Abstract
Aims
Xenin-25 is co-secreted with glucose-dependent insulinotropic polypeptide (GIP) from intestinal K-cells following a meal. Xenin-25 is believed to play a key role in glucose homoeostasis and potentiate the insulinotropic effect of GIP.
Methods
This study investigated the effects of sub-chronic administration of the stable and longer-acting xenin-25 analogue, xenin-25[Lys13PAL] (25 nmol/kg), in diabetic mice fed with a high-fat diet.
Results
Initial studies confirmed the significant persistent glucose-lowering (p < 0.05) and insulin-releasing (p < 0.05) actions of xenin-25[Lys13PAL] compared with native xenin-25. Interestingly, xenin-25 retained significant glucose-lowering activity in GIP receptor knockout mice. Twice-daily intraperitoneal (i.p.) injection of xenin-25[Lys13PAL] for 14 days had no significant effect on food intake or body weight in high-fat-fed mice. Non-fasting glucose and insulin levels were also unchanged, but overall glucose levels during an i.p. glucose tolerance and oral nutrient challenge were significantly (p < 0.05) lowered by xenin-25[Lys13PAL] treatment. These changes were accompanied by significant improvements in i.p. (p < 0.05) and oral (p < 0.001) nutrient-stimulated insulin concentrations. No appreciable changes in insulin sensitivity were observed between xenin-25[Lys13PAL] and saline-treated high-fat mice. However, xenin-25[Lys13PAL] treatment restored notable sensitivity to the biological actions of exogenous GIP injection. Consumption of O2, production of CO2, respiratory exchange ratio and energy expenditure were not altered by 14-day twice-daily treatment with xenin-25[Lys13PAL]. In contrast, ambulatory activity was significantly (p < 0.05 to p < 0.001) increased during the dark phase in xenin-25[Lys13PAL] mice compared with high-fat controls.
Conclusions
These data indicate that sustained administration of a stable analogue of xenin-25 exerts a spectrum of beneficial metabolic effects in high-fat-fed mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Xenin-25 is a 25 amino acid gastrointestinal hormone secreted from enteroendocrine K-cells in response to feeding, generated by post-translational modification involving a parent precursor represented by the alpha-subunit of coatomer [1–3]. Xenin-25 performs a spectrum of biological activities, including classically to inhibit gut motility [4]. However, more recently xenin-25 has been shown to exert beneficial effects on inhibiting food intake [2, 5] and stimulating pancreatic beta-cell insulin release [3]. In addition, xenin-25 is known to potentiate the insulinotropic action of the incretin hormone glucose-dependent insulinotropic polypeptide (GIP) [6, 7]. Given that the action of GIP is severely attenuated in type 2 diabetes mellitus (T2DM) [8], xenin-25 represents an exciting therapeutic drug target for patients with T2DM.
Although the glucose-dependent insulinotropic action of GIP was discovered over 35 years ago [9], antidiabetic therapeutic strategies based on GIP are not yet commercially available. This contrasts sharply with GIP’s sister incretin hormone, glucagon-like peptide-1 (GLP-1), of which several stable, long-acting forms are now available clinically [10]. Therefore, strategies to overcome defective GIP action in T2DM are much sought after and should have significant therapeutic applicability. Despite the promise of xenin-25 in this regard, rapid plasma degradation in the circulation poses a major obstacle in the realisation of any possible clinical use [3, 11]. Plasma-mediated degradation of xenin-25 is poorly understood, and a number of processes have been postulated to be involved including, but not limited to, serine protease-like enzymes [7, 11]. Thus, the use of enzyme inhibitors to prolong xenin-25 action is not feasible at present. To circumvent this problem, we have generated an enzymatic resistant analogue of xenin-25 with an extended biological half-life, namely xenin-25[Lys13Palmitate(PAL)] [7]. Importantly, xenin-25[Lys13PAL] displayed a similar, if not marginally superior, biological action profile to native xenin-25 indicating that acylation does not perturb biological activity [7]. As such, xenin-25[Lys13PAL] represents a potent, stable and long-acting form of xenin-25 with possible antidiabetic actions.
In the light of this, the present study has investigated the biological effects of twice-daily administration of xenin-25[Lys13PAL] in high-fat-fed mice. To date, there is no detailed evidence of normal physiological concentrations of xenin-25 or whether levels change during disease such as obesity or T2DM. However, given that xenin-25 has independent glucose-lowering and insulinotropic properties [3] as well as an ability to potentiate GIP action [6, 7], it is anticipated that the sub-chronic administration of xenin-25[Lys13PAL] would have significant beneficial effects. Initial studies determined the persistent biological effects of xenin-25[Lys13PAL] when compared to the native hormone. We, then, examined the effects of 14-day twice-daily administration of xenin-25[Lys13PAL] on body weight, energy intake, basal glucose and insulin, glucose homoeostasis, peripheral insulin resistance, GIP sensitivity and metabolic response to feeding in high-fat-fed mice. We also assessed the effects of sub-chronic administration of xenin-25[Lys13PAL] on aspects of metabolic rate and locomotor activity. The results illustrate potential antidiabetic actions of stable, long-acting analogues of xenin-25 that merit further consideration.
