Abstract
The purpose of the main research was to investigate the effects of methyl jasmonate (MeJA) (0.05, 0.25, 0.5, and 2.5 mM) on the pollen germination and tube elongation of Pinus nigra. Total pollen germination rate increased after MeJA treatments while the most enhancement was observed at 0.05-mM MeJA. No germination was observed at 2.5-mM MeJA. Although the unipolar and bipolar germination were observed in all groups, no significant changes were observed in unipolar and bipolar pollen germination rates after MeJA treatments. Tube length increased only at 0.05-mM MeJA. Although branched tubes were observed in all groups, branched tube rate increased only at 0.05-mM MeJA. Although two branched, three branched, and consecutive branched tubes were observed in all groups, the most common branching type was two branched type in all groups. Although anisotropy of actin filaments in the shank and apex of unbranched tubes decreased after MeJA treatments, the most decrease was observed at 0.05-mM MeJA. Also, anisotropy of actin filaments in the shank and in pre-branching region of branched tubes decreased only at 0.25-mM MeJA. Anisotropy of both two apexes of a branched tube changed only at 0.25- and 0.5-mM MeJA. Callose accumulation in the apex of unbranched and branched tubes increased in parallel with the increase in MeJA concentration. However, more callose is accumulated in one apex than the other apex of a branched tube. In conclusion, MeJA affected the actin organization, changed the callose distribution, and altered the pollen tube growth of Pinus nigra.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pollen tubes are one of the most important structures of the sexual reproduction process due to their crucial role in the transport of sperm nuclei to the egg cell, in both angiosperms and gymnosperms (Chen et al. 2006). However, most of the studies on pollen tubes have focused on angiosperms; more data is needed on gymnosperm pollen tube physiology (Breygina et al. 2019). Pollen tubes of angiosperm and gymnosperm seem structurally similar; however, there are various differences between them such as tube growth speed, number of elongated tubes, and cell wall properties of tubes (Lora et al. 2016; Breygina et al. 2019). Pollen grains of gymnosperm germinate more slowly than pollen grains of angiosperm, and tube elongation also occurs more slowly (Fernando et al. 2005). In angiosperms, multipolar pollen germination is rare although it is a widespread phenomenon in gymnosperms (Breygina et al. 2019). Researchers have assumed that bipolar germination helps gymnosperm pollen at an earlier stage to obtain nutrients (Lora et al. 2016; Breygina et al. 2019). Moreover, in the case of regular pollen germination of angiosperm, generally, only one regular unbranched tube extends from the pollen grain. However, gymnosperm pollen tubes tend to form branch (Lora et al. 2016). Researchers have assumed that branching helps pollen at an earlier stage to obtain nutrients in the same way as bipolar germination (Lora et al. 2016; Breygina et al. 2019).
Tube growth in angiosperms is highly dependent on the actin cytoskeleton that transports the vesicles containing cell wall pioneer to the apex (Lovy-Wheeler et al. 2005; Zhang et al. 2019). Inhibition of tube elongation by actin disruption in gymnosperms such as Picea abies (Anderhag et al. 2000) and Picea wilsonii (Sheng et al. 2012) has shown that the actin cytoskeleton is also required for growth in gymnosperm pollen tubes. On the other hand, researchers have indicated that cytoskeleton pattern differs between the angiosperms and gymnosperms (Chen et al. 2006). In angiosperms, the tube apex and sub-apex region are characterized by short actin filaments while actin filaments form parallel actin bundles in the shank region (Qu et al. 2015; Zhang et al. 2019). Similarly, researchers have indicated that actin filaments in gymnosperms were located along the tube in a net axial array while they are distributed as short arrays in the elongating tip. However, there is no mention of the presence of the sub-apex region in gymnosperms. Therefore, the structure of the actin cytoskeleton in gymnosperms is a subject that needs more explanation.
Cell wall structure is one of the main distinctions between the angiosperm and gymnosperm pollen tubes. The pollen tube wall of angiosperms has an outer layer containing cellulose and pectin and has an inner wall containing callose (Cai et al. 2011). Although the gymnosperm pollen tube also contains cellulose, pectin, and callose, there is no inner and outer membrane separation (Fernando et al. 2005). In angiosperms, callose is located throughout the pollen tubes, except the apex (Cai et al. 2011). Actually, the apex localized callose is considered an abnormality for angiosperm pollen tubes, because the callose is located in the tips of abnormal or growth-inhibited angiosperm pollen tubes (Hao et al. 2013; Çetinbaş-Genç 2020). However, Derksen et al. (1999) and Fernando et al. (2005) have indicated that the callose is located in the apex of a regular gymnosperm pollen tube.
