Abstract
Diatoms are microalgae that thrive in a range of habitats worldwide including polar areas. Remarkably, non-marine pennate diatoms do not create any morphologically distinct dormant stages that could help them to successfully face unfavourable conditions. Their survival is probably connected with the adaptation of vegetative cells to freezing and desiccation. Here we assessed the freezing tolerance of vegetative cells and vegetative-looking resting cells of 12 freshwater strains of benthic pennate diatoms isolated from polar habitats. To test the effect of various environmental factors, the strains were exposed to −20 °C freezing in four differently treated cultures: (1) vegetative cells growing in standard conditions in standard WC medium and (2) resting cells induced by cold and dark acclimation and resting cells, where (3) phosphorus or (4) nitrogen deficiency were used in addition to cold and dark acclimation. Tolerance was evaluated by measurement of basal cell fluorescence of chlorophyll and determination of physiological cell status using a multiparameter fluorescent staining. Four strains out of 12 were able to tolerate freezing in at least some of the treatments. The minority of cells appeared to be active immediately after thawing process, while most cells were inactive, injured or dead. Overall, the results showed a high sensitivity of vegetative and resting cells to freezing stress among strains originating from polar areas. However, the importance of resting cells for survival was emphasized by a slight but statistically significant increase of freezing tolerance of nutrient-depleted cells. Low numbers of surviving cells in our experimental setup could indicate their importance for the overwintering of diatom populations in harsh polar conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diatoms are microalgae inhabiting a variety of aquatic and terrestrial habitats worldwide, including permanent and temporary freshwater bodies (Round et al. 1990; Mann 1999; Vanormelingen et al. 2008). They also thrive in many polar habitats in both the Arctic (Moore 1974; Antoniades and Douglas 2002; Kim et al. 2008; Pla-Rabés et al. 2016) and Antarctica (Jones 1996; Van de Vijver and Beyens 1999; Cremer et al. 2004; Kopalová et al. 2013) where they have become a dominant part of the algal flora.
The capability to survive seasonal extremes in the polar regions is one of the selection pressures on microorganisms (Vincent 2000). Crucial factors influencing the life of polar microbial communities are freezing and desiccation, which are naturally connected with liquid water availability (Davey 1989; Elster and Benson 2004). These stresses are most significant in unstable terrestrial and hydro-terrestrial habitats. Hydro-terrestrial habitats (e.g. wetlands, shallow lakes and pools, glacial and snow-fed streams) freeze solid in winter, but liquid water is available there during the vegetative season in contrast to terrestrial environments (Elster 2002). However, during the short period of liquid water availability (3 to 5 months), the temperatures are low and fluctuate even in the summer season but rarely fall deeper than −5 °C. During the polar winter, temperatures drop below 0 °C, the water level decreases, and no liquid water is available until spring (Davey et al. 1992; Láska et al. 2012). Moreover winter freezing may be prolonged due to thick snow and ice cover. Water flow maxima are associated with spring snow-melting, and the concentrations of dissolved nutrients are low with the exception of habitats enriched by animal activity (Hawes 1989; Davey et al. 1992; Sheath et al. 1996). In autumn and spring, temperatures are more stable (around 0 °C) but numerous freeze-thaw cycles occur (Davey et al. 1992; Elster and Benson 2004; Láska et al. 2012). It was suggested that spring freeze-thaw cycles could act as a bottleneck for algal populations of Arctic Zygnema (Pichrtová et al. 2014a).
Apart from freezing and desiccation, nutrient starvation represents another stress common in polar habitats (Vincent et al. 2008). Nitrogen and/or phosphorus limitation of polar algae from terrestrial habitats (Davey and Rothery 1992; Arnold et al. 2003) and lakes (Levine and Whalen 2001; Brutemark et al. 2006; Nedbalová et al. 2013; Hogan et al. 2014) are frequently reported. Nutrient starvation is known to be related to cell hardening and increased stress resistance of algae and cyanobacteria (Billi and Caiola 1996; Pichrtová et al. 2014b; Tashyreva and Elster 2015). Diatom dormant stages are often formed under nutrient depletion (Kuwata et al. 1993; McQuoid and Hobson 1996; Kuwata and Takahashi 1999). Nutrient limitation also results in lipid accumulation in microalgae, which is a general response to various stresses (Mock and Kroon 2002a; Fields et al. 2014; Mekhalfi et al. 2014; Arc et al. 2020).
Some laboratory experiments and field studies of polar microalgae have shown an enhanced tolerance to habitat-related stresses such as desiccation (Hawes and Davey 1989; Šabacká and Elster 2006), freezing (Davey 1989; Hawes 1990; Šabacká and Elster 2006; Pichrtová et al. 2016; Trumhová et al. 2019) and osmotic stress (Pichrtová et al. 2014a). However, differences in the survival strategies between species from different habitats were observed (Davey 1991; Šabacká and Elster 2006). Freezing and desiccation tolerance has been also reported in temperate and some polar diatoms; nevertheless, they were shown to be rather sensitive (Souffreau et al. 2010, 2013a; Hejduková et al. 2019, 2020).
Microalgae including diatoms have evolved many diverse mechanisms to protect themselves and prevent freezing injuries (Morgan-Kiss et al. 2006), for instance, the excretion of a variety macromolecular substances that trigger several responses on the intracellular and extracellular levels. Antifreeze proteins (Gwak et al. 2010; Bayer-Giraldi et al. 2011), ice-binding proteins (Janech et al. 2006) and other ice-active substances (Raymond 2000; Raymond and Knight 2003) work through regulation of ice crystal formation such as inhibition of ice recrystallization, modification of single ice crystals morphology and/or decrease of a freezing point (Raymond and Knight 2003; Janech et al. 2006; Gwak et al. 2010; Bayer-Giraldi et al. 2011). Accumulated sugars and other extracellular polymeric substances (EPS) are responsible for the maintenance of liquid environment within habitable pore spaces as sea ice freezes (Krembs et al. 2002) and stabilize phospholipid bilayers and proteins (Crowe et al. 1987; Welsh 2000). The fluidity of membranes is closely connected with optimal metabolic functions at low temperatures, e.g. for ion and gases permeability and electron transport chain during photosynthetic activity. Significant changes in the lipid composition to regulate membrane fluidity are found in direct relation to changes in environmental conditions and consequently physiological status (Morgan-Kiss et al. 2006). The percentage of polyunsaturated fatty acids (PUFA) generally increases with decreasing temperature in many polar diatoms and other microalgae (Nichols et al. 1993; Teoh et al. 2004, 2013). Production rates of several of these substances (e.g. EPS, PUFA) are increased also under other stresses as nutrient limitation and even during extended periods of dark (Smith and Underwood 1998, 2000; Mock et al. 2017).
A widespread strategy to survive adverse environmental conditions reported from different groups of microorganisms is the formation of dormant cells (spores, cysts). Viable stress-resistant stages of diatoms and other algae were reported to accumulate in water columns and/or in sediments where they could persist for a long time and serve as ‘seed banks’ providing an inoculum for a potential recolonization (Lund 1954; Sicko-Goad et al. 1989; Hawes 1990; Poulíčková et al. 2008; Ellegaard and Ribeiro 2018). For instance, snow algae (Chlamydomonadales) are known to form specialized thickened wall spores to survive stressful winter and dry summer seasons (Remias 2012). Cyanobacterial akinetes appeared to be important not only for overwintering but also to ensure long-term survival (Livingstone and Jaworski 1980). Diatom resting spores are well described as a common survival strategy in marine centric species (McQuoid and Hobson 1995). They are formed under nutrient depletion to survive cold winter conditions (Kuwata et al. 1993; McQuoid and Hobson 1996; Kuwata and Takahashi 1999). In contrast, formation of true resting stages in freshwater diatoms is rarely observed. To our knowledge, there are several examples in centric (Edlund et al. 1996) and pennate diatoms (Schmid 1979; von Stosch and Fecher 1979), which are associated with the formation of internal valves under unfavourable conditions. It is assumed that resting spores of freshwater diatoms have been replaced by production of physiological resting cells (Sicko-Goad et al. 1989; McQuoid and Hobson 1996), which are morphologically identical to vegetative cells (Souffreau et al. 2013a) with dense cytoplasmic matter in the centre of the cell (Sicko-Goad et al. 1989) and rounder plastids (Round et al. 1990). Physiological resting cells were observed in pennate diatoms from marine (Morin et al. 2019), freshwater and terrestrial habitats (Souffreau et al. 2013a), and their importance for stress survival has been suggested (Evans 1959; Souffreau et al. 2013a; Morin et al. 2019; Hejduková et al. 2020). However, the role of various environmental factors triggering their formation has not yet been studied in detail.
