Abstract
The antifreeze protein gene (Cn-AFP) from the Antarctic marine diatom, Chaetoceros neogracile was cloned and characterized. The full-length Cn-AFP cDNA contained an open reading frame of 849 bp and the deduced 282 amino acid peptide chain encodes a 29.2 kDa protein, which includes a signal peptide of 30 amino acids at the N terminus. Both the Cn-AFP coding region with and without the signal sequence were cloned and expressed in Escherichia coli. Recombinant Cn-AFPs were shown to display antifreeze activities based on measuring the thermal hysteresis and modified morphology of single ice crystals. Recombinant mature Cn-AFP showed 16-fold higher thermal hysteresis activity than that of pre-mature Cn-AFP at the same concentration. The ice crystal shape changed to an elongated hexagonal shape in the presence of the recombinant mature Cn-AFP, while single ice crystal showed a circular disk shape in absence of Cn-AFP. Northern analysis demonstrated a dramatic accumulation of Cn-AFP transcripts when the cells were subjected to freezing stress. This rapid response to freeze stress, and the antifreeze activity of recombinant Cn-AFPs, indicates that Cn-AFP plays an important role in low temperature adaptation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ocean is the largest biosphere on Earth in regards to area and volume (Morgan-kiss et al. 2006). In addition, 90% of the ocean provides a cold ecosystem, where the water is 5°C or colder (Thomas and Dieckmann 2002), particularly the deep sea and polar regions.
A cold environment influences organisms in many ways, including changes in membrane fluidity (White et al. 2000; Nichols et al. 1993), nutrient availability, reduced biochemical reaction rates (Wiebe et al. 1992; Karasova-Lipovova et al. 2003), energy balancing between absorption and utilization (Parker and Armbrust 2005), and the ability to reproduce successfully (Margesin 2007). Cold-adaptive organisms are able to thrive in these low-temperature environments and have developed a variety of strategies on the molecular level for successful survival (Morgan-Kiss et al. 2006). Cold-adaptive animals, such as fish and insects, use two main adaptive mechanisms to protect them against low temperatures. The first mechanism involves the high accumulation of low-molecular-weight cryoprotectants that suppress the freezing and supercooling point (Storey and Storey 1996), while the second entails the production of an antifreeze protein (AFP) that inhibits the growth of ice crystals and decreases the freezing point of body liquids (Duman 2001; Duman et al. 2004; Davies et al. 2002).
Antifreeze proteins, which decrease the freezing point noncolligatively, referred to as thermal hysteresis (TH) activity, and inhibit ice recrystallization, have been found in numerous freezing-tolerant organisms (Walker et al. 2006; Wilson 1994; Gilbert et al. 2004; DeVries and Chang 1992; Duman and Olsen 1993; Duman 2001). The mechanism of thermal hysteresis activity is presumed to bind to ice surfaces and inhibit ice crystal growth via an adsorption–inhibition mechanism (Raymond and DeVries 1977; Raymond et al. 1989; Knight et al. 1991; Dalal and Sonnichsen 2002; DeVries 1986). Once bound to ice, the AFPs then lower the local freezing point by forcing the ice to grow in curved fronts between the bound AFP molecules (DeVries 1986; Wilson et al. 2006).
Antifreeze proteins have already been applied in various areas. For example, plants transformed with an AFP gene exhibit resistance to freezing and cold weather damage (Davies 1987; Cutler et al. 1989; Meyer et al. 1999). Antifreeze proteins have also been used to help preserve oocytes, red blood cells, and rat livers (Rubinsky et al. 1991; Carpenter and Hansen 1992; Lee et al. 1992), and tested as a chemical adjuvant to facilitate the selective destruction of cells in cryosurgery (Koushafar et al. 1997). Furthermore, antifreeze proteins can be used for food preservation and effective fermentation at low temperatures (Margesin et al. 2007).
Recently, over-expression systems and biotechnological applications of AFPs have been actively studied (Graham et al. 1997; Muryoi et al. 2004; Raymond et al. 2007; Davies 1987; Cutler et al. 1989; Parody-Morreale et al. 1988; Rubinsky et al. 1991; Carpenter and Hansen 1992; Lee et al. 1992; Koushafar et al. 1997; Margesin et al. 2007; Graham et al. 2007; Yue and Zhang 2009; Young and Fletcher 2008; Solomon and Appels 1999; Huang and Duman 2002; Graham et al. 2008; Garnham et al. 2008). Most polar diatoms flourish in the open sea and at the sea ice–water interface at temperatures ranging from −1.8 to 5°C (Mock and Valentin 2004). As a psychrophilic diatom, Chaetoceros neogracile is a major biomass producer that can thrive in extreme environments, like polar oceans (Hwang et al. 2008).
Accordingly, this study aims at understanding of psychrophilic adaptation of polar diatom by analyzing biochemical and biophysical properties of antifreeze protein from C. neogracile. Hence, this study reports on the expression of the C. neogracile AFPs in Escherichia coli and describes the antifreeze activities of this recombinant AFPs demonstrating thermal hysteresis and modification of the morphology of single ice crystals in a solution. Possible applications of this gene and its product were also proposed.
