Abstract
The first objective of this study was to determine normative digital X-ray radiogrammetry (DXR) values, based on original digital images, in a pediatric population (aged 6–18 years). The second aim was to compare these reference data with patients suffering from distal radius fractures, whereas both cohorts originated from the same geographical region and were evaluated using the same technical parameters as well as inclusion and exclusion criteria. DXR-BMD and DXR-MCI of the metacarpal bones II–IV were assessed on standardized digital hand radiographs, without printing or scanning procedures. DXR parameters were estimated separately by gender and among six age groups; values in the fracture group were compared to age- and gender-matched normative data using Student’s t tests and Z scores. In the reference cohort (150 boys, 138 girls), gender differences were found in bone mineral density (DXR-BMD), with higher values for girls from 11 to 14 years and for boys from 15 to 18 years (p < 0.05). Girls had higher normative metacarpal index (DXR-MCI) values than boys, with significant differences at 11–14 years (p < 0.05). In the case–control investigation, the fracture group (95 boys, 69 girls) presented lower DXR-BMD at 15–18 years in boys and 13–16 years in girls vs. the reference cohort (p < 0.05); DXR-MCI was lower at 11–18 years in boys and 11–16 years in girls (p < 0.05). Mean Z scores in the fracture group for DXR-BMD were −0.42 (boys) and −0.46 (girls), and for DXR-MCI were −0.51 (boys) and −0.53 (girls). These findings indicate that the fully digital DXR technique can be accurately applied in pediatric populations ≥ 6 years of age. The lower DXR-BMD and DXR-MCI values in the fracture group suggest promising early identification of individuals with increased fracture risk, without the need for additional radiation exposure, enabling the initiation of prevention strategies to possibly reduce the incidence of osteoporosis later in life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of fractures is characterized by two age peaks, one due to bone loss later in life and the other in early adolescence [1–4]. The most common fractures in childhood and adolescence occur at the distal forearm, particularly at the distal radius, accounting for approximately 25 % of all pediatric fractures, with increasing incidence over the past decades [1, 4–6]. In addition to acute consequences such as immobilization, pediatric distal radius fractures can result in long-term implications, including lasting functional limitations, diminished working capacity, and substantial health care costs [4–6]. Osteoporosis was once considered as solely a disease of the elderly; recently, it is more and more agreed that osteoporosis can have a pediatric origin [7–9]. Individuals who fail to achieve optimal peak bone mass during growth and maturation are predisposed to develop osteoporosis later in their lives [10, 11].
In order to provide effective prevention strategies against the development of osteoporosis, it is important to identify individuals with increased risk as early as possible [9–11]. However, routine measurements of bone mass and bone mineral density (BMD) in children and adolescents are complicated, as their higher sensitivity to ionizing radiation requires strict indications for techniques using radiation exposure [12–14]. Dual-energy X-ray absorptiometry (DXA), the gold standard for diagnosis of osteoporosis in adults, has also become the most widely used osteodensitometric technique in children and adolescents, due to its high safety, good accuracy, relatively low cost, and widespread availability [15–18]. However, in addition to increased radiation exposure, DXA presents further challenges in pediatric populations, as measurement of DXA can be impaired if pediatric bones are too small or too large compared to the calendar age of the children and in the case of obesity [13, 14, 19]. This influence of soft tissue fat becomes more relevant in childhood populations due to their rising incidence of obesity, and high body mass index (BMI) has been proven to increase fracture risk in children [6, 17]. Quantitative ultrasound (QUS) is a promising, radiation-free, possibly portable osteodensitometric technique in pediatric populations; however, as current limitations varying interobserver reproducibility and the possible influence of environment factors during the measurement are discussed [13, 20–23].
Digital X-ray radiogrammetry (DXR) provides automatic radiogeometric bone analysis, and the value of this relatively novel computer-assisted diagnosis (CAD) technique in the assessment of different primary and secondary forms of osteoporosis has been reported in detail for adults, but also for children and adolescents [24–34]. Several investigations have demonstrated that DXR parameters show a close correlation with BMD measurements of the reference standard DXA; DXR allows identification of women at risk for osteoporotic fractures, although DXR calculates BMD at peripheral and not axial skeletal sites [24, 28, 29]. As DXR-BMD can be assessed using hand radiographs, which are commonly acquired in pediatric populations—e.g., for ruling out fractures or for assessing skeletal age—additional ionizing radiation is not necessary [30–34]. Reference values for DXR parameters have been generated in both adult populations and in children and adolescents, whereas hand radiographs had been printed out and subsequently scanned for osteodensitometric CAD analysis [30, 33, 35, 36].
