Abstract
Root-knot nematodes are endoparasites whose mature females lodge and grow inside the root of some cultivated plants, leading to losses in productivity. Herein, we investigated if the infection of okra, Abelmoschus esculentus (Malvaceae), promoted by the root-knot nematode Meloidogyne incognita (Meloidogynidae) changes some agronomic traits of the host plant, as well as the cell wall composition of the root tissues. The okra Santa Cruz 47® cultivar was infected with a suspension of 5000 M. incognita juveniles. The inoculated and non-inoculated okra plants were then submitted to morphological analysis at the end of experiment, as well as histological (at 4, 11, 18, 39, ad 66 days after inoculation) and immunocytochemical analysis (control and 66 days after inoculation). Root-knot nematode infection reduced the dry weight of the stem system but, unexpectedly, the number and weight of fruits increased. At 11 days after inoculation, we detected the presence of giant cells that increased in number and size until the end of the experiment, at 66 days after inoculation. These cells came from the xylem parenchyma and showed intense and moderate labeling for epitopes recognized by JIM5 and JIM7. The presence of homogalacturonans (HGs) with different degrees of methyl esterification seems to be related to the injuries caused by the nematode feeding activity and to the processes of giant cell hypertrophy. In addition, the presence of HGs with high methyl-esterified groups can increase the cell wall porosity and facilitate the flux of nutrients for the root-knot nematode.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The root-knot nematode is one of the most relevant pathogens of cultivated plants around the world, affecting different cultures and leading to great losses in productivity (Jones et al. 2013; Silva et al. 2016). The genus Meloidogyne (Meloidogynidae) stands out as the main disease-causing agent for okra, Abelmoschus esculentus L. Moench (Malvaceae) (Pinheiro et al. 2013), leading to a loss of up to 27% in productivity (Sikora and Fernandez 2005). Okra is highly suitable for small farm agriculture (Filgueira 2008) since it involves low production costs, easy growth, and highly profitable revenue (Mota et al. 2000). It is also a versatile and nutrient-rich vegetable of great potential value, which may be used for its oil, fuel and biomass, and as animal feed (Martin 1982). Despite many benefits, okra culture is exceptionally susceptible to attack by nematodes, such as Meloidogyne incognita root-knot nematode, that could impact the productivity of the crop.
Root-knot nematodes are endoparasites whose globose and sedentary mature females lodge and grow inside the root of plants (Mitkowski and Abawi 2003). These nematodes feed on the stele cells of roots, inducing galls by cell division and hypertrophy of the root cortex (Williamson and Gleason 2003; Galbieri and Belot 2016). During the feeding activity of the root-knot nematodes, 5 to 8 feeding cells, the giant cells, develop in the vascular cylinder (Escobar et al. 2015). Giant cells are induced by the effector proteins secreted by the nematode, which interact with genes and proteins of the host cells (Bellafiore and Briggs 2010; Gheysen and Mitchum 2011; Ali et al. 2017), change their physiological and structural traits (Siddique and Grundler 2015; Juvale and Baum 2018; Smant et al. 2018), and lead to the success of the parasitism. Giant cell formation depends on the continuous feeding stimulus of the gall-inducing nematode, starting with successive nuclear divisions without cytoplasmic division (Mitkowski and Abawi 2003; Abad et al. 2009; Escobar et al. 2015), which makes the cells multinucleated. During giant cell development, the cell cytoplasm turns dense and the cell wall invaginates, increasing the cell surface and the absorption of photoassimilates, minerals, and other substances (Reddigari et al. 1985; Berg et al. 2009; Vilela et al. 2019). For instance, the giant cells induced by the root-knot nematode on Glycine max L. exhibited invaginations on their walls and numerous associated mitochondria (Vilela et al. 2019). The occurrence of numerous mitochondria in giant cells indicates a high energy demand (Golinowski et al. 1996; Hussey and Grundler 1998; Vieira et al. 2013) since giant cells are a specialized sink, supplying resources to the nematode until reproduction.
Few studies using a cellular approach have addressed the changes in cell wall composition promoted by infectious nematodes in host plants. As examples, distinct syncytial cell wall compositions were recorded in infectious processes induced by Heterodera schachtii in Arabidopsis thaliana, by Globodera pallida in potatoes, by Heterodera glycines in soybean, and by Heterodera avenae and H. filipjevi in spring wheat (Davies et al. 2012; Zhang et al. 2016). In the present study, we focused on how the infection by root-knot nematodes changes one of the most important components of the primary cell wall, i.e., pectic polysaccharide (see Albersheim et al. 2011). The main pectic polysaccharides are homogalacturonan (HG), rhamnogalacturonan-I (RG-I), rhamnogalacturonan-II (RG-II), xylogalacturonan (XGA), and apiogalacturonan (AP) (Ridley et al. 2001; Willats et al. 2001; Albersheim et al. 2011). Changes in cross-linking capacity with cations and the degree of methyl-esterification of these polysaccharides, especially HGs, can define the physical and biological properties of the cell walls (Willats et al. 2001; Albersheim et al. 2011; Liu et al. 2013; Oliveira et al. 2014; Martini et al. 2019). The dynamics and impact of Meloidogyne incognita on the host cell wall composition of the host have not been addressed yet. Thus, understanding the cell wall dynamics and composition of giant cells in relation to surrounding cells can provide new insights into the parasite-host interaction and the mechanism of nematode gall formation.