Materials and methods
Peptide synthesis
Xenin-25 and xenin-25[Lys13PAL] were purchased from GL Biochem Ltd. (Shanghai, China). Both peptides were characterised using matrix-assisted laser desorption ionisation time-of-flight (MALDI-TOF) mass spectrometry as described previously [7].
Animals
Young (8-week-old) male NIH Swiss mice (Harlan U.K. Ltd) were housed individually in an air-conditioned room at 22 ± 2 °C with a 12 h light/12 h dark cycle (0800–2000 h). Experimental animals had free access to drinking water and a high-fat diet (45 % fat, 20 % protein and 35 % carbohydrate; per cent of total energy of 26.15 kJ/g; Special Diets Service, Essex, UK). Prior to commencement of experimental studies, animals were maintained on high-fat diet for 145 days. Mice were divided into equivalent groups based on body weight, non-fasting glucose and insulin and glucose tolerance (data not shown). Age-matched control mice from the same source had free access to standard rodent maintenance diet (10 % fat, 30 % protein and 60 % carbohydrate; percentage of total energy of 12.99 kJ per g; Trouw Nutrition, Cheshire, UK) and were used for comparative purposes as appropriate. For GIP receptor knockout (GIPR KO) studies, male C57BL/6 mice with genetic deletion of the GIPR, along with wildtype controls, maintained on standard rodent maintenance diet were used. These mice were derived from an in-house breeding colony originally described elsewhere [12]. All animal experiments were carried out in accordance with the UK Animals (Scientific Procedures) Act 1986.
GIPR KO studies
Groups (n = 8) of C57BL/6 control and GIPR KO mice received an intraperitoneal (i.p.) injection of either glucose alone (18 mmol/kg body wt) or in combination with xenin-25 (25 nmol/kg body wt). Mice were fasted overnight prior to glucose administration and blood glucose concentrations were measured immediately prior to and post-glucose injection at time points indicated in Fig. 1.
Acute glucose-lowering effects of xenin-25 in C57BL/6 control (a) and GIPR KO (b) mice. Blood glucose was measured immediately prior to t = 0 (as indicated by the arrow) and 15, 30, 60 and 105 min after i.p. injection of glucose alone (18 mmol/kg bw) or in combination with native xenin-25 (25 nmol/kg bw) in overnight fasted mice. Blood glucose 0–105 min AUC values are shown in the insets. Values represent mean ± SEM for 8 mice. *p < 0.05 and **p < 0.01 compared with glucose alone
Acute in vivo studies
Groups (n = 8) of high-fat-fed mice received an intraperitoneal (i.p.) injection of either saline vehicle (0.9 %, w/v, NaCl), xenin-25 or xenin-25[Lys13PAL] (both at 25 nmol/kg body wt) 4 h prior to i.p. glucose administration (18 mmol/kg body wt). Mice were fasted 4 h prior to glucose administration, and blood glucose and plasma insulin concentrations were measured immediately prior to and post-glucose injection at time points indicated on Fig. 2.
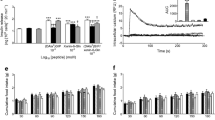
Persistent glucose-lowering (a, b) and insulin-releasing (c, d) effects of xenin-25 and xenin-25[Lys13PAL] in high-fat-fed mice. Blood glucose (a) and plasma insulin (c) were measured immediately prior to t = 0 (as indicated by the arrow) and 15, 30 and 60 min after i.p. injection of glucose alone (18 mmol/kg bw) following the administration of saline vehicle (0.9 % w/v NaCl), xenin-25 or xenin-25[Lys13PAL] (both at 25 nmol/kg bw) 4 h previously. Blood glucose (b) and plasma insulin (d) 0–60 min AUC values are also included. Values represent mean ± SEM for 8 mice. *p < 0.05 and ***p < 0.001 compared with saline alone. Δ p < 0.05 compared with xenin-25
Longer-term in vivo studies
For longer-term studies, separate groups (n = 8) of high-fat-fed mice received twice-daily i.p. injections (09:30 and 17:00 h) of either saline vehicle (0.9 % (w/v), NaCl) or xenin-25[Lys13PAL] (25 nmol/kg body wt) over a 14-day period. Food intake and body weight were recorded daily, whilst glucose and insulin concentrations were monitored at intervals of 3–5 days. Intraperitoneal (i.p.) glucose tolerance (18 mmol/kg body wt) and insulin sensitivity (20 U/kg body wt) tests were conducted in non-fasted mice, whereas GIP tolerance (25 nmol/kg body wt) and metabolic response to feeding tests were examined in 18-h-fasted mice at the end of the study period. All acute experiments were commenced at 10:00 h. Importantly, the daily 09:30 h injection was withheld from all mice before commencement of metabolic tests. For measurement of indirect calorimetry, energy expenditure and locomotor activity on day 14, mice were placed in Complete Laboratory Animal Monitoring System (CLAMS) metabolic chambers (Columbus Instruments, OH, USA) following the normal 09:30 daily injection. Consumption of O2 and production of CO2 were measured for 30 s at 15 min intervals for a total of 22 h. Respiratory exchange ratio (RER) was calculated by dividing VCO2 by VO2. Energy expenditure was calculated using RER with the following Eq. (3.815 + 1.232 × RER) × VO2. Ambulatory locomotor activity of each mouse was measured simultaneously using the optical beams (Opto M3, Columbus Instruments). Consecutive photo-beam breaks were scored as an ambulatory movement. Activity counts in X and Z axes were recorded every minute for 22 h.