Thus, the first aim of this investigation was to determine reference values for bone mineral density (DXR-BMD) and metacarpal index (DXR-MCI) in a pediatric population aged 6–18 years based on original digital hand radiographs, without circuitous printing and scanning processes. The second objective was to compare these reference data with age- and gender-matched patients suffering from distal radius fractures, whereas both cohorts originated from the same geographical region and were evaluated using the same technical parameters as well as inclusion and exclusion criteria. Early identification of children and adolescents at increased risk of experiencing fractures and developing osteoporosis can provide specific prevention strategies, such as dietary consumption and physical activity.
Materials and methods
Subjects
This retrospective analysis of a consecutive enrollment of children and adolescents included Caucasian German subjects aged 6 to 18 years. For analysis of the cortical bone partition, digital hand radiographs were used in anteroposterior projection, performed under standardized technical parameters at the same radiological department. In both, the reference cohort (without any fracture) and the group with distal radius fractures, the clinical indication of the hand radiographs was trauma during daily living or sports activities; some patients received further radiographs—e.g., of the wrist—depending on their clinical symptoms. Each trauma was categorized by orthopedic surgeons as adequate to cause a distal radius fracture. The X-ray examinations of the affected hand were obtained within three days after the trauma, before necessary therapeutic strategies were initiated (e.g., immobilization). All subjects originated from the same geographical region.
The following exclusion criteria were defined:
-
Visible metallic material or splints after osteosynthesis;
-
Previous fractures of the upper extremities;
-
Pathological abnormalities of the hand skeleton;
-
Superposition of the metacarpal bones on X-ray examination;
-
Bone-affecting diseases, early and late puberty;
-
Use of medication with an influence on bone metabolism, e.g., steroids;
-
Incomplete analysis by the DXR system;
-
In the fracture cohort, distal radius fractures involving the epiphyseal growth plate were excluded, as this injury is typically characterized by fragility of the cartilage tissue.
Two experienced radiologists evaluated all radiographs and confirmed or excluded distal radius fractures. All hand radiographs were analyzed as original fully digital images using Sectra Osteoporosis Package (Sectra Medical Systems, Linköping, Sweden) as part of the picture archiving and communication system (PACS) of the hospital, without the influence of printing and/or scanning procedures. All X-ray examinations were performed in the course of routine patient care; no radiographs were obtained solely for study purposes. The study was designed in accordance with local ethics committee guidelines and the Declaration of Helsinki. All patients and their parents gave their consent for the scientific use of their clinical and radiological data.
In the present study, 452 subjects fulfilled the inclusion and exclusion criteria and could be enrolled: 164 patients (95 boys, 69 girls) experienced a distal radius fracture, and 288 children and adolescents (150 boys, 138 girls) had no fracture after adequate trauma, and therefore functioned as the reference cohort. The trauma severity was not significantly different between the fracture and reference cohorts (similar trauma severity associated with fracture graded according to the method described by Landin [4]). All subjects were assigned to one of six subgroups based on their calendar age, and all data were analyzed separately by gender:
-
Group 1: 6–8 years
-
Group 2: 9–10 years
-
Group 3: 11–12 years
-
Group 4: 13–14 years
-
Group 5: 15–16 years
-
Group 6: 17– 18 years
Additionally, skeletal age was evaluated by both radiologists in all subjects according to the method described by Thiemann-Nitz [37]. In all included children and adolescents, skeletal age groups were in concordance with the calendar age groups (+/− one year).
Imaging acquisition and analysis
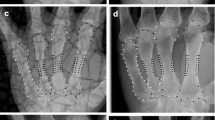
All hand radiographs were obtained in anteroposterior projection using the Philips BuckyDiagnost TH system (Philips Healthcare; Best, The Netherlands), with the following standardized, age-adapted technical parameters: maximum tube voltage 56 kV, maximum exposure level 8 mAs, exposure time 4 ms, film focus distance 100 cm, and filter with 1.0 mm aluminum plus 0.1–0.2 mm copper. The Sectra Osteoporosis Package was used to determine cortical DXR-BMD and DXR-MCI on metacarpal bones II–IV of the original digital hand radiographs. As the DXR technique has been described in detail [24–27], the calculation of DXR-BMD and DXR-MCI is only summarized here. The CAD estimates of cortical bone parameters were performed automatically, without user interaction. DXR-BMD (in g/cm2) was computed considering the total cortical volume per area (VPA), with correction for porosity and relation to DXA measurements of the mid-distal forearm [24–27]. DXR-MCI is a dimensionless parameter, calculated as the ratio of cortical thickness to outer metacarpal width: 2 × cortical thickness/outer metacarpal width [26, 38].