The current study evaluates the tissue and cellular changes promoted by the Meloidogyne incognita root-knot nematode on Abelmoschus esculentus. Cell wall composition was targeted here, with emphasis on protein and pectin modulation during giant cell development, which would help us understand how M. incognita respond to the A. esculentus infestation. Our objectives were (i) to assess the influence of M. incognita root-knot nematodes on the agricultural traits of the okra plant; (ii) determine the changes in the development, morphology, and histology of roots infected by M. incognita; and (iii) evaluate the changes of pectin and protein cell wall composition of root-knot nematode galls, with emphasis on giant cell events.
Material and methods
Experimental setup
The experiment was carried out in the greenhouse of Centro Universitário UniCerrado, Goiatuba municipality (18° 00' 45" S, 49° 21' 17" W), Goiás State, Brazil, between February and May 2019. Abelmoschus esculentus (Malvaceae), Okra Santa Cruz 47® cultivar (Fig. 1a), was selected for the experiment since it is the cultivar most extensively used by small cropping farmers in Brazil (Pinheiro 2017) and because of its high susceptibility to infection with the root-knot nematode Meloidogyne incognita (Meloidogynidae) (Oliveira et al. 2007).
Okra seeds were sown into 98-cell trays filled with Bioplant® substrate. Two weeks after emergence, seedlings were transplanted to 2.8-L vases with a substrate proportion of 1:1 sand/soil sterilized by autoclaving at 120 °C for 1 hour, along with 12 g of the fertilizer Forth Cote NPK (15-09-12). Six days after transplantation, the root-knot nematodes were inoculated into two holes around the roots by pipetting a 5-ml suspension of 5000 M. incognita juveniles (J2). Just the inoculated juvenile, females can induce gall on the roots. A total of 50 okra plants were inoculated and 50 others were kept as controls (i.e., non-inoculated plants) among two treatments with 10 repetitions. Each plot was composed of five vases and each vase contained one plant in a randomized block design.
Structural and agronomical traits of nematode galls
The structural traits resulting from gall infection were analyzed at 4, 11, 18, 39, and 66 days after inoculation (DAI). After evaluating the root changes in each DAI, we selected inoculated (n = 40) and non-inoculated (n = 40) okra plants at 66 DAI for specific agronomical trait analyses. At these stages, all plants were carefully removed from the vases and placed on paper towels to dry. The height of the stem system (from the collar to the terminal bud of the main axis) and the length of the roots (Fig. 1b) were measured. The fresh weight of the stem system, fruits, and roots was weighed on a digital scale (Balmak ELP-10), followed by drying in a kiln for 72 hours and weighing the dry weight.
The fruits were manually counted and weighed using a digital scale (Balmak ELP-10) for the assessment of productivity. Galls were isolated and counted for the estimation of gall index (GI) according to Taylor and Sasser (1978), with plants showing resistant reaction status when they have 0 to 10 galls and susceptible reaction status when they have ≥ 11 galls. The method of Coolen and D’Herde (1972) was used to extract and count the nematodes from 20 roots of okra plants. Galls were classified as large when measuring ≥ 1 cm and as small/medium galls when measuring ≤ 1 cm (Fig. 1c–e).
Histological features
Samples of inoculated okra roots (i.e., 4, 11, 18, 39, and 66 days after inoculation) and non-inoculated roots (i.e., 4 and 66 days after inoculation) were fixed in 4% paraformaldehyde (Roland and Vian 1991), dehydrated in a graded ethanol series (50, 70, 80, 90, and 95%), and embedded in 2-hydroxyethyl methacrylate Historesin® (Leica Instruments, Heidelberg, Germany). Next, all samples were cut into 6-μm transverse and longitudinal sections with a rotary microtome (YD315, ANCAP, Brazil) and stained with 0.05% toluidine blue, pH 4.7 (O'Brien et al. 1964). The sections were mounted with Entellan® and analyzed with a Leica® DM50 light microscope coupled to an HD camera.
Immunocytochemical approach
Roots were selected for immunocytochemistry at 66 days after inoculation and at 66 days after normal non-inoculated growth. The previously described Leica® Historesin protocol was used, but without sample staining or mounting. The samples were then incubated in blocking solution (3% powdered milk solution, Molico® in PBS) for 30 minutes to prevent cross-linking, followed by incubation with primary monoclonal antibodies (JIM 5, JIM 7, LM1, LM5, LM6) at 1:5 dilution in blocking solution for 1 hour in the dark. The samples were washed with PBS three times of 5 min each and incubated with the secondary anti-rat IgG FITC antibody (Sigma-Aldrich®) in blocking solution (1:100) for 2 hours in the dark. Samples of inoculated and non-inoculated roots without primary antibodies were used as controls. Finally, the samples were washed in PBS, mounted in glycerin/distilled water (1:1 v/v), and analyzed with a Leica® DM4000 B fluorescence microscope coupled to a Leica® DFC3000 G camera. We also use a DAPI filter (excitation spectrum 385–400 nm) for autofluorescence localization (Chomick et al. 2014; Joca et al. 2019) in an overlap function of the microscope software to better show the results of monoclonal antibodies.
The monoclonal antibodies labeled epitopes for low methyl-esterified HG (JIM 5), high methyl-esterified HG (JIM 7), extensin (LM1), (1→4) β-D-galactan (LM5), and (1→5) α-L-arabinan (LM6). Based on immunocytochemical images, the intensity of the fluorescence reaction was measured in the different tissues using gray scale methodology (Gy = gray value) and ImageJ version 1.51 k software (http://rsb.info.nih.gov/ij). Fluorescence intensity was measured in triplicate in each tissue and classified into the following three intensity categories: (−) negative (= 0 Gy values); (+) weak (< 15 Gy values); (++) moderate (15–30 Gy values), and (+++) intense (> 30 Gy values).
