Abstract
The plant-parasitic nematode Nacobbus aberrans attacks weeds and cultivated plants, causing drastic crop yield losses. Several tomato cultivars, such as Superman and Mykonos, are produced by Seminis® as nematode resistant and are widely used in Argentina; their specific resistance response to different nematode species, however, is still unknown. In this study we explored the response of tomato cultivars to the infection of N. aberrans isolates, and determined the host status by performing histological analyses and estimating egg mass index (EMI). Two Argentina isolates (from Lules-Tucumán and Río Cuarto-Córdoba) were tested separately in plants of Superman, Mykonos and Platense cultivars. Plants were maintained in a greenhouse for 90 days; then EMI was estimated in root systems and the material was processed to prepare histological slides and for histochemical test. Infected roots exhibited galls with females and a syncytium (feeding site) developed inside them. The vascular tissues were disorganized and displaced to the periphery; xylem percentage was lower than that in the control plants. All the cultivars were susceptible and developed a close plant-parasite relationship, with Mykonos and Platense cultivars infected with the Lules isolate being the most highly affected, as indicated by their highest EMI values. Superman was the least susceptible cultivar, as evidenced by its lowest EMI values, the amount of starch observed, the presence of thickened cell walls around the nematode and the egg mass, and the low percentage of gall occupied by syncytium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
More than 4100 plant-parasitic nematode species have been identified worldwide; all together, they represent an important constraint to global food security (Jones et al. 2013). Some of these species reduce global agricultural production generating economic losses of 115 billion dollars a year (Eves-van den Akker et al. 2014; Yang et al. 2015).
The genus Nacobbus Thorne and Allen, 1944 (known as the false root-knot nematode) is native to the Americas and comprises plant-parasitic nematodes that attack roots of vascular plants. Females are swollen; they have sedentary endoparasitic habits and are characterized by generating galls in the parasitized root tissues (Doucet and Lax 2005). Inside the galls, there is one or several females closely associated with a syncytium (induced by esophageal enzymes of the nematode), which is the feeding site of the parasite. Small mucilaginous protuberances containing eggs deposited by the female are observed externally. The nematode presence produces alterations in the anatomy of roots, affecting their normal functioning and therefore reducing crop yield (Doucet and Lax 2005; Curtis 2008). N. aberrans is present in the United States, Mexico, Ecuador, Bolivia, Peru, Chile and Argentina. In the Andean region of South America, this nematode is the main pathogen affecting potato (Solanum tuberosum L.) (Inserra et al. 1985). Manzanilla-López et al. (2002) reported yield losses of 36 % and 55 % in bean (Phaseolus vulgaris L.) and tomato (S. lycopersicum L.), respectively. According to EPPO (2015), the species should be regulated as a quarantine pest due to the great economic losses causing to agriculture in the Americas. In Argentina, the host range includes important horticultural crops grown both in the field and under greenhouse conditions; in both situations, the nematode can cause important damage (Doucet and Lax 2005). The detection of populations with particular host preferences has led to the differentiation of “physiological groups” or “races” within the species. Host preference has been the main criterion to designate races; however, discrepancies often arise because of the lack of consensus on a consistent system for race determination (Lax et al. 2011).
Phytosanitary problems are among the main factors limiting tomato production worldwide, with damage caused by nematodes being a major one (Sory Toure et al. 2010). In Argentina, estimates provided by some producers and professionals indicate that damage to tomato grown under greenhouse conditions caused by N. aberrans and Meloidogyne sp., produce up to 10 % and 40 % losses in moderate and severe infestations, respectively (Adlercreutz et al. 2008). Using varieties resistant to these nematodes is an environmentally safer and more efficient alternative than using chemicals (Ornat et al. 2001). A gene named Mi conferring resistance to some species of the genus Meloidogyne has been identified in tomato; to-date, however, no gene conferring resistance to N. aberrans has been detected (Veremis et al. 1997; Ornat et al. 2001).
Histopathological analyses are very important in the study of plant-parasitic interactions because they contribute information of the parasite (e.g., stages present, their location in the galls, establishment of a swollen female) and its influence on host development (Doucet et al. 1997; Lorenzo et al. 2001). Some studies have described histological alterations caused by the nematode on tomato roots (Vovlas et al. 2007; Tordable et al. 2010); however, the race of the species has been indicated only in a few cases, and none of the studies have compared different cultivars affected by different isolates of this pathogen. Therefore, further investigations are crucial to elucidate this missing information. In this work we provide specific data on the response of three commercial tomato cultivars to infestation with two N. aberrans isolates, through histological analyses and estimation of the egg mass index (EMI) upon infection.
