Abstract
We investigated whether phytoplankton communities in two lakes in SW Greenland were phosphorus or nitrogen limited. The study lakes have contrasting water chemistry (mean conductivities differ ten fold) and are located near Kangerlussuaq, SW Greenland (~67°N, 51°W). A microcosm nutrient enrichment experiment was performed in June 2003 to determine whether nitrate or phosphate addition stimulated phytoplankton growth. Samples were analysed for species composition, biomass, and alkaline phosphatase activity (APA). Initially, both lakes had extremely low total phosphorus but high total nitrogen concentrations and high APA, suggesting that the phytoplankton were phosphorus limited prior to the start of the experiment. The phytoplankton composition and biomass (mainly Ochromonas spp.) responded to phosphate but not to nitrate addition. In both lakes, chlorophyll a increased significantly when phosphate was added. Furthermore, APA was significantly lower in the two lakes when phosphate was added compared to the control and the nitrogen addition treatment. The dominance of mixotrophic phytoplankton and high DOC values suggest that these lakes may be regulated by microbial loop processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Long-term monitoring and palaeoecological studies revealed that arctic ecosystems have undergone a considerable degree of change in recent decades (Overpeck et al. 1997). Global change models suggest that the effects of global warming will be most pronounced in the Arctic (Serreze et al. 2000) and temperature increases can affect the length of the ice free period as well as alter thermal stratification and microbial processes. Further evidence suggests that the Arctic is affected by long-range atmospheric pollution (Bindler et al. 2001a, b). To address the possible effects of future global change processes on arctic algal communities, a better understanding of contemporary processes in arctic lakes is required, which due to their remote location are often understudied. Because of the lack of long-term monitoring data, palaeolimnological methods are increasingly being used to investigate both short- and long-term changes in arctic lakes (Overpeck et al. 1997). However, meaningful interpretation of the sediment record requires a better understanding of the contemporary ecological processes and biological structure of arctic lakes.
Recent short-core studies in arctic lakes showed striking changes in diatom assemblages over the last 100–150 years, particularly the increase in the abundance of a range of small planktonic Cyclotella species (Gajewski et al. 1997; Sorvari et al. 2002; Rühland et al. 2003). Some of the proposed causal mechanisms are the effects of climate warming on stratification, temperature effects on microbiological activity and nutrient cycling, and long-range nutrient (nitrogen) inputs associated with atmospheric pollution, as has been postulated for the Rocky Mountains (Wolfe et al. 2001). There has been, however, no systematic attempt to determine the possible causal mechanisms using an experimental approach.
A potential scenario is that increased input of atmospheric nitrogen favoured small Cyclotella species. Phytoplankton in arctic lakes, as with freshwaters in general, traditionally was assumed to be nutrient, particularly phosphorus (P) limited. However, nitrogen limitation was also observed in many lakes (Healey and Hendzel 1980; Elser et al. 1990) especially in more pristine areas (Jansson et al. 1996). Nutrient limitation is a dominant control of primary production and community structure in lakes. Consequently an increased availability of nutrients may cause considerable changes to the ecosystem, especially in oligotrophic, arctic systems (Reynolds 1984; Bettez et al. 2002). Phytoplankton and limnological studies of arctic lakes, certainly compared to temperate systems, have been quite limited with more recent studies in Svalbard (Ellis-Evans et al. 2001; Laybourn-Parry and Marshall 2003), Alaska (O’Brien et al. 1997), Canada (Kalff 1991) and Scandinavia (Jeppesen et al. 2003; Bergström et al. 2005).
A number of different methods are used to measure P limitation in phytoplankton, e.g. measurement of phosphate uptake, the loss of cell P content, response to P additions, as well as nutrient ratios (Healey and Hendzel 1979; Pettersson 1980; Vincent 1981). Another common method is the alkaline phosphatase assay. When phosphate concentration in the water drops below a critical threshold level, or when phytoplankton cell P quota decreases, the enzyme alkaline phosphatase is induced in bacteria and phytoplankton, allowing the cells to utilize organically bound phosphorus (see review by Cembella et al. 1984). Alkaline phosphatase is a good indicator of phosphate stress (Kuenzler 1965) and is produced in most taxa (Healey and Hendzel 1979; Cembella et al. 1984; Rengefors et al. 2003).
As relatively little is known about the present status of lakes in Greenland and the dominant processes controlling phytoplankton, the main objective of this project was to investigate whether phytoplankton biomass was nutrient (N or P) limited shortly after ice-out in two contrasting lakes (one oligosaline, one freshwater). Our hypothesis was that the phytoplankton communities would be phosphorus limited as nitrogen is locally more abundant in the Søndre Strømfjord area.
