Abstract
Hymenochirin-1b (Hym-1B; IKLSPETKDNLKKVLKGAIKGAIAVAKMV.NH2) is a cationic, α-helical amphibian host-defense peptide with antimicrobial, anticancer, and immunomodulatory properties. This study investigates the abilities of the peptide and nine analogues containing substitutions of Pro5, Glu6, and Asp9 by either l-lysine or d-lysine to stimulate insulin release in vitro using BRIN-BD11 clonal β cells or isolated mouse islets and in vivo using mice fed a high-fat diet to produce obesity and insulin resistance. Hym-1B produced a significant and concentration-dependent increase in the rate of insulin release from BRIN-BD11 cells without cytotoxicity at concentrations up to 1 µM with a threshold concentration of 1 nM. The threshold concentrations for the analogues were: [P5K], [E6K], [D9K], [P5K, E6K] and [E6K, D9k] 0.003 nM, [E6K, D9K] and [D9k] 0.01 nM, [P5K, D9K] 0.1 nM and [E6k] 0.3 nM. All peptides displayed cytotoxicity at concentrations ≥1 µM except the [P5K] and [D9k] analogues which were non-toxic at 3 µM. The potency and maximum rate of insulin release from mouse islets produced by the [P5K] peptide were significantly greater than produced by Hym-1B. Neither Hym-1B nor the [P5K] analogue at 1 µM concentration had an effect on membrane depolarization or intracellular Ca2+. The [P5K] analogue (1 µM) produced a significant increase in cAMP concentration in BRIN-BD11 cells and stimulated GLP-1 secretion from GLUTag cells. Down-regulation of the protein kinase A pathway by overnight incubation with forskolin completely abolished the insulin-releasing effects of [P5K]hym-1B. Intraperitoneal administration of the [P5K] and [D9k] analogues (75 nmol/kg body weight) to high-fat-fed mice with insulin resistance significantly enhanced glucose tolerance with a concomitant increase in insulin secretion. We conclude that [P5K]hym-1B and [D9k]hym-1B show potential for development into anti-diabetic agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The global epidemic of Type 2 diabetes has intensified the search for new therapeutic options to achieve and sustain glycaemic control and prevent diabetic complications (Peters 2010). Natural occurring bioactive compounds offer boundless opportunities for new anti-diabetic drug discovery and there is an increasing focus on the exploration of these sources (Mandal et al. 2014). Exendin-4 isolated from the venom of the Gila monster lizard was the first of such products to reach the market for the treatment of Type 2 diabetes (Tokuda et al. 2014). Host-defense peptides from the skins of several frog species have also demonstrated potential for development into novel anti-diabetic agents. Several such peptides that were first identified on the basis of their antimicrobial activities have subsequently been found to possess insulinotropic effects. These peptides are generally multifunctional displaying antimicrobial, anticancer, and antiviral activities (reviewed in Conlon et al. 2014).
Clawed frogs of the family Pipidae are distributed into five genera (Hymenochirus, Pseudhymenochirus, Silurana, Xenopus and Pipa). All are indigenous to Africa except members of the genus Pipa which are found in South America. The genus Hymenochirus contains four species: H. boettgeri, H. boulengeri, H. curtipes and H. feae (Frost 2015). Peptidomic analysis of the skin secretions of the Congo dwarf clawed frog H. boettgeri revealed the presence of five structural-related peptides termed hymenochirins that have low structural similarity with the antimicrobial peptides isolated from skin secretions of other frogs (Mechkarska et al. 2012). Hymenochirin-1B (IKLSPETKDNLKKVLKGAIKGAIAVAKMV.NH2) displays multifunctional properties including antimicrobial (Mechkarska et al. 2012, 2013), immunomodulatory (Mechkarska et al. 2013), and anticancer activities (Attoub et al. 2013) but its insulinotropic effects have not yet been reported.
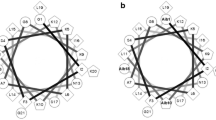
The disadvantages of naturally occurring peptides as therapeutic agents include short-half life in the circulation, toxicity, low potency and bioavailability, and significant immunogenicity (Lewis and Garcia 2003). However, these limitations may be circumvented to varying degrees by the design of appropriate analogues (Avan et al. 2014). The hymenochirins adopt an amphipathic α-helical conformation in a membrane-mimetic solvent (50 % trifluoroethanol–water) (Serra et al. 2014). Studies with analogues of other α-helical, frog skin host-defense peptides have shown that increasing cationicity, while maintaining amphipathicity enhances both antimicrobial (Conlon et al. 2007) and anticancer (Attoub et al. 2013) and produces more potent and effective insulin-releasing peptides (Abdel-Wahab et al. 2008b, 2010; Srinivasan et al. 2014). The aim of the present study was to investigate the insulinotropic actions of hymenochirin-1B, both in vitro using BRIN-BD11 clonal β-cells or isolated mouse islets and in vivo using mice fed a high-fat diet to produce obesity and insulin resistance. The effects of increasing cationicity by substitutions of neutral (Pro5) and acidic (Glu6 and Asp9) amino acids on the hydrophilic face of the α-helix by l-lysine or d-lysine on insulin-releasing and cytotoxic activities of the peptide were also determined.