Materials and methods
Plant material
Two S. lycopersicum cultivars were used, Superman and Mykonos, both produced by Seminis® and widely used in Argentina. These cultivars were selected because they are purported to be resistant to nematodes, but with no specifics on the nematode species. Moreover, there are no records of their response to infection by the studied nematode. In addition, Platense, a cultivar known to be susceptible to N. aberrans, was used as the positive control (Tordable et al. 2010). Seeds of the three cultivars were acquired from seed suppliers and germinated in a 2:1 mixture of autoclaved soil and vermiculite (Lax et al. 2011). The experiment was conducted in the greenhouse of the Centro de Zoología Aplicada (Facultad de Ciencias Exactas, Físicas y Naturales, UNC, Argentina), under controlled light and humidity conditions at a mean temperature of 25 °C.
Nematode inoculum
Nacobbus aberrans individuals were obtained from two populations, one from greenhouses from Río Cuarto (Córdoba, Argentina) and the other from plots located in Lules (Tucumán, Argentina). The material available to develop the isolates consisted of infected soil from the Río Cuarto population and beet roots with galls from the Lules population. According to the proposed physiological races, both isolates belong to the “beet group”, i.e., they do not attack potato but they do attack beet and tomato (Lax et al. 2011). Seeds of the susceptible cultivar Platense were germinated in autoclaved soil and the plants were used as hosts of the parasite. When plants reached the four-leaf stage, they were either transplanted to N. aberrans-infected soil (Rio Cuarto isolate) or placed in pots containing autoclaved soil, to which galled roots from Lules were added (Lules isolate). After four months, portions of the root system showing gall development were collected. The presence of a swollen N. aberrans female inside each gall was confirmed under stereoscopic microscope. Then, egg masses were isolated, placed in micro-sieves (250-μm mesh size) and fastened to a lid of the plastic Petri dish. The sieve was partially immersed in water contained at the base of the dish. Second stage juveniles, J2s, (n = 3600) were collected with a glass Pasteur pipette, transferred to a glass tube and diluted to obtain a concentration of 100 J2/ml. This procedure was equally applied to both isolates to obtain an equal inoculum from each population.
Plant infection and maintenance
Plants of Superman, Mykonos and Platense cultivars at the four-leaf stage were transplanted into 180-ml plastic pots containing 2:1 autoclaved soil and vermiculite. Plants were artificially infected with the two isolates separately. For that purpose, the inocula of Lules and Río Cuarto were homogenized by bubbling with a plastic Pasteur pipette, and 1 ml (100 J2) of each inoculum was applied to the roots of each plant. In addition, some plants were not inoculated (negative control). Plants were kept in the greenhouse for 90 days to allow complete development of the nematode life cycle.
Experimental design
The experiment consisted of a randomized complete block design with two factors (nematodes and cultivars) and a 3 × 3 factorial arrangement (three cultivars and two nematode populations plus negative control), with 12 replications per treatment: Platense-Lules association (Pla-L), Platense-Río Cuarto association (Pla-RC), Platense negative control (PC), Superman-Lules association (Sup-L), Superman-Río Cuarto association (Sup-RC), Superman negative control (SC), Mykonos-Lules association (Myk-L), Mykonos-Río Cuarto association (Myk-RC) and Mykonos negative control (MC).
Estimation of egg mass index (EMI)
Each root system was carefully washed and observed under stereoscopic microscope, and the egg masses were counted. Egg mass index was estimated on the basis of a 0 to 5 scale proposed by Taylor and Sasser (1978) for the sedentary endoparasite Meloidogyne spp., and also used for N. aberrans (Lax et al. 2011; Tordable et al. 2010), where: 0 = no egg mass, 1 = 1–2, 2 = 3–10, 3 = 11–30, 4 = 31–100, 5 = more than 100 egg mass per root system. Values equal to 0 indicate immunity; from 0.1 to 2, resistance; and above 2, susceptibility.
Histological and histochemical studies
Root systems were cut into segments (about 3 cm long) and fixed in FAA to prepare permanent and temporary histological slides (D’Ambrogio de Argüeso 1986). To prepare permanent slides, non-infected 1-cm root portions (control) and galls of the infected plants were taken, dehydrated in a graded series of alcohol and infiltrated with Paraplast®. Transverse sections (10 μm thick) were cut with a rotary microtome model Yidi 7508. After removal of the paraffin, the sections were stained with triple stain (hematoxylin-safranin-fast green). Hematoxylin stains the cell walls blue-violet and safranin confers a red colour to tissues with lignified secondary walls, cutin, suberin and tannins. Fast green stains cytoplasm and nucleus greenish light-blue (Conn et al. 1960; Rodríguez et al. 2010). The slides were mounted in Canada balsam (D’Ambrogio de Argüeso 1986). In addition, the metachromatic dye toluidine blue was used: tissues that are lignified, suberized and with tannins are stained blue green, whereas cellulosic tissues are stained purple red (Sakai 1973).