In this study, a laboratory experiment was conducted which focused on pennate diatoms from freshwater polar habitats. The aim was to test the freezing survival of cells cultivated under various conditions (temperature, light and nutrient supply) simulating natural environmental changes. Nutrient starvation, cold and dark acclimation are hypothesized to increase the survivability. Four differently treated cultures of each strain were tested: (1) vegetative cells grown in standard conditions and physiological resting cells induced by dark and cold, concurrently cultivated using a (2) standard, (3) phosphorus- or (4) nitrogen-depleted medium. The experiments were evaluated using basal chlorophyll fluorescence measurements and multiparameter fluorescent staining, which enabled a detailed insight into the status of the individual cells.
Materials and methods
Experimental strains
Experimental strains were isolated from natural samples of freshwater habitats (lakes, streams, seepages) collected in 2013–2017 in the High Arctic (Spitsbergen) and maritime Antarctic (James Ross Island, Vega Island) (Fig. 1, Table 1). Samples were collected by epilithon scraping from five to ten stones from the littoral zone of the water bodies. Between each location, sampling equipment was cleaned using ethanol to avoid cross-contamination. Material was transferred into plastic tubes and stored in cool (< 10 °C) and dark conditions during transport (max. 1 month). Small quantities of material were incubated in Wright’s cryptophyte (WC) medium (Guillard and Lorenzen 1972) at 15 °C in Q-Cell 60 incubator (Pol-Lab, Wilkowice, Poland), using a fluorescent tube Lumilux G5 8W 430 lm, 4000K (Osram, Munich, Germany) as a light source with photon fluence rate of about 10–30 μmol·m−2·s−1 and light:dark period 12:12h. Monoclonal cultures were established by isolating single diatom cells from the natural samples using a glass micropipette under an inverted Nikon Diaphot 200 microscope (Nikon Corporation, Tokyo, Japan) or Olympus IX51 microscope (Olympus Corporation, Tokyo, Japan). In total, 12 strains (species) belonging to 11 genera were used for the experiments (Figs. 2, S1).
Scanning electron microscopy pictures of the experimental strains: a Achnanthidium lineare, b Caloneis falcifera, c Chamaepinnularia krookiformis, d Encyonopsis descripta, e Gomphonema sp., f Hantzschia abundans, g Hantzschia amphioxys, h Luticola muticopsis, i Mayamaea atomus, j Meridion circulare, k Nitzschia palea, l Pinnularia catenaborealis, scale bar a, c, e, h, i, j = 1 μm, b, d, f, g, k, l = 10 μm
Morphological analysis for taxonomic identification
Diatom cultures were oxidized by adding 69% nitric acid and incubated for 1 week. Samples were subsequently rinsed several times with distilled water alternated with centrifugation (10 min at 2900×g). For light microscopy observation, drops of oxidized material were embedded in Naphrax (Brunel Microscopes Ltd., Chippenham, UK) and observed under an Olympus BX43 light microscope (Olympus Corporation, Tokyo, Japan) equipped with differential interference contrast (Nomarski) optics under oil immersion at 1000× magnification (Fig. S1). Microscopic slides are stored at the Department of Ecology, Charles University, Prague, Czech Republic and available upon request.
For scanning electron microscopy, suspensions of oxidized material were filtered through a Porafil PC 0.4-μm polycarbonate membrane filter (Macherey-Nagel, Dueren, Germany) and fixed on aluminium stubs. The stubs were sputter-coated with a gold layer of approximately 5 nm in a Bal-Tec SCD 050 device (Bal-Tec, Balzers, Liechtenstein) and studied in a FEI Helios NanoLab 660 G3 UC scanning electron microscope (Thermo Scientific, Wilmington, DE, USA) (Fig. 2).
Experimental setup
Diatom strains were exposed to −20 °C freezing as four differently treated cultures: vegetative cells and resting cells induced by three modifications of the cultivation conditions (Table 2). Prior to the experiments, cultures were grown in 12-well plates in WC medium at standard culture conditions of 15 °C, 12:12h light:dark period and photon fluence rate 20–40 μmol·m−2·s−1 depending on the plate position on the experimental shelf. Culture densities were estimated using their basal fluorescence of chlorophyll (F) by a FluorCam 800MF device (Photon Systems Instruments, Brno, Czech Republic) using a default setting, electronic camera shutter = 3 (100 μs), camera sensitivity (gain) = 10, F duration = 2 s and F period = 200 ms, following 15 min of dark adaptation (Consalvey et al. 2005). Fluorescence was measured on eight consecutive days, which allowed for assessing the value at which the cultures became stationary. A 4-day growth period ensured that all cultures were still in the early to mid-exponential phase. Upon recalculation, a relative F value of 0.03 was chosen for reinoculation of all cultures.
To standardize the physiological conditions, exponentially growing cultures for the vegetative treatment (WC-veg) were reinoculated twice at F = 0.03, each time followed by 4 days of exponential growth as per the diagram of the sampling design (Fig. 3), in standard WC medium. Three wells per strain were used as replicates. Prior to each reinoculation, the medium of each well was replaced. For resting cell induction, exponentially growing cultures were inoculated the same way as vegetative using the following media during the second reinoculation: standard WC medium (WC-rest), standard WC medium was substituted with either phosphorus-free (WC-P) medium or nitrogen-depleted WC (WC-N) medium (McQuoid and Hobson 1995). Strains were then kept under standard culture conditions for 7 days to allow the cells to become stationary and exhaust all available phosphorus or nitrogen, after which all these resting cell induction treatments (WC-rest, WC-P, WC-N) were placed for 14 days in the dark at 4 °C to induce dormancy (McQuoid and Hobson 1996). Typical resting cell characteristics were observed: condensed organelles, larger vesicles of storage products, granular cytoplasm, enlarged vacuoles or oil droplets and contracted chloroplasts (McQuoid and Hobson 1996) (Fig. 4). Approximate cell numbers were counted in at least ten randomly chosen microscopic squares and 200 cells per well using an inverted light microscope and ranged from 1000 to 400,000 (median 8887; mean 14,532). The cell densities varied widely between species due to different cell sizes. Both the vegetative and resting cells of the examined strains were frozen the same day. Not all strains could be tested at the same moment; thus, the experiments were conducted as two sets.
Four physiological resting cells of P. catenaborealis with visible condensed organelles, granular cytoplasm, enlarged oil droplets and contracted chloroplasts (McQuoid and Hobson 1996), scale bar = 10 μm
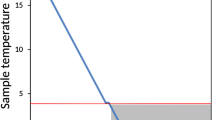
Diatom cultures were exposed to freezing at −20 °C in an ordinary freezer. To slow the temperature change rate (to simulate natural processes), six layers of bubble foil were used as insulation of the experimental plates. Two Minikin Tie dataloggers (EMS Brno, Brno, Czech Republic) were enclosed to monitor the temperature. The freezing procedure took 30 h after which the plates were kept at the same condition for 8/81 days. Thawing was carried out in the dark at 5 °C for 32 h. The rate of temperature changes was less than 1 °C per hour. After thawing, the plates were placed in standard culture condition and covered with paper for 1 h to avoid light stress. Additionally, control plates were inoculated the same way described above in standard WC medium and kept in standard culture conditions to monitor the viability of all the strains without freezing event.
Viability evaluation
The cultures were observed using an inverted light microscope the first, seventh and fourteenth day after the freezing procedure to distinguish dead cells from living ones by their empty silica frustules or shrivelled and colourless cell content. Basal fluorescence of chlorophyll (F) was assessed using default settings (see above) and was measured during ten subsequent days, starting the day of thawing (day 1). The ratio of fluorescence values measured 9 days after thawing to fluorescence values measured immediately after thawing (Fday 9/Fday 1) is referred to as relative increase. The fluorescence value for day 9 was chosen because most cultures were still showing an exponential growth. A positive increase demonstrates recovery of growth after the freezing treatment. A relative increase observed in replicates that did not survive the treatment, but that still gave a fluorescence signal at day 9 due to the presence of residual chlorophyll, was adjusted to zero.