Materials and Methods
Diatom Growth Conditions
The Antarctic marine diatom, C. neogracile, which was provided by the Korea Polar Research Institute and obtained near Ice-cliff, Marian Cove, King George Island, Antarctica, was axenically cultured at approximately 4°C under 20 μmol photon m−2 s−1 (24 h light) in a modified f/2 medium (Jung et al. 2007). The illumination was provided from the top using cool white fluorescent lights. The photon flux density was measured using a quantum meter (Li-Cor, Lincoln, NE), while the cell densities were determined by microscopic counting using hematocytometer (Neubauer, Marienfeld, Germany) and are presented as the means ± SD. To employ freeze stress, the cultures were incubated at −20°C for 20, 40, and 60 min, respectively. The medium started to freeze after 20 min, half of the medium became ice after 40 min passed, and after 60 min, the medium became like ice-sherbet. Then, freeze-stressed cells were thawed in the 4°C for 30 min. After the thawing, the cells were transported to the new culture medium. The viability of the freeze-stressed cells was then determined by measuring the cell concentration after subsequent incubation at 4°C for 4 days by microscopic counting using hematocytometer and the cell concentration was presented as the means ± SD. To assay the antifreeze activity, each cell media was filtered using Nitrocellulose filters with pore diameters of 0.22 μm (Millipore) and then concentrated using an Amicon concentrator (Stirred Ultrafiltration Cell, Millipore). The membrane molecular cutoff was 10,000 Da.
Sequencing and Phylogenetic Analysis
After compiling expressed sequence tag data for C. neogracile and sequencing all the clones with an insert size greater than 0.5 kb (Jung et al. 2007), a Genbank search was conducted to find homologues from other species. One positive clone was identified as the putative C. neogracile AFP (Cn-AFP), which consisted of 994 bp and contained an open reading frame of 849 bp. This sequence was deposited in the GenBank database under accession number FJ505233. The deduced amino acid sequence of the Cn-AFP was aligned with those of AFPs or ice-binding proteins (IBPs) from other psychrophilic organisms, such as fungi, Antarctic soil bacteria, sea ice diatoms, snow mold, and Antarctic plants, using the Clustal W program. The sequences were obtained from the NCBI database (Navicula glaciei, AAZ76251; Fragilariopsis cylindrus, CN212299; Fragilariopsis curta, ACT99634; Chlamydomonas sp., EU190445; Typhula ishkarioensis, AB109748.1; Flammulina populicola, ACL27144; Lentinula edodes, ACL27145; Leucosporidium antarcticum ACX31168; Psychromona ingrahamii, ZP 01349469; Colwellia sp., DQ788793; Deschampsia Antarctica, FJ663038; Lolium perenne, FJ663045 ). On the basis of the alignment a phylogenetic tree was drawn using MEGA4 (www.megasoftware.net) and neighbor-joining clustering algorithm. The bootstrap values obtained with 1,000 repetitions are indicated as percentages at all the branches.
Northern Blot Analysis
The total RNA was extracted from samples and cleaned using a Plant RNeasy mini kit (Qiagen, USA). For a Northern blot analysis, the total RNA (10 µg) was denatured with 50% formamide and 6.3% formaldehyde, and separated on a denaturing agarose. The RNA was then blotted onto a nylon membrane (HybondTM-N+, Amersham) using an alkaline transfer, UV-cross-linked for 10 min, and hybridized to a random primer radio-labeled Cn-AFP cDNA probe for 24 h. The hybridization used the formamide hybridization buffer (KPL, USA). After that, the membrane was washed with 2× wash buffer (2X SSPE and 0.1% sodium dodecyl sulfate (SDS)) for 25 min, two times. The hybridization with the 32P-radio-labeled probe and washing of the gel blot were performed at 42°C. The membrane was then exposed to an X-ray film in −70°C for 16 h. Ethidium bromide (EtBr) staining of ribosomal RNA is included as a loading control. The relative amounts of Cn-AFP mRNA were estimated by densitometric scanning of the autoradiograms.
Southern Blot Analysis
The genomic DNA was isolated according to the protocol modified by Tanksley et al. (1995). The cells were centrifuged, and the pellet re-suspended in 750 µl of a microprep buffer and voltexed. The microprep buffer consisted of a 2.5× extract buffer(0.35 M Sorbitol, 0.1 M Tris/HCl pH 7.5, and 5 mM EDTA), 2.5× nuclei lysis buffer (0.2 M Tris/HCl pH 7.5, 0.05 M EDTA, 2 M NaCl, and 2% (w/v) CTAB), and 1× 5% N-Lauroylsarcosine. The isolated and purified genomic DNA (10 µg) was then digested with restriction enzymes (EcoRV, NcoI, and SacI), size-fractionated on a 0.7% agarose gel, and transferred to a nylon membrane (HybondTM-N+, Amersham). The DNA gel blotting was performed following standard protocols (Sambrook and Russell 2001), and the Cn-AFP labeled 32P-dCTP used as a probe. The Southern hybridizations were performed following the QuikHyb Hybridization protocols (Stratagene, USA). The last wash for the blots after hybridization was in 0.2× SSC containing 0.1% (v/v) SDS at 65°C for 30 min.