Materials and methods
Study area
The ice-free margin of West Greenland contains thousands of lakes, few of which have been systematically studied (Anderson et al. 2001). Limnological surveys are limited (Hansen 1967; Anderson et al. 1999) and there have been only a few studies of the phytoplankton, most of which have been taxonomic in nature and based on spot samples (Kristiansen 1994) or surface sediment samples (Ryves et al. 2002; Pla and Anderson 2005). To date there has been very limited application of “modern” phycological methodology to these lakes. Although the majority of the lakes are dilute, oligotrophic systems there are a number of oligosaline lakes located close to the ice margin, where effective precipitation is very low (Williams 1991; Anderson et al. 2001; McGowan et al. 2003) and appear to have locally enhanced nitrogen concentrations (Table 1). These oligosaline lakes also have very high concentrations of DOC (~50 – 100 mg l–1; N.J. Anderson, unpublished data; Table 1).
The mean annual temperature in the area is –6°C, and the precipitation is low, <150 mm year–1. The lakes in the Kangerlussuaq area are ice-covered for nearly 9 months a year, ice-melt generally occurs in mid-June, and they refreeze around late September to early October (Anderson et al. 2001; Kettle et al. 2004).
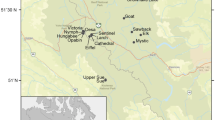
The experiments were performed during the early summer of 2003 (20–26 June) in two oligotrophic lakes, SS2 and Braya Sø (SS4) (Table 1) located in the Kangerlussuaq (Danish = Søndre Strømfjord) area of south-west Greenland (Fig. 1). These two lakes were chosen because of the identical climate, catchment geology (gneiss) and vegetation (dwarf shrub tundra), but differences in water chemistry (Anderson et al. 2001) (Table 1). SS2 (66.99°N, 50.97°W) is a 12-m deep dimictic lake with an area of 0.36 km2 at an altitude of 185 m a.s.l., whereas SS4 (66.99°N, 51.05°W) is a 22-m deep meromictic lake (with a purple sulphur bacteria plate at ~10-m depth) with an area of 0.73 km2 (altitude 170 m a.s.l). SS4 is a closed basin and is slightly saline (conductivity 2636 μS cm–1; Brodersen and Anderson 2002). Both lakes are fishless, as indicated by both gill-netting and the presence of large daphnids in the zooplankton community (Erik Jeppesen, personal communication).
Experimental methods
A microcosm nutrient-enrichment experiment, using cubitainers, was performed in each lake. The design consisted of a phosphate (P) and nitrate (N) treatment, and a control (C) with no nutrient additions, with each treatment having four replicates. Twelve translucent plastic carboys (volume 10 l each) were filled with surface lake water that had been mixed in a 140 l barrel and filtered through a 150 μm sieve to remove zooplankton. Phosphate (K2PO4) was added to a final concentration of 310 μg l–1 PO4−P (10 μmol l–1 ) for the P-treatment. Phosphate additions were designed to achieve an N:P molar ratio of 5:1 to achieve N limitation, based on previous measurements of total nitrogen. Nitrate (NaNO3) was added to a final concentration of 700 μg l–1 NO3−N for the N-treatment. Here, a N:P molar ratio of 300:1 was aimed for, to achieve severe P limitation. The carboys were mixed thoroughly after nutrient additions and supported from a floating frame deployed in the lake at 2-m depth to avoid high surface irradiance. Samples were collected from the barrel and carboys on day 1 and after 6 days.
Conductivity and temperature was measured at the surface in both lakes. Samples for nutrient analyses were filtered through a GF/C filter (Whatman) and stored at –20°C until processed. Soluble reactive phosphorus (SRP) was measured spectrophotometrically according to Murphy and Riley (1962). Samples for total phosphorus (TP) were processed by persulphate digestion and were subsequently analysed spectrophotometrically (Menzel and Corwin 1965). Nitrate was analysed using a cadmium reduction column and analysed spectrophotometrically (Fossing et al. 2003 modified from Grasshof 1983). Total nitrogen (TN) samples were processed by peroxidsulphate digestion (Valderrama 1995).
For chlorophyll a analyses, approximately 2 l of water was collected from the barrel at the start of the experiment and from the carboys on the sixth day. The water was filtered through a GF/F (Whatman) filter although on day 6 a GF/C filter (Whatman) was used. The change in filter was necessitated due to the change from syringe to vacuum pump with a different diameter than the previous filter. Chlorophyll a (Chl a) was determined spectrophotometrically in ethanol extracts (Jespersen and Christoffersen 1987).