Researchers have reported that plant hormone and plant signaling molecule regulate actin organization and cell wall structure (Cao et al. 2010; Lombardo and Lamattina 2018; Li et al. 2018a; b). The researchers have been stated that various plant growth regulators affect some properties of pollen tubes such as cytoskeleton organization or cell wall properties and change the growth dynamics of the tubes (Aloisi et al. 2016; Çetinbaş-Genç 2019). One of these plant growth regulators is jasmonates (Jas). JAs are important plant hormones which derived from linolenic acid in the chloroplast (Fugate et al. 2018). They include jasmonic acid (JA) and its methyl ester named methyl jasmonate (MeJA). MeJA is the most known and studied member of JAs and researchers have indicated that MeJA regulates the signaling networks in plant growth and stress responses (Li et al. 2018a, b). Similar to other hormones, MeJA take place in various processes such as seed germination, root elongation, embryogenesis, senescence, and cellular communication (Gumerova et al. 2015; Li et al. 2018a, b). Researchers have stated that the effects of MeJA on plant growth are versatile such as promoting, preventing, and protecting. It has been stated that the effect of JAs treatment on plants may vary depending on the plant species, type, and application dose of JAs (Ozturk et al. 2015). Contrary to many hormones, the effect of JAs on pollen performance has been little studied. It has been noticed that JA inhibited the pollen germination in strawberry (Yildiz and Yilmaz 2002). However, researchers have shown that MeJA affected pollen germination in a dose-dependent manner and it has been reported that 0.1- and 0.25-mM MeJA promoted while 0.5- and 1-mM MeJA inhibited the pollen germination in apricot (Muradoğlu et al. 2010). Researchers have determined that the tube length decreased as the concentration increases in Bebeco genotypes of apricot. Also, they have determined that 0.1- and 0.25-mM MeJA promoted while 0.5- and 1-mM MeJA inhibited the pollen tube length in Kabaasi genotypes of apricot (Muradoğlu et al. 2010). As understood, the effects of JA have been studied only in angiosperm pollen tubes as in many other plant growth regulators but it is not clear how or with which mechanism MeJA affects pollen tubes. Moreover, their effects on gymnosperm pollen tubes have not been studied before.
The main goal of this study is to investigate the effect of MeJA on pollen germination and tube growth and to find out the effect of MeJA on actin cytoskeleton and callose distribution in pollen tubes of Pinus nigra. The results will also provide a detailed information about growth mechanism of gymnosperm pollen tubes and also present a detailed comparison of angiosperm and gymnosperm pollen tubes.
Material and methods
Plant material
Mature male cones of P. nigra were collected from Marmara University Campus garden in May of 2019. In pollen shedding, the cones are kept at room temperature for 2 days and pollen grains were collected.
In vitro pollen germination and tube growth
Pollen grains were germinated at room temperature for 24 h. Liquid germination medium containing 12% sucrose, 1-mM H3BO3, and 1-mM CaCl2 was used for germination assays (Lazzaro 1999; Maksimov et al. 2018; Breygina et al. 2019). The MeJA-free medium was used for the control group, and 0.05-, 0.25-, 0.5- or 2.5-mM MeJA (Sigma-Aldrich) were added to the medium for the treatment groups. To analyze the germination rates, just about 1500 pollen grains were investigated for each group. The total germination rate was calculated regardless of whether it was unipolar or bipolar. Also, unipolar pollen germination was considered when pollen grains have only one tube and bipolar germination was considered when pollen grains have two tubes longer than the diameter of the grain. The total pollen tube lengths were measured considering randomly selected 150 pollen tubes for each group. However, we found that most of the pollen grains were bipolar germinated and most of the pollen tubes were branched in all groups. So, when calculating the total pollen tube lengths, we measured the length from the tip of the longest branch to the pollen grain in branched pollen tubes. Also, when calculating the total pollen tube lengths, we measured the longest tube of a bipolar germinated pollen grains. Moreover, to make a clear comparison, we measured separately the pollen tube lengths of unipolar and bipolar germinated pollen tubes considering randomly selected 150 tubes for each group. The pollen tube lengths of bipolar germinated pollen grains were measured taking into account the longest tube of a bipolar germinated pollen grains. Branched tube rate, percentages of two branched, three branched and consecutive branched tubes were calculated. Since the most common branched tube morphology is two branched, to make a clear comparison, we measured separately the pollen tube lengths of unbranched and two branched pollen tubes considering randomly selected 150 tubes for each group. We measured the length from the tip of the longest branch to the pollen grain in branched pollen tubes. The Olympus BX-51 light microscope equipped with the KAMERAM software was used for the observations.
Labelling of actin filaments
Actin labelling was performed according to the Lovy-Wheeler method (Lovy-Wheeler et al. 2005). After 30 min of fixation with buffer (pH 6.9) containing 100-mM PIPES, 5-mM MgSO4, 0.5-mM CaCl2, 0.05% (v/v) Triton X-100, 1.5% (m/v) formaldehyde, and 0.05% (m/v) glutaraldehyde, pollen tubes were rinsed with the same buffer containing 10-mM EGTA and 6.6-μM Alexa 488-phalloidin (Thermo Fisher Scientific). Pollen tubes were monitored at 488-nm wavelengths with an Olympus BX-51 fluorescence microscope equipped with the KAMERAM software. To avoid focusing on problems, a single focal plane was used during the observations, and, to measure the anisotropy of actin filaments, 20 tubes with equal length were selected for all groups. Actin filament anisotropy was measured in unbranched and branched tubes using the “Fibril Tool” plugin of ImageJ. Anisotropy in the shank of the unbranched tube was measured in the area after the 0–40 μm from the pollen grain, and anisotropy in the apex of unbranched tube was measured in the area before the 0–10 μm from the tube tip. Anisotropy in the shank of the branched tube was measured in the area after the 0–40 μm from the pollen grain. Anisotropy in the pre-branching region was measured in the area before the 0–10 μm from the branch separation. Also, anisotropy in the apexes of branched tube was measured in the area before the 0–10 μm from the tube tips. The 1st branch is determined as the short branch and the 2nd branch is determined as the long branch.
