Abstract
Actions of esculentin-2CHa(1–30) (GFSSIFRGVAKFASKGLGKDLAKLGVDLVA) and its analogues, ([d-Arg7, d-Lys15, d-Lys23]-esculentin-2CHa(1–30) and [Lys15-octanoate]-esculentin-2CHa(1–30), were evaluated in high-fat fed NIH Swiss mice with impaired glucose tolerance and insulin resistance. Twice-daily i.p. administration of the esculentin-2CHa(1–30) peptides (75 nmol/kg body weight) or exendin-4 (25 nmol/kg) for 28 days reduced body weight, without altering cumulative energy intake. All peptides reduced blood glucose levels by 6–12 mmol/l concomitant with lower plasma insulin levels, with significance evident from day 6. All peptides improved glucose tolerance, insulin sensitivity, blood glucose profile over 24 h and decreased HbA1c to a similar extent as exendin-4. The peptides also reduced high fat diet-induced increases in plasma GLP-1 and glucagon. None of the peptides altered bone mineral density/content or lean mass but decreased fat mass. Islets isolated from peptide-treated mice exhibited improved glucose-, alanine- and GLP-1-stimulated insulin secretion. Islet morphometric analyses revealed that exendin-4 and the esculentin-2CHa(1–30) peptides significantly reduced islet, beta and alpha cell areas compared to high-fat controls. Esculentin-2CHa(1–30) peptides markedly reduced high fat diet-induced increase in beta cell proliferation and apoptosis. Peptide treatments had beneficial effects on expression of islet genes (Ins1, Slc2a2, Pdx1) and skeletal muscle genes involved in insulin action (Slc2a4, Pdk1, Irs1, Akt1). High-fat diet significantly increased LDL cholesterol which was reduced by the acylated esculentin-2CHa(1–30) analogue. Peptide treatments did not alter circulating concentrations of amylase and marker enzymes of liver function, indicating a lack of toxicity. These data indicate that esculentin-2CHa(1–30) and its analogues may be useful for improvement of blood glucose control and weight loss in type 2 diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
After the discovery of exendin-4, first isolated from the salivary secretions of the Gila monster Heloderma suspectum, long-acting glucagon-like peptide-1 (GLP-1) mimetics with improved efficacy have been developed for the treatment of type 2 diabetes (Parkes et al. 2013; Kahn et al. 2014; Irwin and Flatt 2015). As peptide hormone therapeutics often have better specificity, tolerability and safety than small molecule drugs, the hunt for bioactive peptidergic agents with therapeutic potential from natural sources is currently a key area of research.
Skin secretions of amphibians contain a wealth of host defence peptides with antimicrobial, immunomodulatory and chemoattractive properties that may confer survival advantage (Conlon et al. 2014). Selected host defence peptides (brevinin-2-related peptide, alyteserin-2a, magainin AM2, CPF-SE1, tigerinin-1R, hymenochirin 1B) isolated from various frog skin secretions have been shown to exhibit insulinotropic actions in vitro and anti-diabetic actions in vivo (Abdel-Wahab et al. 2010; Ojo et al. 2013a, b, 2015a, b; Srinivasan et al. 2015; Owolabi et al. 2016). Indeed, our previous studies together with those of other investigators have revealed that frog skin secretions contain a multitude of diverse peptide molecules which possess insulinotropic properties (Kim et al. 2010; Mo et al. 2014; Ojo et al. 2013a, b).
One peptide of particular interest is esculentin-2CHa (GFSSIFRGVAKFASKGLGKDLAKLG VDLVACKISKQC; GA30), isolated from the skin secretions of the Chiricahua leopard frog Lithobates chiricahuensis (Ranidae) which exhibits potent antimicrobial activity against multidrug-resistant strains of Staphylococcus aureus, Acinetobacter baumannii, and Stenotrophomonas maltophilia (Conlon et al. 2011). Esculentin-2-CHa has also been reported to stimulate production of the anti-inflammatory cytokine interleukin-10 by mouse lymphoid cells and exert cytotoxicity against human non-small lung adenocarcinoma A549 cells (Attoub et al. 2013). Of particular note, we have demonstrated that the analogue [Lys28]-esculentin-2CHa stimulated insulin secretion and improved glycaemic control in high-fat fed diabetic mice (Ojo et al. 2015c).