Results
Effects of hymenochirin-1B and its analogues on insulin-release from BRIN-BD 11 cells
The basal rate of insulin release from BRIN-BD11 cells in the presence of 5.6 mM glucose alone was 1.05 ± 0.06 ng/106 cells/20 min. In the presence of the well-established insulin secretagogue, 10 mM alanine, the rate of insulin release increased to 6.49 ± 0.24 ng/106 cells/20 min. Incubation with hymenochirin-1B produced a significant (P < 0.05–P < 0.001) stimulatory response at concentrations ≥1 nM (Fig. 1) with a 304 % increase above the basal rate at 1 µM (Table 1). At a concentration of 3 µM, the peptide significantly stimulated the release of LDH from the cells indicating that the integrity of the plasma membrane had been compromised. All analogues tested showed greater insulin-releasing potency than the native peptide. The threshold concentration (minimum concentration producing a significant increase in secretion rate) for the [P5K], [E6K], [D9K], [P5K, E6K] and [E6K, D9K] analogues was 0.003 nM, for the [E6K, D9K] and [D9k] analogues 0.01 nM, for [P5K, D9K]hym-1B 0.1 M, and for [E6k]hym-1B 0.3 nM (Table 1).
A comparison of the effects of a [P5K]-hymenochirin-1B, b [D9k]hymenochirin-1B, and c [E6K]-hymenochirin-1B with hymenochirin-1B on insulin release from BRIN-BD11 cells Values are mean ± SEM for n = 8. *P < 0.05, **P < 0.01, ***P < 0.001 compared to 5.6 mM glucose alone. Δ P < 0.05, ΔΔ P < 0.01, ΔΔΔ P < 0.001 compared to hymenochirin-1B
The di-substituted [P5K, E6K], [P5K, D9K], and [E6K, D9K] analogues showed greater cytotoxicity compared to hym-1B producing a significant increase in the rate of LDH release at concentrations below 1 µM (Table 1). However, the [P5K] and [D9k] analogues were non-toxic at a concentration of 3 µM. Both peptides produced a significantly greater increase in the rate of insulin release at 3 µM compared with hym-1B at its maximum non-toxic concentration of 1 µM (Table 1).
Effects of hym-1B and analogues on insulin release from isolated islets
In the presence of 16.7 mM glucose, Hym-1B and its [P5K] and [D9k] analogues produced a concentration-dependent increase in the rate of insulin secretion from isolated mouse islets (Fig. 2a). Significant stimulatory effects of hym-1B and [D9k]hym-1B were seen at concentrations ≥10 nM while [P5K]hym-1B showed a significant stimulatory effect at 0.1 nM (Table 2). The stimulatory effects of the [P5K] and [D9k] analogues, measured as the % of total insulin content released, were significantly (P < 0.001) greater than GLP-1 when tested at 1 µM concentration (Table 2). These effects were not accompanied by significant release of LDH from isolated islets (Fig. 2b; Table 2).
A comparison of the effects of hymenochirin-1B and [P5K]hymenochirin-1B on a insulin and b LDH release from isolated mouse islets. Values are mean ± SEM (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001 compared to 16.7 mM glucose. + P<0.05, ++ P < 0.01, +++ P < 0.001 compared to hymenochirin-1B. Δ P < 0.05, ΔΔ P < 0.01, ΔΔΔ P < 0.001 compared to GLP-1 (10−6 M)
Insulin-releasing activities of hym-1B and [P5K]hym-1B in the presence of known modulators of insulin release
The mechanism of action of hym-1B and [P5K]hym-1B was investigated by performing incubations in presence of known modulators of insulin release. As shown in Table 3, the stimulatory actions of the peptides were maintained at 16.7 mM ambient glucose concentration and in the presence of K+ channel activator, diazoxide (300 µM) and the blocker of L-type voltage-dependent Ca2+ channels, verapamil (50 μM). A KCl (30 mM) depolarising stimulus increased the rate of insulin release and this increase was significantly (P < 0.001) augmented by hym-1B (1.4-fold) and [P5K]hym-1B (1.9-fold). The stimulatory activities of the peptides were not significantly affected when incubations were performed in the absence of extracellular calcium (Table 4).
Effects of peptides on membrane depolarization and intracellular calcium ([Ca2+]i)
In the presence of 30 mM KCl, a significant (96 %‚ P < 0.001) increase in membrane depolarisation was observed in BRIN-BD11 cells. In contrast to the effects of KCl, hym-1B and [P5K]hym-1B had no effect on membrane depolarisation (Fig. 3). Similarly, alanine caused a significant (87 %, P < 0.001) increase in intracellular calcium concentration but no significant effect was observed with the peptides (Fig. 4).
Effects of [P5K]hym-1B on intracellular concentrations of cyclic AMP
To determine which cell signaling pathway may be involved in the insulin-releasing effects of [P5K]hym-1B, its effects on cAMP production in BRIN-BD11 cells were investigated. As shown in Fig. 5a, [P5K]hym-1B produced a significant (P < 0.01) increase in cAMP concentration compared to 5.6 mM glucose + 200 µM IBMX. The stimulatory effects produced by [P5K]hym-1B (1 µM) was comparable to the effects produced by GLP-1 (10 nM). Concomitantly, [P5K]hym-1B produced a significant (P < 0.01) increase in the rate of insulin release compared to 5.6 mM glucose alone and 5.6 mM glucose + IBMX (200 µM) under the same experimental condition (Fig. 5b).