Construction of C. neogracile AFP Expression Vector and Over-expression of Cn-AFP in E. coli
To obtain a C. neogracile Cn-AFP coding region with or without a signal peptide, the C. neogracile cDNA clone was amplified using two primer sets; the first primer sets for Cn-AFP coding region with signal peptide: 5′-CCGGAATTCATGAGTCTTATTACA-3′ and 5′-TGCCTCGAGTTAGTTTGGTGC-3′, the second primer sets for Cn-AFP coding region without signal peptide: 5′-CCGGAATTCCTCCGTCAGGAGAAA-3′ and 5′-TGCCTCGAGTTAGTTTGGTGC-3′. EcoRI and XhoI site were introduced at 5′ end and 3′ end of Cn-AFP coding region with signal peptide and without signal peptide, respectively. Both amplified Cn-AFPs were purified. The purified DNAs and the pProEX-HTa expression vector (Intvitrogen, USA) were cut with EcoRI and XhoI. Digested inserts and vector DNAs were purified respectively, and were ligated overnight at 16°C using T4 DNA ligase. E. coli BL21 (DE3) competent cells were made by CaCl2 and transformed by standard heat shock method (Sambrook and Russell 2001). The transformed E. coli cells were grown in an LB medium containing 50 μg/ml of ampicillin. At a cell density (OD600) of 0.5, gene expression was induced by adding IPTG to a final concentration of 1 mM. The transformed cells were harvested after 4 h of incubation at 37°C, and then re-suspended in an ice-cold lysis buffer (20 mM Tris, pH 7.9, 0.5 M NaCl) at 10 ml per g wet weight. Thereafter, the cells were sonicated three times for 1 min per burst using a sonicator (Branson, USA) at a setting of 20%. The homogenate was centrifuged at 20,000×g at 4°C for 20 min for separation into the supernatant and pellet fractions. The supernatant was analyzed by SDS-polyacrylamide gel electrophoresis (PAGE).
Purification of Recombinant Cn-AFPs
To purify the expressed C. neogracile AFP protein, the recombinant pre-mature and mature AFPs were both purified by affinity chromatography using Ni-NTA agarose (Qiagen, Germany). Briefly, the crude supernatant fractions were loaded on a Ni-NTA agarose column. After washing the column twice with a wash buffer (50 mM NaH2PO4, pH 8.0, 20 mM imidazole, and 0.3 M NaCl; 4 ml), the recombinant Cn-AFPs were eluted in six fractions (0.5 ml) using an elution buffer (50 mM NaH2PO4, pH 8.0, 250 mM imidazole, and 0.2 M NaCl). The protein concentrations in the samples were determined using a Bio-Rad protein assay according to the manufacturer's instructions (Bio-Rad, USA). The samples were separated by SDS-PAGE (12% polyacrylamide), as described by Laemmli (1970).
Antifreeze Activity
The thermal hysteresis of the cultural filtrates of C. neogracile and purified recombinant Cn-AFPs were measured using a nanoliter osmometer (Otago Nanoliter-osmometer, New Zealand). The recombinant Cn-AFPs were concentrated to 1 mg/ml and then serially diluted. The thermal hysteresis measurements were repeated at least three times.
The antifreeze activity was further assayed by observing the morphology of ice crystals grown in the presence and absence of Cn-AFP, as described and slightly modified by Kobashigawa et al. (2005) and Bravo and Griffith (2005). The protein samples (0.2−0.4 μl) were applied to the center of a temperature-controlled freezing stage (Otago Nanoliter-osmometer, New Zealand) attached to the stage of a conventional microscope (Zeiss Axiolab, Zeiss), then frozen at −20°C for 5 min and warmed. The warming was slowed to 5°C min−1 to thaw the sample until only a single ice crystal was present. Thereafter, the temperature was lowered to a rate of 0.01°C min−1 in order to observe the ice crystal growth. Images of the ice crystals were captured under the microscope using a CCD camera system including a video recorder (Cannon Power Shot A620, Japan). In this assay, ice crystals that are a circular disk shape when grown in solution indicate the absence of antifreeze activity, while hexagonally shaped ice crystals indicate the presence of antifreeze activity.
Statistics
Experiments were conducted at least three times, and the results are presented by the mean ± SD of the raw data. The statistical significance of differences between average values was determined with one-way variance analysis, followed by Tukey’s HSD post hoc test. The level of statistical significance was set at p < 0.05. All statistical analyses were performed using the SPSS 17.0 software (SPSS, USA).
Results and Discussions
Isolation of C. neogracile AFP and Sequence Analysis
An EST database was previously established for C. neogracile and all the clones sequenced (Jung et al. 2007). Thus, the identity of the C. neogracile AFP cDNA clone was assigned by comparing the amino acid sequences in various databases using the alignment search tool (TBLASX) program through NCBI. The Cn-AFP cDNA clone (994 bp) contained an open reading frame of 849 bp, and the deduced 282 amino acid peptide chain encoded a 29.2 kDa protein (Fig. 1). A conventional N-terminal signal peptide was found using the SignalP signal peptide prediction tool to analyze the amino acid sequence (Nielsen et al. 1997), which revealed that the Cn-AFP contained a signal peptide of 30 amino acids at the N terminus. The signal peptide of the Cn-AFP contained a single glycosylation site. Hence, the molecular mass of the mature Cn-AFP was estimated as 26.2 kDa.