Biochemical analysis
Blood samples taken from the cut tip of the tail vein of conscious mice at the times indicated in the figures were immediately centrifuged using a Beckman microcentrifuge (Beckman Instruments, UK) for 30 s at 13,000 g. The resulting plasma was then aliquoted into fresh Eppendorf tubes and stored at −20 °C prior to insulin analysis. Plasma glucose was measured from whole blood using an Ascensia Contour® Blood Glucose Meter (Bayer AG, Leverkusen, Germany). Plasma insulin was assayed by a modified dextran-coated charcoal radioimmunoassay [13].
Statistics
Results are expressed as mean ± SEM. Data were compared using ANOVA, followed by a Student–Newman–Keuls post hoc test. Area under the curve (AUC) analyses were calculated using the trapezoidal rule with baseline subtraction. Groups of data were considered to be significantly different if p < 0.05.
Results
Glucose-lowering effects of xenin-25 in GIPR KO mice
Xenin-25 induced significant (p ≤ 0.05) glucose-lowering effects 60 and 105 min post-injection in C57BL/6 control mice compared with glucose-alone control (Fig. 1a). This glucose-lowering action was substantiated by overall 0- to 105-min AUC values, which were significantly (p < 0.05) decreased in xenin-25-treated mice (Fig. 1a, inset). Similarly, the notable antihyperglycaemic action of xenin-25 was retained in GIPR KO mice in terms of both individual (p < 0.01 to p < 0.05) and overall (p < 0.05) values (Fig. 1b).
Persistent glucose-lowering and insulin-releasing actions of xenin-25[Lys13PAL] in high-fat-fed mice
Administration of native xenin-25 4 h prior to a glucose challenge had no effect on blood glucose or plasma insulin levels when compared to saline-treated controls (b. 2). However, prior application of xenin-25[Lys13PAL] resulted in significantly decreased blood glucose levels 60 min post-glucose injection compared with xenin-25 and saline-treated control mice (p < 0.05 and p < 0.001; respectively) (Fig. 2a). This was corroborated by a significantly (p < 0.05) decreased overall glycaemic excursion in xenin-25[Lys13PAL] mice when compared with all other treatment groups (Fig. 2b). Glucose-stimulated plasma insulin concentrations were significantly (p < 0.05) elevated in mice treated with xenin-25[Lys13PAL] 4 h previously when compared with xenin-25 and saline-treated groups (Fig. 2c). This was highlighted by a significantly (p < 0.05) increased overall insulin secretory response evoked by xenin-25[Lys13PAL] compared with the other treatment groups (Fig. 2d).
Sub-chronic effects of xenin-25[Lys13PAL] on body weight, energy intake, circulating blood glucose and plasma insulin levels in high-fat-fed mice
Administration of xenin-25[Lys13PAL] twice daily for 14 days to high-fat-fed mice resulted in no significant changes in body weight, energy intake, non-fasting blood glucose and plasma insulin concentrations (Fig. 3a–d). Furthermore, body weight, energy intake and non-fasting plasma insulin levels remained significantly (p < 0.05 to p < 0.001) elevated in saline-treated and xenin-25[Lys13PAL]-treated high-fat mice when compared with lean controls (Fig. 3a, b, d). Non-fasting blood glucose levels had a tendency to be increased in all high-fat-fed mice compared with lean controls, but this did not reach significance throughout the study (Fig. 3c).