Effects of [P5K]hym-1B on cAMP production and insulin release in BRIN-BD 11 cells. Cells were incubated for 20 min at 37 °C with control, [P5 k]hym-1B or GLP-1 in KRB buffer supplemented with 5.6 mM glucose + 200 µM 3-isobutyl-1-methylxanthine (IBMX). Values are mean ± SEM for n = 6. ***P < 0.001 compared to 5.6 mM glucose alone. ΔΔ P < 0.01, ΔΔΔ P < 0.001 compared to 5.6 mM glucose + IBMX
Effects of down-regulation of the PKC and PKA pathways on [P5K]hym-1B stimulated insulin release
The experimental protocol made use of the fact that overnight culture of BRIN-BD11 cells with activators of the PKA pathway (forskolin) or the PKC pathway (PMA) blocks the subsequent stimulatory effects of agents that activate these pathways (McClenaghan et al. 2006). Following overnight culture in media alone, [P5K]hym-1B and all control compounds exhibited insulin stimulatory effects which were significantly (P < 0.001) higher than 5.6 mM glucose alone (Fig. 6). Down-regulation of the PKA pathway with 25 µM forskolin completely inhibited the insulin stimulatory activities of [P5K]hym-1B, GLP-1 and forskolin but not CCK8. In a second set of experiment that involved down-regulation of the PKC pathway with PMA (10 nM), [P5K]hym-1B, forskolin and GLP-1 retained their full stimulatory abilities but the effect of CCK8 was abolished. Down-regulation of both the PKA and PKC pathways by forskolin + PMA resulted in the loss of stimulatory response by all agents tested (Fig. 6).
Effects of [P5K]hym-1B on insulin release from BRIN-BD11 cells following down-regulation of the PKA and PKC pathways by overnight culture 25 μM forskolin or 10 nM PMA, respectively. Values are mean ± SEM for n = 8. ***P < 0.001 compared to 5.6 mM glucose, ΔΔΔ P < 0.001 compared to respective incubation with normal culture, ϕϕ P < 0.01, ϕϕϕ P < 0.001 compared to respective incubation with forskolin culture, ++ P < 0.01, +++ P < 0.001, compared to respective incubation with PMA culture
Effects of [P5K]hym-1B on GLP- 1 and LDH release from GLUTag cells
As shown in Fig. 7, [P5K]hym-1B produced a significant (P < 0.001) increase in the rate of GLP-1 release from GLUTag cells. At 1 µM, the peptide elicited a 66 % increase in GLP-1 secretion cells over the basal rate in 2 mM glucose while the positive control (10 mM glutamine) produced a 76 % increase. The observed stimulatory effect on GLP-1 release was without cell toxicity at any concentration tested as evidenced by a lack of increase in the rate of LDH release (data not shown).
Acute effects of [P5K]hym-1B and [D9k]hym-1B on glucose tolerance in high-fat fed mice
No adverse effects were observed in the animals following administration of the peptides. Plasma glucose concentrations in obese, insulin-resistant mice receiving intraperitoneal administration of [P5K]hym-1B (75 nmol/kg body weight) were significantly less at 60 min after intraperitoneal injection of glucose compared to animals receiving vehicle only (Fig. 8a). The integrated response of plasma glucose (area under the curve) was significantly less after administration of vehicle only (Fig. 8b). Plasma insulin concentrations were significantly higher at 15 min after intraperitoneal injection of glucose in animals receiving [P5K]hym-1B (Fig. 8c) and the integrated response (total amount of insulin released over 60 min) was significantly greater (2.2-fold; P < 0.001) (Fig. 8d). Similarly, plasma glucose concentration in animals receiving [D9k]hym-1B were significantly less at 60 min (Fig. 8e) and the integrated response of plasma glucose was also significantly less after administration of vehicle only (Fig. 8f). Plasma insulin concentrations after administration of [D9k]hym-1B were not significantly different at any time point compared with vehicle only (Fig. 8g) but the integrated response was significantly greater (1.7-fold; P < 0.01) (Fig. 8h).
Acute effects of administration of [P5K]hym-1B (75 nmol/kg body weight, a–d) and [D9 k] hym-1B (75 nmol/kg body weight, e–h) on blood glucose and plasma insulin concentrations in high-fat-fed mice after intraperitoneal injection of glucose. Values are mean ± SEM (n = 8). **P < 0.01, ***P < 0.001 compared to glucose alone
Discussion
The present investigation has provided further demonstration that the frog skin host-defense peptide hym-1B shows potential for development into a therapeutically valuable agent. Previous studies have shown that the peptide, and its [E6k, D9k] analogue, display high potency against clinical isolates of multidrug-resistant Gram-positive and Gram-negative bacteria (Mechkarska et al. 2013). A role for the peptides as anti-inflammatory agents in treatment sepsis is suggested by their ability to stimulate the production of the anti-inflammatory cytokines IL-4 and IL-10 by human peripheral blood mononuclear cells without significant effect on production of the pro-inflammatory cytokines TNF-α and IL-17 (Mechkarska et al. 2013). Furthermore, hym-1B and the [D9k] analogue possess high cytotoxic potency against a range of human tumor cells but very low hemolytic activity against human erythrocytes (Attoub et al. 2013). These observations have now been extended to show that hym-1B and its lysine-containing analogues stimulate the rate of insulin release in vitro from both BRIN-BD11 rat clonal β cells or isolated mouse islets and in vivo from mice fed a high-fat diet to induce insulin resistance. Consequently, these peptides show potential for development into agents for the treatment of patients with Type 2 diabetes.