Furthermore, cell viability was evaluated immediately after the thawing process using a multiparameter fluorescent staining protocol originally developed for filamentous cyanobacteria (Tashyreva et al. 2013). Three fluorescent dyes were combined: SYTOX Green nucleic acid stain (S7020, Molecular Probes, Eugene, OR, USA), CTC (5-cyano-2,3-ditolyl tetrazolium chloride, Polysciences Europe GmbH, Eppelheim, Germany) and DAPI (4′,6-diamidino-2-phenylindole, Molecular Probes, Eugene, OR, USA). SYTOX Green is a green-fluorescent nuclear and chromosome counterstain detecting membrane disintegration and serving as an indicator of dead cells, because it does not penetrate living cells. CTC shows respiration activity by accumulation of formazan crystals in the mitochondria of active cells, which appears as a red fluorescence. DAPI stains dsDNA in both living and dead cells and is detected by a blue fluorescence of nuclei. The method was combined with light microscopy and enabled evaluation of the exact physiological state of single cells. The suspension of diatoms was scraped from the bottom of each well into 1.5-ml Eppendorf tubes using a pipette. The material was homogenized and stained by the three fluorescent stains consecutively. SYTOX Green dye was used first, at the final concentration of 1 μM. Stained samples were incubated for 15 min in dark and cold conditions (8 °C) and washed three times afterwards using WC medium. To estimate the respiratory activity, the samples were stained with 5-mM CTC and kept in cold and protected from light by a layer of aluminium foil for 120 min. The dye solutions were removed afterwards, the samples were rinsed once and stained with DAPI at a concentration of 5 μg/ml and incubated for 30 min under dark and cool conditions. The samples were then washed three times, after which the suspension was transferred onto glass microscope slides and studied using an Olympus BX53 fluorescent microscope equipped with a 100-W ultrahigh-pressure mercury arc lamp (Olympus Corporation, Tokyo, Japan) at 200× magnification (Tashyreva et al. 2013). To assess the proportion of the physiological states of the cells, if possible, at least 100 cells per sample were counted on a minimum of 10 random transects. In total, 17,829 cells were studied. According to the staining results, five cell categories were distinguished: active healthy cells, inactive but intact dormant cells (presumably resting cells), injured but active cells, injured and inactive cells and dead cells performing no fluorescence (Table 3). Cells with no fluorescence, but with the presence of visible protoplast remains, were determined to be dead (Fig. 5). The staining process was performed twice: before the freezing event and immediately after thawing.
Data analyses
Fluorescence data were analysed in GraphPad Prism 5.03 (GraphPad Software, La Jolla, CA, USA) using two-way ANOVA with a Bonferroni post hoc test to demonstrate the influence of treatment, strain and their interaction on diatom freezing survival. The analyses were run on data of strains that survived the experiments while excluding the dead strains.
A principal component analysis (PCA) was run to summarize the variability within the multiparameter staining dataset (differences in proportions of cell types before and after each treatment). The effect of freezing event, treatment and strain were tested separately after removing the effects of other factors using partial redundancy analyses (RDA) with permutation tests to show the statistical significance. Prior to running the PCA and RDA, the data were standardized across species (mean variance standardization). The ordination analyses were performed in Canoco 5 (Microcomputer Power, Ithaca, NY, USA).
For graphical visualization of the results, ArcGIS (Esri, Redlands, CA, USA), Zoner Photo Studio 16 (Zoner Software, Brno, Czech Republic), InkScape 0.92.3 (Software Freedom Conservancy, New York, NY, USA) and GraphPad Prism 5.03 (GraphPad Software, La Jolla, CA, USA) were used.
Results
According to light microscopy observation and chlorophyll fluorescence measurements, only all the three replicates for the strains K1-1 (Luticola muticopsis) and EVA-P3 (Pinnularia catenaborealis) survived −20 °C freezing as in all the four treatments used (WC-veg, WC-rest, WC-P and WC-N). Strain H5 (Hantzschia amphioxys) survived treatment WC-rest (all the three replicates) and treatment WC-N (one replicate only). Only one replicate of strain PI9 (Caloneis falcifera) survived the WC-rest treatment. The other eight strains did not survive any of the treatments; nevertheless, all the strains survived the additional control reinoculation of the strains without freezing event (data not shown).
Growth curves for each replicate and treatment based on chlorophyll fluorescence data measured every day during 9 days after the thawing process are shown in Fig. 6. From the initial values (day 1), it is obvious that cultivation conditions for resting cell induction influenced the growth of cultures before the freezing event. The WC-N treatment showed low F values compared to WC-veg, which was cultivated in standard condition without any additional modification. Standard medium and phosphorus-free medium allowed cultures to reach higher initial fluorescence values during the period of resting cell induction. The highest values were measured the last day with maxima reached in the WC-rest and WC-P treatments.
Growth curves for each strain (mean and SEM) and treatment based on measurements of basal chlorophyll fluorescence (F, in absolute units) conducted during 9 days after thawing. Vegetative cells growing in standard WC medium under standard culture conditions (WC-veg), resting cells induced by cold and dark acclimation (WC-rest), concurrent phosphorus (WC-P) or nitrogen deficiency (WC-N). For strain codes, see Table 1
A relative increase in the data calculated from the F values of the surviving strains per replica was the highest in the WC-N treatment (Fig. 7). Values in the other treatments were relatively comparable. Average relative fluorescence increase for all strains is shown in Fig. S2. Statistical analyses of all the surviving strains showed the significant influence of strain, treatment and their interaction (two-way ANOVA, P values < 0.0001, Table 4). If the strains in which all the three replicates survived in all the four treatments were tested, only the influence of treatment was significant (two-way ANOVA, P = 0.0042).
Box plots of relative fluorescence increase for surviving strains 9 days after freezing for each treatment. Replicate values for each strain indicated by shapes: box, first and third quartiles; whiskers, min-max. Vegetative cells growing in standard WC medium under standard culture conditions (WC-veg), resting cells induced by cold and dark acclimation (WC-rest), concurrent phosphorus (WC-P) or nitrogen deficiency (WC-N). For strain codes, see Table 1
Average proportions of the physiological cell statuses of the strains before and after the freezing event for each treatment are shown in Fig. 8. Before the freezing process, the highest number of active cells was observed in cultures from the WC-veg treatment, which were growing in standard conditions. The other treatments showed much lower numbers of active cells, but the proportions of inactive or dead cells were higher which corresponds to the fluorescence data. After thawing, dead and injured inactive cells prevailed in all the treatments in all the strains with the exception of K1-1 from the WC-P treatment and EVA-P3 from WC-N. Surprisingly, there were still dormant inactive and active cells present immediately after thawing, even in the cultures that did not survive according to the fluorescence data. The exception was the EU12 strain, which showed dead cells in all the treatments excluding WC-veg. The effects of freezing, treatment and strain were significant explaining 18.8%, 13.6% and 43.6%, respectively (partial RDAs, test on all axes in all cases P = 0.002).
Average proportion of physiological statuses of the cells per strain before and after each treatment. Vegetative cells growing in standard WC medium under standard culture conditions (WC-veg), resting cells induced by cold and dark acclimation (WC-rest), concurrent phosphorus (WC-P) or nitrogen deficiency (WC-N). For strain codes, see Table 1
Differences in physiological cell status proportions per strains before and after freezing showed that the number of dead cells increased in all the treatments at the expense of active, injured active and/or dormant inactive cells (Figs. 9, 10). The WC-rest and WC-veg treatments were relatively comparable, except that the difference in the number of injured inactive cells slightly increased in WC-veg in comparison to that in WC-rest. The WC-N treatment showed a similar trend, but the difference in injured inactive cells varied a lot depending on strain. The number of injured inactive cells decreased in the WC-P treatment, but the numbers of dormant inactive cells changed almost equally in all strains. According to the statistical analyses, both the effect of treatment (partial RDA, test on all axes P = 0.01) and strain (partial RDA, test on all axes P = 0.002) were significant explaining 9.3% and 26.8%, respectively.
Differences in the proportions (in %) of physiological cell statuses per strains before and after freezing. Average values for each strain indicated by shapes: box, first and third quartiles; whiskers, min-max. Vegetative cells growing in standard WC medium under standard culture conditions (WC-veg), resting cells induced by cold and dark acclimation (WC-rest), concurrent phosphorus (WC-P) or nitrogen deficiency (WC-N). For strain codes, see Table 1
Principal component analysis (PCA) showing the correlation between treatments and the differences in the proportions of the physiological status of cells. The samples are represented by individual strain/treatment. Vegetative cells growing in standard WC medium under standard culture conditions (WC-veg), resting cells induced by cold and dark acclimation (WC-rest), concurrent phosphorus (WC-P) or nitrogen deficiency (WC-N). For strain codes, see Table 1
Discussion
In this study, the impact of four different culture conditions on the freezing tolerance of 12 polar strains of freshwater diatoms was compared in order to disentangle the possible stress factors enhancing survival of vegetative cells. Various environmental aspects have been proved to have a ‘hardening’ impact on stress survival of plants and some algae (e.g. Meryman 1974; Bertrand and Castonguay 2003; Pichrtová et al. 2014a, 2014b). Surprisingly only two strains (EVA-P3, P. catenaborealis and K1-1, L. muticopsis) as all the three replicates survived the −20 °C freezing experiment used in all the four treatments according to long-term light microscopy observation and fluorescence measurements, while the two strains (H5, H. amphioxys and PI9, C. falcifera) only survived some of the treatments as some replicates. Environmental stresses as desiccation, heating and freezing were earlier shown to be harmful for diatoms from temperate (Hostetter and Hoshaw 1970; Souffreau et al. 2010, 2013a) and polar habitats (Hejduková et al. 2019). In this study two more strains exhibited certain freezing tolerance (with only a few surviving replicates) giving a slightly better ratio for polar strain (species) survival in comparison to only three survivors out of 34 examined species originating from temperate habitats (Souffreau et al. 2010). A survival ratio of polar strains in a previous experiment characterized by gradual freezing and fast thawing was 12 out of 14, while it was 2 out of 10 in abrupt freezing and slow thawing settings (Hejduková et al. 2019). It should be noted that in the current experiments, the freezing and thawing procedure was rather slow (approximately < 1 °C·min−1) to simulate a natural process. These results thus support previous findings about the influence of temperature change rate on the survival of microalgae (Cañavate and Lubian 1995, 1997; Hejduková et al. 2019) when rapid changes increase intracellular ice crystals formation and/or recrystallization processes with mechanical damage as a consequence (Mazur 1963, 1984).