Statistical evaluation
The statistical analysis of this investigation was performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). In order to establish digital reference values for DXR-BMD and DXR-MCI in healthy children, the calculations of the means, including standard deviations (SDs), were conducted separately for the six age groups and the genders. As the values of DXR-BMD and DXR-MCI were normally distributed, Student’s t tests for independent samples were performed to indicate possibly significant differences between males and females of the same age groups. The findings of the DXR parameters in boys and girls with distal radius fractures were compared to the normative data, also by using Student’s t tests for independent samples, separately for each age group. Additionally, the DXR-BMD and DXR-MCI of each patient with a distal radius fracture were associated with age- and gender-matched reference values using Z scores. These Z scores were computed as follows:
As the Z scores were also normally distributed, means were calculated for the male and female fracture cohorts and compared. The Z scores for DXR-BMD and DXR-MCI were further categorized as reduced (Z scores between −1 and −2 SD, as well as ≤−2 SD), regular (Z scores ± 1 SD), or increased values (Z scores between 1 and 2 SD, as well as ≥2 SD). For correlation analyses, Pearson′s correlation coefficients were performed. In all statistical analyses, p values less than 0.05 (two-sided) were considered to indicate significance.
Results
Normative DXR values of healthy children and adolescents
The reference values for the 288 healthy children and adolescents are presented in Table 1 (for the 150 boys) and Table 2 (for the 138 girls). Between the age groups 6–8 and 17–18 years for boys, the mean DXR-BMD increased from 0.383 to 0.617 g/cm2 and the mean DXR-MCI from 0.319 to 0.466 (Table 1). Boys showed the highest average elevation of normative DXR-BMD (+26.4 %) and DXR-MCI (+26.3 %) in the age group of 15–16 years compared to 13–14 years (Table 1). In the female reference cohort, the mean DXR-BMD was 0.369 g/cm2 and the mean DXR-MCI 0.325 at 6–8 years, compared to 0.565 g/cm2 and 0.473 at 17–18 years (Table 2). Normative DXR-BMD increased mostly accentuated in girls from 11 to 14 years; the highest average elevation of normative DXR-MCI was 17.2 % in the age group 13–14 years compared to 11–12 years (Table 2). Strong positive correlations between DXR-BMD/DXR-MCI and age were found for both genders: Pearson’s correlation coefficients r = 0.86 (DXR-BMD) and r = 0.73 (DXR-MCI) for boys, and r = 0.81 (DXR-BMD) and r = 0.71 (DXR-MCI) for girls, whereas all correlations were highly significant (p < 0.01). The correlations between DXR-BMD and DXR-MCI were r = 0.88 (boys; p < 0.01) and r = 0.84 (girls; p < 0.01).
In children between 6 and 10 years of age, no significant differences in DXR-BMD were observed between boys and girls. DXR-BMD reference values were significantly higher for girls at ages 11–12 and 13–14 years compared to the corresponding male groups, and boys presented significantly higher DXR-BMD from 15 to 18 years of age (Tables 1 and 2). With regard to DXR-MCI, all female age groups between 6 and 18 years had higher reference values compared to the equivalent male cohorts; these differences reached significance for the age groups 11–12 and 13–14 years (Tables 1 and 2).
DXR values of patients with distal radius fracture
In general, lower values of DXR-BMD and DXR-MCI were seen in children and adolescents with distal radius fractures compared to the reference data across all six age groups. For boys, significant differences between the fracture group and reference cohort were observed from 15 to 18 years for DXR-BMD and from 11 to 18 years for DXR-MCI (Table 3). In comparison, significant differences in DXR-BMD between the fracture group and reference cohort were detected at earlier ages, from 13 to 16 years, in female patients (Table 4). DXR-MCI was significantly lower in girls with distal radius fractures vs. the corresponding reference data between the ages of 11 and 16 years (Table 4).
Comparing DXR-BMD and DXR-MCI in children suffering from distal radius fractures with age- and gender-matched normative data by Z scores, the Z scores were below 0 for both male and female fracture groups. For boys, the mean Z scores were −0.42 (range −3.65 to +3.11, SD 1.31) for DXR-BMD and −0.51 (range −3.08 to +3.75, SD 1.26) for DXR-MCI. The mean Z scores for girls with distal radius fractures compared to the male fracture group were slightly but not significantly lower: Z score = −0.46 (range −3.34 to +2.02, SD 1.04) for DXR-BMD and Z score = −0.53 (range −2.24 to 2.04, SD 0.85) for DXR-MCI.
Among the 95 boys with distal radius fractures, 26 boys had reduced Z scores for DXR-BMD ≤−1 (between −1 and −2: n = 16, 16.8 %; ≤−2: n = 10, 10.5 %; Fig. 1). In 57 male patients (60.0 %), the Z scores for DXR-BMD were within one reference range, and in 12 boys (12.6 %), Z scores for DXR-BMD were above 1. The female fracture cohort presented a similar distribution of Z scores for DXR-BMD compared to the male patients (Fig. 1). With respect to DXR-MCI, more than two-thirds of male and female patients with distal radius fractures showed Z scores within one reference range (Fig. 2). Z scores for DXR-MCI were below −1 in 23 of 95 males (between −1 and −2: n = 15, 15.8 %; ≤−2: n = 8, 8.4 %) and in 18 of 69 girls (between −1 and −2: n = 13, 18.8 %; ≤−2: n = 5, 7.2 %). Increased Z scores for DXR-MCI ≥1 were found in six boys (between 1 and 2: n = 4, 4.2 %; ≥2: n = 2, 2.1 %) and in four girls (between 1 and 2: n = 3, 4.3 %; ≥2: n = 1, 1.4 %; Fig. 2).