Diversity and Phylogenetic Analysis of C. neogracile AFP with Other Psychrophilic Organisms
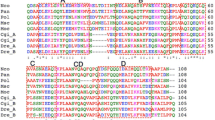
The Cn-AFP exhibited a significant homology with the AFPs or IBPs from other psychrophilic organisms (Fig. 2). IBPs were formerly called ice-active substances (IASs) due to their strong interaction with ice (Raymond et al. 1994; Raymond 2000), causing pitting and other deformities on the surface of growing ice crystals. Later, IASs were renamed as IBPs (Janech et al. 2006). The IBP from the sea ice diatom, N. glaciei showed the highest identity (57.2%) with the Cn-AFP, which also shared a 40.4%, 38.3%, 47.3%, 39.5%, and 41.1%, amino acid sequence identity with the AFPs from F. cylindrus and F. curta (sea ice diatoms), T. ishikariensis (snow mold), Colwellia sp.(Antarctic sea ice bacteria), and P. ingrahamii (psychrophilic bacteria), respectively. However, Cn-AFP showed 11% identity to IBP from psychrophilic green alga, Chlamydomonas sp., which is likely to have ice binding domains found in insect or rye grass AFP (Raymond et al. 2009). A phylogenetic tree was estimated using the sequence alignment shown in Fig. 2, and also included higher plant sequences as out-group sequences. The phylogenetic tree revealed that the Cn-AFP was evolutionally close to the AFP of the Antarctic sea ice diatom, N. glaciei, and somewhat related to eukaryotic AFPs (other sea ice diatoms and snow mold) and snow ice bacterial IBPs; however, it was distinct from higher plant AFP or ice recrystallization inhibition protein (IRIP; Fig. 3).
Phylogenetic tree of selected psychrophilic AFPs, IAFP, IRIP, or IBP amino acid sequences. The sequences were obtained from the NCBI database, and aligned using the Clustal W alignment program. Neighbor-joining tree of amino acid sequences representing AFP from C. neogracile. IAFP ice antifreeze protein, IRIP ice recrystallization inhibition protein
C. neogracile AFP Gene is Encoded by Gene Family and Response to Freezing Stress
C. neogracile genome was examined using the full-length Cn-AFP cDNA as a probe of a Southern blot under very stringent hybridization and washing conditions (Fig. 4). The genomic DNA from C. neogracile was digested using EcoRV, NcoI, and SacI (enzymes that do not cleave within the Cn-AFP gene), transferred to a nylon membrane, and probed with the full-length Cn-AFP cDNA. More than two hybridizing fragments are detected by probing of a Southern blot of C. neogracile genomic DNA with Cn-AFP, indicating that there is a small family of Cn-AFP genes in the C. neogracile genome.
Southern blot analysis. The purified cell nuclear DNA was digested with EcoRV, NcoI, and SacI, fractionated on a 0.7% agarose gel (10 µg per lane), and stained with ethidium bromide. The DNA fragments were transferred to a nylon membrane and hybridized with the Cn-AFP full-length cDNA. The sizes of the DNA markers are shown to the left of the panel in kilobase. E EcoRV, N NcoI, S SacI. Numbers at right indicate estimated lengths of the fragments in kilobases
Previous studies had shown that the IBP gene belongs to a multi-gene family in N. glaciei, with at least five IBP genes in N. glaciei (Genbank accession no. AAZ76251, AAZ76252, AAZ76250, AAZ76253, AAZ76249; Janech et al. 2006). Recently, several isoforms of IBP from Antarctic intertidal Chlamydomonas strain displaying the inhibition of recrystallization of ice were also reported (Raymond et al. 2009). In animals, AFPs are commonly present in multiple isoforms. For example, the AFP has 13 isoforms in the beetle Dendroides canadensis (Andorfer and Duman 2000), 30–50 copies in winter flounder (Scott et al. 1985; Gourlie et al. 1984), and 80 copies in wolffish (Scott et al. 1988). When extensive sequencing has been done at the protein, cDNA, and/or genomic levels in fish or insects, there is evidence of multiple isoforms, many of which only differ by a few conservative amino acid replacements (Graham et al. 2007). In the present study, the result of a Southern blot implied the existence of either more than two copies of the AFP gene or other AFP like genes in the C. neogracile genome. However, only single Cn-AFP gene was isolated and studied further in this study.
A Northern blot analysis was performed to investigate the modulation of steady-state levels of the Cn-AFP mRNA in response to different freezing stresses (Fig. 5). The freezing stress was set at −20°C for 20, 40, and 60 min, respectively, and the stress condition determined according to the status of the medium. The medium started to freeze after 20 min, was half frozen after 40 min, and became like an ice-sherbet after 60 min. Cn-AFP cDNA was used as probe in Northern blot analyses of C. neogracile treated under different freezing conditions. The gene expression from the Northern blot is normalized to total RNA as determined from the EtBr-stained gel. The Cn-AFP transcript increased 1.5-fold after 20 min of freezing stress, was enhanced and peaked after 40 min of freezing stress, and then declined thereafter (Fig. 5b). The reduction in the amount of the Cn-AFP transcript after 60 min of freezing stress was explained by the results of the cell re-growth after thawing (Fig. 5c). The cells that survived from the freezing stress were re-suspended under normal growth conditions and counted. However, after 60 min of freezing stress, the level of Cn-AFP transcripts started to decline, which was due to a 40% reduction in cell growth when the cells subjected to freezing stress for 60 min were thawed. In contrast, the control cell and cells subjected to freezing stress for 20 min grew well after thawing, whereas the cells frozen at −20°C for 40 min exhibit no growth. Thus, the experimental conditions, probably prolonged freezing stress with osmotic imbalance, resulted in a reduction of re-growth by the cells (Fig. 5c) that had been frozen for 60 min and then thawed.