A diatom species complex that has recently captured a lot of attention is Pinnularia borealis Ehrenberg, which appeared to be able to survive freezing (Davey 1991; Souffreau et al. 2010, 2013a) even in liquid nitrogen (Stock et al. 2018; Hejduková et al. 2019), suggesting it is adapted to living in extreme environments. Strain P3 of P. catenaborealis is closely related to lineages within the P. borealis species complex (Pinseel et al. 2017a, 2017b). Its polar lineages from Antarctica have lower optimal growth and lethal temperatures in comparison with temperate ones (Souffreau et al. 2013b), which is believed to be an adaptation to life in extreme environments. Similar preferences were described for H. amphioxys (Souffreau et al. 2013b) represented by the strain H5 in our experiments, which survived the WC-N and WC-rest treatments as a few replicates.
Role of habitat
The freezing tolerance could also be connected to habitat preferences and geographical position of original localities. It is interesting to note that the strains surviving at least some treatments at the minimum of two replicates (EVA-P3, K1-1, H5) originate from Antarctica, which offers an explanation in context of its biogeographical history and limited dispersal capacity, which is supported by large number of endemic diatom species (Vyverman et al. 2010; Verleyen et al. 2021). In contrast Arctic and temperate strains of P. borealis are able to survive similar freezing as well (Souffreau et al. 2010, 2013a; Hejduková et al. 2019). Along the soil-aquatic gradient, some diatoms (including the three surviving strains from the genera of Hantzschia, Luticola and Pinnularia) are largely classified as ‘terrestrial’ (Ettl and Gärtner 2013; Zidarova et al. 2016) but can also be found in the littorals of water bodies (Kopalová et al. 2019). It was recently shown that only terrestrial species survived freezing experiments focused on temperate diatoms. Hardly any aquatic species exhibited resistance to freezing or desiccation indicating that freshwater diatoms in contrast to their soil-inhabiting counterparts do not develop protection against freezing and desiccation stress due to their habitat which is buffered against abrupt fluctuations (Souffreau et al. 2010, 2013a). Habitat dependency of stress survival and differences in overwintering strategies were reported in many other algal groups and cyanobacteria as well (Hawes and Davey 1989; Davey 1989; Hawes et al. 1992; Šabacká and Elster 2006). The freshwater polar strain E16-5 (Mayamaea atomus) did not survive the treatments during this study, whereas it survived a different experimental setting more favourable for survival (gradual freezing and fast thawing) (Hejduková et al. 2019). Interestingly, another strain of the same species from a terrestrial temperate habitat survived abrupt freezing and slow thawing relatively well both as vegetative cells (Souffreau et al. 2010, 2013a) and resting cells induced by N limitation (Souffreau et al. 2013a), suggesting high ecophysiological plasticity within the species.
Resting stages survival
In this study, three ways of vegetative-looking resting cell induction were tested to assess their effect on freezing survival. Higher tolerance to freezing and desiccation has previously been observed in the resting cells of some terrestrial diatoms treated in nitrogen-free medium and kept in dark and cool conditions (Souffreau et al. 2013a). In contrast, comparably induced and nitrogen-starved resting cells of aquatic diatoms from both polar and temperate habitats demonstrated higher freezing tolerance only in mild sub-zero temperatures (Hejduková et al. 2019). According to the differences in physiological cell statuses before and after the freezing event, the lowest increase in the proportion of dead cells was observed in the WC-N treatment. This was also supported by the fluorescence data, which revealed the highest relative increase in this treatment except for the control cultivation without freezing. When we compare the effect of the three resting cell treatments, a relatively high number of dormant non-active cells were detected also in the WC-N treatment before the freezing event implying an important role of nitrogen limitation in freshwater polar diatom resting cell formation.
Nitrogen depletion is quite often used and shown to be effective for diatom resting stage induction in laboratory conditions (Kuwata et al. 1993; McQuoid and Hobson 1995, 1996; Kuwata and Takahashi 1999; Jewson et al. 2008; Sugie and Kuma 2008; Souffreau et al. 2013a; Pelusi et al. 2020). Nevertheless, few examples have been documented concerning enhanced resting stage formation under other nutrient depletions in comparison to nitrogen limitation, e.g. under phosphate limitation from a freshwater environment (Jewson et al. 2008) and iron limitation from marine habitats (Sugie and Kuma 2008). Regarding the freshwater polar environment, it is not easy to provide a clear judgement about nutrient limitations. Field studies from polar environments propose both N and/or P limitations (Davey and Rothery 1992; Levine and Whalen 2001; Arnold et al. 2003; Brutemark et al. 2006; Hogan et al. 2014).
Moreover, the cultivation conditions of the induction itself can influence diatom growth according to measurements of the basal fluorescence of chlorophyll. The higher initial values of the WC-rest and WC-P treatments (day 1) suggest that incubation in dark and cool conditions (WC-rest) and under P limitation (WC-P) resulted in faster growth when compared to the N-limited treatment (WC-N) and vegetative cells. Although only two strains survived all four treatments, these showed a particularly higher tolerance in the WC-rest and WC-N treatments, implying the possible role of resting stage formation in freezing survival.
The ability to withstand freezing stress as vegetative cells without forming specialized dormant stages was observed in several cyanobacteria and algae (Hawes 1990; Hawes et al. 1992; Elster et al. 2008; Gupta and Agrawal 2008; Nagao et al. 2008; Agrawal 2009; Tashyreva and Elster 2016) including diatoms (Souffreau et al. 2010, 2013a; Hejduková et al. 2019). Furthermore, no resting spores of marine diatoms were found to survive prolonged darkness and most presumably survived as vegetative resting cells implying the importance of vegetative resting cells (Peters 1996; Peters and Thomas 1996).
Other factors possibly influencing survival
Under laboratory conditions, nutrient starvation of various algae and cyanobacteria resulted in advanced tolerance to freezing (Nagao et al. 1999; Trumhová et al. 2019) and desiccation stresses (Pichrtová et al. 2014b; Tashyreva and Elster 2015). It should be noted that the resting cells which exhibited higher survival rates in our experiments were induced using nitrogen-free medium and incubated in cool and dark. The effect of cold acclimation (Mock and Valentin 2004; Nagao et al. 2008; Trumhová et al. 2019), light limitation (Peters and Thomas 1996) or desiccation (Hostetter and Hoshaw 1970; Pichrtová et al. 2014a) was also earlier considered to strengthen stress resistance in algae including diatoms. Higher survival rates of cold-acclimated, light-limited or nutrient-starved cultures probably work through the low rate of energy consumption (Sánchez-Saavedra 2006; Morin et al. 2019); increases of various compound syntheses, for instance, PUFA (Nichols et al. 1993; Mock and Kroon 2002b; Teoh et al. 2004, 2013), EPS (Smith and Underwood 1998, 2000), triacylglycerols (Mock and Kroon 2002a; Mekhalfi et al. 2014; Schaub et al. 2017) or eicosapentaenoic acid (Mekhalfi et al. 2014); and cell structure changes such as vacuole reduction, cytoplasmic volume increase, chloroplast enlargement and growing numbers and sizes of starch grains (Nagao et al. 2008; Lyon and Mock 2014).
Overall, a low proportion of strains survived the treatments used in this study. Possibly, this result could have been affected by cell density and culture growth phase. As has been proposed earlier, the stationary phase and other rather matured cultures could exhibit enhanced stress resistance compared to young vegetative cells or intermediate stages (Welsh 2000; Pichrtová et al. 2014b; Trumhová et al. 2019). In contrast, even young cells in their exponential phase showed a certain level of stress tolerance (Hawes 1990). This factor could explain the high survivability of strain P24 Chamaepinnularia krookiformis in the preliminary freezing experiment, but none in the final experiments where lower inoculation densities were used as we attempted to keep all cultures in the same growth phase. Moreover, the ability of this strain to survive freezing is known experimentally (Hejduková et al. 2019).