The middle area of the gall was photographed from transverse sections of the slides using a Nikon Coolpix 5200 digital camera held up to the eyepiece. Photographs were analysed using the Image Tool 3.00 program (Wilcox et al. 2002). The percentage of gall occupied by syncytium and percentage of gall occupied by xylem were determined.
Temporary slides were prepared with galls of all treatments, and the following histochemical tests were performed in hand-made sections: lugol’s solution, which stains starch violet blue, sometimes very dark, almost black (D’Ambrogio de Argüeso 1986); Coomassie brilliant blue R-250, which turns proteins bluish (Bertoia and Magoja 1985); sudan IV, which reveals suberin, cutin, fats and oils by staining them red (D’Ambrogio de Argüeso 1986); and 1 % phloroglucinol HCl, which differentiates the compounds under fluorescence microscope, with suberin retaining its auto-fluorescence, but not lignin (Tordable et al. 2008). For this test, sections were cut using microtome with the aim of obtaining enhanced precision.
The slides were mounted in 50 % glycerine solution. Observations were made under Primo Star-Carl Zeiss optical microscope. Materials stained with phloroglucinol HCl were analysed with a fluorescence microscope (Olympus BX61).
Data analysis
The results of quantitative variables were subjected to an ANOVA using InfoStat software (Di Rienzo et al. 2013). When necessary, the data were transformed to a logarithmic scale to meet the assumptions of independence and normality of residuals, variance homogeneity and block-treatment additive effects. A multiple comparison of means was performed using the Fisher’s LSD test, with a significance level of 0.05.
Results
Egg mass index (EMI)
The highest EMI values corresponded to Myk-L and Pla-L, showing the susceptibility of Mykonos and Platense cultivars to Lules isolate; whereas the other treatments exhibited resistance. Pla-RC showed the lowest value but with a high coefficient of variation with respect to the other treatments (Table 1).
Histological observations
The root system of control plants had a thick primary root and numerous thinner lateral roots. The control roots of the three cultivars showed a normal arrangement of the three (dermal, ground and vascular) tissue systems. The epidermis was composed of a single layer of small cells and an exodermis of rounded and bigger cells towards the interior, with both layers being suberized. The cortex consisted of parenchyma cells, some of them containing crystal sands and others containing starch. Endodermis and pericycle were uniseriate and their cells were smaller and more rectangular than cortical cells. The central cylinder was composed of well-developed xylem and phloem; taproot primary structure was diarchy and the lateral roots had a triarch or tetrarch structure. A recent development of secondary growth was observed (Fig. 1a). The roots of the three cultivars infected with isolates from Río Cuarto and Lules exhibited 2–4 mm long oval galls along the major axis, with many of them showing proliferation of lateral roots (Fig. 1b). Inside the gall, one or two swollen females of N. aberrans with their anterior region embedded in the syncytium/syncitia were observed (Fig. 1c). Syncytia were spindle-shaped (as observed in longitudinal sections); they were located in the central cylinder and were composed of cells with one or more hypertrophied nuclei and evident nucleoli. The cytoplasm was very dense, granular, with numerous vacuoles of variable size. The walls were cellulosic and highly thickened, with frequent ruptures that allowed the confluence of cytoplasm among adjacent cells. They exhibited starch grains, most of which were simple, but some were compound, with central hilum (Fig. 1d). This carbohydrate was also present in the parenchyma cells as compound grains, mostly associated with the nucleus, and sometimes scattered. Cell layers multiplied around the syncytium due to the hyperplastic reaction; this phenomenon, along with the volume occupied by the nematode, generated the gall. Crystal sands were more abundant in the roots of the three analysed cultivars than in control roots and were mainly located in the cortex cells near the periphery.