Data analyses
Statistical analyses were performed only for the morphological results. First, all data were tested for normal distribution of errors and homogeneity of variances and then analyzed by one-way ANOVA and the Tukey test at the 5% level of significance. Analyses were performed using the SISVAR software (Ferreira 2010).
Results
Agronomical traits
After 66 days, inoculated plants were taller than non-inoculated plants, although there were no differences in root length or fresh weight of the stem system and roots (Table 1). The dry weight of the stem system was greater for the non-inoculated treatment, while inoculated plants exhibited larger and more numerous fruits than non-inoculated ones (Table 1). In addition, 66 days after inoculation, there was counted 425 small/medium galls and 15 large galls for each gall system and 169 nematodes per 5 g of roots.
Gall morphology and development
The regular non-inoculated root of A. esculentus was light brown in color with small darkened portions and no pronounced protrusions (Fig. 2a). The roots of inoculated plants did not show any noticeable morphological alteration until day 11, when a slight thickening was first detected (Fig. 2b, c). The root-knot nematode galls greatly increased in size after 18, 39, and 46 days (Fig. 2d, e, f), with new lateral roots developing directly from the galls and the principal roots. After 66 days, we noted an increase in gall number on the root system, with these galls being larger, clustered, and of irregular shape. No changes in morphology were noticed in non-inoculated roots except for the regular thickening caused by normal growth (data not shown).
Morphological changes of okra roots 66 days after inoculation (DAI) of Meloidogyne incognita root-knot nematode. a Non-inoculated root; b 4 DAI, with no morphological signal of galls; c, d after 11 and 18 DAI, respectively, with the beginning of root thickening; e, f larger and numerous galls at 39 and 46 DAI, respectively; g well-developed and grouped galls of varied shapes and sizes at 66 DAI. Abbreviations: Ga = galls, LR = lateral root
Histological changes after nematode induction
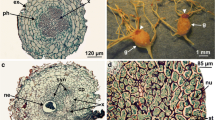
In general, the gall formation disorganized the histological profile of the roots, creating a new and unique structure. At day 4 of the experiment, the non-inoculated plants of A. esculentus showed roots with a uniseriate epidermis, parenchymatic cortex, a vascular cylinder with five protoxylem poles, and no pith (Fig. 3a). On day 66, the vascular cambium had already been established and secondary growth was usual, with the production of more secondary xylem than secondary phloem (Fig. 3b). The main changes seen in inoculated roots were first detected on day 11, with well-formed multinucleated giant cells produced from xylem parenchymal cells, which changed the typical polyarchy organization of the root (Fig. 3c). After giant cell development, the primary phloem was pushed to the periphery of the gall and the cortex grew due to the hypertrophy process (Fig. 3c). In that stage the female of root-knot nemtode are in contact with giant cells that showed thick cell walls and dense cytoplasm (Fig. 3c, detail). On day 18, the giant cells remained in the center of the organ, surrounded by primary xylem (Fig. 3d). On day 39, a large amount of secondary xylem was produced, leading to a lateral enlargement of the gall (Fig. 3e). A periderm was produced in the outer layers and replaced the epidermis (Fig. 3e). On day 66, new giant cells were produced, with a large increase in the cortical parenchyma through hyperplasia and hypertrophy processes (Fig. 3f, g).
Histological features of okra roots 66 days after non-inoculated treatment (DNI) and after different days of inoculation (DAI) with Meloidogyne incognita root-knot nematodes. a, b Non-inoculated roots. c–g Inoculated roots. a 4 DNI with primary axial root and pentarca organization; b 66 DNI with usual secondary growth; c 11 DAI in primary growth and giant cells in formation. In detail, note the presence of the female nematode in touch with the giant cells; d, e 18 and 39 DAI with installation of the vascular cambium and the beginning of secondary growth; f 66 DAI with larger numbers of giant cells and expanded cortical parenchyma. Abbreviations: Ca = vascular cambium, GC = giant cell, Ph = phloem, In inductor, Me metaxylem, Pr protoxylem, Co cortical parenchyma, SPh secondary phloem, SX secondary xylem, PX primary xylem, Pa parenchyma, Xy xylem
Immunocytochemical analyses
The inoculated and non-inoculated A. esculentus roots showed similar results in immunocytochemical analysis (Table 2), which was based on the means and standard deviation present in the supplementary table. However, epitopes of (1→5) α-L-arabinans, recognized by LM6, occurred only in non-inoculated roots (Table 2). These epitopes were intensely labeled in the cortical parenchyma cell walls and moderately in the parenchymal cell wall of xylem, phloem, and vascular cambium (Table 2; Fig. 4a, b). The epitopes of extensin and (1→4) β-D-galactan, recognized by LM1 and LM5, respectively, were not detected in either treatment (Table 2). Epitopes of HGs with high methyl-esterified groups, recognized by JIM7, were intensely to moderately labeled in the cortical parenchyma cell walls and parenchyma cell walls of xylem in both treatments, as well as in giant cells (Table 2; Fig. 4c, d; Fig. 5a, b). Epitopes of HGs with low methyl-esterified groups, recognized by JIM5, were distributed at the same sites as those detected by JIM7, with differences in intensity (Table 2). Epitopes recognized by JIM5 were intensely labeled in the cortical parenchyma cell wall of the non-inoculated root (Table 2; Fig. 4e, f) and in the parenchyma cell wall of xylem and giant cells of inoculated roots (Table 2; Fig. 5c, d).