Labelling of callose
Callose labelling was performed according to Chen et al. (2007). Pollen tubes were labelled with 0.1% Aniline Blue (Sigma-Aldrich) and visualized at 455-nm wavelengths with an Olympus BX-51 fluorescence microscope equipped with the KAMERAM software. To detect the callose accumulations in the apex of unbranched and apexes of branched tubes, fluorescence intensities were calculated in a 100-μm2 area of the tip with the “Rectangle Selection” option of ImageJ. In branched tubes, the 1st branch is determined as the short branch and the 2nd branch is determined as the long branch. We focused on this area corresponding to the growth zone where most of the secretory vesicles fuse; this allowed us to obtain information on the production of callose in the area of highest vesicular secretion. Signals have been reset against the background noise. Fluorescence intensity analysis was performed on 20 pollen tubes with equivalent length for each group.
Analyzing of data
Three technical replicates were conducted for each experiment in each group. Statistical analyses were carried out with the SPSS 16.0 software. Significance of the difference between groups of data was specified by the one-way analysis of variance (ANOVA) with a threshold P value of 0.05. Distinct letters in graphs indicate the statistically significant differences and error bars point out the standard deviations.
Results
The effect of MeJA on in vitro pollen germination and tube growth
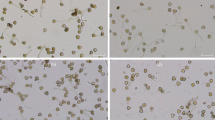
Firstly, pollen germination rates were calculated for the purpose of examining the effects of MeJA on pollen germination. Based on the results, the total pollen germination rate significantly increased by 45.48% at 0.05-mM MeJA and 32.18% at 0.25-mM MeJA when compared with the control (Fig. 1a). No germination was observed after the 2.5-mM MeJA treatment and so it was not included on the graphics in the continuation of the study (Fig. 1i). Besides, since bipolar germination is a very common condition in gymnosperms, the unipolar and bipolar germination rates were calculated to examine whether the MeJA affects unipolar or bipolar germination. Unipolar pollen germination was considered when pollen grains have only one tube and bipolar germination was considered when pollen grains have two tubes (Fig. 1d). Unipolar germination rate was recorded as 26.19% and bipolar germination rate was recorded as 25.41% in the control. After MeJA treatments, no statistically significant changes were observed in unipolar and bipolar pollen germination rates when compared with the control (Fig. 1b, c). However, unipolar and bipolar germinated pollen grains were observed in all groups (Fig. 1e, f, g, h).
Effects of MeJA on pollen germination. a Total pollen germination rate. b Unipolar pollen germination rate. c Bipolar pollen germination rate. d Unipolar (white asterisk) and bipolar (red asterisk) germinated pollen grains. e Germinated pollen grains in the control group. f Germinated pollen grains at 0.05-mM MeJA. g Germinated pollen grains at 0.25-mM MeJA. h Germinated pollen grains at 0.5-mM MeJA. i Ungerminated pollen grains at 2.5-mM MeJA. White asterisk refers to unipolar germinated pollen grains and red asterisk refers to bipolar germinated pollen grains. Distinct letters in graphs indicate significant differences (P < 0.05) and error bars indicate the standard deviations. To analyze the germination rates, just about 1500 pollen grains were investigated for each group. Three technical replicates were conducted for each experiment in each group. Bar, 50 μm
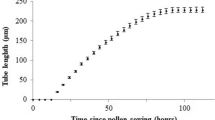
After observing the germination rates, we calculated the total pollen tube lengths for the purpose of examining the effects of MeJA on pollen tube elongation. However, we found that most of the pollen grains were bipolar germinated and most of the pollen tubes were branched in all groups including the control. So, when calculating total pollen tube lengths, we measured the length from the tip of the longest branch to the pollen grain in branched pollen tubes. Also, when calculating the total pollen tube lengths, we measured the longest tube of bipolar germinated pollen grains. The pollen tube length significantly increased by 9.39% at 0.05-mM MeJA, significantly decreased by 12.36% at 0.25-mM MeJA, and significantly decreased by 18.70% at 0.5-mM MeJA when compared with the control (Fig. 2a). Afterwards, to make a clear comparison, we measured separately the pollen tube lengths of unipolar and bipolar germinated pollen tubes. The pollen tube lengths of bipolar germinated pollen grains were measured taking into account the longest tube of a bipolar germinated pollen grains. The pollen tube length of unipolar germinated pollen grains significantly increased by 24.51% at 0.05-mM MeJA and significantly decreased by 16.88% at 0.5-mM MeJA when compared with the control (Fig. 2b). The pollen tube length of bipolar germinated pollen grains significantly increased by 16.35% at 0.05-mM MeJA and by 14.08% at 0.25-mM MeJA when compared with the control (Fig. 2c).