Northern blot analysis of C. neogracile AFP under various freezing stresses. The Cn-AFP gene expression increased under freezing stress. a Autoradiogram of Northern blots and ethidium-bromide-stained gel (to serve as a loading control). b Quantitation of Cn-AFP mRNA based on (a). Quantified and normalized mRNA levels are shown relative to the control for each experiment. Lanes show the accumulation of the mRNAs of the Cn-AFP when the cells were frozen at −20°C for zero minutes (control), 20, 40, and 60 min, respectively. Values are mean ± SD (n = 9). **p < 0.01 compared to control. C re-growth curves of cells at 4°C, when the cells were thawed after each freeze stress for zero minutes (control), 20, 40, and 60 min at −20°C, respectively. Values are mean ± SD (n = 12). **p < 0.01 compared to control
Expression of Recombinant C. neogracile AFP in E. coli
The over-expression of AFPs have been actively studied in relation to many organisms (Graham et al. 1997; Raymond et al. 2007; Davies 1987; Cutler et al. 1989; Parody-Morreale et al. 1988; Rubinsky et al. 1991; Carpenter and Hansen 1992; Lee et al. 1992; Koushafar et al. 1997; Margesin et al. 2007; Graham et al. 2007; Yue and Zhang 2009), except for the case of diatom. To demonstrate that the Cn-AFP encodes a protein with antifreeze activity, recombinant Cn-AFPs were produced using an E. coli expression system. In general, the AFPs from certain organisms, including Antarctic fish and the plant Solanum dulcamara, are known to be heavily glycosylated (Davies and Hew 1990; Duman 1994; Huang and Duman 2002). This characteristic of AFPs hinders expression in E. coli, as post-translational modification is unusual in a bacterial host. However, the Cn-AFP only contains a single putative glycosylation site, which is also located in the signal peptide region (Fig. 1).
In this research, the expressed Cn-AFP containing a signal peptide named as pre-mature Cn-AFP was also subjected to investigate AFP activity. Therefore, a Cn-AFP over-expression vector was constructed by combining the pre-mature Cn-AFP and mature Cn-AFP (mature Cn-AFP:amino acid from 31 to 282) with a protein expression vector, and then transforming the constructed vector into E. coli. As a result, both recombinant pre-mature and mature Cn-AFP was created (Fig. 6) and their AFP activity compared (Table 1 and Fig. 7).
SDS-PAGE analysis of recombinant C. neogracile AFPs. SDS-PAGE analysis M protein marker, 1 un-induced cell lysates, 2 IPTG-induced total cell crude extract including recombinant of pre-mature Cn-AFP, 3 IPTG-induced total cell crude extract including recombinant of mature Cn-AFP, 4 affinity purified recombinant pre-mature Cn-AFP, 5 affinity purified recombinant mature Cn-AFP
The majority of the protein was accumulated in the soluble fraction of the whole-cell extract after induction by IPTG. Plus, SDS-PAGE revealed recombinant protein bands with a molecular mass of approximately 33 and 31 kDa for the pre-mature recombinant and mature Cn-AFP, respectively (Fig. 6a, lanes 2 and 3 from crude extract of E. coli, lanes 4 and 5 from affinity purified protein). The molecular mass of the expected native Cn-AFP was 29.2 kDa for the pre-mature and 26.2 kDa for mature, but the recombinant Cn-AFPs were larger than the native Cn-AFPs, due to the 6 His and spacer amino acid sequences translated from the pProEX vector.
Antifreeze Activity
To assay the antifreeze activity of the recombinant Cn-AFPs, the TH activity, and changes in the morphology of ice crystals were examined using a nanoliter osmometer. To reveal the recombinant Cn-AFP activity, the TH activity was measured, as represented by the difference between the melting point and freezing point, where a higher difference means a higher antifreeze activity. Thermal hysteresis is known to be the only method of providing a quantitative measurement of the antifreeze activity (Yang et al. 1998). The TH values were measured for the recombinant pre-mature and mature Cn-AFP of C. neogracile (Table 1). The recombinant mature Cn-AFP exhibited the highest TH value of 0.8°C, which was 16-fold higher than that of the recombinant pre-mature Cn-AFP at a concentration of 1 mg/ml. Bovine serum albumin was used as the negative control, as it does not show any TH activity.