Immunocytochemistry results for pectic epitopes recognized by LM6 (a, b), JIM7 (c, d) and JIM5 (e, f) antibodies of okra roots at 66 days after non-inoculated treatment (DNI). Abbreviations: Ca = vascular cambium, PX primary xylem, SX secondary xylem, Co cortical parenchyma, Pe periderm, Ep epidermis, LR lateral root, En endodermis. PS.: green labeling on the cell wall indicates a positive result, while blue labeling shows autofluorescence using the DAPI filter
Immunocytochemistry results for pectic epitopes recognized by JIM7 (a, b) and JIM5 (c, d) antibodies of okra roots at 66 days after inoculation (DAI) with Meloidogyne incognita root-knot nematodes. Abbreviations: Ca = vascular cambium, PX primary xylem, SX secondary xylem, Co cortical parenchyma, GC giant cell. PS.: green labeling on the cell wall indicates a positive result, while blue labeling shows negative results using the DAPI filter
Discussion
The root-knot nematode M. incognita impacts some agronomic traits of okra, decreasing, for example, the dry weight of the stem system. However, some traits such as the number and weight of fruits increased unexpectedly, with these changes possibly being related to an increase in the production of lateral roots stimulated by the presence of galls. Giant cells developed from day 11 after inoculation and increased in number and size until 66 days after inoculation. Giant cells came from the parenchyma cells of xylem and showed intense and moderate labeling for epitopes recognized by JIM5 and JIM7. The presence of these HGs with different degrees of methyl esterification seems to be related to the injuries caused by the nematode feeding activity, as well as to the typical hypertrophy process that occurs in giant cells (see Vilela et al. 2019).
Nematode infection determines agronomic traits
Okra is a semi-woody annual shrub that can reach 3 m in height; however, the Santa Cruz 47® cultivar is shorter, reaching up to 2 m on average, a height that facilitates harvest (Filgueira 2008). In the present study, okra ranged from 50 to 70 cm in height at harvesting, with a difference between treatments. Unexpectedly, inoculated plants were taller than non-inoculated ones, a result probably due to the moderate stress caused by the nematodes, as already described in the literature (Abrão and Mazzafera 2001). Nevertheless, the nematode-infected plants usually showed a weaker and shorter development of stem systems (Silva et al. 2006). Meloidogyne spp. infection can lead to root cortex detachment, interruption of root-tip growth, and extensive mutilation of the root system (Brass et al. 2008), something not detected until 66 days after inoculation in our study.
Fresh root weight did not differ between the non-inoculated and inoculated groups, as also observed in parasitic infection promoted by M. javanica in Tectona grandis Linn. F. (Oliveira and Silva 2013) and in banana plantations (Cofcewicz et al. 2004). Due to the large number of galls in the inoculated roots in our study, a greater weight of these roots was expected since, according to Carneiro et al. (1999), the formation of galls and the increase in the number and proliferation of lateral roots can lead to an increase in the weight of inoculated plants. For instance, an increase in the fresh weight of the inoculated roots was reported for passion fruit (Sharma et al. 2005). Meloidogyne spp. infection also typically reduces plant growth and fruit production (Ritzinger and Ritzinger 2003), although in our study the inoculated plants, surprisingly, produced more and heavier fruits than non-inoculated plants despite a decrease in the dry weight of the stem system. According to Pinheiro et al. (2013), nematode-caused stress may directly or indirectly influence the performance and survival of okra plants, a fact that was also observed here with M. incognita infection of okra.
Okra cultivars are highly prone to root-knot nematode infections and are even used to indicate the presence of Meloidogyne spp. (Pinheiro et al. 2013). This expected susceptibility was detected and detailed in the current study. We found over 400 galls per root, including small, medium, and large ones, which, according to the gall index (GI) proposed by Taylor and Sasser (1978), far exceeded the minimum number of 100 for a plant to be considered susceptible. The giant cells are essential for the development of the root-knot nematode, since the nematodes penetrate and establish a feeding site in the root system of plants, thus using their nutrients as food (Kyndt et al. 2013). Galls seem to be good indicators of the presence of the parasite (Davis et al. 2004), with the number of galls in a root permitting the host plant to be classified as susceptible or resistant. Herein, we detected a large number of nematodes in roots and, although the literature suggests that the development and productivity of plants could be affected by the infection, this was observed for okra infection with M. incognita, but, unexpectedly, sometimes in a positive way.
Root-knot gall morphology and histopathology
The root-knot nematode induces a root thickening in okra that may be hard to notice before 11 days of inoculation. On the other hand, the gall reaches its maximum size on day 66 after inoculation. Thus, gall formation is the best-known symptom of Meloidogyne nematode infection since galls develop just after the juveniles infect the roots (Mitkowski and Abawi 2003). According to these authors, galls have an elongated shape and a swollen appearance and occur throughout the root system, as was the case for the galls observed in our study. These galls are a result of cell hypertrophy of the cortex or of adjacent cells surrounding the nematode, as well as tissue hyperplasia usually of the pericycle (Vilela et al. 2019). The development of root-knot nematode galls may be the consequence of secretions from esophageal glands injected by the buccal stylet of the nematode, resulting in the formation of giant cells and of galls. The two structure formations are independent of each other (Ferraz and Brown 2016) and, sometimes, galls may be small or indistinct (Moens et al. 2009) or may be absent (Davis et al. 2004; Ferraz and Monteiro 2011).