Anatomy of the root of tomato, S. lycopersicum L., and alterations caused by the nematode Nacobbus aberrans (Thorne, 1935) Thorne & Allen, 1944 a control lateral root of Mykonos b-d Mykonos-Lules association b external view of galls; arrows indicate egg masses c gall with a female of the nematode associated with the syncytium d detail of the feeding site; red arrows indicate interruptions of the cell wall. Abbreviations: cp cortical parenchyma cells, e epidermis, ex exodermis, g gall, ne nematode, nu nucleus, ph phloem, st simple starch grains, syn functional syncytium, x xylem
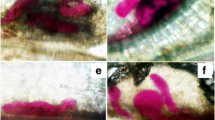
Nematode establishment and syncytium presence caused alterations in the vascular system, with evident reduction and disorganization in galls in all treatments. Xylem and phloem sometimes remained associated, surrounding the syncytium discontinuously, or were separated and displaced to opposite sides; rupture of xylem vessels was also evident (Fig. 2a). As a consequence of these modifications, the endodermis and pericycle became discontinuous, which hindered their identification in galls. Egg masses were often on the surface of the galls (Fig. 2b), but in some cases they were inside the gall. In galls developed in Superman and Mykonos, toluidine blue revealed thickened walls in the cells in contact with those masses (lignin and/or suberine) (Fig. 2c). In both cultivars, but more frequently in Superman, galls with the swollen females surrounded by highly thickened cells were also observed, in some cases with the presence of tannins. This phenomenon was not observed in Platense (Fig. 2d). Histological sections evidenced recent formation of some galls because the syncytium was active (showing cells with the previously mentioned characteristics), whereas other galls looked older. They contained regressive syncytia and cells had a few nuclei and scarce cytoplasm, sometimes with presence of tannins and mucilaginous substance (Fig. 2e-f).
Galls in roots of tomato, Solanum lycopersicum L. produced by Río Cuarto isolate of Nacobbus aberrans (Thorne, 1935) Thorne & Allen, 1944 a Mykonos cultivar b-f Superman cultivar a reduction and disorganization of vascular tissues b egg mass deposited by swollen female (histochemical test with sudan IV) c gall with egg mass stained with toluidine blue, in longitudinal section; thickened walls are indicated d cells with thickened walls around the nematode e regressive syncytium, cells with content of mucilaginous appearance f tannins inside the cells of regressive syncytium and in xylem vessels. Abbreviations: cp cortical parenchyma cells, em egg mass, ne nematode, ph phloem, rs regressive syncytium, sa crystal sands, sch compound starch grains, syn functional syncytium, w thickened cell walls, x xylem
In all treatments, galls contained filiform juvenile stages in the cortical parenchyma, in the area closest to the surface (Fig. 3a). Associated with those galls there were cells with very dense content, hypertrophied nuclei and nucleoli, starch grains and lignified and/or suberized walls (Fig. 3b-c). This phenomenon was also observed in the cortex of lateral roots emerging from galls. Intracellular migration of nematodes generated cavities and cell rupture in the plant tissue (Fig. 3d).
Presence of filiform juvenile stages of Nacobbus aberrans (Thorne, 1935) Thorne & Allen, 1944 in the cortical parenchyma of galls on the roots of tomato, Solanum lycopersicum L., Superman cultivar a Lules isolate b-d Río Cuarto isolate a general view, showing a swollen female and juveniles b detail of cells with dense content c cells with lignified and/or suberized walls (indicated with arrows) d gall and lateral root, showing cavities containing juveniles. Abbreviations: cp cortical parenchyma cells, dc cells with dense content, em egg masses, j juveniles, lr lateral root, ne nematode, nu nucleus, syn functional syncytium, x xylem
The nematode-cultivar interaction was significant for the variable “percentage of gall occupied by syncytium” (F = 4.63; p = 0.0138); the highest values were observed in Myk-L and Pla-RC (Fig. 4a-b). The interaction between factors was not significant for “percentage of gall occupied by xylem” (F = 2.03; p = 0.0972); therefore, only the effects of each factor were analysed separately. There were no differences among cultivars (F = 1.91; p = 0.1539), but there were differences in the factor nematodes (F = 44.90; p < 0.0001), because plants infected with Lules and Río Cuarto isolates exhibited a lower percentage of xylem than non-infected plants.
Percentage of gall of tomato, Solanum lycopersicum L., occupied by syncytium formed by the false root-knot nematode, Nacobbus aberrans (Thorne, 1935) Thorne and Allen, 1944. a the means and standard error are shown; the line intercept reveals nematode-cultivar interaction b mean and standard error in the different treatments; different letters indicate significant differences. Nematode isolates: L Lules, RC Río Cuarto. Tomato cultivars: Myk Mykonos, Pla Platense, Sup Superman
Histochemical tests
Sudan IV revealed the presence of two or more layers of suberized cells in the galls of all treatments. Those cells had highly thickened walls with respect to non-infected roots. This phenomenon was also observed under fluorescence microscope in the slides treated with phloroglucinol HCl (Fig. 5a-b). Lugol’s solution provided a more accurate determination and location of starch than previously observed in permanent slides treated with triple staining. A higher amount of starch was detected in the syncytium cells and cortical parenchyma of the three infected cultivars than in control roots. In addition, starch was more abundant in Superman cultivar than in Mykonos and Platense (Fig. 5c). Finally, Coomassie brilliant blue R 250 revealed proteins in the syncytium cells of the galls from the different treatments (Fig. 5d).