The magnitude of thermal hysteresis, which depends on the specific activity of the AFP and its concentration, can range from 0.7 to 1.5°C in fishes and from 3 to 6°C in insects (Davies and Hew 1990; Duman 2001; Huang and Duman 2002). In the present study, the TH value of the recombinant mature Cn-AFP was 0.8°C when the concentration Cn-AFP was 1 mg/ml, while the typical fish TH value was approximately 1°C with a 10 mg/ml concentration (Garnham et al. 2008). In addition when the concentration of the recombinant Cn-AFP was increased, the TH activity also increased (Table 1). The antifreeze activity was further assayed by observing the morphology of ice crystals grown in the presence and absence of the Cn-AFP (Fig. 7). The single ice crystals grown in dilute solutions of the recombinant mature Cn-AFP in water showed an elongated hexagonal crystal form (Fig. 7b), probably due to the specific binding of the protein to the hexagonal faces, thereby inhibiting the deposition of water on those surfaces. In contrast, single ice crystals grown in water only or bovine serum albumin in water form flat circular disks (Fig. 7a) as seen in previous studies (Raymond et al. 1989; Bravo and Griffith 2005). Parallel experiments conducted with the vector-control culture induced by IPTG showed circular disk shape indicating absence of antifreeze activity (data not shown). The recombinant pre-mature Cn-AFP also formed irregular-shaped ice crystals (Fig. 7c), likely indicating an irregular manner of binding to the surfaces of the ice crystals. The lower TH activity of the recombinant pre-mature Cn-AFP (Table 1) also coincided with the weaker modification of the ice crystal morphology. The lower antifreeze activity of the recombinant pre-mature Cn-AFP relative to the mature Cn-AFP was probably due to the presence of the signal peptide protein that led to improper folding. It has been suggested that TH activity likely involves binding to the ice surfaces and inhibiting ice crystal growth via an adsorption-inhibition mechanism (Raymond and De-Vries 1977; Raymond et al. 1989; Knight et al. 1991; Huang and Duman 2002). The ability of antifreeze proteins to bind to ice causes a characteristic modification of the crystal morphology, resulting in elongated hexagonal forms (Bravo and Griffith 2005).
The examination of cells under freezing stress demonstrated a clear induction of Cn-AFP expression (Fig. 5) and the product of this gene exert antifreeze activity (Fig. 7). These properties may provide broad opportunities in cryopreservation. Cryopreservation has numerous applied uses, and various biotechnological applications are being developed from studies of cold-hardy organisms (Benson et al. 2004; Margesin 2007). Uses of cryopreservation in animal biology include the preservation of gametes for medical use, cell or tissue banking for transplantation, and the preservation of stocks of genetically diverse material for endangered species management (Wildt 2000), and laboratory experimentation (Buchholz et al. 2004). However, a new approach to cryopreservation is now the possible use of transgenics to transfer selected genes from hardy to non-hardy species to improve cold hardiness or freeze tolerance (Margesin et al. 2007). The expressions of AFPs could be applied to protect commercially important crop plants against cold temperatures. It has been reported that expressions of carrot AFP gene in Arabidopsis thaliana (Meyer et al. 1999) and Nicotiana tabacum (Worrall et al. 1998) and an insect AFP in A. thaliana (Huang et al. 2002) result in an accumulation of antifreeze activity (Atici and Nalbantoglu 2003).
In summary, this study investigated the expression and biochemical characterization of recombinant Cn-AFP shown to exhibit typical antifreeze activities measuring TH activity and single ice crystal morphological change. Rapid induction of Cn-AFP gene under freeze stress indicates Cn-AFP could be a key protein that plays an important role in the psychrophilic adaptation of the polar diatom, C. neogracile. Therefore, further exploration of this polar diatom AFP in regards to regulating its ice controlling mechanism and biotechnological research in cryopreservation using this diatom AFP are currently in progress.
References
Andorfer CA, Duman JG (2000) Isolation and characterization of cDNA clones encoding antifreeze proteins of the pyrochroid beetle Dendroides canadensis. J Insect Physiol 46:365–372
Atici O, Nalbantoglu B (2003) Antifreeze proteins in higher plants. Phytochem 64(7):1187–1196
Benson E, Fuller B, Lane N (2004) Life in the frozen state. CRC, Boca Raton
Bravo LA, Griffith M (2005) Characterization of antifreeze activity in Antarctic plants. J Exp Bot 56:1189–1196
Buchholz DR, Fu L, Shi YB (2004) Cryopreservation of Xenopus transgenic lines. Mol Reprod Dev 67:65–69
Carpenter JF, Hansen TN (1992) Antifreeze protein modulated cell survival during cryopreservation: mediation through influence on ice crystal growth. Proc Natl Acad Sci U S A 89:8953–8957