Discussion
Potential of DXR in pediatric populations
The clinical relevance of osteoporosis in childhood and adolescence is gaining increasing attention, among others due to effective prevention strategies and new medical treatments [7, 9, 39, 40]. Bone densitometry can be an effective monitoring tool in pediatric populations for children and adolescents with increased fracture risk, e.g., due to chronic bone-affecting diseases or other factors influencing bone metabolism [13, 31–34]. However, all osteodensitometric methods were initially developed for adults, and thus present drawbacks and challenges for children and adolescents [13, 14, 34]. Digital X-ray radiogrammetry is a user-independent osteodensitometric method with high precision and reproducibility [24, 41]. One major advantage of DXR in pediatric populations is the use of hand radiographs, which are often routinely performed in children—e.g., for estimation of skeletal age—without the need for additional radiation exposure for prospective or retrospective osteodensitometric analysis [30–34]. Furthermore, in contrast to DXA measurements, assessments using DXR seem to be only minimally affected by surrounding fat tissue [13, 14, 42]. In published investigations, DXR parameters have extensively been assessed with hand radiographs that had been printed out and subsequently scanned into the DXR system [24–35]. One benefit of the present study was the sole use of original digital images for direct osteodensitometric analysis, thereby avoiding possible noise-related and smearing effects during the printing process, variations due to differences in print and scanner resolution, or slight alterations in positioning during the scanning process [27, 41]. To our knowledge, this is the first trial, which evaluated the clinical value of DXR in children and adolescents using original digital hand radiographs.
Original digital DXR reference data
The original digital normative values were obtained from healthy boys and girls without fractures after adequate trauma. The DXR-BMD and DXR-MCI normative values were comparable to published reference data generated from printouts, with subsequent scanning to the DXR system, including healthy Caucasian males and females aged 6–18 years [30, 33]. The higher DXR-BMD values in healthy girls vs. boys from 11 to 14 years found in our study is caused by the fact that puberty occurs earlier in girls [30, 33, 35]. In the reference cohorts published by van Rijn et al. [33] and Malich et al. [30], higher DXR-BMD was also observed for girls aged 11 to 14 years, with significant differences only at 11–12 years. In our trial, the increase of normative DXR-BMD was mostly accentuated in girls between 11 and 14 years and in boys aged 15–16 years, i.e., maximum elevation around pubertal growth. At higher age levels, male age groups showed higher DXR-BMD values than corresponding female cohorts, which is in concordance with published reference data [30, 33, 35]. The earlier age of puberty might also be one reason that women reach their peak bone mass—as estimated by DXR—between 30 and 34 years of age, compared to men with peak bone mass at 45–49 years of age [35]. The strong correlation between DXR-BMD and age in our study was similar to corresponding coefficients found by Malich et al. [30]: r = 0.83 (males) and r = 0.84 (females). The excellent correlation between DXR-BMD and DXR-MCI was also similar to the results of Malich et al. [30]: r = 0.85 (males) and r = 0.86 (females). These highly significant associations indicate that the fully digital DXR technique can be applied with clinical confidence in pediatric populations aged ≥6 years.
In all of our evaluated age groups, normative DXR-MCI was higher for females; these differences were significant between the ages of 11 and 14 years. Van Rijn et al. [33] also detected significantly higher DXR-MCI values for healthy girls vs. age-matched boys from 11 to 15 years. Similarly, Böttcher et al. [35] observed a tendency for higher reference DXR-MCI values in healthy premenopausal women vs. men within comparable age groups. The metacarpal index—the ratio of cortical thickness to outer metacarpal width—functions as a relative measurement of cortical bone thickness normalized to outer bone diameter, and is frequently used as a reliable quantification of cortical bone mass [38, 43]. Premenopausal women, therefore, appear to have relatively smaller outer bone diameter of metacarpal bones compared to cortical thickness [38, 43, 44]. According to our findings and those of van Rijn et al. [33], differences in DXR-MCI between boys and girls were not significant between 15/16 and 18 years, presumably due to the accentuated increase of cortical thickness associated with puberty in male adolescents [44]. As DXR-MCI is a relative parameter, its correlation to age was lower than that of DXR-BMD; our correlations obtained from original digital images were slightly higher than corresponding coefficients published by Malich et al. (r = 0.63 [boys] and r = 0.68 [girls]) [30] and van Rijn et al. (r = 0.68 [boys] and r = 0.69 [girls]) [33].