The loss of antimicrobial activity on removal of the cyclic C-terminal domain of esculentin-2CHa (esculentin-2CHa(1–30)) was not associated with abolition of insulinotropic activity in vitro in BRIN BD11 cells (Vasu et al. 2017). Furthermore, we investigated stability and the acute in vitro and in vivo insulinotropic properties of analogues of esculentin-2CHa(1–30), with d-isomer or l-ornithine substitutions or C-8 fatty acid (octanoate) attachment to selected residues (see Table 1). These analogues were designed with a view to conferring resistance to degradation by endopeptidases, thereby enhancing metabolic stability and prolonging half-life in the circulation (Vasu et al. 2017). Peptide analogues with d-isomer substitution at positions 7, 15 and 23 and fatty acid attachment at position 15 exhibited the desired enhanced resistance to degradation by endopeptidases and improved insulinotropic actions in in vitro assays. These insulin-releasing effects were similar to those of established beta-cell activators, such as GLP-1, and were mediated by multiple pathways including stimulation of phospholipase C and influx of Ca2+ ions (Vasu et al. 2017). Notably, acute administration of these peptides improved glucose tolerance in lean NIH Swiss mice. On the basis of these observations, we hypothesised that chronic administration of these analogues of esculentin-2CHa-(1–30) may exert beneficial effects on metabolism and improve glycaemia in high-fat fed mice. These animals show clear manifestation of obesity, impaired glucose tolerance and insulin resistance and so represent a valuable model of type 2 diabetes (T2DM). In the present study, we indeed demonstrate that chronic administration of these peptide analogues to high fat fed mice improves blood glucose profile, glucose tolerance, insulin sensitivity, haemoglobin A1c (HbA1c) and pancreatic islet cell mass and survival. These observations suggest that analogues of esculentin-2CHa may have potential for development into new therapeutic agents for treatment of patients with T2DM.
Materials and methods
Peptide synthesis and purification
Synthetic esculentin-2CHa(1–30) (GA30) and its analogues ([d-Arg7, d-Lys15, d-Lys23]-esculentin(1–30)—(3D-GA30), [Lys15-octanoate]-esculentin(1–30)—acyl-GA30) and exendin-4 (see Table 1) were purchased from GL Biochem Ltd (Shanghai, China) and purified to near homogeneity (>98% pure) by reversed phase HLPC on a (2.2 cm × 25 cm) Vydac 218TP1022 (C18) column (Grace, Deerfield, IL, USA) equilibrated with acetonitrile/water/triflouroacetic acid (21.0/78.9/0.1 v/v) mobile phase at a flow rate of 6 ml/min, with concentration of acetonitrile in the eluting buffer raised to 56% (v/v) over 60 min. The correct molecular masses of the peptides were confirmed using a a Voyager DE-PRO MALDI-TOF mass spectrometry (Applied Biosystems, Foster City, CA) (Table 1).
Animals
Adult (8-week-old), male, National Institutes of Health (NIH) Swiss mice (Harlan Ltd, UK) were housed individually in an air-conditioned room (22 ± 2 °C) with a 12-h light: 12-h dark cycle, with food and water available ad libitum. Mice were maintained for 20 weeks before the experiment on a standard rodent diet (10% fat, 30% protein and 60% carbohydrate; energy density of 12.99 kJ/g; Trouw Nutrition, Cheshire, UK) or high-fat diet (45% fat, 20% protein and 35% carbohydrate; energy density of 25.16 kJ/g; composition:: casein, 26.533; choline bitartrate, 0.296; L-cystine, 0.399; lard, 17.895; rice starch, 28.344; cellulose, 6.171; soya oil, 4.319; sucrose, 10.490; mineral mix, 4.319; and vitamin mix, 1.234; Special Diets Service, Essex, UK) for 20 weeks. Peptides were administered i.p. twice daily at a dose of 25 nmol/kg bw for exendin-4 or 75 nmol/kg bw for analogues of esculentin-2CHa(1–30) for 28 days and parameters including body weight and food intake (daily), blood glucose and plasma insulin (once every 3 days) were monitored. These doses were selected on the basis of previous studies (Vasu et al. 2017; Patterson et al. 2015). Control mice received injections of saline alone. After 28 days, glucose tolerance, HbA1c (A1cNow+, HbA1c test, Chirus Limited, Hertfordshire, UK) and insulin sensitivity (25 U/kg, i.p.) tests were performed as described previously (Martin et al. 2012). Blood samples were collected from a tail vein and blood glucose was determined using an Ascencia Contour Blood Glucose Meter (Bayer, Newbury, UK). At the end of the experimental period, blood and tissue samples were collected together with body fat measurements by dual energy X-ray absorptiometry (DXA) scanning (Primus Densitometer, Inside Outside sales, USA). All animal experiments were carried out in accordance with the UK Animals (Scientific Procedures) Act 1986 and ‘Principles of laboratory animal care’ (NIH publication no. 86–23, revised 1985).
Biochemical analyses
Blood samples were collected in fluoride/heparin microcentrifuge tubes (Sarstedt, Numbrecht, Germany) and centrifuged for 30 s at 13,000×g. Plasma was separated and stored at −80 °C until analysis. Biochemical analyses were carried out for insulin by radioimmunoassay (Flatt and Bailey 1981). Total GLP-1 (GLP-1 total ELISA, EZGLP-1T-36K, Millipore, Billerica, MA, USA), total glucose-dependent insulinotropic polypeptide (GIP) (rat/mouse GIP ELISA, EZRMGIP-55K, Millipore) and glucagon (glucagon chemiluminescent assay, EZGLU-30K, Millipore) were measured by specific enzyme-linked immunoassays following the manufacturers’ instructions. Plasma aspartic acid transaminase (AST), alanine transaminase (ALT) and alkaline phosphatase (AP) concentrations were determined using commercially available kits following manufacturer’s instructions (Randox laboratories, Crumlin, UK). Plasma cholesterol levels were determined using an I-lab 650 automated clinical chemistry analyser (Diamond Diagnostics, Holliston, MA, USA). LDL cholesterol concentrations were computed using the Friedewald equation as described previously (Johnson et al. 1997). Plasma amylase was determined using commercially available assay kit following manufacturer’s instructions (Abcam, ab102523).