Cutler AJ, Saleem M, Kendall E, Gusta LV, Georges F, Fletcher GL (1989) Winter flounder antifreeze proteins improves the cold hardiness of plant tissues. J Plant Physiol 135:351–354
Dalal P, Sonnichsen FD (2002) Source of the ice-binding specificity of antifreeze protein type I. J Chem Inf Comput Sci 40:1276–1284
Davies PL (1987) Antifreeze protein: prospects for transferring freeze resistance. New Biotechnol 1:11–16
Davies PL, Hew CL (1990) Biochemistry of fish antifreeze proteins. FASEB J 4:2460–2468
Davies PL, Baardsnes J, Kuiper MJ, Walker VK (2002) Structure and function of antifreeze proteins. Philos Trans R Soc London Ser B 357:927–933
DeVries AL (1986) Antifreeze glycopeptides and peptides: interactions with ice and water. Methods Enzymol 12:293–303
DeVries AL, Chang CHC (1992) The role of antifreeze glycopeptides and peptides in the survival of cold-water fishes. In: Somero GN, Osmond CB (eds) Water and life: comparative analysis of water relationships at the organismic, cellular and molecular level. Springer, Berlin
Duman JG (1994) Purification and characterization of a thermal hysteresis protein from a plant, the bittersweet nightshade Solanum dulcamara. Biochem Biophys Acta 1206:129–135
Duman JG (2001) Antifreeze and ice nucleator proteins in terrestrial arthropods. Annu Rev Physiol 63:327–357
Duman JG, Olsen TM (1993) Thermal hysteresis protein activity in bacteria, fungi, and phylogenetically diverse plants. Cryobiology 30:322–328
Duman JG, Bennett V, Sformo T, Hochstrasser R, Barnes BM (2004) Antifreeze proteins in Alaskan insects and spiders. J Insect Physiol 50:259–266
Garnham CP, Gilbert JA, Hartman CP, Campbell RL, Laybourn-Parry J, Davies PL (2008) A Ca2+-dependent bacterial antifreeze protein domain has a novel β-helical ice-binding fold. Biochem J 411:171–180
Gilbert JA, Hill PJ, Dodd CE, Laybourn-Parry J (2004) Demonstration of antifreeze protein activity in Antarctic lake bacteria. Microbiology 150:171–180
Gourlie B, Lin Y, Price J, DeVries AL, Powers D, Huang RC (1984) Winter flounder antifreeze proteins: a multigene family. J Biol Chem 259:14960–14965
Graham LA, Liou YC, Walker VK, Davies PL (1997) Hyperactive antifreeze protein from beetles. Nature 388:727–728
Graham LA, Qin W, Lougheed SC, Davies PL, Walker VK (2007) Evolution of hyperactive, repetitive antifreeze proteins in beetles. J Mol Evol 64:387–398
Graham LA, Marshall CB, Lin FH, Campbell RL, Davies PL (2008) Hyperactive antifreeze protein from fish contains multiple ice-binding sites. Biochemistry 47:2051–2063
Huang T, Duman JG (2002) Cloning and characterization of a thermal hysteresis(antifreeze) protein with DNA-binding activity from winter bittersweet nightshade, Solanum dulcamara. Plant Mol Biol 48:339–350
Huang T, Nicodemus J, Zarka DG, Thomashow MF, Wisniewski M, Duman JG (2002) Expression of an insect (Dendroides canadensis) antifreeze protein in Arabidopsis thaliana results in a decrease in plant freezing temperature. Plant Mol Biol 50:333–344
Hwang YS, Jung G, Jin ES (2008) Transcriptome analysis of acclimatory responses to thermal stress in Antarctic algae. Biochem Biophys Res Com 367:635–641
Janech MG, Krell A, Mock T, Kang JS, Raymond JA (2006) Ice-binding protein from sea ice diatom (Bacillariphyceae). J Phycol 42:410–416
Jung G, Lee CG, Kang SH, Jin E (2007) Annotation and expression profile analysis of cDNAs from the Antarctic diatom Chaetoceros neogracile. J Microbiol Biotechnol 17:1330–1337
Karasova-Lipovova P, Strnad H, Spiwok V, Mala S, Kralova B, Russell NJ (2003) The cloning, purification and characterization of a cold-active α-galactosidase from the psychrotolerant Antarctic bacterium Arthrobacter sp. C2-2. Enzyme Microb Technol 33:836–844
Knight CA, Cheng CC, DeVries AL (1991) Adsorption of α-helical antifreeze peptides on specific ice crystal surface planes. Biophys J 59:409–418
Kobashigawa Y, Nishimiya Y, Miura K, Ohgiya S, Miura A, Tsuda S (2005) A part of ice nucleation protein exhibits the ice-binding ability. FEBS Lett 579:1493–1497
Koushafar H, Pham L, Lee C, Rubinsky B (1997) Chemical adjuvant cryosurgery with antifreeze proteins. J Surg Oncol 66:114–121
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lee CY, Rubinsky B, Fletcher GL (1992) Hypothermic preservation of whole mammalian organs with antifreeze proteins. Cryo-Letters 13:59–66
Margesin R (2007) Alpine microorganisms: useful tools for low-temperature bioremediation. J Microbiol 45:281–285
Margesin R, Neuner G, Storey KB (2007) Cold-loving microbes, plants, and animals-fundamental and applied aspects. Naturwissenschaften 94:77–99
Meyer K, Keil M, Naldrett MJ (1999) A leucine-rich repeat protein of carrot that exhibits antifreeze activity. FEBS Lett 447:171–178
Mock T, Valentin K (2004) Photosynthesis and cold acclimation: molecular evidence from a polar diatom. J Phycol 40:732–741
Morgan-Kiss RM, Priscu JC, Pocock T, Gudynaite-Savitch L, Huner NPA (2006) Adaptation and acclimation of photosynthetic microorganisms to permanently cold environments. Microbiol Mol Biol Rev 70:222–252