Alkaline phosphatase (AP) was determined flourometrically using 4-methyl-umbelliferyl phosphate (MUF) according to Petterson (1980). Bulk water samples were stored at –20°C until processed. 0.5 ml 1 mM 4-methyl-umbelliferyl pyrophosphate (MUP) reagent solution was added to 4-ml sample. The samples were measured in a Turner design TD 700 flourometer at 0, 10, 20, 40 and 60 min. Raw fluorescence was converted into nmol MUF l–1 min–1, from the slope of a standard curve.
Phytoplankton samples were preserved with Lugol’s solution and stored in the dark at 4°C. The samples were counted in 10-ml settling chambers using an inverted Nikon eclipse TS 100 microscope. Cells of ~2 μm in diameter were counted as picophytoplankton. Biomass was calculated using geometric formulae provided by Blomqvist and Herlitz (1996).
All statistical analyses were performed in SPSS 11.0 for Windows. Levene’s test was used to test the homogeneity of variances. An ANOVA was used to test differences among the treatments and when significant was followed up by Tukey’s HSD post hoc test.
Results
At the start of the experiment the surface water in lake SS2 had a temperature of 13.2°C and a conductivity of ~350 μS cm–1. In SS4, the temperature was 13.8°C and the conductivity was ~2660 μS cm–1. TP in both lakes was ~2 μg P l−1, while phosphate concentration (SRP) was below detection, TN was 990 μg N l–1 in SS2 and 1575 μg N l–1 in SS4, and nitrate concentrations were ~2 μg NO3 l–1 in both lakes
The P treatments in both lakes had concentrations of 668 and 606 μg PO4−P l–1 in SS2 and SS4 respectively after nutrient additions, which was twice the targeted concentration. In the nitrate treatment, initial concentrations (day 1) were 770 μg NO3−N l–1 in lake SS2 and 690 μg NO3−N l–1 in SS4 (Table 2). The nitrate and phosphate concentrations had decreased by 10–30% by day 6 (Table 2).
Both lakes were initially dominated by small (diameter 5--12 μm) chrysophytes, mainly belonging to the genus Ochromonas. The total biomass was twice as high in SS4 (2×109 μm3 l–1) compared to SS2 (Figs. 2a, 3a). In the oligosaline SS4, the phytoplankton community also consisted of 4% picophytoplankton (~2 μm), whereas in SS2 this group was negligible. Pennate diatoms (predominantly Diatoma elongatum), Rhodomonas spp., Oocystis sp., and various species of filamentous cyanobacteria were also found at low abundance in both lakes. However, Scenedesmus sp. was only encountered in SS4, whereas members of the Rhizoochrysidaceae family, Bitrichia spp., Dinobryon spp., Elakatothrix spp., Cyclotella spp., and Ceratium sp. were found only in SS2. Overall, there was a higher number of taxa in lake SS2 (13 taxa) compared to SS4 (11 taxa).
In SS2, all treatments were dominated by Ochromonas spp. after five days incubation (Fig. 2a) with picophytoplankton contributing ~10% of the biomass in the P treatment. In SS4, Ochromonas spp. was also the dominant taxa in the treatments with nutrient addition (Fig. 3a). In contrast to SS2, no increase was observed in the picophytoplankton fraction of the SS4 community in any of the treatments. In the control treatment, there was a co-dominance of Ochromonas spp. and a pennate diatom, mainly D. elongatum.
