited. In this context, we highlight the most recent developments regarding the regulatory effects of EVs (exosomes) in bone metabolism and the bone microenvironment. As there are still conflicts in the definition and characterization of different vesicles, in most cases it is unclear what types of vesicles have been investigated; therefore, we will use the term as described in the original work.
There is a specific EV type, known as matrix vesicles (30–300 nm), which are secreted by OBs and primarily involved in mineralization of newly forming bone matrix via hydroxyapatite deposition [31]. Some studies have indicated that matrix vesicles contain signaling proteins and growth factors, which are suggested to play a role in intercellular communication [32]. The intercellular communication capacity, rather than the mineralization capacity, of matrix vesicles will also be discussed here.
Extracellular vesicles in osteoblasts or osteoclasts are related to regulation in bone metabolism
Bone is a metabolically active tissue that is continuously remodelled via resorption of mineralized bone by osteoclasts (OCs) and the synthesis of bone matrix by osteoblasts (OBs). OBs of mesenchymal origin are the bone cells that contribute to the strength of the skeletal system through bone matrix production and mineralization. OBs also regulate bone homeostasis via paracrine secretion [33]. Studies have demonstrated that OBs can establish a feedback system in bone growth via secretion of EVs and matrix vesicles (MVs). As well-known OB-derived vehicles, MVs are very important for bone formation and mineralization. A recent study reported that in addition to transport of minerals, MVs also contain signaling proteins and growth factors, including bone morphogenetic proteins (BMP)1–7, vascular endothelial growth factor (VEGF), bone sialoprotein (BSP), osteopontin (OPN), osteocalcin (OC), and osteonectin (ON), and they may play a role in the regulation between bone cells [32]. In an MC3T3 cell model, Min et al. analyzed the content of exosomes derived from mouse OBs. In total, 1069 proteins were found, and many of them, including the EIF family, PP1C, PABP, and Rho GTPases, are involved in regulating osteogenesis and osteogenic differentiation [34]. After OB mineralization, which is affected by osteoblast nucleoside triphosphate pyrophosphatase (NPP1), together with calcium, OBs are embedded into bone matrix and become osteocytes [35]. OBs may play additional regulatory roles in this stage. Exosomes derived from mineralizing OBs (mineralizing pre-osteoblast MC3T3-E1 cells) exert a significant influence on miRNA profiles in bone marrow stromal cells (ST2) and promote their differentiation into OBs. This process is related to activation of the Wnt signaling pathway and is mediated by inhibiting Axin1 expression and increasing β-catenin expression in recipient cells [36].
Morhayim et al. compared and characterized the EVs secreted by human mineralizing (MOB) and non-mineralizing (NMOB) osteoblasts (SV-HFO cell line). Proteomic analysis showed that 97% of the proteins were shared among the OBs derived from EVs under different mineralization conditions, and 30% were novel osteoblast-specific EV proteins. Alkaline phosphatase and RNA binding proteins are at least five times as abundant in EVs from mineralizing osteoblasts as in EVs from non-mineralizing osteoblasts, which are primarily enriched in adhesion proteins [37]. Another study of these groups indicated that human OB-derived EVs contained a high abundance of miRNAs, including critical regulators of hematopoietic proliferation (miR-29a). OB-derived EVs enhanced proliferation of human umbilical cord blood-derived CD34(+) cells. These EV-expanded CD34(+) cells retained their differentiation capacity in vitro and successfully engrafted in vivo. These results indicate that EV-miRNAs should be considered essential for the development of expansion strategies for treating hematological disorders [38]. Similar findings have shown that EVs isolated from pre-osteoblasts are rich in miRNAs (e.g. miR-122-5p, miR-451a, miR-183-5p, miR-144-3p, and miR-142-5p), and they are able to deliver these miRNAs to undifferentiated embryonic stem cells (ESCs), and influence ESC differentiation by effecting persistence of pluripotent gene levels and increasing neuroectoderm differentiation [39]. All these data clarify the regulatory function of OBs in relation to other cells through release of EVs into the bone microenvironment.
OCs are multinucleated bone-resorbing cells formed by cytoplasmic fusion of their mononuclear precursors. The formation and differentiation of OCs is regulated primarily by the receptor activator of nuclear factor κB-ligand (RANKL), its receptor, receptor activator of nuclear factor κB (RANK), and the competitive inhibitor, osteoprotegerin (OPG) regulatory system. Researchers have found that mature OCs are able to express RANK and continue to require RANKL stimulation for bone resorption [40]. The study reported by Deng et al. confirmed that OB-derived MVs contained RANKL [16]. In co-culture, these MVs interacted with and stimulated the differentiation of osteoclast precursors into OCs [16]. In response to particular signals, such as 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] or mechanical stress, OBs express RANKL on their surface, which binds to RANK expressed on the surface of monocytes and triggers monocyte differentiate into OCs. OCs and their precursors also regulate themselves via paracrine signaling mediated by EVs [41]. Research has shown that the EVs derived from OCs and their precursors are enriched in RANK and may be functional by competitively inhibiting the stimulation of RANK on OC surfaces by RANKL. This is similar to osteoprotegerin, which was found to inhibit 1,25(OH)2D3-dependent OC formation in mouse marrow cultures [41].