DXR values in the fracture cohort
The reference collective and the cohort with distal radius fractures originated from the same geographical region and were evaluated using the same technical parameters as well as inclusion and exclusion criteria. Clark et al. [45] performed a meta-analysis of 10 investigations, in which children with fractures were compared with age-matched control subjects. Their meta-analysis found an association between fracture risk and low BMD, which was assessed by DXA in eight trials and by pQCT and QUS in one study each. Lower DXR-BMD values were observed in fracture vs. age-matched control subjects in all age groups in our trial, with significant differences from 15 to 18 years for boys and from 13 to 16 years for girls. Goulding et al. [15] compared BMD, estimated by DXA, in 100 girls with distal forearm fractures vs. 100 Caucasian control females and measured significantly lower BMD in girls with fractures in an overlapping age group of 11–15 years at the following sites: lumbar spine (L2–L4), femur trochanter, and ultradistal radius [15]. In a longitudinal study of 601 children, Jones et al. [46] found a peak age of fracture incidence at 11–12 years for girls and 13–14 years for boys, with the greatest number of fractures in both genders occurring at the wrist/forearm. Children in these age groups with elevated fracture risk may show reduced bone mineral accrual, resulting in lower BMD at later ages. As accentuated increase of normative DXR-BMD occurs earlier in girls (ages 11–14 years) than in boys (ages 15–16 vs. 13–14 years), a substantially lower increase of DXR-BMD can lead to an observed difference between the fracture group and reference cohort in the higher age groups; thus, our findings are feasible.
Comparing DXR parameters of the fracture group with age- and gender-matched reference data using Z scores, the mean Z score for DXR-BMD was below 0. Van Rijn et al. [33] used DXR to evaluate 19 boys and 26 girls with a history of forearm fractures and found a mean Z score of −0.1 for boys and −0.5 for girls; however, the interval considerably varied between onset of fractures and acquisition of hand radiographs, ranging from 0 to 12 years [33]. Ryan et al. [17] compared 76 African American children with forearm fractures to 74 age- and gender-matched control subjects by using DXA and noted a lower mean whole-body Z score for BMD in the fracture cohort; this difference approached but did not achieve statistical significance (p = 0.05). In our trial, more than one-fourth of the children with distal radius fractures showed Z scores for DXR-BMD ≤−1, which is in accordance with published DXA findings. In the study of Goulding et al. [15], 25 % of girls with distal forearm fractures had Z scores for DXA-BMD below −1 at the ultradistal radius of the non-fractured arm within six weeks of cast removal of the fractured arm, while Z scores below −1 were defined by the authors as osteopenia [15]. With a similar study design, Goulding et al. [16] compared 100 boys with distal forearm fractures to 100 control males, and found that 28 % of boys in the fracture group had Z scores for DXA-BMD at the ultradistal radius of ≤−1. With respect to BMD measurements, a certain number of children, approximately one-third to one-fourth of a pediatric population, seem to be prone to fractures. Data from large observational studies have revealed that approximately one-third of patients suffer from recurrent fractures in their childhood and adolescence, most often resulting from slight or moderate trauma [39, 47]. The use of osteodensitometry for the early identification of children with low Z scores and therefore increased fracture risk would address an important public health issue, as strategies for reducing further fractures and the incidence of osteoporosis can be initiated. Prevention strategies are important with regard to the fact that the incidence of fractures, particularly at the site of the distal forearm, has been increased in pediatric populations over the past decades [1, 4–6].
Advantages of our case–control study compared to published investigations were the standardized acquisition of bone parameters directly after the trauma, which avoided possible impacts due to immobilization or increased physical activity of the non-fractured extremity, as well as the separate analysis of DXR-MCI, and the inclusion of Z scores. In order to minimize bone-influencing conditions, strict exclusion criteria were defined that included previous fractures of the upper extremities, as the inclusion of children and adolescents with recurrent fractures of the upper limbs would likely increase the number of Z scores for DXR-BMD ≤−1. In comparison to DXR-BMD, DXR-MCI measurements among the fracture groups generally revealed lower SDs. Therefore, despite modestly lower mean Z scores for DXR-MCI, slightly fewer patients had Z scores ≤−1 for DXR-MCI. One reason for the lower SDs might be the fact that DXR-MCI is a relative parameter. Significantly lower DXR-MCI values were found for the fracture group at the ages of 11–18 years for boys and 11–16 years for girls. These findings are in concordance with the results of Ma and Jones [3], who assessed metacarpal index using morphometry, and measured significantly lower values for children aged 9–16 years with wrist and forearm fractures compared to age- and gender-matched control subjects; however, differences in metacarpal index values were not significant for children with upper arm fractures (higher distance to measurement localization) [3]. In this context, the major limitation of the DXR technique in assessing the peripheral metacarpal bone, and not axial sites associated with high osteoporotic fracture risk in adults, may be of minor relevance for pediatric populations, in whom most fractures occur at the distal forearm/wrist. The excellent correlation of our findings with results of the widely used DXA technique as well as the advantages of DXR in pediatric populations provide promising clinical benefit of DXR in children and adolescents for identifying individuals with increased fracture risk, thereby addressing both individual and public health issues.