Effects of twice-daily administration of xenin-25[Lys13PAL] on a body weight, b energy intake, c blood glucose and d plasma insulin in high-fat-fed mice. Parameters were measured for 5 days prior to and 14 days during (indicated by horizontal black bar) treatment with saline or xenin-25[Lys13PAL] (25 nmol/kg body wt). Values are mean ± SEM for eight mice. *p < 0.05, **p < 0.01 and ***p < 0.001 compared with saline-control group. Δ p < 0.05, ΔΔ p < 0.01 and ΔΔΔ p < 0.001 compared to xenin-25[Lys13PAL]
Sub-chronic effects of xenin-25[Lys13PAL] on metabolic responses and insulin secretion in high-fat-fed mice
Individual glucose levels during glucose tolerance tests were not significantly different in 14-day xenin-25[Lys13PAL]-treated mice compared to high-fat controls (Fig. 4a). However, the overall glycaemic excursion from basal was significantly (p < 0.05) decreased in xenin-25[Lys13PAL] mice, and values were similar to lean controls (Fig. 4b). In addition, whilst individual glucose-stimulated plasma insulin concentrations were not significantly different between xenin-25[Lys13PAL]-treated and high-fat control mice (Fig. 4c), the overall insulin secretory response was significantly (p < 0.05) elevated in these mice (Fig. 5d).
Effects of twice-daily administration of xenin-25[Lys13PAL on (a, b) glucose tolerance and (c, d) plasma insulin response to glucose in high-fat-fed mice. Tests were conducted after twice-daily treatment with saline or xenin-25[Lys13PAL] (25 nmol/kg body wt) for 14 days. Glucose (18 mmol/kg body wt) was administered at the time indicated by the arrow in non-fasted mice. Plasma glucose and insulin 0–105 min AUC values for 0–60 min post-injection are shown. Values are mean ± SEM for eight mice. *p < 0.05 and **p < 0.01 compared with saline control group. Δ p < 0.05 and ΔΔ p < 0.01 compared with xenin-25[Lys13PAL]
Effects of twice-daily administration of xenin-25[Lys13PAL] on glucose (a, b) and insulin (c, d) responses to feeding in high-fat-fed mice. Tests were conducted after twice-daily treatment with saline or xenin-25[Lys13PAL] (25 nmol/kg body wt) for 14 days. Mice were fasted for 18 h and allowed to re-feed for 15 min (black horizontal bar indicates time of feeding). Plasma glucose and insulin 0–105 AUC values are also shown. Values represent mean ± SEM for 8 mice. *p < 0.05 and **p < 0.01 compared with saline control group. Δ p < 0.05, ΔΔ p < 0.01 and ΔΔΔ p < 0.001 compared with xenin-25[Lys13PAL]
Plasma glucose responses to 15 min re-feeding in xenin-25[Lys13PAL] mice were almost identical to lean controls (Fig. 5a). Indeed, both groups of mice had significantly (p < 0.05) lowered blood glucose levels at 30-min post-feeding compared with high-fat controls (Fig. 5a). Similarly, AUC glucose was significantly (p < 0.05) decreased in lean control and xenin-25[Lys13PAL]-treated high-fat mice when compared with high-fat controls (Fig. 5b), despite similar food intakes of 0.3–0.5 g/mouse/15 min in all groups. Furthermore, oral nutrient-stimulated insulin concentrations were significantly (p < 0.05 to p < 0.01) elevated at 30, 60 and 105 min post-feeding in xenin-25[Lys13PAL] mice compared with high-fat controls (Fig. 5c). This was associated with a significant (p < 0.001) elevation of 0–105 min plasma insulin AUC values in xenin-25[Lys13PAL] mice compared with high-fat and lean control mice (Fig. 5d).
As shown in Fig. 6, the hypoglycaemic action of insulin was similar in terms of post-injection and area above the curve (AAC) measures in high-fat mice treated with xenin-25[Lys13PAL] for 14 days when compared to saline-treated high-fat controls. Lean control mice had a significant (p < 0.001) augmentation of the glucose-lowering action of exogenous insulin when compared with all high-fat-fed mice (Fig. 6a).
Effects of twice-daily administration of xenin-25[Lys13PAL] on insulin sensitivity in high-fat-fed mice. Tests were conducted after twice-daily treatment with saline or xenin-25[Lys13PAL] (25 nmol/kg body wt) for 14 days. a Insulin (20 U/kg body wt) was administered at the time indicated by the arrow in non-fasted mice. b Blood glucose 0–60 min AAC values are also shown. Values represent mean ± SEM for 8 mice. *p < 0.05 compared with saline control group. Δ p < 0.05 compared with xenin-25[Lys13PAL]
Effects of exogenous GIP in combination with glucose on glycaemic control and plasma insulin are shown in Fig. 7. Compared with high-fat-fed control mice, xenin-25[Lys13PAL]-treated mice presented with significantly lowered individual (p < 0.05 to p < 0.001) and overall (p < 0.01) glycaemic levels following GIP injection (Fig. 7a). Moreover, the overall 0–105 min glycaemic excursion was significantly (p < 0.05) reduced in xenin-25[Lys13PAL]-treated mice compared with lean controls (Fig. 7b). In harmony with this, the insulin secretory response to GIP was significantly (p < 0.01 to p < 0.001) augmented 15 and 30 min post-injection in xenin-25[Lys13PAL] mice compared to saline-treated high-fat and lean controls (Fig. 7c). This was corroborated by a significantly (p < 0.01) elevated overall insulin release in xenin-25[Lys13PAL] mice compared with high-fat and lean control mice (Fig. 7d).