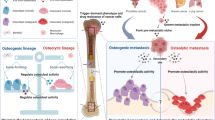
MicroRNA has been demonstrated to be a critical regulator in bone metabolism (reviewed by Shapior et al. [42]) and has been found in OC-derived EVs. In a study by Kagiya et al., miRNAs including let-7e, miR-21, miR-155, miR-210, miR-223, and miR-378 were found in OC (M-CSF stimulated murine bone marrow cell)-derived EVs, with miR-210 and miR-223 expressed at high levels [43]. Among these miRNAs, miR-378 can promote cell survival and participate in blood vessel formation, and plays an important role in osteoclastogenesis during OC differentiation [44, 45]. miR-210 is related to the regulation of various genes involved in cell cycle, differentiation, development, membrane trafficking, and migration/adhesion [46, 47]. miR-223 plays a key role in osteoclastogenesis, and the overexpression of miR-223 blocks osteoclast differentiation [48]. The murine let-7 family is composed of at least 12 members, expressed from eight genomic loci (let-7a-1, let-7a-2, let-7b, let-7c-1, let-7c-2, let-7d, let-7e, let-7f-1, let-7f-2, let-7 g, let-7i, and miR-98), and together with miR-140, they are involved in endochondral bone development. Deficiencies in both let-7 and miR-140 were found to cause a dramatic growth defect, synergistically reducing the mass of proliferating chondrocytes [49]. miR-214-3p is a specific miRNA precursor; it has a crucial role in skeletal disorders and suppresses bone formation. By targeting Osterix, miR-214-3p suppressed osteogenic differentiation of C2C12 myoblast cells [50], and it also interacted with ATF4, an important osteogenic transcriptional factor, to suppress bone formation [51]. Furthermore, miR-214-3p promotes osteoclastogenesis through the PI3K/Akt pathway by targeting phosphatase and tensin homologue (PTEN) [52]. In vitro and in vivo studies by Li et al. demonstrated that an increased osteoclastic miR-214-3p level was associated with both an elevated serum exosomal miR-214-3p level and reduced bone formation. The miR-214-3p in OCs was released in exosome-encapsulated form, and this exosomal miR-214-3p was able to be transferred to osteoblasts, inhibiting osteoblastic bone formation and promoting OC differentiation [53]. A study by Sun et al. also showed that miR-214 and ephrinA2 protein were enriched in OC-derived exosomes. These exosomes can specifically recognize OBs through the interaction between ephrinA2 and EphA2 and can transfer miR-214 into OBs to inhibit their function. Circulating miR-214 can be detected in exosomes from the serum of osteoporotic patients, indicating that it represents a biomarker for bone loss [54] (Fig. 1).
Extracellular vesicles are related to stem cell regulation in bone metabolism
Stem cells (SCs) are undifferentiated and multipotent cells with self-renewal ability and are often used in clinical trials to repair specific tissues [55, 56]. There are many types of SCs. They include mesenchymal stem cells (MSCs), hematopoietic stem cells (HSCs), induced pluripotent stem cells (iPSCs), embryonic stem cells (ESCs), neural stem cells (NSCs), endothelial progenitor cells (EPCs), and cardiac progenitor cells (CPCs). SCs can differentiate into tissue-specific cell types with specialized functions and release a variety of products, such as growth factors, cytokines, and EVs, which help to achieve their relevant effects [57, 58]. Recent studies have indicated that the tissue repair capacity of SCs is related to their paracrine activity rather than direct differentiation into specialized cells in the injured tissue [59], indicating the importance of EVs in this process. In the bone system, OBs derive from MSCs while OCs derive from HSCs, which indicates the close relationship between SCs and bone cells and the possibility that SC-derived EVs are involved in the regulation of bone metabolism. Among the SCs, MSCs are the most efficient producer of secreted EVs [60]. Because of their ability to differentiate into OBs, chondrocytes, and adipocytes [56, 61], and because they can be easily acquired from a patient’s own fat or bone marrow tissue, MSCs are often used clinically for treating bone and cartilage disorders [62, 63]. A study by Perrine et al. showed that RNAs could be transferred between human MSC-derived adipocytes and OBs via EVs. EVs isolated from human MSCs (hMSCs) at different stages of adipocyte differentiation contain PPAR-γ, leptin, adiponectin, and adipogenic RNAs. Co-culturing with these EVs can cause a decrease in osteocalcin and osteopontin expression in hMSC-derived OBs, which indicates that EVs could be a target component for regulating competition between OBs and adipocytes in the prevention or treatment of osteoporosis [64]. A study by Zhao et al. used human marrow stromal cell (HMSC)-derived exosomes to induce osteogenic differentiation of undifferentiated HMSCs. The exosomes isolated from normal HMSCs triggered and increased the expression of the growth factors bone morphogenetic protein 9 (BMP9) and transforming growth factor β1 (TGFβ1)—two effective inducers of osteogenic differentiation of HMSCs [65]. Moreover, the exosomes from osteogenic-activated HMSCs generated a better response in inducing osteogenic differentiation of undifferentiated HMSCs. These results show that exosomes derived from HMSCs can be easily endocytosed by recipient cells, bind to extracellular matrix (ECM) proteins such as type I collagen and fibronectin, and trigger lineage-specific differentiation of undifferentiated HMSCs both in vitro and in vivo [66].
An increasing number of studies have suggested that cargos such as miRNA [67], tRNA [68], and proteins [69] in human bone marrow stromal/stem cell (hBMSC)-secreted EVs include differentiation cues for osteogenic induction [70]. Using miRNA arrays probes, Xu et al. analyzed the miRNA in EVs isolated from bone marrow-derived mesenchymal stem cells (BMSCs). A total of 79 miRNAs were detected in BMSC EVs. Among these, nine EV-related miRNAs were up-regulated and four miRNAs were down-regulated significantly in BMSCs cultured at different time points. Five miRNAs (miR-199b, miR-218, miR-148a, miR-135b, and miR-221) were further validated and differentially expressed in the individual EV samples. Bioinformatic analysis demonstrated that the pathways related to RNA degradation, mRNA surveillance, Wnt signaling, and RNA transport were the most prominent pathways related to osteogenic differentiation, suggesting that miRNAs in BMSC-derived EVs are important regulators of OB differentiation [70]. A study by Qin et al. further demonstrated that BMSC-derived EVs can positively regulate osteogenic genes and osteoblastic differentiation in human OBs (hFOB 1.19). The BMSC-derived EVs promoted bone formation in critical-size calvarial bone defects in Sprague–Dawley (SD) rats, and the miRNA (miR-196a) in these EVs were found to play an essential role in the regulation of osteoblastic differentiation and the expression of osteogenic genes in this model [71].
The use of MSCs and miRNA in bone and cartilage tissue engineering has been well studied (reviewed by Mardones et al. [72]); if the miRNAs with a positive regulatory function in bone metastasis can be packaged into SC-derived EVs and be used in treating bone disorders, actually this strategy has been used. Shi et al. transferred miR-140-5p to synovial mesenchymal stem cells (SMSC-140s) and collected the miR-140-5p-overexpressing SMSC-140-derived exosomes to treat osteoarthritis (OA). They found that these miR-140-5p-enriched exosomes enhanced the proliferation and migration of articular chondrocytes by activating YAP via alternative Wnt signaling, without damaging extracellular matrix (ECM) secretion in vitro. In addition, these exosomes were successful in preventing OA in a rat model [73]. This study shows the broad potential for the modification of SC-derived EVs as a targeted therapeutic strategy for treating bone metabolism disorders in the future.