Effects of MeJA on pollen tube length and branch formation. a Pollen tube length. b Branched tube rate. c Unbranched pollen tube. d Two branched pollen tube. e Three branched pollen tube. f, g, h Consecutive branched pollen tubes. i Two branched pollen tube rate. j Three branched pollen tube rate. k Consecutive branched pollen tube rate. Distinct letters in graphs indicate significant differences (P < 0.05) and error bars indicate the standard deviations. Pollen tube lengths were measured considering randomly selected 150 pollen tubes for each group. Three technical replicates were conducted for each experiment in each group. Bar, 50 μm
To determine whether MeJA-induced changes in tube lengths were related to branch formation and to find out the effect of MeJA on branch formation, the branched tube rate was calculated. The branched tube rate significantly increased by 26.41% at 0.05-mM MeJA and significantly decreased by 42.87% at 0.5-mM MeJA in comparison with the control (Fig. 2d). Unbranched tubes (Fig. 2h) and branched tubes were observed in all groups. Two branched (Fig. 2i), three branched (Fig. 2j), and consecutive branched tubes (Fig. 2k, l, m) were the, respectively, most common branched tube morphologies.
To investigate whether there is a relation between the MeJA application and different branched tube morphologies, the percentages of two branched, three branched, and consecutive branched tubes were calculated. The rate of two branched pollen tubes significantly increased by 60.93% at 0.05-mM MeJA, significantly decreased by 31.91% at 0.25-mM MeJA, and significantly decreased by 44.35% at 0.5-mM MeJA in comparison with the control (Fig. 2e). However, there was no significant change in the rates of three branched and consecutive branched pollen tubes after MeJA treatments when compared with the control (Fig. 2f, g).Since the most common branched tube morphology is two branched, to make a clear comparison, we measured separately the pollen tube lengths of unbranched and two branched pollen tubes. We measured the length from the tip of the longest branch to the pollen grain in branched pollen tubes. The unbranched pollen tube length significantly increased by 21.92% at 0.05-mM MeJA and significantly decreased by 6.71% at 0.25-mM MeJA and 20.28% 0.5-mM MeJA (Fig. 2n). However, there was no significant difference in branched pollen tube length after MeJA treatments (Fig. 2o).
The effect of MeJA on actin filament organization
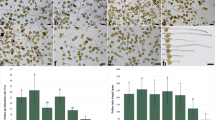
Actin filament distribution was observed to investigate the relationship of actin filament distribution with MeJA-induced changes in tube length. Firstly, we investigated the actin filament distribution in unbranched pollen tubes. In the control, actin filaments were observed as parallel bundles in the shank while they were observed as a dense array of short filaments in the apex (Fig. 3a). After MeJA treatments, actin filament distribution did not show a remarkable difference in the shank and apex when compared with the control (Fig. 3b, c, d). However, to find out the subtler differences of actin filament distribution in both the shank and apex, a software-based anisotropy analysis was performed. Anisotropy in the shank significantly decreased by 36.36% at 0.05-mM MeJA, 13.63% at 0.25-mM MeJA, and 22.72% at 0.5-mM MeJA when compared with the control (Fig. 3i) Also, anisotropy in the apex significantly decreased by 40% at 0.05-mM MeJA and 36% at 0.5-mM MeJA in comparison with the control (Fig. 3j).
Effects of MeJA on actin filament organization. a Unbranched pollen tube at control. b Unbranched pollen tube at 0.05-mM MeJA. c Unbranched pollen tube at 0.25-mM MeJA. d Unbranched pollen tube at 0.5-mM MeJA. e Branched pollen tube at control. f Branched pollen tube at 0.05-mM MeJA. g Branched pollen tube at 0.25-mM MeJA. h Branched pollen tube at 0.5-mM MeJA. i Anisotropy in the shank of unbranched tubes. j Anisotropy in the apex of unbranched tubes. k Anisotropy in the shank of branched tubes. l Anisotropy in pre-branching region. m Anisotropy in the apex of two branches. Distinct letters in graphs indicate significant differences (P < 0.05) and error bars indicate the standard deviations. Anisotropy analysis was performed on 20 pollen tubes with equivalent length for each group. Three technical replicates were conducted for each experiment in each group. Bar, 20 μm
To examine the relationship of actin filament distribution with MeJA-induced changes in branched tube rates, actin filament distribution was observed in branched tubes. Since the most common branched tube morphology is two branched, we made our examinations on two branched tubes. Actin filaments were distributed as long fibrils in both the main shank and branches in all groups (Fig. 3e, f, g, h). However, one of the branches had a denser filament at 0.25-mM MeJA and 0.5-mM MeJA (Fig. 3g, h). Also, it was observed that fibrils formed bundles before branch separation in all groups (Fig. 3e, f, g, h). However, to find out the subtler differences of actin filament distribution in both the shank and pre-branching region, the anisotropy values were measured. According to the results, anisotropy in the shank significantly decreased by 50% at 0.25-mM MeJA and significantly increased by 42.30% at 0.5-mM MeJA compared with the control (Fig. 3k). Also, the anisotropy level in the pre-branching region significantly decreased by 42.30% at 0.25-mM MeJA and significantly increased by 53.84% at 0.5-mM MeJA compared with the control (Fig. 3l).