In vitro insulin secretion and islet immunohistochemistry
Insulin secretory responses of isolated islets and immunohistochemistry of pancreatic tissues from each of the groups of mice were determined as previously described (McKillop et al. 2014). The following primary antibodies were used as appropriate: mouse monoclonal anti-insulin antibody (ab6995, 1:1000; Abcam, Cambridge, UK), guinea-pig anti-glucagon antibody (PCA2/4, 1:200; raised in-house) and rabbit anti-Ki67 antibody (ab15580, 1:200; Abcam). The following secondary antibodies were used as appropriate: Alexa Fluor 488 goat anti-guinea pig IgG—1:400, Alexa Fluor 594 goat anti-mouse IgG—1:400, Alexa Fluor 488 goat anti-rabbit IgG—1:400 or Alexa Fluor 594 goat anti-rabbit IgG—1:400. The slides were viewed under FITC filter (488 nm) or TRITC filter (594 nm) using fluorescent microscope (Olympus system microscope, model BX51) and photographed using the DP70 camera adapter system. TUNEL assay was used to assess beta cell apoptosis, following manufacturer’s instructions (In situ cell death kit, Fluorescein, Roche Diagnostics, Burgess Hill, UK).
Image analysis
Islet parameters including islet, beta cell and alpha cell area, islet size distribution were analysed in a blinded fashion using Cell^F image analysis software (Olympus Soft Imaging Solutions, GmbH). Proliferation or apoptosis frequencies were determined by computing percentage of Ki67 or TUNEL and insulin positive cells of total beta cells analysed (approximately 2000 beta cells per replicate). The balance between proliferation and apoptosis was expressed as ratio of Ki67/TUNEL.
Gene expression studies
mRNA was extracted from skeletal muscle or collagenase isolated islets using TriPure isolation reagent following manufacturer’s instructions and converted to cDNA using SuperScript™ II Reverse Transcriptase (Invitrogen Life Technologies, Carlsbad, CA, USA) (Vasu et al. 2013). Reaction mix for PCR consisted of Quantifast SYBR green PCR mix (Qiagen, Venlo, Netherlands), forward and reverse primers, template cDNA and nuclease-free water and real-time data were monitored using MiniOpticon real-time PCR system (Biorad, Watford UK) as described (Owolabi et al. 2016). Data were normalised to β-actin (Actb) expression and analysed using the ΔΔCt method.
Statistical analysis
Results were analysed using GraphPad PRISM Software (Version 6.0) and presented as mean ± SEM. Statistical analyses were performed using student’s t test (non-parametric) or one-way ANOVA followed by Bonferroni post hoc test wherever applicable. Area under the curve (AUC) analysis was performed using the trapezoidal rule with baseline correction. Results were considered significant if p < 0.05.
Results
Effects of peptide administration on body weight and energy intake
Chronic high-fat feeding for 20 weeks significantly increased body weight (Fig. 1a, b). Treatment over a 28-day period with, significantly decreased body weight (Fig. 1b). High-fat feeding increased cumulative energy intake significantly (p < 0.001, Fig. 1c, d) but this parameter was not reduced by peptide treatment, with the exception of exendin-4 (p < 0.01, Fig. 1d).
Body weight and energy intake during 28-day treatment with GA30, 3D-GA30 and acyl-GA30 in high-fat fed NIH Swiss mice. a Body weight (g), b change in body weight (final–initial, g), c energy intake (kJ/day), d cumulative energy intake (kJ). Values are mean ± SEM (n = 8 mice). *p < 0.05, ***p < 0.001 compared to lean saline control. ΔΔ p < 0.01 compared to high-fat diet saline control. Effects of exendin-4 are shown for comparison
Effects of peptide administration on blood glucose profile, glucose tolerance and insulin sensitivity
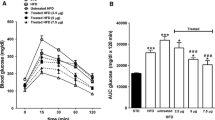
Chronic high-fat feeding for 20 weeks markedly increased blood glucose levels which was gradually reduced by administration of GA30 (p < 0.05), 3D-GA30 (p < 0.01) and acyl-GA30 (p < 0.001) twice daily over 28 days (Fig. 2a). Acyl-GA30 reduced blood glucose levels early while GA30 and 3D-GA30 reduced blood glucose levels from days 21 to 24, with comparable efficacy to exendin-4 (p < 0.05, p < 0.01, p < 0.001, respectively, Fig. 2a, b). Chronic high-fat feeding also increased circulating levels of insulin significantly compared to lean mice (p < 0.001, Fig. 2c). GA30 and acyl-GA30 treatment reduced plasma insulin levels significantly from day 12 while 3D-GA30 reduced plasma insulin levels significantly from day 18 (p < 0.05, p < 0.01, respectively Fig. 2c). These observations were comparable to the effects of exendin-4 (p < 0.05, p < 0.01, respectively, Fig. 2c).