In addition to influencing the function of recipient cells, SCs regulate their own functions via EV-mediated paracrine signaling. Liu et al. found that Fas deficiency caused failure in miR-29b release, thereby elevating intracellular miR-29b levels, and down-regulated the expression of DNA methyltransferase 1 (Dnmt1) in MRL/lpr bone marrow MSCs. The decrease in Dnmt1 caused Notch1 promoter hypomethylation and activated Notch signaling, in turn leading to impaired osteogenic differentiation. The authors also demonstrated via MSC transplantation that EVs could transfer Fas to recipient MRL/lpr bone marrow MSCs and reduce intracellular levels of miR-29b, resulting in the recovery of Dnmt1-mediated Notch1 promoter hypomethylation and thereby improving MRL/lpr bone marrow MSC function [74]. A study by Martins et al. validated the osteoinductive potential of hBMSC-derived EVs. Under osteogenic stimulus (standard chemical cocktail or RUNX2 cationic lipid transfection), hBMSC-derived EVs were used to stimulate homotypic uncommitted cells, inducing an osteogenic phenotype characterized by marked early induction of BMP2, SP7, SPP1, BGLAP/IBSP, and alkaline phosphatase. These data showed that naturally secreted EVs could guide the osteogenic commitment of hBMSCs in the absence of other chemical or genetic osteoinductors [75]. A similar study investigated the effects of secreted factors from umbilical cord-derived mesenchymal stem cells (hUCMSCs) on osteogenic differentiation of MSCs. The results indicated that secreted hUCMSC factors could initiate osteogenic differentiation and increase the amount of calcium deposited in BMSCs. Moreover, the expression of osteogenesis-related genes, including ALP, BMP2, OCN, Osterix, Col1α, and RUNX2, was significantly up-regulated in the recipient cells. The study also found that factors secreted by hUCMSCs together with hBMSCs promoted ectopic bone formation in nude mice. Although the authors did not indicate whether these hUCMSC factors derived from EVs, it is likely that they are delivered via EVs and may be potential sources for promoting bone regeneration [76].
Regulation between cells in the intercellular microenvironment is a bidirectional process. SCs can also be affected by other cell-derived EVs. The monocyte/macrophage system plays a central role in host defense, immune regulation, and wound healing, and closely interacts with bone cells. The recruitment of MSCs and their osteogenic differentiation is a crucial step for bone formation at the bone–biomaterial interface. Monocytes/macrophages and/or their products play a very important role in regulating the recruitment and differentiation of MSCs. Research on the role of EVs in the interaction between monocytes and bone cells is still needed, but some studies have used conditioned media (CM) to explore these interactions. A study by Omar et al. demonstrated that monocytes isolated from human blood activated by LPS or IL-4 could influence the osteogenic gene expression of hMSCs via the production of cytokines and possibly other soluble factors. After treatment of hMSCs with monocyte CM, bone morphogenetic protein-2 (BMP-2) and runt-related transcription factor 2 (RUNX2) genes were significantly up-regulated in hMSCs. However, whether this regulatory effect of activated monocytes on hMSCs is mediated by EVs requires further investigation [77]. A study by Ekstrom et al. showed that under given experimental conditions, exosomes isolated from LPS-stimulated human monocytes were positive for CD9, CD63, CD81, TSG101, and Hsp70, and contained a wide size distribution of RNA. These exosomes interacted with MSCs and caused increased expression of the osteogenic markers RUNX2 and BMP-2 in MSCs [78]. Wang et al. found that DC-derived exosomes expressed surface molecules specific for DCs (CD83, CD86, CD80, and HLA-DR). These exosomes caused high expression of RUNX2 and increased the ALP activity in MSCs, indicating that DC-derived exosomes can induce MSCs to differentiate into OBs [79]. To sum up, the above data suggest that exosomes constitute an additional mode of cell–cell signaling interaction, with an effect on MSC differentiation during the transition from injury and inflammation to bone regeneration.
Extracellular vesicles mediate regulation of bone metabolism under disease conditions
Bone resorption and remodeling is in an ideal balance under normal conditions. However, under some pathological conditions, this balance is perturbed, which causes osteolytic lesions, bone pain, hypercalcemia and renal failure. The role of EVs in various bone-related pathological processes has been gradually revealed. To explore the mechanism of abnormal bone metabolism caused by multiple myeloma (MM), Raimondi et al. investigated the effect of MM cell-derived exosomes on OC differentiation. MM cell (U266, MM1S, and OPM2 cell lines)-derived exosomes induced the expression of OC markers including cathepsin K (CTSK), matrix metalloproteinase 9 (MMP-9), and tartrate-resistant acid phosphatase (TRAP) in RAW 264.7 cells and human primary OCs. By increasing CXCR4 expression and triggering a survival pathway, MM exosomes positively modulated the migration of these pre-OCs [80]. Similar results were obtained with exosomes derived from MM patient sera [80].
Osteoarthritis (OA) is a highly prevalent joint disease that involves progressive degradation of articular cartilage, synovial hyperplasia, bone remodeling, and angiogenesis in various joint tissues [81]. An early study found that the articular cartilage of the knees and hips from OA patients contained more MVs (50–250 nm) than normal. These OA-derived MVs exhibited high alkaline phosphatase (ALP) enzymatic activity and originated from the surface membranes of articular chondrocytes [82]. However, it was still not clear what role those MVs played in OA. Kato et al. examined the communication function of exosomes among joint tissue cells. The exosomes isolated from human synovial fibroblasts (SFBs) and interleukin-1β (IL-1β)-stimulated SFBs were analyzed and used to treat normal articular chondrocytes. NanoString analysis showed that 50 miRNAs were differentially expressed in exosomes from IL-1β-stimulated SFBs compared to non-stimulated SFBs. Compared with the non-stimulated SFB-derived exosomes, exosomes isolated from IL-1β-stimulated SFBs significantly up-regulated MMP-13 and ADAMTS-5 expression and down-regulated COL2A1 and ACAN in articular chondrocytes. These exosomes also significantly promoted migration and tube formation activity in human umbilical vein endothelial cells (HUVECs) and enhanced the release of proteoglycan from cartilage explants [83].