The results from Lake SS2 shown for the four different treatments, initial (I), control (C), phosphate (P) and nitrate (N) addition. Vertical error bars show standard deviation (n=4). a Biomass expressed as μm3 l–1 shows an increase for all treatments, especially for the phosphate treatment, when compared to initial. b Chlorophyll a (Chl a) in μg l–1. A significant (P<0.05) increase for all treatments compared to initial value. The phosphate treatment is significantly (P<0.05) differed from the control and nitrate treatment. c The control and nitrate treatment shows a significant (P<0.05) increase in alkaline phosphatase activity (APA), nmol MUF l–1 min–1 compared to initial. The phosphate treatment is significantly (P<0.05) lower than the other treatments
The results from Lake SS4 shown for the four different treatments, initial (I), control (C), phosphate (P) and nitrate (N) addition. Vertical error bars show standard deviation (n=4). a Biomass expressed as μm3 l–1 shows an increase for all treatments, especially for the phosphate treatment, when compared to initial. b Chlorophyll a (Chl a) in μg l–1 A significant (P<0.05) increase for all treatments compared to initial value. The phosphate treatment is significantly (P<0.05) differed from the control and nitrate treatment. c The control and nitrate treatment shows a significant (P<0.05) increase in APA, nmol MUF l–1 min–1 compared to initial. The phosphate treatment is significantly (P<0.05) lower than the other treatments
In both lakes, the microcosms showed a significant (ANOVA, P<0.05) increase in chlorophyll a concentration in all treatments by day 6, compared to the initials (Figs. 2b, 3b). While there was no significant difference between the control and nitrate treatment in lake SS2, the chlorophyll a doubled in the P treatment (ANOVA, P<0.05), compared to both the control and nitrate treatment. The same pattern was observed for biomass as calculated from the phytoplankton counts (Fig. 2a). Lake SS4 responded similarly to SS2. There was no significant difference (ANOVA; P>0.05) between the chlorophyll a concentration in the control and N treatment, while in the P treatment chlorophyll a was three to four times higher (ANOVA P<0.05) than in the other treatments. Even though SS2 and SS4 had the same initial chlorophyll a level, the response to the P treatment was much greater in lake SS4, where it increased from 0.1 to 4.9 μg l–1(Fig. 3b) compared to an increase from 0.1 to 1.6 μg l–1 in SS2 (Fig. 2b).
Initially, APA was similar in the two lakes, amounting to 2.5 nmol MUF l−1 min–1 or 38 and 27 nmol MUF l−1 min–1 (μg Chl a)−1 respectively for SS2 and SS4 (Table 2). Following nutrient enrichment, APA was significantly (ANOVA; P<0.05) lower in the P treatment than in the control and N treatment in both lakes (Figs. 2c, 3c, respectively). In lake SS2, APA in the P treatment was also significantly lower than the initial value (ANOVA; P<0.05). In both lakes, APA increased significantly (ANOVA; P<0.05) in the control and N treatments, compared to the initial value, although there was no significant difference between the two (ANOVA; P>0.05).
Discussion
Experimental work on remote arctic lakes is quite limited and mainly confined to areas with more extensive logistic support, e.g. Toolik Lake (O’Brien et al. 1997) and lakes on Svalbard (Laybourn-Parry and Marshall 2003; Jeppesen et al. 2003). This study confirms our hypothesis that the spring phytoplankton communities in two lakes in SW Greenland were severely phosphorus limited. The results from the two contrasting lakes are probably typical representatives for the two main lake groups at the head of the fjord, freshwater and oligosaline (Anderson et al. 2001; Pla and Anderson 2005).
Soluble reactive phosphorus (SRP), TP and nitrate concentrations were close to the detection-limit in both lakes shortly after ice-out, confirming that the lakes are oligotrophic. In contrast, Brodersen and Anderson (2002) found that TP was 10.5 μg l–1 in lake SS2, and 10.8 μg l–1 in lake SS4 (based on a 4 year average (1996–2000) samples taken between April and September). This discrepancy may be due to our single sampling approach at a time when the phytoplankton biomass was very low. However, higher TN concentrations than expected were observed in this study. In Brodersen and Anderson (2002), the TN concentrations reported for the two lakes averaged 653 and 963 μg l–1 respectively, which compares with 990 and 1575 μg l–1 in the present study. These differences are probably due to interannual variability and are not considered further.
The molar N:P ratio in healthy, growing phytoplankton is 16:1, with higher ratios indicating phosphorus limitation (Redfield 1958). Guildford and Hecky (2000) analyzed a large lake data set and concluded that TN:TP >50 (molar) was consistent with P deficient growth, while TN:TP<20 indicated N deficiency. In the present study, the TN:TP (molar ratio) was 298 for lake SS2 and 419 for lake SS4, indicating severe phosphorus limitation (Guildford and Hecky 2000). In Brodersen and Anderson (2002) the TN:TP ratios were 28:1 and 40:1 (molar ratio) for each respective lake, indicating an alternation of the limiting nutrient, or neither N or P limitation.