In both two apexes of a branched tube, actin filaments were observed as a dense array of short filaments in all groups (Fig. 3e, f, g, h). The software-based anisotropy analysis was performed to observe both the difference between the groups and the difference between the branches of the same group. According to the results, significant changes in the anisotropy of the apex were observed only at 0.25-mM MeJA and 0.5-mM MeJA groups in comparison with the control (Fig. 3m). The anisotropy measured in both apexes of a branched tube was not statistically different from each other at the control and 0.05-mM MeJA groups. However, the anisotropy measured in both apexes of a branched tube was statistically different from each other at 0.25-mM MeJA and 0.5-mM MeJA (Fig. 3m). The anisotropy in the apex was 3.25 times higher than the other apex at 0.25-mM MeJA and 1.94 times higher than the other apex at 0.5-mM MeJA, significantly (Fig. 3m).
The effect of MeJA on callose distribution
Callose distribution was examined to determine the relation between callose accumulation and MeJA induction in pollen tubes. Initially, we investigated the callose distribution in unbranched pollen tubes. In the control, the callose is located in the apex but not presented throughout the shank (Fig. 4a). After MeJA treatments, similar callose pattern with the control appeared in the tubes. However, it was found that the callose accumulation in the apex increased with increasing MeJA concentrations (Fig. 4b, c, d). To find out the clear difference of callose accumulation, fluorescence intensity of the apex localized callose was measured in 100-μm2 segments of the apex. Fluorescence intensity significantly increased by 34.71% at 0.05-mM MeJA, 118.13% at 0.25-mM MeJA and 131.34% at 0.5-mM MeJA in comparison with the control (Fig. 4i).
Effects of MeJA on callose accumulation a Unbranched pollen tube at control. b Unbranched pollen tube at 0.05-mM MeJA. c Unbranched pollen tube at 0.25-mM MeJA. d Unbranched pollen tube at 0.5-mM MeJA. e Branched pollen tube at control. f Branched pollen tube at 0.05-mM MeJA. g Branched pollen tube at 0.25-mM MeJA. h Branched pollen tube at 0.5-mM MeJA. i Fluorescence intensity of callose in the apex of unbranched tubes. j Fluorescence intensity of callose in the apexes of branched tubes. Distinct letters in graphs indicate significant differences (P < 0.05) and error bars indicate the standard deviations. Fluorescence intensity analysis was performed on 20 pollen tubes with equivalent length for each group. Three technical replicates were conducted for each experiment in each group. Bar, 20 μm
To investigate the relation between callose distribution and MeJA induction in branched tube rates, callose distribution was observed in branched tubes. Since the most common branched tube morphology is two branched, we made our examinations on two branched tubes. In all groups, callose is located in both apexes of a branched tube while one of the apexes of a branched tube had a denser callose accumulation (Fig. 4e, f, g, h). Fluorescence intensity of the apex localized callose was calculated in 100-μm2 segments of the both apexes of a branched tube to observe both the difference between the groups and the difference between apexes of a branched tube. Fluorescence intensity significantly increased in only one apex of 0.05-mM MeJA while significantly increased in both apexes of a tube at 0.25- and 0.5-mM MeJA in comparison with the control. Fluorescence intensity in the apex was 1.49 times higher than the other apex at 0.05-mM MeJA, 1.23 times higher than the other apex at 0.25-mM MeJA, and 1.16 times higher than the other apex at 0.5-mM MeJA, significantly (Fig. 4j).
Discussion
The effects of plant growth regulators on pollen germination and pollen tube elongation have been frequently studied (Aloisi et al. 2016; Çetinbaş-Genç 2019). JA and MeJA are the plant growth regulators whose effects on pollen germination and tube elongation are the least studied. Also, pollen grains of angiosperms were investigated in most of these studies (Yildiz and Yilmaz 2002; Muradoğlu et al. 2010). Therefore, our study reveals important and original findings about the effects of MeJA on gymnosperm pollen tubes.
Researchers have indicated that 0.5-mM JA inhibited the pollen germination in strawberry (Yildiz and Yilmaz 2002). Also, Muradoğlu et al. (2010) have reported that 0.1- and 0.25-mM MeJA promoted while 0.5- and 1-mM MeJA inhibited the pollen germination in apricot. Similar with these results, the 0.05- and 0.25-mM MeJA increased the total pollen germination while 0.5 mM had no significant effect according to our results. Also, it was quite interesting that there was no germination after the 2.5-mM MeJA treatment. When evaluated the literature together with our findings, it was thought that JA and MeJA have similar effects on both angiosperm and gymnosperm pollen tubes. Bipolar germination is very common in gymnosperms, unlike most angiosperms (Breygina et al. 2019). Bipolar germination has been reported in some angiosperms such as Impatiens and Oenothera (Derksen et al. 1995) and Conospermum endemic to Australia (Stone et al. 2004). Researchers have stated that bipolar germination is a typical feature in gymnosperms, especially in the Pinaceae family. For instance, bipolar pollen germination has been reported in Pinus sylvestris (deWin et al. 1996) and Picea pungens (Breygina et al. 2019). According to our results, bipolar germination was a very common phenomenon and observed in all groups, including control. Although the MeJA affected the total germination rate in a dose-dependent manner, there were no significant effects on unipolar or bipolar germination rates.