Tumor-derived EVs have been characterized as playing a pivotal role in cancer growth, development, and metastasis. Some specific tumor cells, including prostate, lung, and breast cancer cells, are prone to bone metastasis and have substantial crosstalk with bone cells in the bone microenvironment. Whether tumor-derived exosomes are involved in tumor and bone cell interactions is an attractive research problem. Karlsson et al. explored the inhibitory effect of prostate tumor-derived exosomes on OCs. Exosomes isolated from the murine prostate cancer cell line TRAMP-C1 dramatically reduced fusion and differentiation of monocytic osteoclast precursors into mature, multinucleated osteoclasts. They also reduced the expression of established markers, including DC-STAMP, TRAP, cathepsin K, and MMP-9, for OC fusion and differentiation [84]. Another study indicated that the EVs derived from PC3 cells (an advanced prostate cancer cell line) could be internalized by OC precursor RAW264.7 cells and primary human OBs (hOBs), stimulating osteoclastogenesis and hOB proliferation [85]. Reciprocally, OB-derived EVs can also be taken up by prostate cancer cells (PC3) and stimulate their growth by regulating the expression of related genes in vitro [37]. Osteosarcoma is another type of tumor that causes severe bone destruction, reducing overall bone quality and bone strength. Rama et al. found that extracellular membrane vesicles (EMVs) isolated from human osteosarcoma cells (143B and HOS) contained bioactive pro-osteoclastic cargo, including matrix metalloproteinase-1 and metalloproteinase-13 (MMP-1, MMP-13), transforming growth factor-β (TGF-β), CD-9, and RANKL, indicating that they play important roles in stimulating osteoclastogenesis and the activation of MMPs [86]. Studies regarding the role of EVs in other bone metastasis-prone tumors are still limited, but it is conceivable that tumor-derived EVs may be involved in the bone metastasis process and could be detected as a marker of bone metastasis in the future. In addition, the possible regulation of tumors by bone cells via secretion of EVs in the tumor-bone microenvironment is an attractive and promising area of study.
The effect of other EVs on bone metabolism
Milk has been considered to promote bone growth and density because it is rich in calcium and contains components that enhance intestinal calcium uptake or directly affect bone metabolism. Oliveira et al. examined the effect of bovine-derived milk 100,000-g pellet (P100), which contained nanoparticles (<220 nm) including EVs, on OC differentiation and bone resorption. The results showed that milk P100 increased the formation of small OCs with increased expression of TRAP, NFATc1, and c-Fos. P100 also increased the number of OCs in a mouse model, but it did not lead to greater bone resorption, likely due to reduced acid secretion [87]. Another study by the same group showed that milk-derived EVs increased the number of OCs and markedly increased brittle woven bone tissue. These EVs also up-regulated many osteogenic genes but decreased the production of type I collagen. To conclude, milk-derived EVs accelerate osteoblastogenesis but impair bone matrix formation, which indicates that milk-derived EVs may have negative effects on bone formation [88] (Fig. 2).
Discussion
After more than 30 years of research, the production, secretory pathway, and function of EVs are being increasingly revealed. EVs are derived from the late endosomal system; their predecessors are endosomes. Portions of the endosome membrane bud off into the lumen to form intraluminal vesicles. These late endosomes are called multivesicular bodies (MVBs). On one hand, MVBs fuse with lysosomes to degrade their intraluminal cargo, and on the other hand, MVBs can also fuse with the plasma membrane to release their intraluminal vesicles as EVs into the extracellular space [89, 90]. Thus far, the process by which cellular proteins and RNAs are targeted to endosomes and subsequently uploaded into EVs has not been elucidated. Some proteins have been shown to be targeted to exosomes in an ESCRT (endosomal sorting complex required for transport)-independent manner via higher-order oligomerization or a ceramide-dependent process [91,92,93]. Nevertheless, it is obvious that the biogenesis of EVs requires cellular expenditure of energy and resources, implying a functional importance for these vesicles rather than simply the discharge of waste. Using a specific fluorescent dye (PKH-26) as a label, Carrie et al. demonstrated that the uptake of exosomes was an active and specific process, and overnight storage at 4 or −20 °C did not impact exosome uptake. However, the uptake of exosomes by recipient cells can be partially blocked by heparin treatment [94].
As specific communication carriers between cells, there are two possible ways that EVs act on recipient cells. They may act as signaling complexes and stimulate recipient cells directly, or by transferring receptors between cells and delivering proteins and genetic information to the recipient cells (reviewed by Camussi et al. [7]). This indicates that EVs interact with specific target cells rather than random cells in the intercellular microenvironment. EVs are natural liposomes, which are non-toxic and have a long half-life, and they can escape immune surveillance and be taken up efficiently by recipient cells [8]. EVs also represent a population of membrane vesicles that can protect miRNAs from RNase-induced degradation [95]. As such, EVs represent the most promising drug carriers for use in future research. Many studies have attempted to remold EVs derived mainly from SCs or other cells, including by inducing expression of specific receptors to enhance the ability of EVs to bind to target recipient cells, in order to transfer anti-tumor drugs, anti-inflammatory drugs, and miRNA with therapeutic action, and then use the modified EVs for treating various diseases. Current methods used to upload drugs into EVs include chemical transfection of the host cells with the drugs (compounds, proteins, nucleic acids, etc.), and subsequent host cell secretion of EVs containing the drugs; isolation of EVs and co-incubation with the drugs; or increasing electroporation steps to upload more drugs into EVs. Moreover, as EVs contain special proteins or nucleic acids from the host cells, their use as excellent molecular biomarkers for diagnosis and prognosis of clinical diseases is very promising. For example, the detection of EVs has been experimentally used for tumor diagnosis (reviewed by Schorey et al. and Oltra et al. [96, 97].