Blood glucose and plasma insulin during 28-day treatment with GA30, 3D-GA30 and acyl-GA30 in high-fat fed NIH Swiss mice. a Blood glucose (mmol/l), b blood glucose change (final–initial, mmol/l), c plasma insulin (ng/ml), d Final plasma insulin (ng/ml). Values are mean ± SEM (n = 8 mice). *p < 0.05, **p < 0.01, ***p < 0.001 compared to lean saline control. ∆ p < 0.05, ∆∆ p < 0.01, ∆∆∆ p < 0.001 compared to high-fat diet saline control. Effects of exendin-4 are shown for comparison
On administration of intraperitoneal glucose to fasted mice, blood glucose and plasma insulin levels remained elevated until 90 min in high-fat fed mice compared to lean mice (p < 0.05, Fig. 3a–d). Chronic peptide administration improved glucose tolerance, with GA30 significantly reducing blood glucose levels (p < 0.05, Fig. 3b) while all peptides significantly raised plasma insulin levels (p < 0.001, Fig. 3d). To test insulin sensitivity, insulin was administered to mice which resulted in rapid glucose clearance from the circulation of lean and peptide-treated high-fat fed mice but not in saline-treated high-fat fed mice (p < 0.05, p < 0.01, p < 0.001, Fig. 4a). Thus insulin sensitivity was significantly improved in peptide-treated mice compared with high-fat fed mice (p < 0.001, Fig. 4a, b). High-fat fed mice exhibited raised HbA1c compared to lean mice, with blood glucose levels being constantly elevated over a 24-h period (p < 0.05, p < 0.01 Fig. 4c). In contrast, twice daily peptide treatment for 28 days normalised HbA1c and resulted in near-normal blood glucose levels over 24 h period (p < 0.05, Fig. 4d).
Glucose tolerance and plasma insulin response to glucose after 28-day treatment with GA30, 3D-GA30 and acyl-GA30 in high-fat fed NIH Swiss mice. a Blood glucose AUC (mmol/l), b blood glucose (mmol/l min), c plasma insulin (ng/ml), d plasma insulin (ng/ml min). Values are mean ± SEM (n = 8 mice). *p < 0.05, **p < 0.01, ***p < 0.001 compared to lean saline control. Δ p < 0.05, ΔΔ p < 0.01, ΔΔΔ p < 0.001 compared to high-fat diet saline control. Effects of exendin-4 are shown for comparison
Insulin sensitivity after 28-day treatment with GA30, 3D-GA30 and acyl-GA30 in high-fat fed NIH Swiss mice. a Blood glucose (mmol/l) after injection of insulin (25 U/ml), b blood glucose AUC (mmol/l min), c HbA1C (%), d blood glucose profile over 24 h; arrows indicate time at which peptides were administered. Values are mean ± SEM (n = 8 mice). *p < 0.05, **p < 0.01, ***p < 0.001 compared to lean saline control. Δ p < 0.05, ΔΔ p < 0.01, ΔΔΔ p < 0.001 compared to high-fat diet saline control. Effects of exendin-4 are shown for comparison
Effects of peptide administration on hormone levels and liver enzymes
Exendin-4 and 3D-GA30 significantly reduced the elevated plasma GLP-1 levels associated with high-fat feeding (p < 0.01, Fig. 5a). High-fat diet significantly increased plasma GIP and glucagon levels (p < 0.05) which were not significantly affected by any of the peptide treatments (Fig. 5b, c). Consumption of high-fat diet or peptide administration did not affect liver function, as evident from normal range plasma ALT, AST and AP levels (Fig. 5d–f).
Plasma GLP-1, GIP, glucagon and markers of liver function in high-fat fed NIH Swiss mice after 28-day treatment with GA30, 3D-GA30 and acyl-GA30. a Plasma GLP-1 (pmol/l), b plasma GIP (pmol/l), c plasma glucagon (pmol/l), d plasma ALT (U/l), e plasma AST (U/l, f plasma ALP (U/l). Values are mean ± SEM (n = 4). *p < 0.05, **p < 0.01, ***p < 0.001 compared to lean saline control. ΔΔ p < 0.01 compared to high-fat diet saline control. Effects of exendin-4 are shown for comparison
Effects of peptide administration on plasma amylase and lipid profile
High-fat diet or peptide treatment had no effect on plasma amylase levels, indicating no adverse effect on exocrine pancreas (Fig. 6a). High-fat diet markedly increased LDL cholesterol which was significantly reduced by peptide treatment (p < 0.05, Fig. 6b). HDL cholesterol and total cholesterol were similar in all groups (Fig. 6c, d).
Plasma amylase and lipid profile after 28-day treatment with GA30, 3D-GA30 and acyl-GA30 in high-fat fed NIH Swiss mice. a Plasma amylase activity (mU/ml), b LDL (mmol/l), c total cholesterol (mmol/l), d HDL cholesterol (mmol/l). Values are mean ± SEM (n = 4). **p < 0.01 compared to lean saline control. Δ p < 0.05 compared to high-fat diet saline control. Effects of exendin-4 are shown for comparison
Effects of peptide administration on bone density and body mass
High-fat diet or peptide treatment did not affect bone mineral density, content and lean mass (Fig. 7a–c). High-fat diet markedly increased fat mass and body fat percentage (p < 0.01, p < 0.001, respectively, Fig. 7d, e) which were reduced by peptide treatment, but not significantly (Fig. 7d, e).