The clinical usefulness of hym-1B as an anti-diabetic agent is limited by the fact that significant cytotoxicity against BRIN-BD11 was measured at concentrations greater than 1 µM although this concentration is 1000 times greater than the minimum concentration producing a significant increase in the rate of insulin release. The three-dimensional structure of hym-1B has not been determined but a nuclear magnetic resonance investigation of a structurally similar peptide from the same family, hymenochirin-1P has demonstrated that the peptide has a random coil conformation in water but, in the membrane-mimetic solvent 50 % (v/v) trifluoroethanol–water adopts a well-defined conformation characterized by two α-helical domains from residues 6 to 17 and from 21 to 28 with the N-terminal region unfolded (Serra et al. 2014). To develop analogues of hym-1B with increased insulinotropic potency and efficacy, the Pro5, Glu6 and Asp9 amino acid residues in the first α-helical domain were substituted by one or more l-lysine or d-lysine residues. All analogues tested showed great potency than the native peptide indicating that an increase in cationicity promotes insulin-releasing activity. This conclusion is consistent with the increased insulinotropic potency observed with [L18K]pseudin-2 (Abdel-Wahab et al. 2008b), [D4K]B2RP (Abdel-Wahab et al. 2010) and [G11K]alyteserin-2a (Ojo et al. 2013a). The [P5K, E6K], [P5K, D9K], and [E6K, D9K] analogues were more cytotoxic to BRIN-BD11 cells and the [E6K], [D9K], [D6k], and [E6k, D9k] peptides were equally cytotoxic (Table 1). However, the present study has identified two analogues, [P5K]hym-1B and [D9k]hym-1B that display greater potency and efficacy (response at the maximum non-toxic concentration) than hym-1B and are not cytotoxic at concentrations up to and including 3 µM.
Insulin secretion is regulated by two major signaling pathways: the KATP channel-dependent and KATP channel-independent augmentation pathway (Henquin 2000). In the former pathway, insulin secretion is triggered by an increased [ATP]/[ADP] ratio, closure of ATP-sensitive potassium channels and opening of voltage-dependent calcium channels. The influx of extracellular calcium and elevation of cytoplasmic intracellular calcium induce exocytosis of insulin. Previous studies with the frog skin peptides alyteserin-2a (Ojo et al. 2013a), tigerinin-1R (Ojo et al. 2013b), and CPF-6 (Srinivasan et al. 2013) using BRIN-BD11 cells and with a brevinin-1 family peptide using RINm5F rat insulinoma-derived cells (Kim et al. 2010) have shown that the peptides produce cellular depolarization and increase intracellular calcium concentration consistent with their insulin-releasing activity being mediated by the KATP channel-dependent pathway. In contrast, the insulin-releasing activities of brevinin-2GUb (Conlon et al. 2008), phylloseptin-L2 (Abdel-Wahab et al. 2008a), pseudin-2 (Abdel-Wahab et al. 2008b), and several temporin peptides (Abdel-Wahab et al. 2007) do not appear to involve an increase in intracellular Ca2+ concentrations. The maintenance of the insulin-releasing activity of [P5K]hym-1B in Ca2+-free media and in the presence of potassium channel activator, diazoxide and the blocker of L-type voltage-dependent calcium channels, verapamil, suggests that the effects of the peptide are not mediated primarily by the KATP channel-dependent pathway. The augmentation by [P5K]hym-1B of the rate of insulin release produced by a depolarizing stimulus (30 mM KCl) may indicate that the peptide may be acting by an, as yet uncharacterized, KATP channel-independent pathway.
Earlier studies by Green et al. (2004a, b, c) demonstrated that incubation of BRIN-BD11 cells with GLP-1 stimulated cAMP production and it has been speculated that signaling via the PKA pathway may contribute to the modulation of KATP independent pathway of insulin secretion by GLP-1 (McClenaghan et al. 2006). [P5K]hym-1B also increases intracellular cAMP concentration with concomitant insulin release from BRIN-BD11 cells (Fig. 5) and, in line with this observation, down-regulation of PKA pathway by forskolin abolishes the insulinotropic activity of the peptide (Fig. 6) In contrast, down-regulation of the PKC pathway by PMA had no significant effect on insulin release. It is concluded, therefore, that insulinotropic activities of [P5K]hym-1B may involve the activation of PKA pathway.
The use of long-acting analogues of GLP-1 in the treatment of Type 2 diabetes is well established (Green et al. 2005). In addition to its potent insulinotropic properties, GLP-1 improves of glucose uptake in peripheral tissues, augments of insulin gene transcription and reverses the islet cell deterioration seen in type 2 diabetes (Green et al. 2004c, 2005; Meier et al. 2003; Pospisilik et al. 2003). Moreover, insulin release in response to GLP-1 is glucose dependent, a characteristic which places it at an advantage over other agents that may produce rebound hypoglycemia (Green et al. 2004c, 2005; Nattrass and Bailey 1999). Previous studies have demonstrated the ability of several amphibian host-defense peptides such as magainin-AM2, PGLa and tigerinin-1R to stimulate GLP-1 release from GLUTag cells (Ojo et al. 2013b). GLUTag cells are a useful model with which to identify candidates that stimulate GLP-1 release (Gribble et al. 2003). The ability of glutamine to stimulate GLP-1 secretion from GLUTag cells is well documented (Reimann et al. 2004; Tolhurst et al. 2011) so that it has been used it as positive control in this study. At 1 µM concentration [P5K]hym-1B produces a significant increase in the rate of GLP-1 release from GLUTag cells that is comparable to that produced by 10 mM glutamine. Consequently, in addition to its direct insulinotropic action on the β cells of the pancreas, [P5K]hym-1B may also promote the secretion of the incretin hormone GLP-1 from the L-cells of the intestine.