In the field of bone microenvironment research, further exploration of the role of EVs is very important for elucidating the process of bone metabolism under physiological and pathological conditions, and clarifying the pathogenesis of certain bone-related diseases. It will also be helpful for revealing other biological processes related to the bone system. The use of EVs as disease biomarkers or tailored drug delivery vehicles in bone-related diseases can be expected. For example, OB-derived MVs are the natural vehicle for the transfer of hydroxyapatite and calcium to the growth interface of bone. It is easy to envision the use of these natural carriers for calcium supplementation, enhanced bone matrix formation, or treatment of bone metabolic diseases by appropriately transforming EVs and uploading the relevant drugs. At the present time, however, the manufacture of EVs, including purification, isolation, and cloning, is still expensive. Because of their nanoscale size, the detection and classification of EVs is also challenging [98], and thus the development of relevant separation, tracing, and detection techniques is critical. Moreover, before EVs can be applied in clinical therapies, the difference between in vitro and in vivo experimental systems should be carefully considered.
References
Qin Y, Sun R, Wu C, Wang L, Zhang C (2016) Exosome: a novel approach to stimulate bone regeneration through regulation of osteogenesis and angiogenesis. Int J Mol Sci 17:712. doi:10.3390/ijms17050712
Nolte-’t Hoen E, Cremer T, Gallo RC, Margolis LB (2016) Extracellular vesicles and viruses: are they close relatives?. Proc Natl Acad Sci USA 113:9155–9161. doi:10.1073/pnas.1605146113
Morhayim J, Baroncelli M, van Leeuwen JP (2014) Extracellular vesicles: specialized bone messengers. Arch Biochem Biophys 561:38–45. doi:10.1016/j.abb.2014.05.011
Cocucci E, Racchetti G, Meldolesi J (2009) Shedding microvesicles: artefacts no more. Trends Cell Biol 19:43–51. doi:10.1016/j.tcb.2008.11.003
Simons M, Raposo G (2009) Exosomes–vesicular carriers for intercellular communication. Curr Opin Cell Biol 21:575–581. doi:10.1016/j.ceb.2009.03.007
Hristov M, Erl W, Linder S, Weber PC (2004) Apoptotic bodies from endothelial cells enhance the number and initiate the differentiation of human endothelial progenitor cells in vitro. Blood 104:2761–2766. doi:10.1182/blood-2003-10-3614
Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L (2010) Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int 78:838–848. doi:10.1038/ki.2010.278
van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R (2012) Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev 64:676–705. doi:10.1124/pr.112.005983
Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9:654–659. doi:10.1038/ncb1596
Hannafon BN, Carpenter KJ, Berry WL, Janknecht R, Dooley WC, Ding WQ (2015) Exosome-mediated microRNA signaling from breast cancer cells is altered by the anti-angiogenesis agent docosahexaenoic acid (DHA). Mol Cancer 14:133. doi:10.1186/s12943-015-0400-7
Shurtleff MJ, Temoche-Diaz MM, Karfilis KV, Ri S, Schekman R (2016) Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. eLife. doi:10.7554/eLife.19276
Nakamachi Y, Kawano S, Takenokuchi M, Nishimura K, Sakai Y, Chin T, Saura R, Kurosaka M, Kumagai S (2009) MicroRNA-124a is a key regulator of proliferation and monocyte chemoattractant protein 1 secretion in fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Rheum 60:1294–1304. doi:10.1002/art.24475
Wilusz JE, Sunwoo H, Spector DL (2009) Long noncoding RNAs: functional surprises from the RNA world. Genes Dev 23:1494–1504. doi:10.1101/gad.1800909
Sims NA, Walsh NC (2012) Intercellular cross-talk among bone cells: new factors and pathways. Curr Osteoporos Rep 10:109–117. doi:10.1007/s11914-012-0096-1
Chiu YH, Ritchlin CT (2016) DC-STAMP: a key regulator in osteoclast differentiation. J Cell Physiol 231:2402–2407. doi:10.1002/jcp.25389
Deng L, Wang Y, Peng Y, Wu Y, Ding Y, Jiang Y, Shen Z, Fu Q (2015) Osteoblast-derived microvesicles: a novel mechanism for communication between osteoblasts and osteoclasts. Bone 79:37–42. doi:10.1016/j.bone.2015.05.022
Bronisz A, Godlewski J, Chiocca EA (2016) Extracellular vesicles and microRNAs: their role in tumorigenicity and therapy for brain tumors. Cell Mol Neurobiol 36:361–376. doi:10.1007/s10571-015-0293-4
Kosaka N, Yoshioka Y, Fujita Y, Ochiya T (2016) Versatile roles of extracellular vesicles in cancer. J Clin Investig 126:1163–1172. doi:10.1172/JCI81130
Ciardiello C, Cavallini L, Spinelli C, Yang J, Reis-Sobreiro M, de Candia P, Minciacchi VR, Di Vizio D (2016) Focus on extracellular vesicles: new frontiers of cell-to-cell communication in cancer. Int J Mol Sci 17:175. doi:10.3390/ijms17020175
Lopatina T, Gai C, Deregibus MC, Kholia S, Camussi G (2016) Cross talk between cancer and mesenchymal stem cells through extracellular vesicles carrying nucleic acids. Front Oncol 6:125. doi:10.3389/fonc.2016.00125
Desrochers LM, Antonyak MA, Cerione RA (2016) Extracellular vesicles: satellites of information transfer in cancer and stem cell biology. Dev Cell 37:301–309. doi:10.1016/j.devcel.2016.04.019
Kramer-Albers EM, Hill AF (2016) Extracellular vesicles: interneural shuttles of complex messages. Curr Opin Neurobiol 39:101–107. doi:10.1016/j.conb.2016.04.016