Bone and body mass profiles after 28-day treatment with GA30, 3D-GA30 and acyl-GA30 in high-fat fed NIH Swiss mice. a Bone mineral density (g/cm2), b bone mineral content (g/cm), c lean mass (g), d fat mass (g), e fat (%).Values are mean ± SEM (n = 8). *p < 0.05, **p < 0.01, ***p < 0.001 compared to lean saline control. Effects of exendin-4 are shown for comparison
Effects of peptide administration on ex vivo insulin secretion and pancreatic islet morphology
Islets isolated from high-fat fed mice displayed blunted insulin secretory responses to stimulatory concentrations of glucose, alanine or GLP-1 which were fully restored by the peptide treatments (Fig. 8). Representative images showing islets from lean and high-fat fed mice with or without peptide treatment, stained using haematoxylin (nucleus) and eosin (cytoplasm) (top panel) or for insulin (red) and glucagon (green) (bottom panel) (Fig. 9a). High-fat diet significantly increased islet, beta cell and alpha cell areas (p < 0.001, Fig. 9b–d), which were reduced significantly by peptide treatments (p < 0.001, Fig. 9b–d). Representative images of Ki67/TUNEL (green) positive, insulin positive (red) cells in islets of lean or high-fat fed treated with saline or peptide analogues are shown in Fig. 10a. High-fat diet significantly increased beta cell proliferation and apoptosis frequencies (p < 0.001, Fig. 10b, c), which were reduced by the peptide treatments (p < 0.05, p < 0.01, p < 0.001, respectively, Fig. 10b, c). Proliferation to apoptosis ratio was raised in high-fat fed saline-treated mice (p < 0.05, Fig. 10d), which was reduced to normal by 3D-GA30 and acyl-GA30 treatment (p < 0.05, Fig. 10d).
Insulin secretory responses to glucose, GLP-1 and alanine by pancreatic islets isolated from high-fat fed NIH Swiss mice after 28-day treatment with GA30, 3D-GA30 and acyl-GA30. Values are mean ± SEM (n = 4). *p < 0.05 compared to 20 mM glucose, Δ p < 0.05, ΔΔ p < 0.01 compared to 3 mM glucose. Effects of exendin-4 are shown for comparison
Islet morphometry after 28-day treatment with GA30, 3D-GA30 and acyl-GA30 in high-fat fed NIH Swiss mice. a Representative images showing haematoxylin and eosin, insulin (red) and glucagon (green) staining of islets. Arrows indicate islets. b Islet area (µm2), c alpha cell area (µm2), d beta cell area (µm2). *p < 0.05, **p < 0.01, ***p < 0.001 compared to lean saline control. ΔΔΔ p < 0.001 compared to high-fat diet saline control. Effects of exendin-4 are shown for comparison (colour figure online)
Beta cell proliferation and apoptosis frequencies in islets after 28-day treatment with GA30, 3D-GA30 and acyl-GA30 in high-fat fed NIH Swiss mice. a Representative images show Ki67 (green), TUNEL (green) and insulin (red) staining of islets. Arrows indicate cells positive for both Ki67/TUNEL and insulin. b Beta cell proliferation frequency, c beta cell apoptosis frequency, expressed as percentage of total beta cells analysed, d ratio of Ki67:Tunel positive beta cells. *p < 0.05, **p < 0.01, ***p < 0.001 compared to lean saline control. Δ p < 0.05, ΔΔ p < 0.01, ΔΔΔ p < 0.001 compared to high-fat diet saline control. Effects of exendin-4 are shown for comparison (colour figure online)
Effects of peptide administration on gene expression in islets and skeletal muscle
High-fat feeding increased islet expression of Ins1 (mouse insulin 1), but decreased Slc2a2 (glucose transporter 2; GLUT2) and Pdx1 [insulin promoter factor 1 (Fig. 11a–c)]. Peptide treatments generally reversed these trends and the level of expression was significantly different in islets of exendin-4 compared with high-fat fed control mice (p < 0.001, Fig. 11a–c). High-fat diet also decreased skeletal muscle expression of Slc2a4 (glucose transporter 4; GLUT4), Irs1 (insulin receptor substrate 1), and Pdk1 (3-phosphatidylinositol 3-kinase) (Fig. 11d–f). Slc2a4 was significantly increased by 3D-GA30 and acyl-GA30 (p < 0.05, p < 0.01 Fig. 10d); Akt1 (protein kinase B) was increased by acyl-GA30 (p < 0.05, Fig. 11g) and Pdk1 (3-phosphoinositide dependent protein kinase 1) was increased by exendin-4 (p < 0.001, Fig. 11e). Decreased expression of Irs1 induced by high-fat feeding was not observed in any of the peptide treated groups (Fig. 10f).