Phosphorus limitation was also confirmed by the significant increase in chlorophyll a in both lakes SS2 and SS4 as a response to phosphate addition. Chlorophyll a in the lakes at the time of the initial sampling was 0.1 μg l–1 in both SS2 and SS4 (Figs. 2b, 3b), representing the chlorophyll a values typically found in arctic lakes (Guildford and Hecky 2000). Jeppesen et al. (2003) found equally low chlorophyll (from 0.2 μg l–1) in some Greenlandic lakes. The volumetric phytoplankton biomass as calculated from microscopic measurements followed the same patterns as chlorophyll a in lake SS2. In SS4, however, the volumetric biomass and control were relatively higher than the chlorophyll measurements. A partial explanation is that some of the diatom frustules counted may have lacked, or contained only low amounts of chlorophyll a. Moreover, the biovolume of small Ochromonads may not necessarily correlate perfectly with chlorophyll a, since many of them are mixotrophic and may thereby vary considerably in chlorophyll a content (Pålsson and Daniel 2004).
Initial values of specific community APA, i.e. the ratio between APA and chlorophyll a, was 100-fold higher (38 in SS2 and 27 in SS4) than for extremely deficient phytoplankton communities (Istvánovics et al. 1992), suggesting that the phytoplankton communities were severely P stressed. In the nutrient addition experiment, as expected, APA was lower in the P treatments compared to the control and N treatments in both lakes. These results showed that P addition alleviated the P stress, although some AP was still produced. These results agree with Rengefors et al. (2003) that AP cannot be completely turned off in 5 days. APA increased in the N treatments, as expected, indicating that phosphorus deficiency was enhanced. The equivalent increase of APA in the control treatments add further evidence that the lake communities were severely P stressed at the start of the experiment.
In both lakes the phytoplankton composition was dominated both initially and throughout the nutrient incubation experiment by Ochromonas spp. Flagellates, such as Ochromonas spp., dominates phytoplankton communities frequently when DOC is high (Drakare et al. 2003). The dominance of Ochromonas spp. is probably due to its mixotrophic nutrition mode (Andersson et al. 1989; Sanders et al. 2001), which provides additional nutrient by phagotrophy (bacterivory) (e.g. Caron et al. 1990; Rothhaupt 1996). This is particularly true for P-limitation, as bacteria tend to be rich in P due to their higher P assimliation efficiency at low P concentrations (Currie and Kalff 1984; Jansson et al. 1996). We did not test whether Ochromonas spp. utilized the phosphate directly or indirectly via bacteria, i.e. if they grew phototrophically or by mixotrophy. Nonetheless, the biomass of Ochromonas spp. increased in the P treatments in both lakes by ~100% (SS2) and by ~270% (SS4), compared to day 1. Given the extremely high DOC concentrations in these lakes (Table 1) the microbial loop is probably important and hence bacterivory and mixotrophy might be a very successful strategy (Jansson et al. 1999).
In our study, the biomass of picophytoplankton (<2 μm) contributed initially only 0.4% of total biomass in lake SS2, and 4% of total biomass in lake SS4. Two major classes of phytoplankton contribute to the picophytoplankton, i.e. cyanobacteria and chlorophyta (Stockner and Antia 1986) and might be favoured relative to large autotrophic phytoplankton in nutrient poor systems (Stockner 1991). We did not separate heterotrophic picoplankton from photosynthetic picoplankton, hence the possibility of bacterial growth cannot be neglected. Nevertheless the nutrient additions in this study favoured the growth of picophytoplankton (and possibly bacterial growth). In SS2, the picophytoplankton biomass increased in all treatments compared to initial, by 50x in the control, 28x in the N treatment and 150x in the P treatment. The biomass increase in the control also suggests that there was an increase in picophytoplankton in the lake during the experimental period. Nevertheless, the 150-fold increase in biomass in the P treatment, suggests that the picophytoplankton were initially phosphorus limited. In lake SS4, the response of picophytoplankton was minimal, but as the samples were combined we cannot tell whether the differences were significant. The different response between the lakes may in part be explained by the presence of diatoms in SS4, which are also known to have high nutrient uptake rates. An alternative explanation is that the high DOC inhibited the response of picophytoplankton, as was observed by Drakare et al. (2003). Drakare et al. (2003) concluded that being small does not give a general advantage neither in brown-water or clear-water lakes, and picophytoplankton are not necessarily better competitors for nutrients than bacterioplankton (Rhee 1972) or larger phytoplankton.
The biomass of the third most common taxa in lake SS2, Dinobryon spp., was very low in all treatments (<0.5% of total biomass). Like Ochromonas spp., Dinobryon spp. is also mixotrophic (Bird and Kalff 1987), hence, they can utilize bacteria to satisfy their nutrient demand. Laybourn-Parry and Marshall (2003) found that a low temperature (3°C) was preferred for Dinobryon spp. growth in the Arctic. Furthermore, they found that Dinobryon spp. grew mixotrophically during the ice covered period. There are abundant resting cysts of Dinobryon spp. in the sediments of SS4 (Pla and Anderson 2005), suggesting, that it has been common in the phytoplankton in the lake for hundreds of years (McGowan et al. 2003). D. elongatum the co-dominant taxa in lake SS4, seemed to contribute substantially to the biomass in the control treatment (Fig. 3a). However, no major response could be observed when phosphate or nitrate was added, suggesting that Diatoma elongatum is limited by other factors.