Herein, we first noted the formation of a feeding site with giant cells on day 11 after inoculation, differently from the findings of Vilela et al. (2019), who noticed giant cells induced on soybean by M. javanica on day 18 after inoculation. Also, root-knot galls induced by M. graminicola and M. incognita on rice (Oryza sativa L.) showed different times of giant cell formation, which developed 2 and 6 days, respectively, after inoculation (Nguyen et al. 2014). Giant cells are essential for the development and reproduction of the parasite since they act as a metabolic drain of the plant, taking nutrients from the host plant organs to feed the nematodes (Galbieri and Belot 2016). These worms do not kill host cells (Abad et al. 2003), but rather form a feeding tube (Davis et al. 2004) from the secretions of the esophageal glands towards the cytoplasm of the plant cell, filtering the cytosol during ingestion (Mitkowski and Abawi 2003). Giant cells typically have a dense cytoplasm, numerous nuclei, and thick cell walls, as observed by Castañeda (2015) in an investigation of banana roots (Musa acuminata subgroup Cavendish, cv. Grande Naine) infested with M. incognita. Using electron microscopy to study Glycine max root galls induced by M. javanica, Vilela et al. (2019) revealed that the walls of giant cells were thicker and exhibited invaginations towards the cell lumen. The cell walls undergo thickening and loosening processes to expand and support the absorption of nutrients by the nematodes (Escobar et al. 2015; Bohlmann and Sobczak 2014). The presence of giant cells at 11 days after okra inoculation with M. incognita disorganized the central vascular cylinder of the roots and could compromise water transport and nutrient uptake, leading to plant wilt, yellowing, and reduced growth (Dorhout et al. 1991; Anwar and Javed 2010; Premachandra and Gowen 2015). Herein, based on agronomic traits, the growth of the okra plants was not compromised even though giant cells and gall formation disorganized the histological profile of the roots.
Cell wall composition of parenchyma cells of xylem and giant cells
In general, galls develop from tissue hyperplasia and cellular hypertrophy (Oliveira et al. 2016), and these processes are dependent on changes in cell wall dynamics and composition, as discussed for insect galls (Oliveira et al. 2014; Carneiro et al. 2015; Teixeira et al. 2018; Martini et al. 2019). The development of root-knot nematode galls also requires a sequence of destructive and constructive cell wall modifications (Bohlmann and Sobczak 2014), especially driven by the action of different pectolytic enzymes (see Wieczorek 2015). The detection of pectic epitopes by monoclonal antibodies has been used as a tool to detect pectolytic activities in gall tissues (Oliveira et al. 2014). Herein, cell hypertrophy was a striking feature of the root cortex, leading to gall formation on okra induced by M. incognita. Giant cell development also requires changes in the distribution and dynamics of epitopes of high and low methyl-esterified HGs.
HGs with high methyl-esterified groups were intensely detected in the parenchyma cells of xylem in both non-inoculated and inoculated plants. Giant cells showed moderate labeling for HG epitopes with a high degree of methyl-esterification since these cells come from the parenchyma cells of xylem. HGs are linear homopolymers with approximately 100 molecules of (1-4)–α-linked-D-galacturonic acid, synthesized in the Golgi apparatus in a high methyl-esterified form (Albersheim et al. 2011). The presence of HGs with high methyl-esterified groups is usually detected on young tissues during the processes of growth and elongation (Knox 1992; Albersheim et al. 2011; Wolf and Greiner 2012) and, in galls, it has been associated with the processes of cell hypertrophy during development (Carneiro et al. 2015; Oliveira et al. 2016; Teixeira et al. 2018; Martini et al. 2019). The presence of epitopes with high methyl-esterified groups in the cell walls of giant cells may guarantee cell growth and elongation and, therefore, the establishment of root-knot nematodes.
HGs with low methyl-esterified groups were weakly detected in the parenchyma cells of xylem in non-inoculated plants and intensely in inoculated ones and also detected intensely in the giant cells. The process of HG de-methyl-esterification depends on the action of pectin methylesterases (PME) or polygalacturonases (PG) (Willats et al. 2000; Hongo et al. 2012). Since the random de-methyl-esterification of HGs leads to whole cell wall degradation and cell death (Hongo et al. 2012), we suppose that these processes in the root-knot nematode gall on okra occur by the action of PMEs. Our results support an intense process of synthesis and de-methyl-esterification of parenchyma cell walls of xylem and giant cells on the roots of inoculated plants. The de-methyl-esterification process and the presence of low methyl-esterified epitopes of HG in the parenchyma cells of xylem and giant cells may increase cell stiffening and porosity, as already detected in galls induced by insects (Oliveira et al. 2014; Carneiro et al. 2015; Martini et al. 2019). Herein, the increase in porosity may have favored the nutrient exchange between host tissues and nematode galls induced by M. incognita in okra.
Conclusions
The root-knot nematode Meloidogyne incognita induces galls and giant cells on the roots of okra, disorganizing the histological profile of root tissues. However, the disorganization of the anatomical structure of the root did not affect the growth and development of okra until 66 days after inoculation; on the contrary, the inoculated plants even grew more. Giant cell formation occurs at 11 days after inoculation and is essential for the establishment of root-knot nematodes. As a feeding site for the root-knot nematode, giant cell formation depends on changes in the degree of HG methyl-esterification of the xylem parenchyma cell wall in order to elongate and in nutrients flux due to increased porosity.