Study limitations
There were certain limitations of this investigation. First, DXR provides estimates only of cortical bone mineralization, not trabecular bone architecture. However, isolated cortical bone fractures occur more frequently in children compared to adults [1, 4–6]. Another limitation of our study was that the number in each age subgroup of the normative and fracture cohorts was small, although 452 children and adolescents were in total enrolled; to obtain a minimum of 10 subjects in each subgroup, age intervals of 2–3 years were used. Despite the small number of subjects in the subgroups, however, the relatively low SDs suggest reliable findings. The reference cohort, which did not experience a distal radius fracture after trauma (trauma severity similar to the fracture group), may had higher DXR-BMD and DXR-MCI values compared to the general Caucasian population aged 6–18 years, which could be a study bias. However, the normative fully digital DXR values were comparable to published reference data obtained from printouts with subsequent scanning to the DXR system [30, 33]. The DXR technique was initially developed for adults, and a minimum metacarpal size is required to correctly identify the cortical edges of the diaphysis [30]. Therefore, the DXR method does not provide reliable results in subjects aged <6 years, which limits the application of DXR in young children [30–34]. Furthermore, a greater number of hand radiographs are not recognizable by the automatic DXR system in pediatric populations, even for children over 6 years of age, compared to adults [30–34]. In our study, 10 hand radiographs in the fracture group (5.7 %) and 12 images in the reference cohort (4.0 %) could not be correctly analyzed by the fully digital DXR system, and these patients had to been excluded. Van Rijn et al. [33] described a failure rate of 4.3 % in 535 healthy Caucasian children and adolescents. Thus, further technological improvements are needed for a more reliable use of the DXR system in younger children. The BoneXpert device is a beneficial, computer-assisted tool for the automatic evaluation of bone age in pediatric populations. Several bone parameters, including metacarpal index, thickness, width, and length, can be determined, only the assessment of BMD is actually not possible with this CAD method [44, 48]. Optimal adaptation of osteodensitometric techniques to pediatric populations is important, particularly for these CAD methods, which can be easily integrated into routine clinical diagnosis, in order to obtain as much information as possible in children and adolescents from radiographs, which have been acquired for clinical purpose.
Conclusions
The estimation of bone mineral density and metacarpal index in children with distal radius fractures yielded significantly different results, with lower DXR-BMD and DXR-MCI values compared to age-matched control subjects, who experienced no fracture after trauma of similar severity. The early identification of children and adolescents with reduced bone mineralization and bone mass can aid in preventing recurrent fractures and in reducing the risk of osteoporosis later in life. With further technological improvement, DXR might be used for routine screening—e.g., in the case of chronic bone-affecting diseases—in order to identify children at high risk of fracture and to initiate early preventive strategies, including medication and dietary regimens as well as increased physical activity.
References
Cooper C, Dennison EM, Leufkens HG, Bishop N, van Staa TP (2004) Epidemiology of childhood fractures in Britain: a study using the general practice research database. J Bone Miner Res 19:1976–1981
Marshall D, Johnell O, Wedel H (1996) Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 312:1254–1259
Ma D, Jones G (2003) The association between bone mineral density, metacarpal morphometry, and upper limb fractures in children: a population-based case-control study. J Clin Endocrinol Metab 88:1486–1491
Landin LA (1983) Fracture patterns in children: analysis of 8,682 fractures with special reference to incidence, etiology and secular changes in a Swedish urban population 1950–1979. Acta Orthop Scand Suppl 202:1–109
Khosla S, Melton LJ 3rd, Dekutoski MB, Achenbach SJ, Oberg AL, Riggs BL (2003) Incidence of childhood distal forearm fractures over 30 years: a population-based study. JAMA 290:1479–1485
Ryan LM (2010) Forearm fractures in children and bone health. Curr Opin Endocrinol Diabetes Obes 17:530–534
Carrié Fässler AL, Bonjour JP (1995) Osteoporosis as a pediatric problem. Pediatr Clin N Am 42:811–824