To conclude, our main findings were that the phytoplankton communities in both an oligosaline and a freshwater Greenlandic lake were severely phosphorus limited during early summer. The total phosphorus to total nitrogen ratios were extremely high and so was alkaline phosphatase activity, furthermore the phytoplankton biomass responded to P but not N addition. Mixotrophic phytoplankton dominated the phytoplankton community in these lakes, suggesting that these lakes are based on a heterotrophic food chain (Jansson et al. 2000). Given the high DOC concentrations in lakes in this area (Table 1), the potential importance of mixotrophic/hetereotrophic flagellates in general and chrysophytes in particular, further studies on the microbial loop are probably warranted.
References
Anderson NJ, Bennike O, Christoffersen K, Jeppesen E, Markager S, Miller G, Renberg I (1999) Limnological and paleolimnological studies of lakes in south-western Greenland. Geol Greenland Surv Bull 183:68–74
Anderson NJ, Harriman R, Ryves DB, Patrick ST (2001) Dominant factors controlling variability in the ionic composition of West Greenland lakes. Arctic Antarct Alp Res 33:418–425
Andersson A, Falk S, Samuelsson G, Hagström Å (1989) Nutritional characteristics of a mixotrophic nanoflagellate, Ochromonas sp. Microb Ecol 17:251–262
Bergström A-K, Blomqvist B, Jansson M (2005) Effects of atmospheric nitrogen deposition on nutrient limitation and phytoplankton biomass in unproductive Swedish lakes. Limnol Oceanogr 50:987–994
Bettez ND, Rublee PA, O’Brien J, Millers MC (2002) Changes in abundance, composition and controls within the plankton of a fertilised arctic lake. Freshw Biol 47:303–311
Bindler R, Renberg I, Anderson NJ, Appleby PG, Emteryd O, Boyle J (2001a) Pb isotope ratios of lake sediments in West Greenland: inferences on pollution sources. Atmos Environ 35:4675–4685
Bindler R, Renberg I, Appleby PG, Anderson NJ, Rose NL (2001b) Mercury accumulation rates and spatial patterns in lake sediments from West Greenland: A coast to ice margin transect. Environ Sci Technol 35:1736–1741
Bird DF, Kalff J (1987) Algal phagotrophy---regulating factors and importance relative to photosynthesis in Dinobryon (Chrysophyceae). Limnol Oceanogr 32:277–284
Blomqvist P, Herlitz E (1996) Methods for quantitative assessment of phytoplankton in freshwaters. Part 2. Literature and its use for determination of planktonic Volvocales, Tetrasporales, Chlorococcales, and Ulotrichales, and formulas for calculation of biovolume of the organisms. Naturvårdsverkets förlag, Rapport 4861, pp 1–70
Brodersen KP, Anderson J (2002) Distribution of chironomids (Diptera) in low arctic West Greenland lakes: trophic conditions, temperature and environmental reconstruction. Freshw Biol 47:1137–1157
Caron DA, Porter KG, Sanders RW (1990) Carbon, nitrogen and phosphorus budgets for the mixotrophic flagellate Poteriochromonas malhamensis (Chrysophyceae) during bacterial ingestion. Limnol Oceanogr 35:433–443
Cembella AD, Antia NJ, Harrison PJ (1984) The utilization of inorganic and organic phosphorous compounds as nutrients by eukaryotic microalgae: a multidisciplinary perspective: part 1. CRC Crit Rev Microbiol 10:317–391
Currie DJ, Kalff J (1984) A comparison of the abilities of freshwater algae and bacteria to acquire and retain phosphorus. Limnol Oceanogr 29:298–310
Drakare S, Blomqvist P, Bergström A-K, Jansson M (2003) Relationships between picophytoplankton and environmental variables in lakes along a gradient of water colour and nutrient content. Freshw Biol 48:729–740
Ellis-Evans JC, Galchenko V, Laybourn-Parry J, Mylnikov AP, Petz W (2001) Environmental characteristics and microbial plankton activity of freshwater environments at Kongsfjorden, Spitsbergen (Svalbard). Arch Hydrobiol 152:609–632
Elser JJ, Marzolf ER, Goldman CR (1990) Phosphorus and nitrogen limitation of phytoplankton growth in the freshwaters of North America: a review and critique of experimental enrichments. Can J Fish Aquat Sci 47:1468–1477