References
Abad P, Favery B, Rosso MN, Castagnone-Sereno P (2003) Root-knot nematode parasitism and host response: molecular basis of a sophisticated interaction. Mol Plant Pathol 4:217–224. https://doi.org/10.1046/j.1364-3703.2003.00170.x
Abad P, Castagnone-Sereno P, Rosso MN, Engler JA, Bo F (2009) Invasion, feeding and development. In: Perry RN, Moens M, Starr JL (eds) In Root-knot nematodes. CABI International Press, Cambridge, pp 163–181
Abrão MM, Mazzafera P (2001) Fitossanidade. Efeitos do nível de inoculo de Meloidogyne incognita em algodoeiro. Bragantia 60:19–26. https://doi.org/10.1590/S0006-87052001000100003
Albersheim P, Darvill A, Roberts K, Sederoff R, Staehelin A (2011) Plant cell walls. Garland Sci, New York 108(1):viii–ix. https://doi.org/10.1093/aob/mcr128
Ali MA, Azeem FLH, Bohlmann H (2017) Smart parasitic nematodes use multifaceted strategies to parasitize plants. Front Plant Sci 8:1699. https://doi.org/10.3389/fpls.2017.01699
Anwar SA, Javed N (2010) Meloidogyne incognita infecting Dahlia. Pakistan J Zool 42:348–350
Bellafiore S, Briggs SP (2010) Nematode effectors and plant responses to infection. Curr Opin Plant Biol 13:442–448. https://doi.org/10.1016/j.pbi.2010.05.006
Berg RH, Fester T, Taylor CG (2009) Development of the Root-Knot Nematode Feeding Cell. In: Berg RH, Taylor CG (eds) Cell Biology of Plant Nematode Parasitism. Plant Cell Monographs, vol 15. Springer, Berlin. 15: 115–152. https://doi.org/10.1007/978-3-540-85215-5_5
Bohlmann H, Sobczak M (2014) The plant cell wall in the feeding sites of cyst nematodes. Front Plant Sci Mar 19:89. https://doi.org/10.3389/fpls.2014.00089
Brass FEB, Veronezze NC, Pacheco E, Bosquê GG (2008) Aspectos biológicos do Meloidogyne spp. relevantes à cultura de café. Revista cientifica Eletrônica de Agronomia. Editora FAEF, São Paulo
Carneiro RG, Ferraz LCCB, Mazzafera P (1999) Carbon partitioning in soybean infected with Meloidogyne incognita and M. javanica. J. Nematol 31:348–355
Carneiro RGS, Pacheco P, Isaias RMS (2015) Could the extended phenotype extend to the cellular and subcellular levels in insect-induced galls? PLoS ONE 10:e0129331. https://doi.org/10.1371/journal.pone.0129331
Castañeda NEN (2015) Estudo da interação Musa acuminata- Meloidogyne incognita. Thesis, Universidade de Brasília.
Chomick G, Bidel LPR, Jay-Allemand C (2014) Exodermis structure controls fungal invasion in the leafless epiphytic orchid Dendrophylax lindenii (Lindl.) Benth. ex Rolfe. Flora 209:88–94. https://doi.org/10.1016/j.flora.2014.01.001
Cofcewicz ET, Carneiro RMDG, Cordeiro CMT, Quénéhervé P, Faria JJC (2004) Reação de cultivares de bananeira a diferentes espécies de nematóides das galhas. Nematol Bras 28:11–22
Coolen WA, D’Herde CJ (1972) A method for the quantitative extraction of nematodes from plant tissue. State Agricultural Research Centre, Ghent
Davies LJ, Lilley CJ, Knox JP, Urwin PE (2012) Syncytia formed by adult female Heterodera schachtii in Arabidopsis thaliana roots have a distinct cell wall molecular architecture. New Phytol 196:238–246. https://doi.org/10.1111/j.1469-8137.2012.04238.x
Davis EL, Hussey RS, Baum TJ (2004) Getting to the roots of parasitism by nematodes. Trends Parasitol 20:134–141. https://doi.org/10.1016/j.pt.2004.01.005
Dorhout R, Gommers FJ, Kollöffel C (1991) Water transport through tomato roots infected with Meloidogyne incognita. Phytopathology 81:379–385. https://doi.org/10.1094/Phyto-81-379
Escobar C, Barcala M, Cabrera J, Fenoll C (2015) Plant Nematode interactions - A view on compatible interrelationships. In: Escobar C, Fenoll C (eds) Advances in Botanical Research. Elsevier, Oxford, pp 1–32
Ferraz LCCB, Brown DJF (2016) Nematologia de plantas: fundamentos e importância. Norma editora, Manaus
Ferraz LCCB, Monteiro AR (2011) Nematoides. In: Amorim L, Rezende JAM, Bergamin Filho A (eds) Manual de fitopatologia: princípios e conceitos. Agronômica Ceres, Piracicaba, pp 277–305
Ferreira DF (2010) SISVAR - Sistema de análise de variância. Versão 5.3. UFLA, Lavras
Filgueira FAR (2008) Novo manual de olericultura: agrotecnologia moderna na produção e comercialização de hortaliças. UFV, Viçosa
Galbieri R, Belot JL (2016) Nematoides fitoparasitas do algodoeiro nos Cerrados brasileiros: biologia e medidas de controle. IMAmt, Cuiabá
Gheysen G, Mitchum MG (2011) How nematodes manipulate plant development pathways for infection. Curr Opin Plant Biol 14:415–421. https://doi.org/10.1016/j.pbi.2011.03.012