The high-fat-fed mouse is a useful model for understanding the effect of an energy-dense high-fat western diet on the onset of obesity and insulin resistance (Wang and Liao 2012) and the development of metabolic syndrome and Type 2 diabetes (Islam and du Loots 2009; Zhang et al. 2009). Previous studies have shown that several host-defense peptides including brevinin-2-related peptide (Abdel-Wahab et al. 2010), alyteserin-2a (Ojo et al. 2013a), magainin-AM2 (Ojo et al. 2015a) and tigerinin-1R (Ojo et al. 2015b) improve glucose tolerance in these mice. The present study has demonstrated that the potent but non-toxic analogues [P5K] and [D9k] analogues of hym-1B peptides also lowered blood glucose and enhanced insulin secretion in high-fat-fed mice. Consistent with data from in vitro studies, [P5K]hym-1B displayed a greater effect on glucose tolerance than [D9k]hym-1B. Further studies are underway to study the effects of daily administration of [P5K]hym-1B over a period of 28 days on glucose tolerance and lipid profile in high-fat-fed mice. Future studies will also investigate effects of [P5K]hym-1B administration on islet cells morphology in obese mice.
Materials and methods
Peptide synthesis and purification
Hym-1B (IKLSPETKDNLKKVLKGAIKGAIAVAKMV.NH2) and its l-lysine- and d-lysine-containing analogues were supplied in crude form by GL Biochem Ltd (Shanghai China) and were purified to near homogeneity (>98 % purity) using reversed-phase HPLC as previously described (Mechkarska et al. 2013). The identity of all peptides was confirmed by MALDI-TOF mass spectrometry using a Voyager DE PRO instrument (Applied Biosystems, Foster City, USA).
In vitro insulin release studies using BRIN-BD11 cells
BRIN-BD11 rat clonal β cells were cultured at 37 °C in an atmosphere of 5 % CO2 and 95 % air in RPMI-1640 tissue culture medium containing 10 % (v/v) fetal calf serum, antibiotics (100 U/mL penicillin, 0.1 mg/mL streptomycin) and 1.1 mM glucose as previously described (Srinivasan et al. 2014). Cells were seeded into 24-multi-well plates and allowed to attach during overnight incubation at 37 °C. After incubation, the culture medium was removed and replaced with 1 ml Krebs–Ringer bicarbonate (KRB) buffer containing 115 mM NaCl, 4.7 mM KCl, 1.28 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 20 mM HEPES, 25 mM NaHCO3 and 0.1 % bovine serum albumin (BSA), supplemented with 1.1 mM glucose (pH 7.4) and incubated for 40 min at 37 °C. After pre-incubation, incubations with purified synthetic peptides (10−12–3 × 10−6 µM; n = 6) were carried out for 20 min at 37 °C using KRB buffer supplemented with 5.6 mM glucose. After incubation, aliquots of cell supernatant were removed for insulin radioimmunoassay (Flatt and Bailey 1981).
Additional incubations (n = 6) with hym-1B (1 µM) and [P5K]hym-1B (1 µM) were carried out in the presence of known modulators of insulin release: (a) 16.7 mM glucose, (b) 5.6 mM glucose + diazoxide (300 µM), (c) 5.6 mM glucose + verapamil (50 µM), and (d) 16.7 mM glucose + 30 mM KCl. The role of extracellular calcium in stimulated insulin release from BRIN-BD11 cells was investigated by pre-incubating cells for 40 min with calcium-free KRB buffer pH 7.4 containing 1.1 mM glucose and 1 mM EGTA followed by incubation for 20 min with hym-1B (1 µM) and [P5K]hym-1B (1 µM) in calcium-free KRB buffer containing 5.6 mM glucose .
Insulin-release studies using dispersed mouse islets
Pancreatic islets were isolated from adult C57BL/6 mice as previously described (Lacy and Kostianovsky 1967; Goto et al. 1985) by digestion with collagenase P obtained from Clostridium histolyticum (Sigma-Aldrich, Dorset, UK). After 48 h of culture, islets were pre-incubated with 500 µL KRB containing 0.1 % bovine serum albumin, and 1.4 mM glucose (pH 7.4) for 1 h at 37 °C. After pre-incubation, incubations with hym-1B and [P5K]hym-1B (0.1 nM–1 µM; n = 4) were carried out for 1 h at 37 °C using KRB buffer supplemented with 16.7 mM glucose. Control incubations were carried out in the presence of GLP-1 (1 µM). Aliquots of supernatant were removed for insulin radioimmunoassay. Islets were retrieved for later determination of islet insulin content following acid–ethanol extraction as previously described (Ojo et al. 2015a, b).
Cytotoxicity assay
The effects of peptides on the rate of lactate dehydrogenase (LDH) release from BRIN-BD11 cells were measured using the cell supernatants obtained from the acute insulin-release experiments. LDH concentrations were determined using a CytoTox 96 non-radioactive cytotoxicity assay kit (Promega, Southampton, UK) according to the manufacturer’s instructions as previously described (Ojo et al. 2013a).
Effects of peptides on membrane depolarization and intracellular calcium ([Ca2+]i)
Effects of hym-1B (1 µM) and [P5K]hym-1B (1 µM) on membrane depolarization and intracellular Ca2+ concentrations were determined fluorimetrically with monolayers of BRIN-BD11 cells using membrane potential and intracellular Ca2+ assay kits Molecular Devices, Sunnyvale, CA, USA according to the manufacturer’s recommended protocols as previously described (Abdel-Wahab et al. 2008a, b). Data were acquired using a FlexStation scanning fluorimeter with integrated fluid transfer workstation (Molecular Devices). The cells were incubated at 37 °C for 10 min with peptides. Control incubations in the presence of 5.6 mM glucose only, 5.6 mM glucose + 30 mM KCl, and 5.6 mM glucose + 10 mM alanine were also carried out.