Fossing H, Cristensen PB, Dalsgaard T. Rysgaard S, Risgaard-Petersen N (2003) Sediment – intern belastning. In: Teknisk anvisning for marin overvågning-Bentiske parametre. Miljøministeriet Danmarks Miljøundersøgelser, pp 14, 1–14, 32
Gajewski K, Hamilton PB, McNeely R (1997) A high resolution proxy-climate record from an arctic lake with annually-laminated sediments on Devon Island, Nunavut, Canada. J Paleolimnol 17:215–225
Grasshoff K (1983) Determination of nitrate. In: Grasshof K, Ehrhardt M, Kremling K (eds) Methods of Seawater analysis. Verlag Chemie, Weinheim, pp 143–150
Guildford SJ, Hecky RE (2000) Total nitrogen, total phosphorus, and nutrient limitation in lakes and oceans: Is there a common relationship. Limnol Oceanogr 45:1213–1223
Hansen K (1967) The general limnology of arctic lakes as illustrated by examples from Greenland. Meddelelser om Grønland 178:1–77
Healey FP, Hendzel LL (1979) Indicators of phosphorus and nitrogen deficiency in five algae in culture. J Fish Res Board Can 36:1364–1369
Healey FP, Hendzel LL (1980) Physiological indicators of nutrient deficiency in lake phytoplankton. Can J Fish Aquat Sci 37:442–453
Istvánovics V, Petterson K, Pierson D, Bell R (1992) Evaluation of phosphorus deficiency indicators for summer phytoplankton in Lake Erken. Limnol Oceanogr 37:890–900
Jansson M, Blomqvist P, Jonsson A, Bergström A-K (1996) Nutrient limitation of bacterioplankton, autotrophic andmixotrophic phytoplankton, and heterotrophic nanoflagellates in Lake Örträsket. Limnol Oceanogr 41:1552–1559
Jansson M, Bergström A-K, Blomqvist P, Isaksson A, Johansson A (1999) Impact of allochtonous organic carbon on microbial food web dynamics and structure in Lake Örträsket. Arch Hydrobiol 144:409–428
Jansson M, Bergström A-K, Blomqvist P, Drakare S (2000) Allochtonous organic carbon and phytoplankton/bacterioplankton production relationships in lakes. Ecology 81:3250–3255
Jespersen A-M, Christoffersen K (1987) Measurements of chlorophyll a from phytoplankton using ethanol as extraction solvent. Arch Hydrobiol 109:445–454
Jeppesen E, Jensen JP, Jensen C, Faafeng B, Hessen DO, Søndergaard M, Lauridsen T, Brettum P, Christoffersen K (2003). The impact of nutrient state and lake depth on top-down control in the pelagic zone of lakes: A study of 466 lakes from the temperate zone to the Arctic. Ecosystems 6:313–325
Kalff J (1991) Phytoplankton dynamics in an arctic lake. J Fish Res Board Can 24:1861–1971
Kettle H, Thompson R, Anderson NJ, Livingstone DM (2004) Empirical modeling of summer lake surface temperatures in southwest Greenland. Limnol Oceanogr 49:271–282
Kristiansen J (1994) Preliminary studies on the distribution of silica-scaled chrysophytes in Greenland. Verh Internat Verein Limnol 25:2234–2236
Kuenzler EJ (1965) Glucose-6-phosphate utilization by marine algae. J Phycol 1:156–164
Laybourn-Parry J, Marshall WA (2003) Photosynthesis, mixotrophy and microbial plankton dynamics in two high arctic lakes during summer. Polar Biol 26:517–524
McGowan SM, Ryves DB, Anderson NJ (2003) Holocene records of effective precipitation in West Greenland. Holocene 13:239–249
Menzel DW, Corwin N (1965) The measurement of total phosphorus in seawater based on the liberation of organically bound fractions by persulphate oxidation. Limnol Oceanogr 10:280–282
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
O’Brien WJ, Bahr M, Hershey AE, Hobbie JE, Kipphut GW, Kling GW, Kling H, McDonald M, Miller MC, Rublee P, Vestal JR (1997) The limnology of Toolik Lake. In: Milner A, Oswood MW (eds) Alaskan freshwaters. Springer, Berlin Heidelberg New York, pp 61–106
Overpeck J, Hughen K, Hardy D, Bradley R, Case R, Douglas M, Finney B, Gajewski K, Jacoby G, Jennings A, Lamoureux S, Lasca A, MacDonald G, Moore J, Retelle M, Smith S, Wolfe A, Zielinski G (1997) Arctic environmental change of the last four centuries. Science 278:1251–1256