Golinowski W, Grundler FMW, Sobczak M (1996) Changes in the structure of Arabidopsis thaliana during female development of the plant-parasitic nematode Heterodera schachtii. Protoplasma 194:103–116. https://doi.org/10.1007/BF01273172
Hongo S, Sato K, Yokoyama R, Nishitani K (2012) Demethylesterification of the primary wall by pectin methylesterase 35 provides mechanical support to the Arabidopsis stem. Plant Cell 24:2624–2634. https://doi.org/10.1105/tpc.112.099325
Hussey RS, Grundler FMW (1998) Nematode parasitism of plants. In: Perry RN, Wright DJ (eds) Physiology and biochemistry of free-living and plant parasitic nematodes. CAB International Press, England, pp 213–243
Joca TAC, Oliveira DC, Zotz G, Cardoso JCF, Moreira ASFP (2019) Chemical composition of cell walls in velamentous roots of epiphytic Orchidaceae. Protoplasma 257:103–118
Jones JT, Haegeman A, Danchin EGJ, Hari SG, Helder J, Jones MGK, Kikuchi T, Manzanilla-López R, Palomares-Rius JE, Wesemael WM, Perry RN (2013) Top 10 plant-parasitic nematodes in molecular plant pathology. Mol Plant Pathol 14:946–961. https://doi.org/10.1111/mpp.12057
Juvale PS, Baum TJ (2018) 'Cyst-ained' research into Heterodera parasitism. PLoS Pathog 14:e1006791. https://doi.org/10.1371/journal.ppat.1006791
Knox JP (1992) Cell adhesion, cell separation and plant morphogenesis. Plant J 2:137–141. https://doi.org/10.1111/j.1365-313X.1992.00137.x
Kyndt T, Vieira P, Gheysen G, de Almeida-Engler J (2013) Nematode feeding sites: unique organs in plant roots. Planta 238:807–818. https://doi.org/10.1007/s00425-013-1923-z
Liu L, ShangGuan K, Zhang B, Liu X, Yan M, Zhang L, Shi Y, Zhang M, Qian Q, Li J, Zhou Y (2013) Brittle Culm1, a COBRA-Like Protein, Functions in Cellulose Assembly through Binding Cellulose Microfibrils. PLoS Genet 9:e1003704. https://doi.org/10.1371/journal.pgen.1003704
Martin FW (1982) Okra, potential multiple-purpose crop for the temperate zones and tropics. Econ Bot 36:340–345. https://doi.org/10.1007/BF02858558
Martini VC, Moreira ASFP, Kuster VC, Oliveira DC (2019) Galling insects as phenotype manipulators of cell wall composition during the development of galls induced on leaves of Aspidosperma tomentosum (Apocynaceae). S Afr J Bot 127:226–233. https://doi.org/10.1016/j.sajb.2019.09.006
Mitkowski NA, Abawi GS (2003) Nematoide de galhas. The Plant Health Instructor, EUA
Moens M, Perry RN, Starr JL (2009) Meloidogyne species - a diverse group of novel and important plant parasites. In: Perry RN, Starr JL, Moens M (eds) Nemátodos das galhas. CABI International, Wallingford
Mota WF, Finger FL, Casali UWD (2000) Olericultura: melhoramento genético do quiabeiro. UFV, Viçosa
Nguyen PV, Bellafiore S, Petitot AS, Haidar R, Bak A, Fernandez D (2014) Meloidogyne incognita - rice (Oryza sativa) interaction: a new model system to study plant-root-knot nematode interactions in monocotyledons. Rice 7:23. https://doi.org/10.1186/s12284-014-0023-4
O'Brien TP, Feder N, McCully ME (1964) Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59:368–373. https://doi.org/10.1007/BF01248568
Oliveira AS, Silva RA (2013) Ocorrência e patogenicidade de Meloidogyne javanica sobre plantas de teca (Tectona grandis Linn. F.). Ciênc Florest 23:563–569. https://doi.org/10.5902/1980509812340
Oliveira RDL, Silva MB, Aguiar NDC, Bergamo FLK, Costa ASV, Prezotti L (2007) Nematofauna associada à cultura do quiabo na região leste de Minas Gerais. Hortic Bras 25:88–93. https://doi.org/10.1590/S0102-05362007000100017
Oliveira DC, Magalhães TA, Ferreira BG, Teixeira CT, Formiga AT, Fernandes GW, Isaias RMS (2014) Variation in the degree of pectin methylesterification during the development of Baccharis dracunculifolia kidney-shaped gall. PLoS One 9:e94588. https://doi.org/10.1371/journal.pone.0094588
Oliveira DC, Isaias RMS, Fernandes GW, Ferreira BG, Carneiro RGS (2016) Manipulation of host plant cells and tissues by gall-inducing insects and adaptive strategies used by different feeding guilds. J Insect Physiol 84:103–113. https://doi.org/10.1016/j.jinsphys.2015.11.012
Pinheiro JB (2017) Nematoides em hortaliças Brasília. Embrapa, Embrapa
Pinheiro JB, Pereira RB, Carvalho ADF, Rodrigues CS (2013) Manejo de nematoides na cultura do quiabeiro. https://www.embrapa.br/busca-de-publicacoes/-/publicacao/960522/manejo-de-nematoides-na-cultura-do-quiabeiro Accessed 20 March 2020.