Histochemical tests in galls of tomato, Solanum lycopersicum L., cultivar Superman, produced by the Lules isolate of Nacobbus aberrans (Thorne, 1935) Thorne & Allen, 1944 a gall stained with sudan IV b longitudinal section of gall treated with phloroglucinol HCL under fluorescence microscope c staining with lugol’s solution, transverse section d treatment with Coomassie brilliant blue Abbreviations: cp cortical parenchyma cells, sc suberized cells, sch compound starch grains, syn functional syncytium, x xylem
Discussion
Treatments Pla-L and Myk-L exhibited the highest EMI values, indicating high susceptibility of Platense and Mykonos to Lules isolate. According to the scale of Taylor and Sasser (1978), the other treatments were resistant; however, according to the observed histological alterations, all the treatments should be considered susceptible to infection, with Pla-L and Myk-L being the most affected ones. While Pla-RC presented the lowest EMI values, some plants from this treatment showed little development of the root system (they had few lateral roots of reduced thickness). This explains the high coefficient of variation, since plants with limited root growth had no galls and therefore no egg masses. Therefore, the low EMI value of Pla-RC does not mean that this cultivar is resistant to the nematode; in fact, the susceptibility of Platense cultivar to N. aberrans is well known (Tordable et al. 2010). Although the two isolates used belong to “the beet group”, Lules generated a higher number of egg masses in the evaluated cultivars, showing greater reproductive potential than Río Cuarto. Therefore, experiments with more than one population should be conducted to evaluate the response of new cultivars to nematode attack, since entities of different geographical origin may differ in aggressiveness level on the same host (Lax et al. 2006). Information on the race to which a given nematode population belongs is very important because it supports decision making about the crops that should be included in a rotation scheme, and contributes to the implementation of appropriate management strategies (Manzanilla-López et al. 2002; Lax et al. 2011).
The histological characteristics of both functional and regressive syncytia are consistent with previous descriptions of tomato roots attacked by this nematode (Lorenzo et al. 2001; Tordable et al. 2010). The presence of syncytia, both in the center and the cortex of roots, was reported previously (Lorenzo et al. 2001). In the present work, syncytia were found only in the central cylinder, as reported by Tordable et al. (2010). This location might be due to the abundant nutrient availability in that area (Freire and Dos Santos 1978). The presence of proteins in the cytoplasm of syncytial cells was also reported in beans infected with this parasite (Martínez-Fuentes et al. 2010), indicating the role of these sites as a source of nutrients for nematode feeding. The amount of starch observed in syncytial and cortical parenchyma cells of galls was much higher in roots of treatments than in control roots. The presence of starch is characteristic of syncytia formed by N. aberrans, since this nematode induces starch production in the infected plant (Harveson 2008). Starch accumulation provides an important amount of nutrients that are used by the nematode during the reproductive stage (Souza 2001). In roots of S. tuberosum cv. Revolución infected with N. aberrans, galls had starch in the cells surrounding the syncytium; however, a higher amount of starch was found in the clone 7,601,217.7 of wild potato S. sparsipilum (Britt.) Juz. et Buk., which is less susceptible than Revolución (Finetti Sialer 1990). Therefore, a greater amount of starch in Superman cultivar than in Mykonos and Platense indicates a lower susceptibility to the evaluated isolate. Indeed, the resistant tobacco (Nicotiana tabacum L.) cultivar NC-89 infected with M. javanica exhibited an accumulation of starch grains in cortical cells with respect to other susceptible cultivars (Sosa-Moss et al. 1983). Here, a higher amount of crystal sands were present in the cortex of galls of infected roots than in that of non-infected roots. This compound is the final product of cell metabolism (Fahn 1974), and its presence suggests that the metabolic activity in cells increases in the presence of the nematode. The presence of juveniles in the cortical parenchyma of galls was also reported by Vovlas et al. (2007) and Lorenzo et al. (2001) in tomato and pepper, respectively. The characteristics reported in the present work coincide with intracellular invasion by J2 reported by Manzanilla-López et al. (2002), who observed rupture of cell walls and cells with dense content and hypertrophied nuclei as a result of feeding and migration of juveniles. Remarkably, those cells did not form a syncytium and, unlike the latter, they had lignified and/or suberized walls. Since in this experiment all plants were inoculated with N. aberrans infective (physiologically active) J2s, the post-infection (J3 and J4 stages) behaviour is unknown, as well as the behaviour of young females, which are also capable of penetrating roots and induce syncytium. Therefore, similar experiments should be conducted that include transplanting of inoculated plants to soil free from nematodes 2–4 days after inoculation, with the aim of ensuring that all galls are formed at the same time.