Pålsson C, Daniel C (2004). Effects of prey abundance and light intensity on nutrition of a mixotrophic flagellate and its competitive relationship with an obligate heterotroph. Aquat Microb Ecol 36:247–256'
Pettersson K (1980) Alkaline phosphatase activity and algal surplus phosphorus as phosphorus-deficiency indicators in Lake Erken. Arch Hydrobiol 89:54–87
Pla S, Anderson NJ (2005) Environmental factors correlated with chrysophyte cyst assemblages in low arctic lakes of south-west Greenland. J Phycol 41:957–974
Redfield AC (1958) The biological control of chemical factors in the environment. Am Sci 46:205–221
Rengefors K, Ruttenberg KC, Haupert CL, Taylor C, Howes BL, Anderson DM (2003) Experimental investigation of taxon-specific response of alkaline phosphatase activity in natural freshwater phytoplankton. Limnol Oceanogr 48:1167–1175
Reynolds CS (1984) The ecology of freshwater phytoplankton. Cambridge University Press, Cambridge
Rhee G-Y (1972) Competition between an algae and an aquatic bacterium for phosphate. Limnol Oceanogr 17:505–514
Rothhaupt KO (1996) Utilization of substitutable carbon and phosphorus sources by the mixotrophic chrysophyte Ochromonas sp. Ecology 77:706–715
Rühland K, Priesnitz A, Smol JP (2003) Paleolimnological evidence from Diatoms for recent environmental changes in 50 lakes across Canadian arctic treeline. Arctic Antarct Alp Res 35:110–123
Ryves DB, McGowan S, Anderson NJ (2002) Development and evaluation of a diatom-conductivity model from lakes in West Greenland. Freshw Biol 47:995–1014
Sanders RW, Caron DA, Davidson JM, Dennet MR, Moran DM (2001) Nutrient acquisition and population growth of a mixotrophic alga in axenic and bacterized cultures. Microb Ecol 42:513–523
Serreze MC, Walsb JE, Chapin FS, Osterkamp T, Dyurgerov M, Romanovsky V, Oechel WC, Morison J, Zhang T, Barry RG (2000) Observational evidence of recent change in the northern high-latitude environment. Clim Change 46:159–207
Sorvari S, Korhola A, Thompson R (2002) Lake diatom response to recent Arctic warming in Finnish Lapland. Global Change Biol 8:171–181
Stockner JG (1991) Autotrophic picoplankton in freshwater ecosystems: the view from the summit. Int Rev ges Hydrobiol 76:483–492
Stockner JG, Antia NJ (1986) Algal picophytoplankton from marine and freshwater ecosystems: a multidisciplinary perspective. Can J Fish Aquat Sci 43:2472–2503
Valderrama JC (1995) Methods of nutrient analysis. In: Hallegraeff GM, Anderson DM, Cembella AD (eds) Manual of harmful marine microalgae. IOC Manuals and guides No 33, UNESCO, pp 251–268
Vincent WF (1981) Rapid physiological assays for nutrient demand by the plankton. II. Phosphorus. J Plankton Res 3:699–710
Williams WD (1991) Comments on the so-called salt lakes of Greenland. Hydrobiologia 210:67–74
Wolfe AP, Baron JS, Cornett RJ (2001) Anthropogenic nitrogen deposition induces rapid ecological changes in alpine lakes of the Colorado Front Range (USA). J Paleolimnol 25:1–7
Acknowledgements
The fieldwork was supported by a grant to NJA by the Danish Natural Science Research Council (SNF). The project was sponsored by the Biology Education Program for Master’s Theses, Lund University. H. Perren, B. Perren, P. Christiansen are gratefully thanked for assistance in the field. M. Svensson is acknowledged for help with APA analyses, and C. Pålsson for identification of mixotrophic flagellates. Dr P. Carlsson helped with nitrogen analysis and provided helpful comments on the manuscript. We are grateful for the constructive comments provided by the reviewers.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brutemark, A., Rengefors, K. & Anderson, N.J. An experimental investigation of phytoplankton nutrient limitation in two contrasting low arctic lakes. Polar Biol 29, 487–494 (2006). https://doi.org/10.1007/s00300-005-0079-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-005-0079-0