Premachandra DWTS, Gowen SR (2015) Influence of pre-plant densities of Meloidogyne incognita on growth and root infestation of spinach (Spinacia oleracea L.) (Amaranthaceae) – an important dimension towards enhancing crop production. Future of Food: Journal on Food. Agric Soc 3:18–26
Reddigari SR, Sundermann CA, Hussey RS (1985) Isolation of subcellular granules from second-stage juveniles of Meloidogyne incognita. J Nematol 17:482–488
Ridley BL, O’Neill MA, Mohnen D (2001) Pectins: structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 57:929–967. https://doi.org/10.1016/S0031-9422(01)00113-3
Ritzinger CHSP, Ritzinger R (2003) Acerola, Ataque às raízes. Rev Cult Hort Frut 19:36–38
Roland JC, Vian B (1991) General preparation and staining of thin sections. In: Hall JL, Hawes C (eds) Electron microspcopy of plant cells. Academic Press, London, pp 1–66
Sharma RD, Valéria IA, Calvante MJB, Gomes AC (2005) Reação de genótipos de pimenta-longa aos nematóides Meloidogyne javanica, M. incognita raça 1 e Rotylenchulus reniformes. Nematol Bras 29:83–86
Siddique S, Grundler FMW (2015) Metabolism in Nematode Feeding Sites. In: Escobar C, Fenoll C (eds) Plant Nematode Interactions: A View on Compatible Interrelationships, vol 73. Elsevier, Nova Iorque, pp 119–138. https://doi.org/10.1016/bs.abr.2015.02.001
Sikora RA, Fernandez E (2005) Nematodes parasites of vegetables. In: Luc M, Sikora RA, Bridge J (eds) Plant-parasitic nematodes in subtropical and tropical agriculture. Cabi, Wallingford, pp 319–392
Silva JBC, Giordano LB, Furumoto O, Boiteux LS, França FH, Bôas GLV, Branco MC, Medeiros MA, Marouelli W, Silva WLC, Lopes CA, Ávila AC, Nascimento WM, Pereira W (2006) Cultivo de tomate para industrialização. https://sistemasdeproducao.cnptia.embrapa.br/FontesHTML/Tomate/TomateIndustrial_2ed/autores.htm Accessed: 15 march 2020.
Silva MCL, Santos CDG, Silva GS (2016) Espécies de Meloidogyne associada a vegetais em microrregiões do estado do Ceará. Rev Ciênc Agron 47:710–719. https://doi.org/10.5935/1806-6690.20160085
Smant G, Helder J, Goverse A (2018) Parallel adaptations and common host cell responses enabling feeding of obligate and facultative plant parasitic nematodes. Plant J 93:686–702. https://doi.org/10.1111/tpj.13811
Taylor AL, Sasser JN (1978) Biology, identification and control of root-knot nematodes (Meloidogyne species). NCSU & USAID Coop Publ, Raleigh
Teixeira CT, Oliveira DC, Kuster VC, Isaias RMS (2018) Immunocytochemical demonstration of cell wall components related to tissue compartments in the globoid galls induced by Clinodiplosis sp. (Cecidomyiidae) on Croton floribundus Spreng. (Euphorbiaceae). Botany 96:9–18. https://doi.org/10.1139/cjb-2017-0123
Vieira P, Escudero C, Rodiuc N, Boruc J, Russinova E, Glab N et al (2013) Ectopic expression of Kip-related proteins restrains root-knot nematode-feeding site expansion. New Phytol 199:505–5019. https://doi.org/10.1111/nph.12255
Vilela RMIF, Martini VC, Gonçalves L d A, Kuster VC, Oliveira DC (2019) Structure and development of root gall induced by Meloidogyne javanica in Glycine max L. Semina: Série botânica 40:1033–1048. https://doi.org/10.5433/1679-0359.2019v40n3p1033
Wieczorek K (2015) Cell wall alterations in nematode-infected roots. Adv Bot Res 73:61–90. https://doi.org/10.1016/bs.abr.2014.12.002
Willats WGT, LimBerg G, Bucholt HC, Van Alebeek GJ, Benen J, Christensen TMIE et al (2000) Analysis of pectic epitopes recognised by hybridoma and phage display monoclonal antibodies using defined oligosaccharides, polysaccharides, and enzymatic degradation.charides e degradação enzimática. Carbohydr Res 327:309–320. https://doi.org/10.1016/S0008-6215(00)00039-2
Willats WGT, McCartney L, Mackie W (2001) Pectin: Cell biology and prospects for functional analysis. Plant Mol Biol 47:9–27. https://doi.org/10.1023/A:1010662911148
Williamson VM, Gleason CA (2003) Plant-nematode interactions. Curr Opin Plant Biol 6:327–333. https://doi.org/10.1016/S1369-5266(03)00059-1
Wolf S, Greiner S (2012) Growth control by cell wall pectins. Protoplasma 249:169–175. https://doi.org/10.1007/s00709-011-0371-5
Zhang R, Feng YLH, Yuan HX, Dai JL, Cao AZ, Xing LP, Li HL (2016) Cereal cyst nematode resistance gene CreV effective against Heterodera filipjevi transferred from chromosome of 6VL Dasypyrum villosum to bread wheat. Mol Breed 36:122. https://doi.org/10.1007/s11032-016-0549-9
Availability of data and material
All data generated or analyzed during this study are included in this published article.
Funding
This study was financed in part Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG). The authors also thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for a DCO fellowship.
Author information
Authors and Affiliations
Contributions
DCO and RMIFV conceived and designed the research. RMIFV, CAM, and ACPF conducted the cultivation experiments and collected the agricultural traits. RMIFV, VCK, and DCO conducted the immunocytochemistry experiments. All the authors wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Consent to participate
All authors agree with the participation.
Consent to publication
All authors agree with the publication.
Additional information
Handling Editor: Handling Editor: David McCurdy
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Vilela, R.M.I.F., Kuster, V.C., Magalhães, T.A. et al. Impact of Meloidogyne incognita (nematode) infection on root tissues and cell wall composition of okra (Abelmoschus esculentus L. Moench, Malvaceae). Protoplasma 258, 979–990 (2021). https://doi.org/10.1007/s00709-021-01618-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-021-01618-0