Muryoi N, Sato M, Kaneko S, Kawahara H, Obata H, Yaish MWF, Griffith M, Glick BR (2004) Cloning and expression of afpA, a gene encoding an antifreeze protein from the arctic plant growth-promoting rhizobacterium pseudomonas putida GR12-2. J Bacteriol 186:5661–5671
Nichols DS, Nichols PD, Sullivan CW (1993) Fatty acid, sterol and hydrocarbon composition of Antarctic sea ice diatom communities during the spring bloom in McMurdo Sound. Antarc Sci 5:217–278
Nielsen H, Engelbrecht J, Brunak S, Heijne GV (1997) Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng 10:1–6
Parker MS, Armbrust EV (2005) Synergistic effects of light, temperature, and nitrogen source on transcription of genes for carbon and nitrogen metabolism in the centric diatom Thalassiosira pseudonana (Bacillariophyceae). J Phycol 4:1142–1153
Parody-Morreale A, Murphy KP, Cera ED, Fall R, DeVries AL, Gill SJ (1988) Inhibition of bacterial ice nucleators by fish antifreeze glycoproteins. Nature 333:782–783
Raymond JA (2000) Distribution and partial characterization of ice-active molecules associated with sea-ice diatoms. Polar Biol 23:721–729
Raymond JA, DeVries AL (1977) Adsorption inhibition as a mechanism of freezing resistance in polar fishes. Proc Natl Acad Sci U S A 86:881–885
Raymond JA, Wilson PW, DeVries AL (1989) Inhibition of growth on nonbasal planes in ice by fish antifreeze. Proc Natl Acad Sci U S A 86:881–885
Raymond JA, Sullivan CW, DeVries AL (1994) Release of an ice-active molecule associated with sea ice diatoms. Polar Biol 14:71–75
Raymond JA, Fritsen CH, Shen K (2007) An ice-binding protein from an Antarctic sea ice bacterium. FEMS Microbiol Ecol 61:214–221
Raymond JA, Janech MG, Fritsen CH (2009) Novel ice-binding proteins from a psychrophilic Antarctic alga (Chlamydomonadaceae). J Phycol 45:130–136
Rubinsky B, Arav A, Fletcher GL (1991) Hypothermic protection—fundamental property of antifreeze proteins. Biochem Biophys Res Comm 180:566–571
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor
Scott GK, Hew CL, Davies PL (1985) Antifreeze protein genes are tandemly linked and clustered in the genome of the winter flounder. Proc Natl Acad Sci U S A 82:2613–2617
Scott GK, Davies PL, Kao MH, Fletcher GL (1988) Differential amplification of antifreeze protein genes in the pleuronectinae. J Mol Evol 27:29–35
Solomon RG, Appels R (1999) Stable high-level expression of a type I antifreeze protein in Escherichia coli. Protein Expr Purif 16:53–62
Storey KB, Storey JM (1996) Natural freezing survival in animals. Ann Rev Ecolog Syst 27:365–386
Tanksley SD, Ganal MW, Martin GB (1995) Chromosome landing: a paradigm for map-based gene cloning in plants with large genomes. Trends Genet 11:63–68
Thomas DN, Dieckmann GS (2002) Antarctic sea ice—a habitat for extremophiles. Science 295:641–644
Walker VK, Palmer GR, Voordouw G (2006) Freeze-thaw tolerance and clues to the winter survival of soil community. Appl Environ Microbiol 72:1784–1792
White PL, Wynn-Williams DD, Russell NJ (2000) Diversity of thermal responses of lipid composition in the membranes of the dominant culturable members of an Antarctic fellfield soil bacterial community. Antarc Sci 12:386–393
Wiebe WJ, Sheldon WM, Pomeroy LR (1992) Bacterial growth in the cold: evidence for an enhanced substrate requirement. Appl Environ Microbiol 58:359–364
Wildt DE (2000) Genome resource banking for wildlife research, management, and conservation. ILAR J 41:228–234
Wilson PW (1994) A model for thermal hysteresis utilizing the anisotropic interfacial energy of ice crystals. Cryobiology 31:406–412
Wilson SL, Kelley DL, Walker VK (2006) Ice-active characteristics of soil bacteria selected by ice-affinity. Environ Microbiol 8:1816–1824
Worrall D, Elias L, Ashford D, Smallwood M, Sidebottom C, Lilford P, Telford J, Holt C, Bowles D (1998) A carrot leucine-rich-repeat protein that inhibits ice recrystallization. Science 282:115–117
Yang DS, Hon WC, Bubanko S, Xue Y, Seetharaman J, Hew CL, Sicheri F (1998) Identification of the ice-binding surface on a type III antifreeze protein with a “flatness function” algorithm. Biophys J 74:2142–2151
Young HM, Fletcher GL (2008) Antifreeze protein gene expression in winter flounder pre-hatch embryos: implications for cryopreservation. Cryobiology 57:84–90
Yue CW, Zhang YZ (2009) Cloning and expression of Tenebrio molitor antifreeze protein in Escherichia coli. Mol Biol Rep 36:529–536
Acknowledgements
This work was supported by Grant No. R01-2006-000-10856-0 from the Korean Science and Engineering Foundation (KOSEF) funded by the Korean Government (MOST).
Author information
Authors and Affiliations
Corresponding author
Additional information
In Gyu Gwak and Woong sic Jung contributed equally to the paper.
Rights and permissions
About this article
Cite this article
Gwak, I.G., sic Jung, W., Kim, H.J. et al. Antifreeze Protein in Antarctic Marine Diatom, Chaetoceros neogracile . Mar Biotechnol 12, 630–639 (2010). https://doi.org/10.1007/s10126-009-9250-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-009-9250-x