Effects of twice-daily administration of xenin-25[Lys13PAL] on glucose (a, b) and insulin (c, d) responses to exogenous GIP administration in high-fat-fed mice. Tests were conducted after twice-daily treatment with saline or xenin-25[Lys13PAL] (25 nmol/kg body wt) for 14 days. Glucose (18 mmol/kg body wt) in combination with native GIP (25 nmol/kg body wt) was administered at the time indicated by the arrow in 18-h-fasted mice. Plasma glucose and insulin 0–105 min AUC values for 0–105 min post-injection are shown. Values are mean ± SEM for eight mice. *p < 0.05, **p < 0.01 and ***p < 0.001 compared with saline control group. Δ p < 0.05, ΔΔ p < 0.01 and ΔΔΔ p < 0.001 compared with xenin-25[Lys13PAL]
Sub-chronic effects of xenin-25[Lys13PAL] on locomotor activity in high-fat-fed mice
Figure 8 depicts the effects of 14-day treatment with xenin-25[Lys13PAL] on locomotor activity. There were no significant differences between all groups in terms of ambulatory activity during the light phase (Fig. 8a, b). However, xenin-25[Lys13PAL] significantly (p < 0.05) increased ambulatory activity in high-fat-fed mice as assessed by X beam breaks during the dark phase (Fig. 8c), returning values towards that of lean controls. In addition, rearing and jumping episodes during the dark phase, as assessed by Z beam breaks, were similar in lean control and xenin-25[Lys13PAL]-treated high-fat mice and significantly (p < 0.001) elevated compared with high-fat controls (Fig. 8d).
Effects of twice-daily administration of xenin-25[Lys13PAL] on locomotor activity in high-fat-fed mice. Parameters were measured after twice-daily treatment with saline or xenin-25[Lys13PAL] (25 nmol/kg body wt) for 14 days. Mice were placed in CLAMS metabolic chambers and locomotor activity measured using optical beams. Activity counts in X and Z axes were recorded every minute for 22 h. Values are mean ± SEM for six mice. *p < 0.05, **p < 0.01 and ***p < 0.001 compared with saline control group
Sub-chronic effects of xenin-25[Lys13PAL] on metabolic rate and energy expenditure in high-fat-fed mice
Administration of xenin-25[Lys13PAL] had no significant effect on O2 consumption or CO2 production when compared to high-fat controls (Fig. 9a, b). Similarly, RER and energy expenditure were not significantly altered in terms of individual measurements or overall responses during the 22-h observation period in mice treated with xenin-25[Lys13PAL] (Fig. 9c, d). However, lean control mice did have significantly (p < 0.001) elevated CO2 production and RER when compared with saline-treated or xenin-25[Lys13PAL]-treated high-fat-fed mice (Fig. 9b, c).
Effects of twice-daily administration of xenin-25[Lys13PAL] on O2 consumption (a), CO2 production (b), respiratory exchange ratio (c) and energy expenditure (d). Parameters were measured after twice-daily treatment with saline or xenin-25[Lys13PAL] (25 nmol/kg body wt) for 14 days. Mice were placed in CLAMS metabolic chambers, and O2 consumption or CO2 production were measured for 30 s at 15 min intervals. RER was calculated by dividing VCO2 by VO2. Energy expenditure was calculated using RER with the following Eq. (3.815 + 1.232 × RER) × VO2. The dark phase is indicated by the black horizontal bar. Insets depict the consequence of combining light and dark phase data. Values are mean ± SEM for six mice. ***p < 0.001 compared with saline control group. ΔΔ p < 0.01 and ΔΔΔ p < 0.001 compared with xenin-25[Lys13PAL]
Discussion
Initially, we confirmed the persistence of the biological actions of xenin-25[Lys13PAL] in comparison with native xenin-25 in high-fat-fed mice. This is a commonly studied animal model of obesity–diabetes displaying several abnormalities characteristic of human T2DM, including moderate obesity, glucose intolerance and insulin resistance [14]. In addition, these mice have been used previously to assess the acute and sub-chronic actions of similar synthetic GIP and GLP-1 compounds [15, 16]. In the present study, even 4 h after a single injection of xenin-25[Lys13PAL], the glucose-lowering and insulin-releasing actions were still clearly evident. This is presumably related to binding to serum albumin and slow release of active peptide, thus, preventing enzymatic degradation and subsequent removal from the body. This also conforms well to other studies that have utilised acylated versions of GIP and GLP-1 peptides [15, 17]. Indeed, fatty acid derivation has been shown to extend the biological action of regulatory hormones well beyond 4 h [15, 17], which is likely the case for xenin-25[Lys13PAL]. As such, development of a specific assay to directly measure xenin-25[Lys13PAL] in plasma would provide more precise details of circulating half-life. In contrast, similar injection of xenin-25 had little metabolic consequence, reflecting rapid plasma degradation and elimination [7]. Similarly, native xenin-25 lacked significant insulinotropic effects in type 2 diabetes [18]. Indeed, recent studies suggest that the naturally produced degradation products of xenin-25 could function as inhibitors of the native peptide [11]. Based on the evidence above, all further studies were conducted with xenin-25[Lys13PAL], rather than the rapidly degraded native peptide, as a potentially useful agent for alleviation of obesity-related diabetes.