Even though polar areas are considered as an extreme environment for life, the real conditions in natural diatom habitats can be often less harsh than those tested by the experimental setting in this study. For example, winter temperatures recorded in Antarctic lakes and Arctic streams rarely fell below −20 °C and −15 °C, respectively, and mostly oscillated around −10 °C (Váczi et al. 2011; Hejduková et al. 2020). Furthermore, the under ice temperature of Antarctic streams was only −4 °C, while air temperature fell to almost −30 °C (Hawes 1989) documenting that ice and snow cover become an important thermal insulation (Hawes 1989; Davey et al. 1992). Likewise, some deep lakes are capped by ice but water on the bottom remains liquid (Vincent et al. 2008). However, a field study focused on annual diatom population development in the High Arctic stated a high number of dead and injured cells in winter samples from frozen freshwater streams where the winter temperature dropped to −5 and −15 °C. A small portion of viable cells was also present implying importance of surviving cells (viable and dormant) for spring population growth (Hejduková et al. 2020).
Another aspect when considering possible survival mechanisms that could increase freezing tolerance under natural conditions is the possible vertical migration into the sediment. This has been documented in the context of various environmental factors (Consalvey et al. 2004; Perkins et al. 2010) but could not be tested in our experiments. Moreover, heterotrophic growth was described in various freshwater and marine diatoms (Lewin 1953; Tuchman et al. 2006; Pahl et al. 2010) and could play an important role in improving diatom survival in the conditions of the long polar winter nights (McKnight et al. 2000; Morin et al. 2019).
The combination of two methodological approaches used in this study enabled a deeper insight into the physiological changes that occurred following the freezing event. Even though the presence of at least some dormant inactive cells was detected by multiparameter staining in almost all the samples, the real number of surviving strains documented by the fluorescence measurements and light microscopy observation was low. This could be influenced by the fact that the staining procedure was performed immediately after the thawing process, while the fluorescence data represent a ratio of values measured over 9 days of potential cell recovery. This indicates that multiparameter staining provides a detailed insight into cell physiology. However, the survival rate immediately after the freezing and thawing processes can substantially differ from the survival on a longer timescale, suggesting the cells were not able to recover and died soon afterwards. Correspondingly, a study on the green alga Zygnema sp., which used a comparable experimental setup, demonstrated that a decline in fluorescence efficiency (effective quantum yield of photo system II) observed after 24 h of recovery reflected the physiological status of the cells more realistically than fluorescent staining performed just after the thawing process (Trumhová et al. 2019).
Our study confirms the sensitivity of vegetative and resting cells of polar benthic freshwater diatoms to freezing stress, which agrees with previous findings about temperate and some polar diatoms and their tolerance to freezing and desiccation (Souffreau et al. 2010, 2013a; Hejduková et al. 2019). Two strains out of 12 survived all the four −20 °C freezing treatments simulating changes in environmental conditions, and two more strains exhibited a certain freezing tolerance in some of the treatments, suggesting the possible importance of resting cells for freezing survival. Our study further supports current knowledge about the success of diatom cryopreservation, which could largely depend on cryoprotectants and species ecology and seems to be species specific. Such sensitivity could affect diatom dispersal effectivity and could explain the number of regionally endemic species from isolated areas. Though our study indicates that cells of at least some polar diatoms are capable of surviving freezing, the reasons for such rare survival are not clear and should be further investigated. However, a slightly improved trend in tolerance was seen in nitrogen-depleted resting cells. It is believed nitrogen starvation, together with dark and cold resting cell induction, could result in more resistant cells in comparison to phosphorus or no nutrient-limited resting cells and cells in the vegetative state. It should be noted that the experimental conditions were artificial and of course differentiated from the natural environment in many potentially important factors. Additional studies could include some natural conditions into their scope and/or focus on the molecular mechanisms underlying diatom stress tolerance.
References
Agrawal SC (2009) Factors affecting spore germination in algae - review. Folia Microbiol 54:273–302. https://doi.org/10.1007/s12223-009-0047-0
Antoniades D, Douglas MSV (2002) Characterization of high arctic stream diatom assemblages from Cornwallis Island, Nunavut, Canada. Can J Bot 80:50–58. https://doi.org/10.1139/b01-133
Arc E, Pichrtová M, Kranner I, Holzinger A (2020) Pre-akinete formation in Zygnema sp. from polar habitats is associated with metabolite re-arrangement. J Exp Bot 71:3314–3320. https://doi.org/10.1093/jxb/eraa123
Arnold RJ, Convey P, Hughes KA, Wynn-Williams DD (2003) Seasonal periodicity of physical factors, inorganic nutrients and microalgae in Antarctic fellfields. Polar Biol 26:396–403. https://doi.org/10.1007/s00300-003-0503-2
Bayer-Giraldi M, Weikusat I, Besir H, Dieckmann G (2011) Characterization of an antifreeze protein from the polar diatom Fragilariopsis cylindrus and its relevance in sea ice. Cryobiology 63:210–219. https://doi.org/10.1016/j.cryobiol.2011.08.006
Bertrand A, Castonguay Y (2003) Plant adaptations to overwintering stresses and implications of climate change. Can J Bot 81:1145–1152. https://doi.org/10.1139/b03-129
Billi D, Caiola MG (1996) Effects of nitrogen limitation and starvation on Chroococcidiopsis sp. (Chroococcales). New Phytol 133:563–571. https://doi.org/10.1111/j.1469-8137.1996.tb01925.x
Brutemark A, Rengefors K, Anderson NJ (2006) An experimental investigation of phytoplankton nutrient limitation in two contrasting low Arctic lakes. Polar Biol 29:487–494. https://doi.org/10.1007/s00300-005-0079-0
Cañavate JP, Lubian LM (1995) Relationship between cooling rates, cryoprotectant concentrations and salinities in the cryopreservation of marine microalgae. Mar Biol 124:325–334. https://doi.org/10.1007/BF00347136
Cañavate JP, Lubian LM (1997) Effects of slow and rapid warming on the cryopreservation of marine microalgae. Cryobiology 35:143–149. https://doi.org/10.1006/cryo.1997.2031
Consalvey M, Paterson DM, Underwood GJC (2004) The ups and downs of life in a benthic biofilm: migration of benthic diatoms. Diatom Res 19:181–202. https://doi.org/10.1080/0269249X.2004.9705870
Consalvey M, Perkins RG, Paterson DM, Underwood GJC (2005) PAM fluorescence: a beginners guide for benthic diatomists. Diatom Res 20:1–22. https://doi.org/10.1080/0269249X.2005.9705619
Cremer H, Gore D, Hultzsch N, Melles M, Wagner B (2004) The diatom flora and limnology of lakes in the Amery Oasis, East Antarctica. Polar Biol 27:513–531. https://doi.org/10.1007/s00300-004-0624-2
Crowe JH, Crowe LM, Carpenter JF, Aurell Wistrom C (1987) Stabilization of dry phospholipid bilayers and proteins by sugars. Biochem J 242:1–10. https://doi.org/10.1042/bj2420001
Davey MC (1989) The effects of freezing and desiccation on photosynthesis and survival of terrestrial Antarctic algae and cyanobacteria. Polar Biol 10:29–36. https://doi.org/10.1007/BF00238287