Effects of [P5K]hym-1B on cyclic AMP production
Cells were seeded at a density of 2 × 105 per well into 24 multi-well plate and allowed to attach overnight at 37 °C. Medium was discarded and cells were pre-incubated with KRB containing 1.1 mM glucose (1 ml) for 40 min at 37 °C. Pre-incubation buffer was replaced by KRB buffer supplemented with 5.6 mM glucose and 200 µM of the phophodiesterase inhibitor, 3-isobutyl-1-methylxanthine (IBMX) (1 ml), and the cells incubated for 20 min. The supernatant was removed and stored for measurement of insulin concentration by radioimmunoassay. Thereafter, 200 µl of lysis buffer (1:5 dilution) was added to each well and cells were lysed by repeated freezing and thawing cycles. Cyclic AMP concentrations in the cell lysate were measured using a R&D Systems Parameter kit (Abingdon, UK) following the manufacturer’s recommended protocol.
Effects of down-regulation of the PKA and PKC pathways on insulin release
BRIN-BD11 cells were seeded at a density of 1.5 × 105 cells per well into 24 multi-well plate and allowed to attach during an 18-h culture at 37 °C in an atmosphere of 5 % CO2 and 95 % air. Cells were cultured with forskolin (25 µM; Sigma-Aldrich, UK) in experiments involving down-regulation of the protein kinase A (PKA) pathway, with phorbol 12-myristate 13-acetate (PMA; 10 nM; Sigma-Aldrich, UK) for down-regulation of the protein kinase C (PKC) pathway, or with a combination of 25 µM forskolin + 10 nM PMA for down-regulation of both pathways. Prior to the acute tests, cells were pre-incubated in 1 ml KRB buffer (1 ml) supplemented with 1.1 mM glucose and 0.1 % bovine serum albumin (pH 7.4) for 40 min at 37 °C. Thereafter, the cells were incubated for 20 min in KRB buffer supplemented with 5.6 mM glucose (1 ml) solution containing (a) [P5K]hym-1B (1 µM), (b) GLP-1 (10 nM) and (c) CCK8 (10 nM). Control incubations with forskolin (25 µM), PMA (10 nM) and forskolin (25 µM) + PMA (10 nM) were also carried out.
Effects of [P5K]-hymenochirin-1B on GLP- 1 release from GLUTag cells
GLUTag cells (Drucker et al. 1992) were cultured and maintained at 37 °C and atmosphere of 5 % CO2 and 95 % air in Dulbecco’s Modified Eagle’s medium (DMEM) media supplemented with 5.6 mM glucose, 2 mM l-glutamine, and 10 % fetal bovine serum as previously described (Ojo et al. 2013b). Cells were allowed to attach to 24 multi-well matrigel-coated plates at a density of 1.5 × 105 cells per well during a 24-h incubation at 37 °C. Prior to the experiment, cells were pre-incubated in KRB buffer supplemented with 25 mM HEPES, 1 % BSA and 1 mM glucose (1 ml) for 45 min at 37 °C. Test solutions were made in KRB buffer supplemented with 2 mM glucose. After pre-incubation, the cells were incubated for 2 h at 37 °C in KRB buffer containing 2 mM glucose (1 ml) containing [P5K]hym-1B (10−12–10−6 M) or 10 mM glutamine as a positive control. The concentrations of GLP-1 in the supernatants were determined using a GLP-1 ELISA kit (Millipore, MA, USA) according to the manufacturer’s instructions.
In vivo insulin release studies
High-fat fed mice with clear manifestations of obesity, glucose intolerance and insulin resistance and age-matched lean mice (control) were used in the study. Adult (8-week-old), male, National Institutes of Health Swiss mice (Harlan Ltd, UK), were housed separately in an air-conditioned room (22 ± 2 °C) with a 12-h light:12-h dark cycle. Animals were maintained on a high-fat diet (45 % kcal fat, 20 % kcal protein, and 35 % kcal carbohydrate) (Dietex International Ltd, Witham, UK) or on a standard rodent pellet diet (Trouw Nutrition, Northwich, UK) for 3 months before the experiment. All animal experiments were carried out in accordance with the UK Animals (Scientific Procedures) Act 1986 and EU Directive 2010/63EU for animal experiments and approved by Ulster University Animal Ethics Review Committee. All necessary steps were taken to ameliorate any potential animal suffering.
Overnight fasted high-fat-fed mice (n = 8) were injected intraperitoneal with glucose alone (18 mmol/kg body weight) or together with [P5K]hym-1B (75 nmol/kg body weight) or [D9k]hym-1B (75 nmol/kg body weight). This peptide dose was chosen as a result of a preliminary study that determined acute effects of various concentrations of the peptides on glucose tolerance. Blood samples were collected as previously described (Srinivasan et al. 2014) before and after peptide administration at the different time points shown in Fig. 8. Blood glucose concentrations were measured using an Ascencia Contour Blood Glucose Meter (Bayer, Newbury, UK). Plasma insulin concentrations were measured by radioimmunoassay.
Statistical analysis
Data were compared using unpaired Student’s t test (non-parametric, with two-tailed P values and 95 % confidence interval) and one-way ANOVA with Bonferroni post hoc test wherever applicable. Area under the curve (AUC) analysis was carried out using the trapezoidal rule with baseline correction. Values are presented as mean ± SEM. Results are considered significant if P < 0.05.