Twice-daily administration of our enzymatically stable xenin-25[Lys13PAL] analogue to high-fat-fed mice resulted in significant improvements of intraperitoneal and oral glucose tolerance by day 14. Notably, all injections were administered during the light cycle when mice are less active, thus more pronounced beneficial effects may have be observed if xenin-25[Lys13PAL] was administered during the dark cycle. As expected, a key component of the beneficial action of xenin-25[Lys13PAL] concerned the stimulation of insulin secretion [3, 7]. Thus, both intraperitoneal and oral nutrient-stimulated insulin concentrations were significantly raised in high-fat-fed mice receiving the novel fatty acid-derivatised analogue. This is consistent with the action of xenin-25 as an independent dose-dependent stimulator of insulin secretion [3] and acute potentiator of the GIP-induced incretin effect [6, 7]. We did not measure endogenous GIP concentrations in the current study, but given that xenin-25 is co-secreted with GIP form a subset of enteroendocrine K-cells [1], it would seem unlikely that they would be elevated in xenin-25[Lys13PAL]-treated mice. Moreover, using GIPR KO mice, we confirmed that xenin-25 has significant glucose-lowering actions independent of the GIP receptor [3], as well as potentiating the insulinotropic action of GIP [7]. In keeping with this, the biological actions of native GIP were completely restored in xenin-25[Lys13PAL]-treated high-fat-fed mice. Thus, in the current study, the bioactivity of GIP was severely limited in saline-treated control high-fat mice, a phenomenon also observed in patients with T2DM [8]. Notably, the improvement of GIP action by xenin-25[Lys13PAL] was not simply a reflection of reduced glucose toxicity, since basal glucose levels were not altered in these mice. As such, GIP resistance in diabetic rats has been shown to be reversed by a reduction of hyperglycaemia [19, 20]. Furthermore, given that high circulating concentrations of GIP are present in obesity-diabetes [21–23] and that fat is a potent stimulus for GIP secretion [24], it is tempting to link a major part of the positive effects of xenin-25[Lys13PAL] to augmentation of GIP action [6, 7]. However, in vitro studies in our laboratory revealed no effect of native xenin-25 or xenin-25[Lys13PAL] on GIP receptor gene expression following prolonged 18-h culture [data not shown]. As such, xenin-25[Lys13PAL] does clearly have independent glucose-lowering and insulin-releasing actions [7].
Further studies are needed to assess the contribution of possible non-beta actions, such as stimulation of glucagon secretion or inhibition of gastric emptying [25, 26], to the metabolic effects of xenin-25[Lys13PAL]. However, these are likely to be small given the opposing metabolic actions of glucagon and the observation that glucose homoeostasis was improved when nutrients were administered by either the intraperitoneal or oral route. In agreement with this, energy intake and body weight were unchanged during the study ruling out the possibility that improvements of glucose homoeostasis were merely the consequence of body weight loss. Thus, although native xenin-25 does have some homology with C-terminal region of the anorectic neuropeptide neurotensin [2], they are unlikely to function via identical pathways. Further confirmation of body weight neutral effects of xenin-25[Lys13PAL] were gained at the end of the study by lack of enhanced insulin-induced reductions in blood glucose levels in xenin-25[Lys13PAL]-treated mice. In addition, aspects of metabolic rate were not altered by sub-chronic xenin-25[Lys13PAL] administration. Although modulation of GIP receptor signalling can affect respiratory quotient, it does not change oxygen consumption [16, 27], as such xenin and GIP may have further similarities here. In addition, the observed lack of effect on respiratory exchange ratio (RER) could be one explanation for the weight neutral effects of xenin-25[Lys13PAL]. However, we also failed to observe suppressive effects of xenin-25[Lys13PAL] on feeding at the dose employed, which necessitates further evaluation [2]. This could be related to factors such as the palatability of the high-fat diet used, age of mice, duration of study and circulating levels of active xenin achieved. In this regard, we have previously observed a satiety effect of xenin-25, but only at highly elevated concentrations [3, 11].