Davey MC (1991) Effects of physical factors on the survival and growth of Antarctic terrestrial algae. Br Phycol J 26:315–325. https://doi.org/10.1080/00071619100650281
Davey MC, Rothery P (1992) Factors causing the limitation of growth of terrestrial algae in maritime Antarctica during late summer. Polar Biol 12:595–601. https://doi.org/10.1007/BF00236982
Davey MC, Pickup J, Block W (1992) Temperature variation and its biological significance in fellfield habitats on a maritime Antarctic island. Antarct Sci 4:383–388. https://doi.org/10.1017/S0954102092000567
Edlund MB, Stoermer EF, Taylor CM (1996) Aulacoseira skvortzowii sp. nov. (Bacillariophyta), a poorly understood diatom from Lake Baikal, Russia 1. J Phycol 32:165–175. https://doi.org/10.1111/j.0022-3646.1996.00165.x
Ellegaard M, Ribeiro S (2018) The long-term persistence of phytoplankton resting stages in aquatic ‘seed banks’. Biol Rev 93:166–183. https://doi.org/10.1111/brv.12338
Elster J (2002) Ecological classification of terrestrial algal communities in polar environments. In: Geoecology of Antarctic Ice-Free Coastal Landscapes. Springer-Verlag, Berlin, pp 303–326
Elster J, Benson EE (2004) Life in the polar terrestrial environment with a focus on algae and cyanobacteria. In: Fuller B, Lane N, Benson EE (eds) Life in the Frozen State. Taylor and Francis, London, pp 111–150
Elster J, Degma P, Kováčik L, Valentová L, Šramková K, Batista Pereira A (2008) Freezing and desiccation injury resistance in the filamentous green alga Klebsormidium from the Antarctic, Arctic and Slovakia. Biologia 63:843–851. https://doi.org/10.2478/s11756-008-0111-2
Ettl H, Gärtner G (2013) Syllabus der Boden-, Luft- und Flechtenalgen. Springer-Verlag, Berlin
Evans JH (1959) The survival of freshwater algae during dry periods: Part II. Drying experiments: Part III. Stratification of algae in pond margin litter and mud. J Ecol 47:55–81. https://doi.org/10.2307/2257248
Fields MW, Hise A, Lohman EJ, Bell T, Gardner RD, Corredor L, Moll K, Peyton BM, Characklis GW, Gerlach R (2014) Sources and resources: importance of nutrients, resource allocation, and ecology in microalgal cultivation for lipid accumulation. Appl Microbiol Biotechnol 98:4805–4816. https://doi.org/10.1007/s00253-014-5694-7
Guillard RRL, Lorenzen CJ (1972) Yellow-green algae with chlorophyllide c. J Phycol 8:10–14. https://doi.org/10.1111/j.1529-8817.1972.tb03995.x
Gupta S, Agrawal SC (2008) Vegetative survival of some wall and soil blue-green algae under stress conditions. Folia Microbiol 53:343–350. https://doi.org/10.1007/s12223-008-0053-7
Gwak IG, sic Jung W, Kim HJ et al (2010) Antifreeze protein in Antarctic marine diatom, Chaetoceros neogracile. Mar Biotechnol 12:630–639. https://doi.org/10.1007/s10126-009-9250-x
Hawes I (1989) Filamentous green algae in freshwater streams on Signy Island, Antarctica. Hydrobiologia 172:1–18. https://doi.org/10.1007/BF00031608
Hawes I (1990) Effects of freezing and thawing on a species of Zygnema (Chlorophyta) from the Antarctic. Phycologia 29:326–331. https://doi.org/10.2216/i0031-8884-29-3-326.1
Hawes I, Davey MC (1989) Use of the fluorochrome Auramine O for determination of cell viability in filamentous and thalloid algae. Phycologia 28:518–520. https://doi.org/10.2216/i0031-8884-28-4-518.1
Hawes I, Howard-Williams C, Vincent WF (1992) Desiccation and recovery of Antarctic cyanobacterial mats. Polar Biol 12:587–594. https://doi.org/10.1007/BF00236981
Hejduková E, Pinseel E, Vanormelingen P, Nedbalová L, Elster J, Vyverman W, Sabbe K (2019) Tolerance of pennate diatoms (Bacillariophyceae) to experimental freezing: comparison of polar and temperate strains. Phycologia 58:1–11. https://doi.org/10.1080/00318884.2019.1591835
Hejduková E, Elster J, Nedbalová L (2020) Annual cycle of freshwater diatoms in the High Arctic revealed by multiparameter fluorescent staining. Microb Ecol 80:559–572. https://doi.org/10.1007/s00248-020-01521-w
Hogan EJ, McGowan S, Anderson NJ (2014) Nutrient limitation of periphyton growth in Arctic lakes in south-west Greenland. Polar Biol 37:1331–1342. https://doi.org/10.1007/s00300-014-1524-8
Hostetter HP, Hoshaw RW (1970) Environmental factors affecting resistance to desiccation in the diatom Stauroneis anceps. Am J Bot 57:512. https://doi.org/10.2307/2441048
Janech MG, Krell A, Mock T, Kang JS, Raymond JA (2006) Ice-binding proteins from sea ice diatoms (Bacillariophyceae). J Phycol 42:410–416. https://doi.org/10.1111/j.1529-8817.2006.00208.x
Jewson DH, Granin NG, Zhdanov AA, Gorbunova LA, Bondarenko NA, Gnatovsky RY (2008) Resting stages and ecology of the planktonic diatom Aulacoseira skvortzowii in Lake Baikal. Limnol Oceanogr 53:1125–1136. https://doi.org/10.4319/lo.2008.53.3.1125
Jones VJ (1996) The diversity, distribution and ecology of diatoms from Antarctic inland waters. Biodivers Conserv 5:1433–1449. https://doi.org/10.1007/BF00051986
Kim GH, Klochkova TA, Kang SH (2008) Notes on freshwater and terrestrial algae from Ny-Ålesund, Svalbard (high Arctic sea area). J Environ Biol 29:485–491
Kopalová K, Nedbalová L, Nývlt D, Elster J, van de Vijver B (2013) Diversity, ecology and biogeography of the freshwater diatom communities from Ulu Peninsula (James Ross Island, NE Antarctic Peninsula). Polar Biol 36:933–948. https://doi.org/10.1007/s00300-013-1317-5
Kopalová K, Soukup J, Kohler TJ, Roman M, Coria SH, Vignoni PA, Lecomte KL, Nedbalová L, Nývlt D, Lirio JM (2019) Habitat controls on limno-terrestrial diatom communities of Clearwater Mesa, James Ross Island, Maritime Antarctica. Polar Biol 42:1595–1613. https://doi.org/10.1007/s00300-019-02547-8
Krembs C, Eicken H, Junge K, Deming JW (2002) High concentrations of exopolymeric substances in Arctic winter sea ice: implications for the polar ocean carbon cycle and cryoprotection of diatoms. Deep Sea Res Part I Oceanogr Res Pap 49:2163–2181. https://doi.org/10.1016/S0967-0637(02)00122-X
Kuwata A, Takahashi M (1999) Survival and recovery of resting spores and resting cells of the marine planktonic diatom Chaetoceros pseudocurvisetus under fluctuating nitrate conditions. Mar Biol 134:471–478. https://doi.org/10.1007/s002270050563
Kuwata A, Hama T, Takahashi M (1993) Ecophysiological characterization of two life forms, resting spores and resting cells, of a marine planktonic diatom, Chaetoceros pseudocurvisetus, formed under nutrient depletion. Mar Ecol Prog Ser 102:245–256. https://doi.org/10.3354/meps102245
Láska K, Witoszová D, Prošek P (2012) Weather patterns of the coastal zone of Petuniabukta, central Spitsbergen in the period 2008–2010. Polish Polar Res 33:297–318. https://doi.org/10.2478/v10183
Levine MA, Whalen SC (2001) Nutrient limitation of phytoplankton production in Alaskan Arctic foothill lakes. Hydrobiologia 455:189–201. https://doi.org/10.1023/A:1011954221491
Lewin JC (1953) Heterotrophy in diatoms. J Gen Microbiol 9:305–313. https://doi.org/10.1099/00221287-9-2-305
Livingstone D, Jaworski GHM (1980) The viability of akinetes of blue-green algae recovered from the sediments of Rostherne Mere. Br Phycol J 15:357–364. https://doi.org/10.1080/00071618000650361
Lund JWG (1954) The seasonal cycle of the plankton diatom, Melosira italica (Ehr.) Kutz. subsp. subarctica O. Müll. J Ecol 42:151–179. https://doi.org/10.2307/2256984
Lyon BR, Mock T (2014) Polar microalgae: new approaches towards understanding adaptations to an extreme and changing environment. Biology 3:56–80. https://doi.org/10.3390/biology3010056
Mann DG (1999) The species concept in diatoms. Phycologia 38:437–495. https://doi.org/10.2216/i0031-8884-38-6-437.1
Mazur P (1963) Kinetics of water loss from cells at subzero temperatures and the likelihood of intracellular freezing. J Gen Physiol 47:347–369. https://doi.org/10.1085/jgp.47.2.347
Mazur P (1984) Freezing of living cells: mechanisms and implications. Am J Phys 247:125–142. https://doi.org/10.1152/ajpcell.1984.247.3.c125