Abbreviations
- LDH:
-
Lactate dehydrogenase
- Hym-1B:
-
Hymenochirin-1b
- [Ca2+]i:
-
Intracellular calcium concentration
- IBMX:
-
3-Isobutyl-1-methylxanthine
- PKC:
-
Protein kinase C
- PKA:
-
Protein Kinase A
- GLP-1:
-
Glucagon-like peptide 1
- TNF-α:
-
Tumor necrosis factor α
- IL-17:
-
Interleukin 17
- CPF-6:
-
Caerulein precursor fragment 6
- PMA:
-
Para-methoxyamphetamine
- MALDI-TOF:
-
Matrix-assisted laser desorption/ionization time of flight
- EGTA:
-
Ethylene glycol tetraacetic acid
- CCK8:
-
Cholecystokinin 8
- HEPES:
-
4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid
References
Abdel-Wahab YH, Marenah L, Flatt PR, Conlon JM (2007) Insulin releasing properties of the temporin family of antimicrobial peptides. Protein Pept Lett 14:702–707
Abdel-Wahab YH, Power GJ, Flatt PR, Woodhams DC, Rollins-Smith LA, Conlon JM (2008a) A peptide of the phyllos/eptin family from the skin of the frog Hylomantis lemur (Phyllomedusinae) with potent in vitro and in vivo insulin-releasing activity. Peptides 29:2136–2143
Abdel-Wahab YHA, Power GJ, Ng MT, Flatt PR, Conlon JM (2008b) Insulin-releasing properties of the frog skin peptide pseudin-2 and its [Lys (18)]-substituted analogue. Biol Chem 389:143–148
Abdel-Wahab YHA, Patterson S, Flatt PR, Conlon JM (2010) Brevinin-2-related peptide and its [D4K] analogue stimulate insulin release in vitro and improve glucose tolerance in mice fed a high fat diet. Horm Metab Res 42:652–656
Attoub S, Arafat H, Mechkarska M, Conlon JM (2013) Anti-tumour activities of the host-defense peptide hymenochirin-1B. Regul Pept 187:51–56
Avan C, Hall D, Katritzky AR (2014) Peptidomimetics via modifications of amino acids and peptide bonds. Chem Soc Rev 43:3575–3594
Conlon JM, Al-Ghaferi N, Abraham B, Leprince J (2007) Strategies for transformation of naturally-occurring amphibian antimicrobial peptides into therapeutically valuable anti-infective agents. Methods 42:349–357
Conlon JM, Power GJ, Abdel-Wahab YHA, Flatt PR, Jiansheng H, Coquet L, Leprince J, Jouenne T, Vaudry H (2008) A potent, non-toxic insulin-releasing peptide isolated from an extract of the skin of the Asian frog, Hylarana guntheri (Anura:Ranidae). Regul Pept 151:153–159
Conlon JM, Mechkarska M, Lukic ML, Flatt PR (2014) Potential therapeutic applications of multifunctional host-defense peptides from frog skin as anti-cancer, anti-viral, immunomodulatory, and anti-diabetic agents. Peptides 57:67–77
Drucker DJ, Lee YC, Asa SL, Brubaker PL (1992) Inhibition of pancreatic glucagon gene expression in mice bearing a subcutaneous glucagon-producing GLUTag transplantable tumor. Mol Endocrinol 6:2175–2184
Flatt PR, Bailey CJ (1981) Abnormal plasma glucose and insulin responses in heterozygous lean (ob/+) mice. Diabetologia 20:573–574
Frost DR (2015) Amphibian species of the world: an online reference. Version 6.0. Electronic database accessible at http://research.amnh.org/herpetology/amphibia/index.php. American Museum of Natural History, New York, USA
Goto M, Maki T, Kiyoizumi T, Satomi S, Monaco AP (1985) An improved method for isolation of mouse pancreatic islets. Transplantation 40:437–438
Green BD, Gault VA, Flatt PR, Harriott P, Greer B, O’Harte FPM (2004a) Comparative effects of GLP-1 and GIP on cAMP production, insulin secretion, and in vivo antidiabetic actions following substitution of Ala8/Ala2 with 2-aminobutyric acid. Arch Biochem Biophys 428:136–143
Green BD, Mooney MH, Gault VA, Irwin N, Bailey CJ, Harriott P, Greer B, O’Harte FPM, Flatt PR (2004b) N-terminal His7-modification of glucagon-like peptide-1(7–36) amide generates dipeptidyl peptidase IV-stable analogues with potent antihyperglycaemic activity. J Endocrinol 180:379–388
Green BD, Mooney MH, Gault VA, Irwin N, Bailey CJ, Harriott P, Greer B, Flatt PR, O’Harte FPM (2004c) Lys9 for Glu9 substitution in glucagon-like peptide-1(7–36)amide confers dipeptidylpeptidase IV resistance with cellular and metabolic actions similar to those of established antagonists glucagon-like peptide-1(9–36) amide and exendin (9–39). Metabolism 53:252–259
Green BD, Gault VA, O’Harte FPM, Flatt PR (2005) A comparison of the cellular and biological properties of DPP-IV resistant N-glucitol analogues of glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide. Diabetes Obes Metab 7:595–604
Gribble FM, Williams L, Simpson AK, Reimann F (2003) A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes 52:1147–1154
Henquin JC (2000) Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 49:1751–1760
Islam MS, du Loots T (2009) Experimental rodent models of type 2 diabetes: a review. Methods Find Exp Clin Pharmacol 31:249–261
Kim JH, Lee JO, Jung JH, Lee SK, You GY, Park SH, Kim HS (2010) Gaegurin-6 stimulates insulin secretion through calcium influx in pancreatic beta Rin5mf cells. Regul Pept 159:123–128