Zappulli V, Friis KP, Fitzpatrick Z, Maguire CA, Breakefield XO (2016) Extracellular vesicles and intercellular communication within the nervous system. J Clin Investig 126:1198–1207. doi:10.1172/JCI81134
Teixeira JH, Silva AM, Almeida MI, Barbosa MA, Santos SG (2016) Circulating extracellular vesicles: their role in tissue repair and regeneration. Transfus Apheresis Sci 55:53–61. doi:10.1016/j.transci.2016.07.015
Koniusz S, Andrzejewska A, Muraca M, Srivastava AK, Janowski M, Lukomska B (2016) Extracellular vesicles in physiology, pathology, and therapy of the immune and central nervous system, with focus on extracellular vesicles derived from mesenchymal stem cells as therapeutic tools. Front Cell Neurosci 10:109. doi:10.3389/fncel.2016.00109
Robbins PD, Dorronsoro A, Booker CN (2016) Regulation of chronic inflammatory and immune processes by extracellular vesicles. J Clin Investig 126:1173–1180. doi:10.1172/JCI81131
Merino-Gonzalez C, Zuniga FA, Escudero C, Ormazabal V, Reyes C, Nova-Lamperti E, Salomon C, Aguayo C (2016) Mesenchymal stem cell-derived extracellular vesicles promote angiogenesis: potencial clinical application. Front Physiol 7:24. doi:10.3389/fphys.2016.00024
Kinoshita T, Yip KW, Spence T, Liu FF (2017) MicroRNAs in extracellular vesicles: potential cancer biomarkers. J Hum Genet 62:67–74. doi:10.1038/jhg.2016.87
Gillet V, Hunting DJ, Takser L (2016) Turing revisited: decoding the microRNA messages in brain extracellular vesicles for early detection of neurodevelopmental disorders. Curr Environ Health Rep 3:188–201. doi:10.1007/s40572-016-0093-0
Vader P, Mol EA, Pasterkamp G, Schiffelers RM (2016) Extracellular vesicles for drug delivery. Adv Drug Deliv Rev 106:148–156. doi:10.1016/j.addr.2016.02.006
Anderson HC, Garimella R, Tague SE (2005) The role of matrix vesicles in growth plate development and biomineralization. Front Biosci: J Virtual Libr 10:822–837
Nahar NN, Missana LR, Garimella R, Tague SE, Anderson HC (2008) Matrix vesicles are carriers of bone morphogenetic proteins (BMPs), vascular endothelial growth factor (VEGF), and noncollagenous matrix proteins. J Bone Miner Metab 26:514–519. doi:10.1007/s00774-008-0859-z
Proff P, Romer P (2009) The molecular mechanism behind bone remodelling: a review. Clin Oral Investig 13:355–362. doi:10.1007/s00784-009-0268-2
Ge M, Ke R, Cai T, Yang J, Mu X (2015) Identification and proteomic analysis of osteoblast-derived exosomes. Biochem Biophys Res Commun 467:27–32. doi:10.1016/j.bbrc.2015.09.135
Valenti MT, Dalle Carbonare L, Mottes M (2016) Hypophosphatasia and mesenchymal stem cells: a therapeutic promise. Int J Stem Cell Res Ther 3:1–3. doi:10.3390/ijms18010041
Cui Y, Luan J, Li H, Zhou X, Han J (2016) Exosomes derived from mineralizing osteoblasts promote ST2 cell osteogenic differentiation by alteration of microRNA expression. FEBS Lett 590:185–192. doi:10.1002/1873-3468.12024
Morhayim J, van de Peppel J, Demmers JA, Kocer G, Nigg AL, van Driel M, Chiba H, van Leeuwen JP (2015) Proteomic signatures of extracellular vesicles secreted by nonmineralizing and mineralizing human osteoblasts and stimulation of tumor cell growth. FASEB J 29:274–285. doi:10.1096/fj.14-261404
Morhayim J, van de Peppel J, Braakman E, Rombouts EW, Ter Borg MN, Dudakovic A, Chiba H, van der Eerden BC, Raaijmakers MH, van Wijnen AJ, Cornelissen JJ, van Leeuwen JP (2016) Osteoblasts secrete miRNA-containing extracellular vesicles that enhance expansion of human umbilical cord blood cells. Sci Rep 6:32034. doi:10.1038/srep32034
Nair R, Santos L, Awasthi S, von Erlach T, Chow LW, Bertazzo S, Stevens MM (2014) Extracellular vesicles derived from preosteoblasts influence embryonic stem cell differentiation. Stem Cells Dev 23:1625–1635. doi:10.1089/scd.2013.0633
Burgess TL, Qian Y, Kaufman S, Ring BD, Van G, Capparelli C, Kelley M, Hsu H, Boyle WJ, Dunstan CR, Hu S, Lacey DL (1999) The ligand for osteoprotegerin (OPGL) directly activates mature osteoclasts. J Cell Biol 145:527–538
Huynh N, VonMoss L, Smith D, Rahman I, Felemban MF, Zuo J, Rody WJ Jr, McHugh KP, Holliday LS (2016) Characterization of regulatory extracellular vesicles from osteoclasts. J Dent Res 95:673–679. doi:10.1177/0022034516633189
Shapiro IM, Landis WJ, Risbud MV (2015) Matrix vesicles: are they anchored exosomes?. Bone 79:29–36. doi:10.1016/j.bone.2015.05.013
Kagiya T, Taira M (2013) Expression of microRNAs in the extracellular microvesicles of murine osteoclasts. J Oral Tissue Eng 10:142–150
Kagiya T, Nakamura S (2013) Expression profiling of microRNAs in RAW264.7 cells treated with a combination of tumor necrosis factor alpha and RANKL during osteoclast differentiation. J Periodontal Res 48:373–385. doi:10.1111/jre.12017
Lee DY, Deng Z, Wang CH, Yang BB (2007) MicroRNA-378 promotes cell survival, tumor growth, and angiogenesis by targeting SuFu and Fus-1 expression. Proc Natl Acad Sci USA 104:20350–20355. doi:10.1073/pnas.0706901104
Fasanaro P, Greco S, Lorenzi M, Pescatori M, Brioschi M, Kulshreshtha R, Banfi C, Stubbs A, Calin GA, Ivan M, Capogrossi MC, Martelli F (2009) An integrated approach for experimental target identification of hypoxia-induced miR-210. J Biol Chem 284:35134–35143. doi:10.1074/jbc.M109.052779