The external zone of galls of all cultivars had suberized cells with highly thickened walls compared to galls of control roots. Suberin is deposited in the cell wall as a response to wounding and pathogen infection, and as a barrier against the loss of water and solutes (Taiz and Zeiger 2006). Galls of the cultivar Superman and, to a lesser degree, of Mykonos, exhibited cells with tannins and walls strengthened with lignin and/or suberin surrounding the female. These compounds serve as a defence mechanism against microorganisms and are frequent in the occurrence of an invasion or wound (Akai and Fukutomi 1980; Taiz and Zeiger 2006). Although this phenomenon did not prevent nematode establishment or development, a notable histological modification was evident in Superman, whereas in Platense, the walls of the cells surrounding the parasite were cellulosic and did not show any visible alteration that might benefit the plant. Our results showed a notable disorganization of endodermis in galls; Jones (1981) confirmed metabolic leakage from the central cylinder to the cortex in tomato roots infected with M. incognita, suggesting the loss of apoplastic barrier provided by endodermis in the presence of gall formation. The pericycle was also altered, showing an abnormal proliferation of lateral roots in the galled areas. This phenomenon was previously reported for tomato and quinoa (Chenopodium album L.) infected with N. aberrans (Vovlas et al. 2007; Tordable et al. 2010). The root system facilitates water and nutrient availability to plants; hence, any modification to its normal development may alter crop vigor (Dickison 2000). Previous works studying tomato roots infected with this nematode reported displacement and reduction of vascular tissues (Doucet et al. 1997; Tordable et al. 2010); however, unlike the present work, in these no quantitave analyses is presented. Here, xylem percentage, an anatomical character defining water transport capacity (Sory Toure et al. 2010), was significantly lower in infected than in non-infected plants. Furthermore, Pla-RC and Myk-L treatments exhibited the greatest percentages of gall occupied by syncytium, meaning that normal root tissues, including vascular ones, were notably reduced. These results support previous studies reporting that alterations caused by N. aberrans reduce water and nutrient absorption and transport (Doucet and Lax 2005; Curtis 2008). The presence of the nematode evidently alters plant development by damaging vascular tissues and, therefore, reducing nutrient availability. Plant structure and physiology are the result of several processes involved in a complex interaction, forming a dynamic system; hence, any disturbance in a specific area may affect the entire organism (Dropkin 1979).
Based on the present results, we conclude that all cultivars infected with either isolate (Lules or Río Cuarto) underwent alterations in root anatomy caused by the establishment of the nematode and formation of the feeding site. The presence of egg masses indicates that the pathogen successfully completed the life cycle. While the three cultivars established a close plant-parasite relationship, allowing nematode development, Superman was the least susceptible cultivar to the evaluated isolates, which makes it a promising cultivar to be assessed in the field. This phenomenon was demonstrated by the amount of starch observed, the presence of highly thickened cell walls around the nematode and egg masses, and the lowest values of EMI and percentage of gall occupied by syncytium with respect to the other cultivars. Mykonos and Platense cultivars infected with the Lules isolate were the most highly affected, as indicated by their highest EMI values. The present work provides specific data on the response of commercial tomato cultivars and identifies a cultivar of low susceptibility to the evaluated isolate. Future studies should assess the interaction between other cultivars of this horticultural crop (as well as other plants of economic interest) and different isolates of the nematode.
References
Adlercreutz, E. G., Chaves, E., Mondino, E., & Szczesny, A. (2008). Fluctuación poblacional de juveniles del segundo estadío de Nacobbus aberrans y Meloidogyne sp. bajo condiciones de invernáculos (Período sept. 2004/oct. 2007). Congreso Argentino de Horticultura 31. Mar del Plata, Buenos Aires, Argentina.
Akai, S., & Fukutomi, M. (1980). Preformed internal physical defenses. In J. G. Horsfall & E. B. Cowling (Eds.), Plant Disease: An Advanced Treatise. Volume V. How Plants Defend Themselves (pp 139–158). Academic press, Inc. London.