Although research has been conducted on the beta-cell secretory function and potential antidiabetic actions of xenin-based peptides [3, 6, 7, 25], there is no information on their possible effects on locomotor activity. In the present study, xenin-25[Lys13PAL]-treated mice displayed significantly increased locomotor activity during the dark phase, but this was not associated with more feeding bouts. Nonetheless, this is of interest given that locomotor activity of mice is already generally elevated during the dark phase. Increased locomotor activity did not result in reduced body weight gain, which may reflect the fact that activity was unaltered during the light phase. Our observations on locomotor activity also contrast with previous studies using long-acting GIP analogues, where locomotor activity was enhanced only during the light phase [16]. This indicates that there may be further interactions between GIP-based and xenin-based peptides other than their effects on insulin secretion [6, 7], which have not been considered to date.
In conclusion, the present study has demonstrated for the first time that twice-daily administration of the stable xenin-25 analogue, xenin-25[Lys13PAL], improves glucose tolerance, GIP sensitivity and insulin secretory responsiveness in mice with environmentally induced obesity–diabetes. More detailed and prolonged studies are still needed, but xenin-25[Lys13PAL] may prove to be useful as a new class of drugs for the treatment of T2DM.
References
Feurle GE, Ikonomu S, Partoulas G, Stoschus B, Hamscher G (2003) Xenin plasma concentrations during modified sham feeding and during meals of different composition demonstrated by radioimmunoassay and chromatography. Regul Pept 111:153–159
Cooke JH, Patterson M, Patel SR, Smith KL, Ghatei MA, Bloom SR, Murphy KG (2009) Peripheral and central administration of xenin and neurotensin suppress food intake in rodents. Obesity (Silver Spring) 17:1135–1143
Taylor AI, Irwin N, McKillop AM, Patterson S, Flatt PR, Gault VA (2010) Evaluation of the degradation and metabolic effects of the gut peptide xenin on insulin secretion, glycaemic control and satiety. J Endocrinol 207:87–93
Kamiyama Y, Aihara R, Nakabayashi T, Mochiki E, Asao T, Kuwano H (2007) The peptide hormone xenin induces gallbladder contractions in conscious dogs. Neurogastroenterol Motil 19:33–240
Leckstrom A, Kim ER, Wong D, Mizuno TM (2009) Xenin, a gastrointestinal peptide, regulates feeding independent of the melanocortin signaling pathway. Diabetes 58:87–94
Wice BM, Wang S, Crimmins DL, Diggs-Andrews KA, Althage MC, Ford EL, Tran H, Ohlendorf M, Griest TA, Wang Q, Fisher SJ, Ladenson JH, Polonsky KS (2010) Xenin-25 potentiates glucose-dependent insulinotropic polypeptide action via a novel cholinergic relay mechanism. J Biol Chem 2285:19842–19853
Martin CM, Gault VA, McClean S, Flatt PR, Irwin N (2012) Degradation, insulin secretion, glucose-lowering and GIP additive actions of a palmitate-derivatised analogue of xenin-25. Biochem Pharmacol 84:312–319
Holst JJ, Knop FK, Vilsbøll T, Krarup T, Madsbad S (2011) Loss of incretin effect is a specific, important, and early characteristic of type 2 diabetes. Diabetes Care 34:S251–S257
Pederson RA, Brown JC (1976) The insulinotropic action of gastric inhibitory polypeptide in the perfused isolated rat pancreas. Endocrinology 99:780–785
Gallwitz B (2011) Glucagon-like peptide-1 analogues for Type 2 diabetes mellitus: current and emerging agents. Drugs 71:1675–1688
Martin CM, Parthsarathy V, Pathak V, Gault VA, Flatt PR, Irwin N (2014) Characterisation of the biological activity of xenin-25 degradation fragment peptides. J Endocrinol 221:193–200
Mieczkowska A, Irwin N, Flatt PR, Chappard D, Mabilleau G (2013) Glucose-dependent insulinotropic polypeptide (GIP) receptor deletion leads to reduced bone strength and quality. Bone 56:337–342
Flatt PR, Bailey CJ (1981) Abnormal plasma glucose and insulin responses in heterozygous lean (ob/+) mice. Diabetologia 20:573–577
Irwin N, Flatt PR (2009) Therapeutic potential for GIP receptor agonists and antagonists. Best Pract Res Clin Endocrinol Metab 23:499–512
Porter DW, Kerr BD, Flatt PR, Holscher C, Gault VA (2010) Four weeks administration of Liraglutide improves memory and learning as well as glycaemic control in mice with high fat dietary-induced obesity and insulin resistance. Diabetes Obes Metab 12:891–899