Frost HM (2002) Emerging views about “osteoporosis”, bone health, strength, fragility, and their determinants. J Bone Miner Metab 20:319–325
Gordon CM, Leonard MB, Zemel BS (2014) 2013 Pediatric position development conference: executive summary and reflections. J Clin Densitom 17:219–224
Rizzoli R, Bianchi ML, Garabédian M, McKay HA, Moreno LA (2010) Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone 46:294–305
De Smet S, Michels N, Polfliet C, D’Haese S, Roggen I, De Henauw S, Sioen I (2014) The influence of dairy consumption and physical activity on ultrasound bone measurements in Flemish children. J Bone Miner Metab (Epub ahead of print)
Njeh CF, Fuerst T, Hans D, Blake GM, Genant HK (1999) Radiation exposure in bone mineral density assessment. Appl Radiat Isot 50:215–236
van Rijn RR, van der Sluis IM, Link TM, Grampp S, Guglielmi G, Imhof H, Glüer C, Adams JE, van Kuijk C (2003) Bone densitometry in children: a critical appraisal. Eur Radiol 13:700–710
van Rijn RR, Van Kuijk C (2009) Of small bones and big mistakes; bone densitometry in children revisited. Eur J Radiol 71:432–439
Goulding A, Cannan R, Williams SM, Gold EJ, Taylor RW, Lewis-Barned NJ (1998) Bone mineral density in girls with forearm fractures. J Bone Miner Res 13:143–148
Goulding A, Jones IE, Taylor RW, Williams SM, Manning PJ (2001) Bone mineral density and body composition in boys with distal forearm fractures: a dual-energy X-ray absorptiometry study. J Pediatr 139:509–515
Ryan LM, Teach SJ, Singer SA, Wood R, Freishtat R, Wright JL, McCarter R, Tosi L, Chamberlain JM (2012) Bone mineral density and vitamin D status among African American children with forearm fractures. Pediatrics 130:e553–e560
Guo B, Xu Y, Gong J, Tang Y, Xu H (2013) Age trends of bone mineral density and percentile curves in healthy Chinese children and adolescents. J Bone Miner Metab 31:304–314
Leonard MB, Propert KJ, Zemel BS, Stallings VA, Feldman HI (1999) Discrepancies in pediatric bone mineral density reference data: potential for misdiagnosis of osteopenia. J Pediatr 135:182–188
Xu Y, Guo B, Gong J, Xu H, Bai Z (2014) The correlation between calcaneus stiffness index calculated by QUS and total body BMD assessed by DXA in Chinese children and adolescents. J Bone Miner Metab 32:159–166
Gkogka C, Christoforidis A, Printza N, Kollios K, Kazantzidou E, Papachristou F (2014) Longitudinal assessment of bone quality in pediatric patients with chronic kidney disease in relation to treatment modality. J Bone Miner Metab (Epub ahead of print)
Mentzel HJ, Reusch R, Kaiser WA (2009) Seasonal dependence of the parameters of quantitative ultrasonic measurements on the peripheral skeleton. Rofo 181:760–766
Njeh CF, Fuerst T, Diessel E, Genant HK (2001) Is quantitative ultrasound dependent on bone structure? A reflection. Osteoporos Int 12:1–15
Rosholm A, Hyldstrup L, Bæcksgaard L, Grunkin M, Thodberg HH (2001) Estimation of bone mineral density by digital X-ray radiogrammetry: theoretical background and clinical testing. Osteoporos Int 12:961–969
Pfeil A, Haugeberg G, Hansch A, Renz DM, Lehmann G, Malich A, Wolf G, Böttcher J (2011) Value of digital X-ray radiogrammetry in the assessment of inflammatory bone loss in rheumatoid arthritis. Arthritis Care Res Hoboken 63:666–674
Schäfer ML, Böttcher J, Pfeil A, Hansch A, Malich A, Maurer MH, Streitparth F, Röttgen R, Renz DM (2012) Comparison between amputation-induced demineralization and age-related bone loss using digital X-ray radiogrammetry. J Clin Densitom 15:135–145
Böttcher J, Pfeil A, Rosholm A, Schäfer ML, Malich A, Petrovitch A, Seidl B, Lehmann G, Mentzel HJ, Hein G, Wolf G, Kaiser WA (2006) Computerized digital imaging techniques provided by digital X-ray radiogrammetry as new diagnostic tool in rheumatoid arthritis. J Digit Imaging 19:279–288
Bouxsein ML, Palermo L, Yeung C, Black DM (2002) Digital X-ray radiogrammetry predicts hip, wrist and vertebral fracture risk in elderly women: a prospective analysis from the study of osteoporotic fractures. Osteoporos Int 13:358–365
Ozçakar L, Guven GS, Unal S, Akinci A (2005) Osteoporosis in Turkish HIV/AIDS patients: comparative analysis by dual energy X-ray absorptiometry and digital X-ray radiogrammetry. Osteoporos Int 16:1363–1367
Malich A, Freesmeyer MG, Mentzel HJ, Sauner D, Boettcher J, Petrovitch A, Behrendt W, Kaiser WA (2003) Normative values of bone parameters of children and adolescents using digital computer-assisted radiogrammetry (DXR). J Clin Densitom 6:103–111