Tsuchiya S, Fujiwara T, Sato F, Shimada Y, Tanaka E, Sakai Y, Shimizu K, Tsujimoto G (2011) MicroRNA-210 regulates cancer cell proliferation through targeting fibroblast growth factor receptor-like 1 (FGFRL1). J Biol Chem 286:420–428. doi:10.1074/jbc.M110.170852
Sugatani T, Hruska KA (2007) MicroRNA-223 is a key factor in osteoclast differentiation. J Cell Biochem 101:996–999. doi:10.1002/jcb.21335
Papaioannou G, Inloes JB, Nakamura Y, Paltrinieri E, Kobayashi T (2013) let-7 and miR-140 microRNAs coordinately regulate skeletal development. Proc Natl Acad Sci USA 110:E3291–E3300. doi:10.1073/pnas.1302797110
Shi K, Lu J, Zhao Y, Wang L, Li J, Qi B, Li H, Ma C (2013) MicroRNA-214 suppresses osteogenic differentiation of C2C12 myoblast cells by targeting Osterix. Bone 55:487–494. doi:10.1016/j.bone.2013.04.002
Wang X, Guo B, Li Q, Peng J, Yang Z et al (2013) miR-214 targets ATF4 to inhibit bone formation. Nat Med 19:93–100. doi:10.1038/nm.3026
Zhao C, Sun W, Zhang P, Ling S, Li Y, Zhao D, Peng J, Wang A, Li Q, Song J, Wang C, Xu X, Xu Z, Zhong G, Han B, Chang YZ (2015) miR-214 promotes osteoclastogenesis by targeting Pten/PI3k/Akt pathway. RNA Biol 12:343–353. doi:10.1080/15476286.2015.1017205
Li D, Liu J, Guo B, Liang C, Dang L et al (2016) Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation. Nat Commun 7:10872. doi:10.1038/ncomms10872
Sun W, Zhao C, Li Y, Wang L, Nie G et al (2016) Osteoclast-derived microRNA-containing exosomes selectively inhibit osteoblast activity. Cell Discov 2:16015. doi:10.1038/celldisc.2016.15
Zhang B, Yeo RW, Tan KH, Lim SK (2016) Focus on extracellular vesicles: therapeutic potential of stem cell-derived extracellular vesicles. Int J Mol Sci 17:174. doi:10.3390/ijms17020174
Phinney DG, Prockop DJ (2007) Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells 25:2896–2902. doi:10.1634/stemcells.2007-0637
Ronne WYY, Ruenn CL, Kok HT, Sai KL (2013) Exosome: a novel and safer therapeutic refinement of mesenchymal stem cell. J Circ Biomark 1:1–12. doi:10.5772/57460
Thery C (2011) Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep 3:15. doi:10.3410/B3-15
Baglio SR, Pegtel DM, Baldini N (2012) Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol 3:359. doi:10.3389/fphys.2012.00359
Yeo RW, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ, Lim SK (2013) Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev 65:336–341. doi:10.1016/j.addr.2012.07.001
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Maumus M, Guerit D, Toupet K, Jorgensen C, Noel D (2011) Mesenchymal stem cell-based therapies in regenerative medicine: applications in rheumatology. Stem Cell Res Ther 2:14. doi:10.1186/scrt55
Ranganath SH, Levy O, Inamdar MS, Karp JM (2012) Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell 10:244–258. doi:10.1016/j.stem.2012.02.005
Martin PJ, Haren N, Ghali O, Clabaut A, Chauveau C, Hardouin P, Broux O (2015) Adipogenic RNAs are transferred in osteoblasts via bone marrow adipocytes-derived extracellular vesicles (EVs). BMC cell Biol 16:10. doi:10.1186/s12860-015-0057-5
Zhao L, Jiang S, Hantash BM (2010) Transforming growth factor beta1 induces osteogenic differentiation of murine bone marrow stromal cells. Tissue Eng Part A 16:725–733. doi:10.1089/ten.TEA.2009.0495
Narayanan R, Huang CC, Ravindran S (2016) Hijacking the cellular mail: exosome mediated differentiation of mesenchymal stem cells. Stem Cells Int 2016:3808674. doi:10.1155/2016/3808674
Collino F, Deregibus MC, Bruno S, Sterpone L, Aghemo G, Viltono L, Tetta C, Camussi G (2010) Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One 5:e11803. doi:10.1371/journal.pone.0011803
Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Perez Lanzon M, Zini N, Naaijkens B, Perut F, Niessen HW, Baldini N, Pegtel DM (2015) Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther 6:127. doi:10.1186/s13287-015-0116-z
Kim HS, Choi DY, Yun SJ, Choi SM, Kang JW, Jung JW, Hwang D, Kim KP, Kim DW (2012) Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J Proteome Res 11:839–849. doi:10.1021/pr200682z
Xu JF, Yang GH, Pan XH, Zhang SJ, Zhao C, Qiu BS, Gu HF, Hong JF, Cao L, Chen Y, Xia B, Bi Q, Wang YP (2014) Altered microRNA expression profile in exosomes during osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. PLoS One 9:e114627. doi:10.1371/journal.pone.0114627
Qin Y, Wang L, Gao Z, Chen G, Zhang C (2016) Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci Rep 6:21961. doi:10.1038/srep21961
Mardones R, Jofre CM, Minguell JJ (2015) Cell therapy and tissue engineering approaches for cartilage repair and/or regeneration. Int J Stem Cells 8:48–53. doi:10.15283/ijsc.2015.8.1.48
Tao SC, Yuan T, Zhang YL, Yin WJ, Guo SC, Zhang CQ (2017) Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 7:180–195. doi:10.7150/thno.17133
Liu S, Liu D, Chen C, Hamamura K, Moshaverinia A, Yang R, Liu Y, Jin Y, Shi S (2015) MSC transplantation improves osteopenia via epigenetic regulation of notch signaling in lupus. Cell Metab 22:606–618. doi:10.1016/j.cmet.2015.08.018
Martins M, Ribeiro D, Martins A, Reis RL, Neves NM (2016) Extracellular vesicles derived from osteogenically induced human bone marrow mesenchymal stem cells can modulate lineage commitment. Stem Cell Rep 6:284–291. doi:10.1016/j.stemcr.2016.01.001
Wang KX, Xu LL, Rui YF, Huang S, Lin SE, Xiong JH, Li YH, Lee WY, Li G (2015) The effects of secretion factors from umbilical cord derived mesenchymal stem cells on osteogenic differentiation of mesenchymal stem cells. PLoS One 10:e0120593. doi:10.1371/journal.pone.0120593