Gault VA, Porter DW, Irwin N, Flatt PR (2011) Comparison of sub-chronic metabolic effects of stable forms of naturally occurring GIP(1-30) and GIP(1-42) in high-fat fed mice. J Endocrinol 208:265–271
Irwin N, Clarke GC, Green BD, Greer B, Harriott P, Gault VA, O’Harte FP, Flatt PR (2006) Evaluation of the antidiabetic activity of DPP IV resistant N-terminally modified versus mid-chain acylated analogues of glucose-dependent insulinotropic polypeptide. Biochem Pharmacol 72:719–728
Wice BM, Reeds DN, Tran HD, Crimmins DL, Patterson BW, Dunai J, Wallendorf MJ, Ladenson JH, Villareal DT, Polonsky KS (2012) Xenin-25 amplifies GIP-mediated insulin secretion in humans with normal and impaired glucose tolerance but not type 2 diabetes. Diabetes 61:1793–1800
Pathak V, Vasu S, Flatt PR, Irwin N (2014) Effects of chronic exposure of clonal β-cells to elevated glucose and free fatty acids on incretin receptor gene expression and secretory responses to GIP and GLP-1. Diabetes Obes Metab 16:357–365
Piteau S, Olver A, Kim SJ, Winter K, Pospisilik JA, Lynn F, Manhart S, Demuth HU, Speck M, Pederson RA, McIntosh CH (2007) Reversal of islet GIP receptor down-regulation and resistance to GIP by reducing hyperglycemia in the Zucker rat. Biochem Biophys Res Commun 362:1007–1012
Salera M, Giacomoni P, Pironi L, Cornia G, Capelli M, Marini A, Benfenati F, Miglioli M, Barbara L (1982) Gastric inhibitory polypeptide release after oral glucose: relationship to glucose intolerance, diabetes mellitus, and obesity. J Clin Endocrinol Metab 55:329–336
Marks V (1988) In: James WPT, Parker SW (eds) Current approaches: obesity. Southampton, Duphar Medical Relations, pp 13–19
Yip RG, Wolfe MM (2000) GIP biology and fat metabolism. Life Sci 66:91–103
Kwasowski P, Flatt PR, Bailey CJ, Marks V (1985) Effects of fatty acid chain length and saturation on gastric inhibitory polypeptide release in obese hyperglycaemic (ob/ob) mice. Biosci Rep 5:701–705
Silvestre RA, Rodríguez-Gallardo J, Egido EM, Hernández R, Marco J (2003) Stimulatory effect of xenin-8 on insulin and glucagon secretion in the perfused rat pancreas. Regul Pept 115:25–29
Chowdhury S, Reeds DN, Crimmins DL, Patterson BW, Laciny E, Wang S, Tran HD, Griest TA, Rometo DA, Dunai J, Wallendorf MJ, Ladenson JH, Polonsky K, Wice BM (2014) Xenin-25 delays gastric emptying and reduces postprandial glucose levels in humans with and without type 2 diabetes. Am J Physiol Gastrointest Liver Physiol 306:G301–G309
Miyawaki K, Yamada Y, Ban N, Ihara Y, Tsukiyama K, Zhou H, Fujimoto S, Oku A, Tsuda K, Toyokuni S, Hiai H, Mizunoya W, Fushiki T, Holst JJ, Makino M, Tashita A, Kobara Y, Tsubamoto Y, Jinnouchi T, Jomori T, Seino Y (2002) Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med 8:738–742
Acknowledgments
These studies were supported by the Department of Education and Learning, Northern Ireland, University of Ulster Proof of Principle Funding Programme, the European Foundation for the Study of Diabetes and a research grant from Invest Northern Ireland Proof of Concept funding.
Conflict of interest
V.A.G., P.R.F. and N.I. hold stock in Diabetica Ltd. which has patents for exploitation of incretin-based drugs and other peptide therapeutics. C.M.A.M. and V.P. declare that they have no conflict of interest.
Ethical standard
All animal experiments were carried out in accordance with the UK Animals (Scientific Procedures) Act 1986 and approved by the University of Ulster Animal Ethics Review Committee. All necessary steps were taken to ameliorate any potential animal suffering.
Human and animal rights disclosure
This article does not contain any studies with human subjects. All animal experiments were carried out in accordance with the UK Animals (Scientific Procedures) Act 1986.
Author information
Authors and Affiliations
Corresponding author
Additional information
Managed by Massimo Porta.
Rights and permissions
About this article
Cite this article
Gault, V.A., Martin, C.M.A., Flatt, P.R. et al. Xenin-25[Lys13PAL]: a novel long-acting acylated analogue of xenin-25 with promising antidiabetic potential. Acta Diabetol 52, 461–471 (2015). https://doi.org/10.1007/s00592-014-0681-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-014-0681-0