Bertoia, L. M., & Magoja, J. L. (1985). Constancia del tamaño de los cuerpos de zeína en el maíz (Zea mays) y sus parientes silvestres: Zea perennis, Zea diploperennis y Tripsacum dactyloides (Gramineae). Boletín de la Sociedad Argentina de Botánica, 24(1–2), 95–106.
Conn, H. J., Darrow, M. A., & Emmel, V. M. (1960). Staining procedures. Baltimore: I-XII. Ed. Williams Wilkins Co.
Curtis, R. H. C. (2008). Plant-nematode interactions: environmental signals detected by the nematode’s chemosensory organs control changes in the surface cuticle and behaviour. Parasite, 15, 310–316.
D’Ambrogio de Argüeso, A. (1986). Manual de técnicas en histología (vegetal. ed.). Argentina: Hemisferio Sur.
Di Rienzo, J. A., Casanoves, F., Balzarini, M. G., Gonzalez, L., Tablada, M., & Robledo, C. W. (2013). InfoStat. Argentina. URL: Grupo InfoStat, FCA, Universidad Nacional de Córdoba http://www.infostat.com.ar.
Dickison, W. C. (2000). Integrative plant anatomy. USA: Harcourt/Academic Press Massachusetts.
Doucet, M. E., & Lax, P. (2005). El género Nacobbus Thorne & Allen, 1944 en Argentina. 6. La especie N. aberrans (Thorne, 1935) Thorne & Allen, 1944 (Nematoda: Tylenchida) y su relación con la agricultura. Anales de la Academia Nacional de Agronomía y Veterinaria, 59, 5–45.
Doucet, M. E., Ponce de León, E. L., Tordable, M. C., & Poloni, N. (1997). Nacobbus aberrans y su asociación con vegetales en Argentina. Nematologia Mediterranea, 25, 279–285.
Dropkin, V. H. (1979). How nematodes induce disease. In: J. G. & E. B. Cowling (Eds), Plant Disease-An Advanced Treatise. Volume IV. How pathogens induce disease. Academic Press, New York. USA. INC. 219–238.
EPPO (2015). EPPO A1 and A2 lists of pests recommended for regulation as quarantine pests. European and Mediterranean plant protection organization. Paris: France.
Eves-van den Akker, S., Lilley, C., Danchin, E., Rancurel, C., Cock, P., Urwin, P., & Jones, J. (2014). The transcriptome of Nacobbus aberrans reveals insights into the evolution of sedentary endoparasitism in plant-parasitic nematodes. Genome Biology and Evolution, 6(9), 2181–2194.
Fahn, A. (1974). Anatomía Vegetal. España: H. Blume Ediciones. Madrid.
Finetti Sialer, M. (1990). Histopathological changes induced by Nacobbus aberrans in resistant and susceptible potato roots. Révue de Nématologie, 13(2), 155–160.
Freire, F., & Dos Santos, A. V. P. (1978). Histopatologia de raízes de pimenta-do-reino (Piper nigrum L.) parasitadas por Meloidogyne incognita. Acta Amazonica, 8(1), 19–24.
Harveson, R. (2008). False Root-Knot Nematode. NebGuide. University of Nebraska-Lincoln Extension, Institute of Agriculture and Natural Resources. G1857.
Inserra, R. N., Griffin, G. D., & Anderson, J. L. (1985). The false root-knot nematode Nacobbus aberrans. In Research bulletin 510. Utah Agricultural Experiment Station, Logan Utah: USA.
Jones, M. G. K. (1981). Host cell responses to endoparasitic nematode attack: structure and function of giant cells and syncytia. Annals of Applied Biology, 91, 353–372.
Jones, J. T., Haegeman, A., & Danchin, E. G. J. (2013). Top 10 plant-parasitic nematodes in molecular plant pathology. Molecular Plant Pathology, 14(9), 946–961.
Lax, P., Doucet, M. E., Braga, R., & Gioria, R. (2006). Response of different pepper varieties to the attack by two populations of Nacobbus aberrans. Nematologia Brasileira, 30, 259–265.
Lax, P., Rondan Dueñas, J. C., Coronel, N. B., Gardenal, C. N., Bima, P., & Doucet, M. E. (2011). Host range study of argentine Nacobbus aberrans Sensu Sher populations and comments on the differential host test. Crop Protection, 30, 1414–1420.