mRNA expression of Slc2a2 (a), Ins1 (b), Pdx1 (c) in isolated islets and Slc2a4 (d), Pdk1 (e), Irs1 (f) and Akt (g) in skeletal muscle after 28-day treatment of high-fat fed NIH Swiss mice with GA30, 3D-GA30 and acyl-GA30. Values are mean ± SEM (n = 4). *p < 0.05, ** p < 0.01, ***p < 0.001 compared to lean control. Δ p < 0.05, ΔΔ p < 0.01, ΔΔΔ p < 0.001 compared to high-fat diet saline control. Effects of exendin-4 are shown for comparison
Discussion
Ever increasing incidence of T2DM, difficulty in achieving tight glycaemic control and inability to prevent diabetic complications are driving research into development of new therapeutics that target pancreatic beta cell function and insulin resistance that demonstrate improved potency and efficacy over existing therapies (Bailey 2009; Irwin and Flatt 2015). Search for bioactive compounds in skin secretions of amphibians has led to isolation of numerous compounds with antimicrobial, anti-inflammatory and anti-tumour properties (Conlon et al. 2014). Some of these components have been shown to possess insulinotropic properties (Ojo et al. 2013a, b). One such peptide, esculentin-2CHa was identified with strong insulinotropic actions in vitro, together with an analogue—[Lys28]-esculentin-2CHa which exerted anti-diabetic actions in vivo (Ojo et al. 2015a, b, c). Importantly, we recently demonstrated that esculentin-2CHa(1–30), a truncated analogue more readily synthesised and appropriate for drug development, retained insulin-releasing and glucose-lowering activity. The second-generation analogues of this peptide—[d-Arg7, d-Lys15, d-Lys23]-esculentin-2CHa(1–30) (3D-GA30) and [Lys15]-octanoate-esculentin-2CHa(1–30) (acyl-GA30) demonstrated resistance to degradation by endopeptidases and positive effects on glucose tolerance and insulin secretion when administered to lean mice (Vasu et al. 2017).
In the present study, we have compared the anti-diabetic potential of these analogues in high-fat fed mice with the established antidiabetic agent, exendin-4. Consistent with previous studies, high-fat fed mice exhibited hyperglycaemia, hyperlipidaemia, glucose intolerance, hyperinsulinaemia and insulin resistance (Winzell and Ahren 2004; Ojo et al. 2015a, b, c). Twice daily administration of esculentin-2CHa(1–30) and its analogues for 28 days progressively reduced body weight without effecting cumulative energy intake. This might reflect positive effects on energy expenditure, possibly also involving a shift in fuel utilisation towards burning of fat. It is noteworthy that we did not observe an effect of [Lys28]-esculentin-2CHa on body weight or food intake in previous chronic studies using high-fat fed mice (Ojo et al. 2015a, b, c). This suggests that these designer analogues of the shorter esculentin-2CHa(1–30) peptide exert superior actions on body weight than the full-length peptide possibly due to increased potency, enzyme resistance and/or superior pharmacokinetic properties. However, further investigations of these aspects, involving administration over a period longer than 28 days, together with more detailed studies on energy balance, are required to confirm these speculations.
Chronic peptide administration reduced the high fat diet-induced elevation in LDL cholesterol, non-fasted blood glucose and plasma insulin levels, with obvious beneficial effects on glucose tolerance at the end of the study. The effects of esculentin-2CHa(1–30) analogues were at least as good as those produced by exendin-4. Blood glucose profile over a period 24 h and HbA1c, both measured at the end of the study, were significantly improved by peptide treatment, whereas high-fat fed mice receiving saline alone displayed sustained hyperglycaemia. Treatment with peptide analogues improved insulin sensitivity and decreased insulin demand as evidenced by decreased circulating insulin levels both in the fed state and following a glucose load. This effect on insulin sensitivity was accompanied by partial reversal of the high fat diet-induced down-regulation of regulatory genes in skeletal muscle involved in insulin action (Slc2a4, Irs1 and Pdk1). Peptide treatments, most notably with [Lys15]-octanoate-esculentin-2CHa(1–30), also countered hyperglucagonaemia suggesting a possible inhibitory effect on hepatic glucose production. High-fat diet increased circulating concentrations of GLP-1 and GIP consistent with increased activity of the enteroinsular axis (Bailey et al. 1986). Plasma GIP was unaltered by peptide treatments, while exendin-4 and [d-Arg7, d-Lys15, d-Lys23]-esculentin-2CHa(1–30) decreased circulating GLP-1 concentrations, indicating that secondary effects mediated via GLP-1 do not contribute to the positive actions of these peptides.