Omar OM, Graneli C, Ekstrom K, Karlsson C, Johansson A, Lausmaa J, Wexell CL, Thomsen P (2011) The stimulation of an osteogenic response by classical monocyte activation. Biomaterials 32:8190–8204. doi:10.1016/j.biomaterials.2011.07.055
Ekstrom K, Omar O, Graneli C, Wang X, Vazirisani F, Thomsen P (2013) Monocyte exosomes stimulate the osteogenic gene expression of mesenchymal stem cells. PLoS One 8:e75227. doi:10.1371/journal.pone.0075227
Wang Z, Ding L, Zheng XL, Wang HX, Yan HM (2014) [DC-derived exosomes induce osteogenic differentiation of mesenchymal stem cells] (in Chinese). Zhongguo shi yan xue ye xue za zhi 22:600–604. doi:10.7534/j.issn.1009-2137.2014.03.005
Raimondi L, De Luca A, Amodio N, Manno M, Raccosta S, Taverna S, Bellavia D, Naselli F, Fontana S, Schillaci O, Giardino R, Fini M, Tassone P, Santoro A, De Leo G, Giavaresi G, Alessandro R (2015) Involvement of multiple myeloma cell-derived exosomes in osteoclast differentiation. Oncotarget 6:13772–13789. doi:10.18632/oncotarget.3830
Anderson HC, Mulhall D, Garimella R (2010) Role of extracellular membrane vesicles in the pathogenesis of various diseases, including cancer, renal diseases, atherosclerosis, and arthritis. Lab Investig J Tech Methods Pathol 90:1549–1557. doi:10.1038/labinvest.2010.152
Ali SY, Griffiths S (1983) Formation of calcium phosphate crystals in normal and osteoarthritic cartilage. Ann Rheum Dis 42:45–48
Kato T, Miyaki S, Ishitobi H, Nakamura Y, Nakasa T, Lotz MK, Ochi M (2014) Exosomes from IL-1beta stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Res Ther 16:R163. doi:10.1186/ar4679
Karlsson T, Lundholm M, Widmark A, Persson E (2016) Tumor cell-derived exosomes from the prostate cancer cell line TRAMP-C1 impair osteoclast formation and differentiation. PLoS One 11:e0166284. doi:10.1371/journal.pone.0166284
Inder KL, Ruelcke JE, Petelin L, Moon H, Choi E, Rae J, Blumenthal A, Hutmacher D, Saunders NA, Stow JL, Parton RG, Hill MM (2014) Cavin-1/PTRF alters prostate cancer cell-derived extracellular vesicle content and internalization to attenuate extracellular vesicle-mediated osteoclastogenesis and osteoblast proliferation. J Extracell Vesicles 3:23784. doi:10.3402/jev.v3.23784
Garimella R, Washington L, Isaacson J, Vallejo J, Spence M, Tawfik O, Rowe P, Brotto M, Perez R (2014) Extracellular membrane vesicles derived from 143B osteosarcoma cells contain pro-osteoclastogenic cargo: a novel communication mechanism in osteosarcoma bone microenvironment. Transl Oncol 7:331–340. doi:10.1016/j.tranon.2014.04.011
Oliveira MC, Di Ceglie I, Arntz OJ, van den Berg WB, van den Hoogen FH, Ferreira AV, van Lent PL, van de Loo FA (2017) Milk-derived nanoparticle fraction promotes the formation of small osteoclasts but reduces bone resorption. J Cell Physiol 232:225–233. doi:10.1002/jcp.25414
Oliveira MC, Arntz OJ, Blaney Davidson EN, van Lent PL, Koenders MI, van der Kraan PM, van den Berg WB, Ferreira AV, van de Loo FA (2016) Milk extracellular vesicles accelerate osteoblastogenesis but impair bone matrix formation. J Nutr Biochem 30:74–84. doi:10.1016/j.jnutbio.2015.11.017
Scita G, Di Fiore PP (2010) The endocytic matrix. Nature 463:464–473. doi:10.1038/nature08910
Ludwig AK, Giebel B (2012) Exosomes: small vesicles participating in intercellular communication. Int J Biochem Cell Biol 44:11–15. doi:10.1016/j.biocel.2011.10.005
Fang Y, Wu N, Gan X, Yan W, Morrell JC, Gould SJ (2007) Higher-order oligomerization targets plasma membrane proteins and HIV gag to exosomes. PLoS Biol 5:e158. doi:10.1371/journal.pbio.0050158
Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, Simons M (2008) Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319:1244–1247. doi:10.1126/science.1153124
Raiborg C, Rusten TE, Stenmark H (2003) Protein sorting into multivesicular endosomes. Curr Opin Cell Biol 15:446–455
Franzen CA, Simms PE, Van Huis AF, Foreman KE, Kuo PC, Gupta GN (2014) Characterization of uptake and internalization of exosomes by bladder cancer cells. Biomed Res Int 2014:619829. doi:10.1155/2014/619829
Mause SF, Weber C (2010) Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ Res 107:1047–1057. doi:10.1161/CIRCRESAHA.110.226456
Schorey JS, Bhatnagar S (2008) Exosome function: from tumor immunology to pathogen biology. Traffic 9:871–881. doi:10.1111/j.1600-0854.2008.00734.x
Oltra E (2014) Relevance of splicing on tumor-released exosome landscape: implications in cancer therapeutics. Front Endocrinol 5:194. doi:10.3389/fendo.2014.00194
van der Pol E, Hoekstra AG, Sturk A, Otto C, van Leeuwen TG, Nieuwland R (2010) Optical and non-optical methods for detection and characterization of microparticles and exosomes. J Thromb Haemost 8:2596–2607. doi:10.1111/j.1538-7836.2010.04074.x
Acknowledgements
We gratefully acknowledge financial support from the National Natural Science Foundation of China (NSFC; Grant No. 81502465).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflict of interest.
About this article
Cite this article
Li, Q., Huang, QP., Wang, YL. et al. Extracellular vesicle-mediated bone metabolism in the bone microenvironment. J Bone Miner Metab 36, 1–11 (2018). https://doi.org/10.1007/s00774-017-0860-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-017-0860-5