It is known that most plant growth regulators affect tube elongation depending on the dose (Aloisi et al. 2016, Çetinbaş-Genç 2019). It has been reported that 0.1- and 0.25-mM MeJA promoted while 0.5- and 1-mM MeJA inhibited the pollen tube length in apricot (Muradoğlu et al. 2010). Similar with this finding, we detected that tube length increased at 0.05-mM MeJA while decreased at 0.25- and 0.5-mM MeJA. During our examinations, most of the tubes were found to be branched in all groups including the control. The researchers stated that gymnosperm pollen tubes tend to form branch (Lora et al. 2016) and that this trend may be concerned with the slow elongation of gymnosperm tubes (Mogami et al. 1999; Hao et al. 2005; Owens et al. 2005). Many researchers have stated that branching in pollen tubes of both angiosperm and gymnosperm may be related to pollen tubes functioning as haustorium (Johri 1992). It has been known that the tip of the pollen tubes is branched in some angiosperms such as Cucurbita and Onagraceae (Tilquin et al. 1983). Branching in angiosperm pollen tubes usually means that the growth is inhibited. For instance, branch formation blocked the pollen tube elongation in Trifolium pretense (Büyükkartal 2002). In addition, Cheung et al. (2003) have noticed the pollen tube elongation blocked after branch formation. Moreover, it has been known that branching usually occurs as a result of stress in angiosperms. For instance, pollen tubes of Echinopsis chamaecereus have branched after high-temperature treatments (Çetinbaş-Genç 2020). According to our results, branched tube rate increased at 0.05-mM MeJA while decreased at 0.5-mM MeJA. However, the 0.25-mM MeJA had no effect on branch formation. Two branched tubes were very common in all groups including the control. Two branched tube rates increased at 0.05-mM MeJA while decreased at 0.25- and 0.5-mM MeJA. Also, the three branched or consecutive branched tubes were observed in all groups including the control. But MeJA concentrations had no effect on the three branched or consecutive branched tube rates. Similar with this, deWin et al. (1996) have reported the two branched, three branched, and consecutive branched tubes in Pinus sylvestris and have stated that the most common branching type was two branched formation.
Proper construction of actin cytoskeleton is essential for pollen tube elongation in both angiosperms and gymnosperms (Qu et al. 2015) because actin filaments carry newly synthesized cell wall materials to the tube tip, allowing the tube to extend properly (Cai et al. 2011). In angiosperms, pollen tube is classified into three zones. Actin filaments exist as short and dynamic arrays in the apex zone while as long parallel arrays in the shank. The sub-apex zone is located before the apex zone and actin filaments form a fringe structure in this zone (Cai et al. 2015). In studies performed in gymnosperm pollen tubes, there is no mention of the presence of a fringe-like actin organization and a sub-apex region. Researchers have indicated that actin filaments were located along the pollen tube in a net axial array while they distributed as short arrays in the elongating tip in gymnosperms such as Pinus densiflora (Terasaka and Niitsu 1994), Pinus sylvestris (deWin et al. 1996), Picea mayeri (Chen et al. 2007), Pinus thunbergii (Li et al. 2008), Pinus bungeana (Wang et al. 2009), and Picea wilsonii (Sheng et al. 2012). The terms shank and apex were also not used in these studies. However, as a result of both the literature and our findings, we detected that the actin organization in the main tube body and the tip were similar in both angiosperms and gymnosperms. Therefore, we divided the pollen tubes into two regions as the shank and apex in order to examine the actin filament organizations more clearly. So, we used the shank and apex terms in this study. According to our results, actin filaments of unbranched tubes were observed as parallel bundles in the shank while they were observed as a dense array of short filaments in the apex in the control groups in parallel with previous studies conducted on Pinus densiflora (Terasaka and Niitsu 1994), Pinus sylvestris (deWin et al. 1996), Picea mayeri (Chen et al. 2007), Pinus thunbergii (Li et al. 2008), Pinus bungeana (Wang et al. 2009), and Picea wilsonii (Sheng et al. 2012). To investigate the detailed changes in actin filament organization after MeJA treatment, the anisotropy values in the shank and apex were measured. the anisotropy value is a count between 0 and 1 (Boudaoud et al. 2014). And decreasing of anisotropy refers to the increase in proper actin organization (Parrotta et al. 2016). So, less anisotropy means more proper actin organization; more proper actin organization refers to the proper transfer of organelles and vesicles at the apex and leads to higher germination rate and longer pollen tubes. Actin filament anisotropy in the shank decreased after 0.05-, 0.25-, and 0.5-mM MeJA treatment. Also, the anisotropy in the apex decreased after 0.05- and 0.5-mM MeJA treatment. The fact that actin filaments at 0.05-mM MeJA have the less anisotropy in both the apex and shank explained the high germination rate and high tube length at 0.05-mM MeJA.