Lacy PE, Kostianovsky M (1967) Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes 16:35–39
Lewis RJ, Garcia ML (2003) Therapeutic potential of venom peptides. Nat Rev 2:790–802
Mandal SM, Roy A, Ghosh AK, Hazra TK, Basak A, Franco OL (2014) Challenges and future prospects of antibiotic therapy: from peptides to phages utilization. Front Pharmacol 5:105
McClenaghan NH, Flatt PR, Ball AJ (2006) Actions of glucagon-like peptide-1 on KATP channel-dependent and -independent effects of glucose, sulphonylureas and nateglinide. J Endocrinol 190:889–896
Mechkarska M, Prajeep M, Coquet L, Leprince J, Jouenne T, Vaudry H, King JD, Conlon JM (2012) The hymenochirins: a family of host-defense peptides from the Congo dwarf clawed frog Hymenochirus boettgeri (Pipidae). Peptides 35:269–275
Mechkarska M, Prajeep M, Radosavljevic GD, Jovanovic IP, Al Baloushi A, Sonnevend A, Lukic ML, Conlon JM (2013) An analog of the host-defense peptide hymenochirin-1B with potent broad-spectrum activity against multidrug-resistant bacteria and immunomodulatory properties. Peptides 50:153–159
Meier JJ, Gallwitz B, Nauck MA (2003) Glucagon-like peptide-1 and gastric inhibitory polypeptide: potential applications in type 2 diabetes mellitus. Biodrugs 2:93–102
Nattrass M, Bailey CJ (1999) New agents for type 2 diabetes. Best Pract Res Clin Endocrinol Metab 13:309–329
Ojo OO, Abdel-Wahab YHA, Flatt PR, Conlon JM (2013a) Insulinotropic actions of the frog skin host-defense peptide alyteserin-2a: a structure-activity study. Chem Biol Drug Des 82:196–204
Ojo OO, Conlon JM, Flatt PR, Abdel-Wahab YH (2013b) Frog skin peptides (tigerinin-1R, magainin-AM1, -AM2, CPF-AM1, and PGla-AM1) stimulate secretion of glucagon-like peptide 1 (GLP-1) by GLUTag cells. Biochem Biophys Res Commun 431:14–18
Ojo OO, Srinivasan DK, Owolabi BO, Conlon JM, Flatt PR, Abdel-Wahab YHA (2015a) Magainin-AM2 improves glucose homeostasis and beta cell function in high-fat fed mice. Biochim Biophys Acta 1850:80–87
Ojo OO, Srinivasan DK, Owolabi BO, Flatt PR, Abdel-Wahab YH (2015b) Beneficial effects of tigerinin-1R on glucose homeostasis and beta cell function in mice with diet-induced obesity-diabetes. Biochimie 109:18–26
Peters A (2010) Incretin-based therapies: review of current clinical trial data. Amer J Med 123:S28–S37
Pospisilik JA, Martin J, Doty T, Ehses JA, Pamir N, Lynn FC, Teau S, Demuth H-U, McIntosh CHS, Pederson RA (2003) Dipeptidyl peptidase IV inhibitor treatment stimulates cell survival and islet neogenesis in streptozotocin-induced diabetic rats. Diabetes 52:741–750
Reimann F, Williams L, da Silva Xavier G, Rutter GA, Gribble FM (2004) Glutamine potently stimulates glucagon-like peptide-1 secretion from GLUTag cells. Diabetologia 47:1592–1601
Serra I, Scorciapino MA, Manzo G, Casu M, Rinaldi AC, Attoub S, Mechkarska M, Conlon JM (2014) Conformational analysis and cytotoxic activities of the frog skin host-defense peptide, hymenochirin-1Pa. Peptides 61:114–121
Srinivasan D, Mechkarska M, Abdel-Wahab YHA, Flatt PR, Conlon JM (2013) Caerulein precursor fragment (CPF) peptides from the skin secretions of Xenopus laevis and Silurana epitropicalis are potent insulin-releasing agents. Biochimie 95:429–435
Srinivasan D, Ojo OO, Abdel-Wahab YHA, Flatt PR, Guilhaudis L, Conlon JM (2014) Insulin-releasing and cytotoxic properties of the frog skin peptide, tigerinin-1R: a structure-activity study. Peptides 55:23–31
Tokuda M, Katsuno T, Ochi F, Miyakoshi K, Kusunoki Y, Murai K, Miuchi M, Hamaguchi T, Miyagawa J, Namba M (2014) Effects of exenatide on metabolic parameters/control in obese Japanese patients with type 2 diabetes. Endocr J 61:365–372
Tolhurst G, Zheng Y, Gribble FM (2011) Glutamine triggers and potentiates glucagon-like peptide-1 secretion by raising cytosolic Ca2+ and cAMP. Endocrinology 152:405–413
Wang CY, Liao JK (2012) A mouse model of diet-induced obesity and insulin resistance. Methods Mol Biol 821:421–433
Zhang HJ, Zhou F, Ji BP, Li B, Luo YC, Yu HQ, Zhang GZ (2009) Effects of fructose and/or fat in the diet on developing the type 2 diabetic-like syndrome in CD-1 mice. Horm Metab Res 41:40–45
Acknowledgments
This study was supported by the University of Ulster Research Strategy Funding and an award of a University Vice Chancellor Research Studentship to DKS. We thank Professor D. Drucker for access to GLUTag cells.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest declared.
Additional information
Handling Editor: M. S. Palma.
Rights and permissions
About this article
Cite this article
Owolabi, B.O., Ojo, O.O., Srinivasan, D.K. et al. In vitro and in vivo insulinotropic properties of the multifunctional frog skin peptide hymenochirin-1B: a structure–activity study. Amino Acids 48, 535–547 (2016). https://doi.org/10.1007/s00726-015-2107-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-015-2107-x