Mentzel HJ, Blume J, Boettcher J, Lehmann G, Tuchscherer D, Pfeil A, Kramer A, Malich A, Kauf E, Hein G, Kaiser WA (2006) The potential of digital X-ray radiogrammetry (DXR) in the assessment of osteopenia in children with chronic inflammatory bowel disease. Pediatr Radiol 36:415–420
Mentzel HJ, John U, Boettcher J, Malich A, Pfeil A, Vollandt R, Misselwitz J, Kaiser WA (2005) Evaluation of bone-mineral density by digital X-ray radiogrammetry (DXR) in pediatric renal transplant recipients. Pediatr Radiol 35:489–494
van Rijn RR, Grootfaam DS, Lequin MH, Boot AM, van Beek RD, Hop WC, van Kuijk C (2004) Digital radiogrammetry of the hand in a pediatric and adolescent Dutch Caucasian population: normative data and measurements in children with inflammatory bowel disease and juvenile chronic arthritis. Calcif Tissue Int 74:342–350
van Rijn RR, Boot A, Wittenberg R, van der Sluis IM, van den Heuvel-Eibrink MM, Lequin MH, de MuinckKeizer-Schrama SM, Van Kuijk C (2006) Direct X-ray radiogrammetry versus dual-energy X-ray absorptiometry: assessment of bone density in children treated for acute lymphoblastic leukaemia and growth hormone deficiency. Pediatr Radiol 36:227–232
Böttcher J, Pfeil A, Schäfer ML, Petrovitch A, Seidl BE, Mentzel HJ, Lehmann G, Malich A, Heyne JP, Hein G, Wolf G, Kaiser WA (2006) Normative data for digital X-ray radiogrammetry from a female and male German cohort. J Clin Densitom 9:341–350
Arvidsson B, Bodin L, Rask E, Schvarcz E, Möller M (2013) Reference data for bone mineral density in Swedish women using digital X-ray radiometry. J Clin Densitom 16:183–188
Schmidt S, Nitz I, Ribbecke S, Schulz R, Pfeiffer H, Schmeling A (2013) Skeletal age determination of the hand: a comparison of methods. Int J Legal Med 127:691–698
Hyldstrup L, Nielsen SP (2001) Metacarpal index by digital X-ray radiogrammetry: normative reference values and comparison with dual X-ray absorptiometry. J Clin Densitom 4:299–306
Manias K, McCabe D, Bishop N (2006) Fractures and recurrent fractures in children: varying effects of environmental factors as well as bone size and mass. Bone 39:652–657
Yi KH, Hwang JS, Kim EY, Lee JA, Kim DH, Lim JS (2014) Reference values for bone mineral density according to age with body size adjustment in Korean children and adolescents. J Bone Miner Metab 32:281–289
Böttcher J, Pfeil A, Rosholm A, Malich A, Petrovitch A, Heinrich B, Lehmann G, Mentzel HJ, Hein G, Linss W, Kaiser WA (2005) Influence of image-capturing parameters on digital X-ray radiogrammetry. J Clin Densitom 8:87–94
Colt E, Kälvesten J, Cook K, Khramov N, Javed F (2010) The effect of fat on the measurement of bone mineral density by digital X-ray radiogrammetry (DXR-BMD). Int J Body Compos Res 8:41–44
Shepherd JA, Meta M, Landau J et al (2005) Metacarpal index and bone mineral density in healthy African-American women. Osteoporos Int 16:1621–1626
Martin DD, Heckmann C, Jenni OG, Ranke MB, Binder G, Thodberg HH (2011) Metacarpal thickness, width, length and medullary diameter in children—reference curves from the First Zürich Longitudinal Study. Osteoporos Int 22:1525–1536
Clark EM, Tobias JH, Ness AR (2006) Association between bone density and fractures in children: a systematic review and meta-analysis. Pediatrics 117:e291–e297
Jones IE, Williams SM, Dow N, Goulding A (2002) How many children remain fracture-free during growth? A longitudinal study of children and adolescents participating in the Dunedin multidisciplinary health and development study. Osteoporos Int 13:990–995
Yeh FJ, Grant AM, Williams SM, Goulding A (2006) Children who experience their first fracture at a young age have high rates of fracture. Osteoporos Int 17:267–272
Thodberg HH, van Rijn RR, Tanaka T, Martin DD, Kreiborg S (2010) A paediatric bone index derived by automated radiogrammetry. Osteoporos Int 21:1391–1400
Acknowledgments
The authors thank Monika Arens (Managing Director, Arewus GmbH, Germany), Jakob Algulin (Managing Director, Sectra Medical Systems, Germany), and Anders Rosholm, PhD, for the use of digital X-ray radiogrammetry equipment. The authors also thank Bettina Herwig (Berlin, Germany) for editorial assistance.
Conflict of interest
None of the authors have any personal or financial conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Renz, D.M., Malich, A., Ulrich, A. et al. Reference values for digital X-ray radiogrammetry parameters in children and adolescents in comparison to estimates in patients with distal radius fractures. J Bone Miner Metab 34, 55–64 (2016). https://doi.org/10.1007/s00774-014-0641-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-014-0641-3