To investigate the relation between actin filament distribution and MeJA induction in branched tube rates, the actin filament distribution was also observed in branched tubes. After the 0.05-mM MeJA treatment, there was no change in the anisotropy of the shank, apex, and pre-branching region. Also, there was no difference between the levels of two apexes of a tube. It was observed that the anisotropy of actin filaments decreased in the shank and pre-branching region after the 0.25-mM MeJA treatment, while it was observed that the anisotropy increased in the shank and apex after the 0.5-mM MeJA treatment. After the 0.25- and 0.5-mM MeJA treatment, anisotropy showed a remarkable difference between the apexes of a tube. It was observed that anisotropy increased in one apex and decreased in the other apex of a tube. Also, one of the branches had a denser actin filament at 0.25- and 0.5-mM MeJA. It was thought that this difference may be related to the low branching rate at 0.25- and 0.5-mM MeJA and branch formation might have been prevented as a result of the increased anisotropy of actin filament in one apex of a tube. deWin et al. (1996) have stated that actin filaments were evident at both apexes of a tube in Pinus sylvestris. Anderhag et al. (2000) have reported that actin filament disruption has led to the formation of numerous short branches in Picea abies. Also, Fernando et al. (2005) have indicated that actin filament disruption-induced branch formation is unique to gymnosperm pollen tubes due to the branching which is not a widespread response to microfilament degradation in angiosperms. The researchers mentioned that branching may be related to the fact that pollen tubes play the role of haustoria in gymnosperms. The authors discussing the evolution of pollen tube growth assume the “haustorial” nature of multiple tubes: they could obtain nutrients more effectively (Lora et al. 2016; Breygina et al. 2019). Terasaka and Niitsu (1994) have noticed that actin filament disruption after colchicine treatments inhibits tube growth and induces additional branching in Pinus densiflora. Therefore, it can be thought that branching is closely related to the actin filament disruption and changes in the filament disruption as a result of various stresses affecting the branching rate. Moreover, researchers have stated that actin filament disruption may cause swelling, curling of tube, or rarely branch formation in angiosperms (Parrotta et al. 2019; Çetinbaş-Genç et al. 2019).
Cell wall structure is closely related with the actin filament organization in both angiosperms and gymnosperms because the actin cytoskeleton carries newly synthesized cell wall materials to the tube apex (Cai et al. 2011; Cai et al. 2015). For instance, the modified actin organization changed the cell wall construction in angiosperms such as Nicotiana tabacum (Parrotta et al. 2016) and Camellia sinensis (Çetinbaş-Genç 2019). Also, the disturbed actin organization affected the cell wall architecture in gymnosperms such as Picea meyeri (Chen et al. 2007) and Picea wilsonii (Sheng et al. 2012). One of the most obvious differences between angiosperm and gymnosperm pollen tube cell wall is the callose distribution. Callose is distributed throughout the pollen tubes, except the apex in angiosperms (Cai et al. 2011) because the apex localized callose is related to the abnormal or blocked tube in angiosperms (Hao et al. 2013; Çetinbaş-Genç 2020). However, in gymnosperms, callose is located in the apex of young pollen tube and it cannot be observed throughout the tube in later stages (Derksen et al. 1999; Fernando et al. 2005). We investigated the callose accumulation in both unbranched and branched tubes. According to our results, callose only appeared in the apex of the unbranched tubes in all groups. Similarly, researchers have indicated that callose accumulates at the tips of young pollen tubes in Pinus sylvestris (Fernando et al. 2005), Picea meyeri (Chen et al. 2007), and Pinus bungeana (Hao et al. 2013). Based on our results, callose accumulation in the apex of unbranched tubes increased with the increasing concentration of MeJA. The intense accumulation of callose in the apex after 0.25- and 0.5-mM MeJA was thought to be related to the increase of actin anisotropy in these groups. Similarly, Sheng et al. (2012) have reported that callose signal in the apex increased after the disruption of actin filament by lead stress in Picea wilsonii. In branched tubes, callose accumulation increased only in one apex of a branched tube at 0.05-mM MeJA. However, callose accumulation increased at both apexes of a branched tube after 0.25- and 0.5-mM MeJA treatment. After all concentration of MeJA treatments, it was determined that different accumulations of callose were seen in both apexes of a branched tube. This suggested that one of the branches was young and the other was older. Derksen et al. (1999) have reported that young apex contains denser callose than the older apex in Pinus sylvestris.
There is a relationship between JAs and ethylene and this relationship has been shown in many plants (Farmer et al. 2003). It has been known that JA treatments increase the ethylene production (Kondo et al. 2007; Mukkun and Singh 2009). For instance, researchers stated that JA and MeJA increased the ethylene production in fruit or leaves of strawberry, apple, tomato, kiwifruit, and olive (Perez et al. 1997; Hudgins and Franceschi 2004; Wu et al. 2020). Ethylene signal is required for pollen germination and tube elongation (Jia et al. 2018). Holden et al. (2003) have stated that ethylene production in the pistil after pollination promotes the pollen germination and tube elongation in Petunia inflata and that a decrease in ethylene amount reduced the pollen germination and tube elongation. Moreover, researchers have reported that ethylene affects the actin cytoskeleton (Kushwah et al. 2011; Li and Jia 2013). Jia et al. (2018) have reported that exogenously ethylene treatment promoted the pollen germination and tube elongation by modifying the actin cytoskeleton and changing the cell wall structure.
Conclusion
In conclusion, it was shown that exogenous MeJA alters the pollen tube elongation of Pinus nigra by reorganizing the actin construction and callose distribution. It has been identified that 0.05-mM MeJA was more effective on pollen germination and tube elongation. These findings provide new insights into the gymnosperm pollen tube elongation and mechanism of MeJA treatment in the tip growth of pollen tubes. Also, our experiments provided new insight into the comparison of tube elongation between angiosperm and gymnosperms.