Lorenzo, E., Doucet, M. E., Tordable, M. C., & Poloni, N. (2001). Anatomía de raíces de pimiento y tomate atacadas por Nacobbus aberrans. Boletín de la Sociedad Argentina de Botánica, 36, 97–103.
Manzanilla-López, R. H., Costilla, M. A., Doucet, M. E., Franco, J., Inserra, R. N., Lehman, P. S., et al. (2002). The genus Nacobbus Thorne & Allen, 1944 (Nematoda: Pratylenchidae): systematics, distribution, biology and management. Nematropica, 32, 149–227.
Martínez-Fuentes, R., Tovar-Soto, A., Laguna-Hernández, G., & Torres-Coronel, R. (2010). Histopatología de las agallas inducidas por Nacobbus aberrans Thorne et Allen en frijol (Phaseolus vulgaris L.). Nematologia Mediterranea, 38, 45–52.
Ornat, C., Verdejo-Lucas, S., & Sorribas, F. J. (2001). A population of Meloidogyne javanica in Spain virulent to the Mi resistance Gene in tomato. Plant Disease, 85(3), 271–276.
Rodríguez, M., Arjona, H., Fischer, G., Campos, H., & Chaparro de Valencia, M. (2010). Aspectos Anatómicos del Desarrollo del Fruto de Feijoa [Acca sellowiana (O.Berg) Burret]. Revista Facultad Nacional de Agronomía, Medellín, 63(1), 5267–5273.
Sakai, W. S. (1973). Simple method for differential staining of paraffin embedded plant material using toluidine blue O. Stain Technology, 48(3), 247–249.
Sory Toure, A., Nieto-Ángel, R., Rodríguez-Pérez, J. E., Barrientos-Priego, A. F., Ibáñez-Castillo, L. A., Romanchik, K. E., & Núñez-Colín, C. A. (2010). Variación anatómica del xilema en tallo de cultivares de tomate injertados en un tipo criollo. Revista Chapingo Serie Horticultura, 16(1), 67–76.
Sosa-Moss, C., Barker, K. R., & Daykin, M. E. (1983). Histopathology of selected cultivars of tobacco infected with Meloidogyne species. Journal of Nematology, 15(3), 392–397.
Souza, R. M. (2001). O falso nematoide das galhas. Revisão anual de patologia de plantas, 9, 237–266.
Taiz, L., & Zeiger, E. (2006). Fisiología vegetal (Vol. 1). Castelló de la Plana. Publicacions de la Universitat Jaume I., D. L. España.
Taylor, A. I., & Sasser, J. N. (1978). Biology, identification and control of root-knot nematode (Meloidogyne species). North Carolina State University graphics. NC: Raleigh.
Tordable, M. C., Lax, P., & Doucet, M. E. (2008). Análisis histopatológico en tubérculos de dos variedades de papa andina (Solanum tuberosum subsp. andigenum) infectadas por especies del género Meloidogyne. Nematropica, 38(1), 95–103.
Tordable, M. C., Lax, P., Doucet, M. E., Bima, P., Ramos, D., & Vargas, L. (2010). Response of roots of different plants to the presence of the false root-knot nematode Nacobbus aberrans. Russian Journal of Nematology, 18(1), 31–39.
Veremis, J. C., Cap, G. B., & Roberts, P. A. (1997). A search for resistance in Lycopersicon spp. to Nacobbus aberrans. Plant Disease, 81, 217–221.
Vovlas, N., Nico, A. I., De Luca, F., De Giorgi, C., & Castillo, P. (2007). Diagnosis and molecular variability of an Argentinean population of Nacobbus aberrans with some observations on histopathology in tomato. Journal of Nematology, 39(1), 17–26.
Wilcox, D., Dove, B., McDavid, D., & Greer, D. (2002). Imagen Tool for Windows Version 3.00. UTHSCSA. The University of Texas Health Science Center in San Antonio.
Yang, F., Abdelnabby, H., & Xiao, Y. (2015). Novel point mutations in β-tubulin gene for carbendazim resistance maintaining nematode pathogenicity of Paecilomyces lilacinus. European Journal of Plant Pathology, 143, 57–68.
Acknowledgments
This work was supported by the Consejo Nacional de Investigaciones Científicas y Técnicas and the Secretaría de Ciencia y Tecnología, Universidad Nacional de Córdoba, Argentina.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cabrera, V.A., Dottori, N. & Doucet, M.E. Histopathology of roots of three tomato cultivars infected with two separate isolates of the false root-knot nematode Nacobbus aberrans . Eur J Plant Pathol 148, 393–403 (2017). https://doi.org/10.1007/s10658-016-1097-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-016-1097-1