McKnight DM, Howes BL, Taylor CD, Goehringer DD (2000) Phytoplankton dynamics in a stably stratified Antarctic lake during winter darkness. J Phycol 36:852–861. https://doi.org/10.1046/j.1529-8817.2000.00031.x
McQuoid MR, Hobson LA (1995) Importance of resting stages in diatom seasonal succession. J Phycol 31:44–50. https://doi.org/10.1111/j.0022-3646.1995.00044.x
McQuoid MR, Hobson LA (1996) Diatom resting stages. J Phycol 32:889–902. https://doi.org/10.1111/j.0022-3646.1996.00889.x
Mekhalfi M, Amara S, Robert S, Carrière F, Gontero B (2014) Effect of environmental conditions on various enzyme activities and triacylglycerol contents in cultures of the freshwater diatom, Asterionella formosa (Bacillariophyceae). Biochimie 101:21–30. https://doi.org/10.1016/j.biochi.2013.12.004
Meryman HT (1974) Freezing injury and its prevention in living cells. Annu Rev Biophys Bioeng 3:341–363. https://doi.org/10.1146/annurev.bb.03.060174.002013
Mock T, Kroon BMA (2002a) Photosynthetic energy conversion under extreme conditions - I: important role of lipids as structural modulators and energy sink under N-limited growth in Antarctic sea ice diatoms. Phytochemistry 61:41–51. https://doi.org/10.1016/S0031-9422(02)00216-9
Mock T, Kroon BMA (2002b) Photosynthetic energy conversion under extreme conditions - II: the significance of lipids under light limited growth in Antarctic sea ice diatoms. Phytochemistry 61:53–60. https://doi.org/10.1016/S0031-9422(02)00215-7
Mock T, Valentin K (2004) Photosynthesis and cold acclimation: molecular evidence from a polar diatom. J Phycol 40:732–741. https://doi.org/10.1111/j.1529-8817.2004.03224.x
Mock T, Otillar RP, Strauss J, McMullan M, Paajanen P, Schmutz J, Salamov A, Sanges R, Toseland A, Ward BJ, Allen AE, Dupont CL, Frickenhaus S, Maumus F, Veluchamy A, Wu T, Barry KW, Falciatore A, Ferrante MI, Fortunato AE, Glöckner G, Gruber A, Hipkin R, Janech MG, Kroth PG, Leese F, Lindquist EA, Lyon BR, Martin J, Mayer C, Parker M, Quesneville H, Raymond JA, Uhlig C, Valas RE, Valentin KU, Worden AZ, Armbrust EV, Clark MD, Bowler C, Green BR, Moulton V, van Oosterhout C, Grigoriev IV (2017) Evolutionary genomics of the cold-adapted diatom Fragilariopsis cylindrus. Nature 541:536–540. https://doi.org/10.1038/nature20803
Moore JW (1974) Benthic algae of Southern Baffin Island: II. The epipelic communities in temporary ponds. J Ecol 62:809–819. https://doi.org/10.1111/j.1529-8817.1974.tb02739.x
Morgan-Kiss RM, Priscu JC, Pocock T, Gudynaite-Savitch L, Huner NPA (2006) Adaptation and acclimation of photosynthetic microorganisms to permanently cold environments. Microbiol Mol Biol Rev 70:222–252. https://doi.org/10.1128/MMBR.70.1.222
Morin PI, Lacour T, Grondin PL, Bruyant F, Ferland J, Forget MH, Massicotte P, Donaher N, Campbell DA, Lavaud J, Babin M (2019) Response of the sea-ice diatom Fragilariopsis cylindrus to simulated polar night darkness and return to light. Limnol Oceanogr 65:1041–1060. https://doi.org/10.1002/lno.11368
Nagao M, Arakawa K, Takezawa D, Yoshida S, Fujikawa S (1999) Akinete formation in Tribonema bombycinum Derbes et Solier (Xanthophyceae) in relation to freezing tolerance. J Plant Res 112:163–174. https://doi.org/10.1007/PL00013870
Nagao M, Matsui K, Uemura M (2008) Klebsormidium flaccidum, a charophycean green alga, exhibits cold acclimation that is closely associated with compatible solute accumulation and ultrastructural changes. Plant Cell Environ 31:872–885. https://doi.org/10.1111/j.1365-3040.2008.01804.x
Nedbalová L, Nývlt D, Kopáček J, Šobr M, Elster J (2013) Freshwater lakes of Ulu Peninsula, James Ross Island, north-east Antarctic Peninsula: origin, geomorphology and physical and chemical limnology. Antarct Sci 25:358–372. https://doi.org/10.1017/S0954102012000934
Nichols DS, Nichols PD, Sullivan CW (1993) Fatty acid, sterol and hydrocarbon composition of Antarctic sea ice diatom communities during the spring bloom in McMurdo Sound. Antarct Sci 5:271–278. https://doi.org/10.1017/S0954102093000367
Norwegian Polar Institute Map data Svalbard 1:1 000 000 (2019) https://data.npolar.no. Accessed 10 Oct 2019
Pahl SL, Lewis DM, Chen F, King KD (2010) Heterotrophic growth and nutritional aspects of the diatom Cyclotella cryptica (Bacillariophyceae): effect of some environmental factors. J Biosci Bioeng 109:235–239. https://doi.org/10.1016/j.jbiosc.2009.08.480
Pelusi A, Santelia ME, Benevenuto G et al (2020) The diatom Chaetoceros socialis: spore formation and preservation. Eur J Phycol 55:1–10. https://doi.org/10.1080/09670262.2019.1632935
Perkins RG, Lavaud J, Serôdio J, Mouget JL, Cartaxana P, Rosa P, Barille L, Brotas V, Jesus BM (2010) Vertical cell movement is a primary response of intertidal benthic biofilms to increasing light dose. Mar Ecol Prog Ser 416:93–103. https://doi.org/10.3354/meps08787
Peters E (1996) Prolonged darkness and diatom mortality: II. marine temperate species. J Exp Mar Biol Ecol 207:43–58. https://doi.org/10.1016/0022-0981(95)02519-7
Peters E, Thomas DN (1996) Prolonged darkness and diatom mortality I: marine Antarctic species. J Exp Mar Biol Ecol 207:25–41. https://doi.org/10.1016/S0022-0981(96)02520-8
Pichrtová M, Hájek T, Elster J (2014a) Osmotic stress and recovery in field populations of Zygnema sp. (Zygnematophyceae, Streptophyta) on Svalbard (High Arctic) subjected to natural desiccation. FEMS Microbiol Ecol 89:270–280. https://doi.org/10.1111/1574-6941.12288
Pichrtová M, Kulichová J, Holzinger A (2014b) Nitrogen limitation and slow drying induce desiccation tolerance in conjugating green algae (Zygnematophyceae, Streptophyta) from polar habitats. PLoS One 9:1–12. https://doi.org/10.1371/journal.pone.0113137
Pichrtová M, Hájek T, Elster J (2016) Annual development of mat-forming conjugating green algae Zygnema spp. in hydro-terrestrial habitats in the Arctic. Polar Biol 39:1653–1662. https://doi.org/10.1007/s00300-016-1889-y
Pinseel E, Hejduková E, Vanormelingen P, Kopalová K, Vyverman W, de Vijver BV (2017a) Pinnularia catenaborealis sp. nov. (Bacillariophyceae), a unique chain-forming diatom species from James Ross Island and Vega Island (maritime Antarctica). Phycologia 56:94–107. https://doi.org/10.2216/16-18.1
Pinseel E, Van de Vijver B, Kavan J et al (2017b) Diversity, ecology and community structure of the freshwater littoral diatom flora from Petuniabukta (Spitsbergen). Polar Biol 40:533–551. https://doi.org/10.1007/s00300-016-1976-0
Pla-Rabés S, Hamilton PB, Ballesteros E, Gavrilo M, Friedlander AM, Sala E (2016) The structure and diversity of freshwater diatom assemblages from Franz Josef Land Archipelago: a northern outpost for freshwater diatoms. PeerJ 2016:1–22. https://doi.org/10.7717/peerj.1705
Poulíčková A, Lysáková M, Hašler P, Lelková E (2008) Fishpond sediments - the source of palaeoecological information and algal ‘seed banks’. Nova Hedwigia 86:141–153. https://doi.org/10.1127/0029-5035/2008/0086-0141
Raymond JA (2000) Distribution and partial characterization of ice-active molecules associated with sea-ice diatoms. Polar Biol 23:721–729. https://doi.org/10.1007/s003000000147
Raymond JA, Knight CA (2003) Ice binding, recrystallization inhibition, and cryoprotective properties of ice-active substances associated with Antarctic sea ice diatoms. Cryobiology 46:174–181. https://doi.org/10.1016/S0011-2240(03)00023-3
Remias D (2012) Cell structure and physiology of alpine snow and ice algae. In: Lütz C (ed) Plants in Alpine Regions. Springer-Verlag, Vienna, pp 175–185
Round FE, Crawford RM, Mann DG (1990) Biology of diatoms. In: Round FE, Crawford RM, Mann DG (eds) Diatoms: Biology and Morphology of the Genera. Cambridge University Press, Cambridge, pp 1–130
Šabacká M, Elster J (2006) Response of cyanobacteria and algae from Antarctic wetland habitats to freezing and desiccation stress. Polar Biol 30:31–37. https://doi.org/10.1007/s00300-006-0156-z