Beneficial actions of these esculentin-2CHa(1–30) peptide analogues on blood glucose control may also be attributed to positive actions on pancreatic beta cell function and survival. Consistent with previous published studies, high-fat diet induced compensatory beta cell mass expansion, with marked increase in beta cell proliferation frequency (Moffett et al. 2015). However, beta cell exhaustion due to existing hyperglycaemia and hyperlipidaemia was evident from a significant increase in the frequency of beta cell apoptosis. Chronic peptide treatment reduced both beta cell proliferation and apoptosis frequency, resulting in reduced islet area and beta cell area. Such effects may be consequential to improved metabolic control or beta cell rest (Pathak et al. 2015). Further investigations on whether these peptide analogues confer protection from glucotoxicity and lipotoxicity are warranted. Nevertheless, chronic treatment with the peptide analogues over 28 days improved beta cell survival, expression of key functional genes (insulin, Glut2 and Pdx1), in vitro insulin secretory responses to glucose, alanine or GLP-1 and exerted clear beneficial effects on glucose homeostasis with no evidence of pancreatitis or insulitis. Esculentin-2CHa(1–30) has been shown to exert direct stimulatory actions on beta cells involving peptide internalisation, KATP-independent membrane depolarisation and elevation of intracellular Ca2+ which culminates in insulin exocytosis (Vasu et al. 2017). The internalisation of the peptide may also contribute to the observed changes in gene transcription in target tissues. Such action has been noted for the amphibian histone H2A-derived buforin II (Elmore. 2012) which traverses the cell membrane via transient toroidal pores and accumulates in the nucleus to alter cellular function (Lee et al. 2008).
To conclude, analogues of esculentin-2CHa(1–30), [d-Arg7, d-Lys15, d-Lys23]-esculentin-2CHa(1–30) and [Lys15]-octanoate-esculentin-2CHa(1–30), exert anti-diabetic actions in high-fat fed mice, with clear beneficial and protective effects on pancreatic beta cells, insulin sensitivity and glucose homeostasis. Further studies investigating energy homeostasis, cellular actions, effects on adipocyte and liver metabolism and the relapse with cessation of peptide administration using different animal models are needed to explore further the potential of esculentin-2CHa(1–30) analogues for therapy of diabetes in patients.
References
Abdel-Wahab YH, Flatt PR, Patterson S, Conlon JM (2010) Insulin-releasing properties of the frog skin peptide B2RP (brevinin-2 related peptide) and its analogues both in vitro and in vivo. Regul Pept 164:51
Attoub S, Mechkarska M, Sonnevend A, Radosavljevic G, Jovanovic I, Lukic ML, Conlon JM (2013) Esculentin-2CHa: a host-defense peptide with differential cytotoxicity against bacteria, erythrocytes and tumor cells. Peptides 39:95–102
Bailey CJ (2009) New therapies for diabesity. Curr Diab Rep 9:360–367
Bailey CJ, Flatt PR, Kwasowski P, Powell CJ, Marks V (1986) Immunoreactive gastric inhibitory polypeptide and K cell hyperplasia in obese hyperglycaemic (ob/ob) mice fed high fat and high carbohydrate cafeteria diets. Acta Endocrinol (Copenh) 112:224–229
Conlon JM, Mechkarska M, Coquet L, Jouenne T, Leprince J, Vaudry H, Kolodziejek J, Nowotny N, King JD (2011) Characterization of antimicrobial peptides in skin secretions from discrete populations of Lithobates chiricahuensis (Ranidae) from central and southern Arizona. Peptides 32(4):664–669 (United States: 2011 Elsevier Inc.)
Conlon JM, Mechkarska M, Lukic ML, Flatt PR (2014) Potential therapeutic applications of multifunctional host-defense peptides from frog skin as anti-cancer, anti-viral, immunomodulatory, and anti-diabetic agents. Peptides 57:67–77
Elmore DE (2012) Insights into buforin II membrane translocation from molecular dynamics simulations. Peptides 38:357–362
Flatt PR, Bailey CJ (1981) Abnormal plasma glucose and insulin responses in heterozygous lean (ob/+) mice. Diabetologia 20:573–577
Irwin N, Flatt PR (2015) New perspectives on exploitation of incretin peptides for the treatment of diabetes and related disorders. World J Diabetes 6:1285–1295
Johnson R, McNutt P, MacMahon S, Robson R (1997) Use of the Friedewald formula to estimate LDL-cholesterol in patients with chronic renal failure on dialysis. Clin Chem 43:2183–2184
Kahn SE, Cooper ME, Del Prato S (2014) Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet 383:1068–1083
Kim JH, Lee JO, Jung JH, Lee SK, You GY, Park SH, Kim HS (2010) Gaegurin-6 stimulates insulin secretion through calcium influx in pancreatic beta Rin5mf cells. Regul Pept 159(1–3):123–128 (Netherlands)
Lee HS, Park CB, Kim JM, Jang SA, Park IY, Kim MS, Cho JH, Kim SC (2008) Mechanism of anticancer activity of buforin IIb, a histone H2A-derived peptide. Cancer Lett 271(1):47–55 (Ireland)
Martin CM, Gault VA, McClean S, Flatt PR, Irwin N (2012) Degradation, insulin secretion, glucose-lowering and GIP additive actions of a palmitate-derivatised analogue of xenin-25. Biochem Pharmacol 84(3):312–319 (England: 2012 Elsevier Inc.)