Sánchez-Saavedra MDP (2006) The effect of cold storage on cell viability and composition of two benthic diatoms. Aquac Eng 34:131–136. https://doi.org/10.1016/j.aquaeng.2005.06.004
Schaub I, Wagner H, Graeve M, Karsten U (2017) Effects of prolonged darkness and temperature on the lipid metabolism in the benthic diatom Navicula perminuta from the Arctic Adventfjorden, Svalbard. Polar Biol 40:1425–1439. https://doi.org/10.1007/s00300-016-2067-y
Schmid AM (1979) Influence of environmental factors on the development of the valve in diatoms. Protoplasma 99:99–115. https://doi.org/10.1007/BF01274072
Scientific Committee on Antarctic Research Antarctic Digital Database Version 7.0 (2020) https://www.add.scar.org/. Accessed 27 Mar 2020
Sheath RG, Vis ML, Hambrook JA, Cole KM (1996) Tundra stream macroalgae of North America: composition, distribution and physiological adaptations. Hydrobiologia 336:67–82. https://doi.org/10.1007/BF00010820
Sicko-Goad L, Stoermer EF, Kociolek JP (1989) Diatom resting cell rejuvenation and formation: time course, species records and distribution. J Plankton Res 11:375–389. https://doi.org/10.1093/plankt/11.2.375
Smith DJ, Underwood GJC (1998) Exopolymer production by intertidal epipelic diatoms. Limnol Oceanogr 43:1578–1591. https://doi.org/10.4319/lo.1998.43.7.1578
Smith DJ, Underwood GJC (2000) The production of extracellular carbohydrates by estuarine benthic diatoms: the effects of growth phase and light and dark treatment. J Phycol 36:321–333. https://doi.org/10.1046/j.1529-8817.2000.99148.x
Souffreau C, Vanormelingen P, Verleyen E, Sabbe K, Vyverman W (2010) Tolerance of benthic diatoms from temperate aquatic and terrestrial habitats to experimental desiccation and temperature stress. Phycologia 49:309–324. https://doi.org/10.2216/09-30.1
Souffreau C, Vanormelingen P, Sabbe K, Vyverman W (2013a) Tolerance of resting cells of freshwater and terrestrial benthic diatoms to experimental desiccation and freezing is habitat-dependent. Phycologia 52:14–24. https://doi.org/10.2216/12-087.1
Souffreau C, Vanormelingen P, Van de Vijver B et al (2013b) Molecular evidence for distinct Antarctic lineages in the cosmopolitan terrestrial diatoms Pinnularia borealis and Hantzschia amphioxys. Protist 164:101–115. https://doi.org/10.1016/j.protis.2012.04.001
Stock W, Pinseel E, De Decker S et al (2018) Expanding the toolbox for cryopreservation of marine and freshwater diatoms. Sci Rep 8:1–9. https://doi.org/10.1038/s41598-018-22460-0
Sugie K, Kuma K (2008) Resting spore formation in the marine diatom Thalassiosira nordenskioeldii under iron- and nitrogen-limited conditions. J Plankton Res 30:1245–1255. https://doi.org/10.1093/plankt/fbn080
Tashyreva D, Elster J (2015) Effect of nitrogen starvation on desiccation tolerance of Arctic Microcoleus strains (cyanobacteria). Front Microbiol 6:1–11. https://doi.org/10.3389/fmicb.2015.00278
Tashyreva D, Elster J (2016) Annual cycles of two cyanobacterial mat communities in hydro-terrestrial habitats of the High Arctic. Microb Ecol 71:887–900. https://doi.org/10.1007/s00248-016-0732-x
Tashyreva D, Elster J, Billi D (2013) A novel staining protocol for multiparameter assessment of cell heterogeneity in Phormidium populations (cyanobacteria) employing fluorescent dyes. PLoS One 8:1–12. https://doi.org/10.1371/journal.pone.0055283
Teoh M-L, Chu W-L, Marchant H, Phang S-M (2004) Influence of culture temperature on the growth, biochemical composition and fatty acid profiles of six Antarctic microalgae. J Appl Phycol 16:421–430. https://doi.org/10.1007/s10811-004-5502-3
Teoh M-L, Phang S-M, Chu W-L (2013) Response of Antarctic, temperate, and tropical microalgae to temperature stress. J Appl Phycol 25:285–297. https://doi.org/10.1007/s10811-012-9863-8
Trumhová K, Holzinger A, Obwegeser S, Neuner G, Pichrtová M (2019) The conjugating green alga Zygnema sp. (Zygnematophyceae) from the Arctic shows high frost tolerance in mature cells (pre-akinetes). Protoplasma 256:1681–1694. https://doi.org/10.1007/s00709-019-01404-z
Tuchman NC, Schollett MA, Rier ST, Geddes P (2006) Differential heterotrophic utilization of organic compounds by diatoms and bacteria under light and dark conditions. Hydrobiologia 561:167–177. https://doi.org/10.1007/s10750-005-1612-4
Váczi P, Barták M, Nedbalová L, Elster J (2011) Comparative analysis of temperature courses in Antarctic lakes of different morphology: study from James Ross Island, Antarctica. Czech Polar Reports 1:78–87. https://doi.org/10.5817/cpr2011-2-7
Van de Vijver B, Beyens L (1999) Biogeography and ecology of freshwater diatoms in Subantarctica: a review. J Biogeogr 26:993–1000. https://doi.org/10.1046/j.1365-2699.1999.00358.x
Vanormelingen P, Verleyen E, Vyverman W (2008) The diversity and distribution of diatoms: from cosmopolitanism to narrow endemism. Biodivers Conserv 17:393–405. https://doi.org/10.1007/s10531-007-9257-4
Verleyen E, Van de Vijver B, Tytgat B et al (2021) Diatoms define a novel freshwater biogeography of the Antarctic. Ecography 44:1–13. https://doi.org/10.1111/ecog.05374
Vincent WF (2000) Evolutionary origins of Antarctic microbiota: invasion, selection and endemism. Antarct Sci 12:374–385. https://doi.org/10.1017/s0954102000000420
Vincent WF, Hobbie JE, Laybourn-Parry J (2008) Introduction to the limnology of high-latitude lake and river ecosystems. In: Vincent WF, Laybourn-Parry J (eds) Polar Lakes and Rivers: Limnology of Arctic and Antarctic Aquatic Ecosystems. Oxford University Press, New York, pp 1–23
von Stosch HA, Fecher K (1979) ‘Internal thecae’ of Eunotia soleirolii (Bacillariophyceae): development, structure and function as resting spores. J Phycol 15:233–243. https://doi.org/10.1111/j.0022-3646.1979.00233.x
Vyverman W, Verleyen E, Wilmotte A, Hodgson DA, Willems A, Peeters K, van de Vijver B, de Wever A, Leliaert F, Sabbe K (2010) Evidence for widespread endemism among Antarctic micro-organisms. Polar Sci 4:103–113. https://doi.org/10.1016/j.polar.2010.03.006
Welsh DT (2000) Ecological significance of compatible solute accumulation by micro-organisms: from single cells to global climate. FEMS Microbiol Rev 24:263–290. https://doi.org/10.1111/j.1574-6976.2000.tb00542.x
Zidarova R, Kopalová K, Van de Vijver B (2016) Diatoms from the Antarctic region: Maritime Antarctica. In: Lange-Bertalot H (ed) Iconographia Diatomologica. Koeltz Botanical Books, pp 1–509
Acknowledgements
We acknowledge Kateřina Kopalová and Petra Vinšová for collecting some field samples and Eveline Pinseel for providing one of the strains. We are grateful to Prof. Bart Van de Vijver for identification of the diatom strains. We thank the two anonymous reviewers whose comments and suggestions helped to improve and clarify this manuscript.
Funding
The study was supported by the Grant Agency of Charles University (Project No. 20217), the Ministry of Education, Youth and Sports of the Czech Republic (INTER-ACTION project LTAIN19139) and by the institutional long-term research plan of the Institute of Botany of Czech Academy of Sciences (RVO 67985939).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Andreas Holzinger
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
In memory of Ursula Lütz-Meindl.
Supplementary Information
ESM 1
Light microscopy pictures of the experimental strains: a Mayamaea atomus, b Achnanthidium lineare, c Meridion circulare, d Chamaepinnularia krookiformis, e Gomphonema sp., f Caloneis falcifera, g Luticola muticopsis, h Hantzschia abundans, i Pinnularia catenaborealis, j Hantzschia amphioxys, k Nitzschia palea, l Encyonopsis descripta, scale bar = 10 μm (PNG 10.2 mb)

Rights and permissions
About this article
Cite this article
Hejduková, E., Nedbalová, L. Experimental freezing of freshwater pennate diatoms from polar habitats. Protoplasma 258, 1213–1229 (2021). https://doi.org/10.1007/s00709-021-01648-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-021-01648-8