McKillop AM, Ng MT, Abdel-Wahab YH, Flatt PR (2014) Evidence for inhibitory autocrine effects of proinsulin C-peptide on pancreatic beta-cell function and insulin secretion. Diabetes Obes Metab 16:937–946
Mo GX, Bai XW, Li ZJ, Yan XW, He XQ, Rong MQ (2014) A novel insulinotropic peptide from the skin secretions of amolops loloensis frog. Nat Prod Bioprospect 4:309–313
Moffett RC, Vasu S, Flatt PR (2015) Functional GIP receptors play a major role in islet compensatory response to high fat feeding in mice. Biochim Biophys Acta 1850:1206–1214
Ojo OO, Abdel-Wahab YH, Flatt PR, Conlon JM (2013a) Insulinotropic actions of the frog skin host-defense peptide alyteserin-2a: a structure-activity study. Chem Biol Drug Des 82:196–204
Ojo OO, Flatt PR, Abdel-Wahab YHA, Conlon JM (2013b) Insulin-releasing peptides. In: Kastin A (ed) Handbook of biologically active peptides, section: amphibian skin peptides, 2nd edn. Elsevier Academic, Amsterdam, pp 364–370
Ojo OO, Srinivasan DK, Owolabi BO, Conlon JM, Flatt PR, Abdel-Wahab YH (2015a) Magainin-AM2 improves glucose homeostasis and beta cell function in high-fat fed mice. Biochim Biophys Acta 1850:80–87
Ojo OO, Srinivasan DK, Owolabi BO, Flatt PR, Abdel-Wahab YH (2015b) Beneficial effects of tigerinin-1R on glucose homeostasis and beta cell function in mice with diet-induced obesity-diabetes. Biochimie 109:18–26
Ojo OO, Srinivasan DK, Owolabi BO, Vasu S, Conlon JM, Flatt PR, Abdel-Wahab YH (2015c) Esculentin-2CHa-related peptides modulate islet cell function and improve glucose tolerance in mice with diet-induced obesity and insulin resistance. PLoS One 10(10):e0141549 (United States)
Owolabi BO, Ojo OO, Srinivasan DK, Conlon JM, Flatt PR, Abdel-Wahab YH (2016) In vitro and in vivo insulinotropic properties of the multifunctional frog skin peptide hymenochirin-1B: a structure-activity study. Amino Acids 48(2):535–547 (Austria)
Parkes DG, Mace KF, Trautmann ME (2013) Discovery and development of exenatide: the first antidiabetic agent to leverage the multiple benefits of the incretin hormone, GLP-1. Expert Opin Drug Discov 8:219–244
Pathak V, Vasu S, Gault VA, Flatt PR, Irwin N (2015) Sequential induction of beta cell rest and stimulation using stable GIP inhibitor and GLP-1 mimetic peptides improves metabolic control in C57BL/KsJ db/db mice. Diabetologia 58:2144–2153
Patterson S, de Kort M, Irwin N, Moffett RC, Dokter WH, Bos ES, Miltenburg AM, Flatt PR (2015) Pharmacological characterization and antidiabetic activity of a long-acting glucagon-like peptide-1 analogue conjugated to an antithrombin III-binding pentasaccharide. Diabetes Obes Metab 17:760–770
Srinivasan D, Ojo OO, Owolabi BO, Conlon JM, Flatt PR, Abdel-Wahab YH (2015) The frog skin host-defense peptide CPF-SE1 improves glucose tolerance, insulin sensitivity and islet function and decreases plasma lipids in high-fat fed mice. Eur J Pharmacol 764:38–47
Vasu S, McClenaghan NH, McCluskey JT, Flatt PR (2013) Cellular responses of novel human pancreatic beta-cell line, 1.1B4 to hyperglycemia. Islets 5(4):170–177 (United States)
Vasu S, McGahon MK, Moffett RC, Curtis TM, Conlon JM, Abdel-Wahab YH, Flatt PR (2017) Esculentin-2CHa(1–30) and its analogues: stability and mechanisms of insulinotropic action. J Endocrinol 232(3):423–435 (England: 2017 Society for Endocrinology)
Winzell MS, Ahren B (2004) The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 53(Suppl 3):S215–S219 (United States)
Author information
Authors and Affiliations
Contributions
SV and RCM performed the experiments, analysed data and prepared the manuscript. OO, YHAA, JMC and PRF conceived and designed the study and prepared the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. Funding for this study was provided by a proof of concept project grant from Invest NI (Grant Number POC 418) and project grant from Diabetes UK. Ulster University has patent filings in the area of frog skin peptides and diabetes.
Research involving humans and/or animals
All procedures performed in studies involving animals were in accordance with UK Animals (Scientific Procedures) Act 1986 and ‘Principles of laboratory animal care’ (NIH publication no. 86–23, revised 1985). This article does not contain any studies with human participants performed by any of the authors.
Additional information
Handling Editor: T. Langer.
Rights and permissions
About this article
Cite this article
Vasu, S., Ojo, O.O., Moffett, R.C. et al. Anti-diabetic actions of esculentin-2CHa(1–30) and its stable analogues in a diet-induced model of obesity-diabetes. Amino Acids 49, 1705–1717 (2017). https://doi.org/10.1007/s00726-017-2469-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-017-2469-3