References
Aloisi I, Cai G, Serafini-Fracassini D, Del Duca S (2016) Polyamines in pollen: from microsporogenesis to fertilization. Front Plant Sci 7:155. https://doi.org/10.3389/fpls.2016.00155
Anderhag P, Hepler PK, Lazzaro MD (2000) Microtubules and microfilaments are both responsible for pollen tube elongation in the conifer Picea abies (Norway spruce). Protoplasma 214:141–157. https://doi.org/10.1007/BF01279059
Boudaoud A, Burian A, Borowska-Wykret D, Uyttewaal M, Wrzalik R, Kwiatkowska D, Hamant O (2014) FibrilTool, an ImageJ plug-in to quantify fibrillar structures in raw microscopy images. Nat Protoc 9:457–463. https://doi.org/10.1038/nprot.2014.024
Breygina M, Maksimov N, Polevova S, Evmenyeva A (2019) Bipolar pollen germination in blue spruce (Picea pungens). Protoplasma 256:941–949. https://doi.org/10.1007/s00709-018-01333-3
Büyükkartal HN (2002) In vitro pollen germination and pollen tube characteristics in tetraploid red clover (Trifolium pratense L.). Turk J Bot 27:57–61
Cai G, Faleri C, Del Casino C, Emons AMC, Cresti M (2011) Distribution of callose synthase, cellulose synthase, and sucrose synthase in tobacco pollen tube is controlled in dissimilar ways by actin filaments and microtubules. Plant Physiol 155(3):1169–1190. https://doi.org/10.1104/pp.110.171371
Cai G, Parrotta L, Cresti M (2015) Organelle trafficking, the cytoskeleton, and pollen tube growth. J Integr Plant Biol 57:63–78. https://doi.org/10.1111/jipb.12289
Cao S, Zheng Y, Wang K, Rui H, Tang S (2010) Effect of methyl jasmonate on cell wall modification of loquat fruit in relation to chilling injury after harvest. Food Chem 118(3):641–647. https://doi.org/10.1016/j.foodchem.2009.05.047
Çetinbaş-GenÇ A (2019) Putrescine modifies the pollen tube growth of tea (Camellia sinensis) by affecting actin organization and cell wall structure. Protoplasma 257:89–101. https://doi.org/10.1007/s00709-019-01422-x
Çetinbaş-Genç A (2020) Adverse effects of heat stress in relation to actin cytoskeleton on pollen performance of Echinopsis chamaecereus (Cactaceae). Braz J Bot 43:29–34. https://doi.org/10.1007/s40415-020-00580-0
Çetinbaş-Genç A, Cai G, Vardar F, Ünal M (2019) Differential effects of low and high temperature stress on pollen germination and tube length of hazelnut (Corylus avellana L.) genotypes. Sci Hort 255:61–69. https://doi.org/10.1016/j.scienta.2019.05.024
Chen Y, Chen T, Shen S, Zheng M, Guo Y, Lin J, Baluska F, Samaj J (2006) Differential display proteomic analysis of Picea meyeri pollen germination and pollen-tube growth after inhibition of actin polymerization by latrunculin B. Plant J 47:174–195. https://doi.org/10.1111/j.1365-313X.2006.02783.x
Chen T, Teng N, Wu X, Wang Y, Tang W, Samaj J, Baluska F, Lin J (2007) Disruption of actin filaments by latrunculin B affects cell wall construction in Picea meyeri pollen tube by disturbing vesicle trafficking. Plant and Cell Physiol 48:19–30. https://doi.org/10.1093/pcp/pcl036
Cheung AY, Chen CYH, Tao LZ, Andreyeva T, Twell D, Wu HM (2003) Regulation of pollen tube growth by Rac-like GTPases. J Exp Bot 54:73–81. https://doi.org/10.1093/jxb/erg044
Derksen J, Rutten T, vanAmstel T, deWin A, Doris F, Steer M (1995) Regulation of pollen tube growth. Acta Bot Neerl 44:93–119
Derksen J, Li YQ, Knuiman B, Geurts H (1999) The wall of Pinus sylvestris L. pollen tubes. Protoplasma 208:26–36. https://doi.org/10.1007/BF01279072
deWin AH, Knuiman B, Pierson ES, Geurts H, Kengen HM, Derksen J (1996) Development and cellular organization of Pinus sylvestris pollen tubes. Sex Plant Reprod 9:93–101. https://doi.org/10.1007/BF02153056
Farmer EE, Almeras E, Krishnamurthy V (2003) Jasmonates and related oxylipins in plant responses to pathogenesis and herbivory. Curr Opin Plant Biol 6:372–378. https://doi.org/10.1016/s1369-5266(03)00045-1
Fernando DD, Lazzaro MD, Owens JN (2005) Growth and development of conifer pollen tubes. Sex Plant Reprod 18:149–162. https://doi.org/10.1007/s00497-005-0008-y
Fugate KK, Lafta AM, Eide JD, Li G, Lulai EC, Olson LL, Deckard EL, Khan MFR, Finger FL (2018) Methyl jasmonate alleviates drought stress in young sugar beet (Beta vulgaris L.) plants. J Agron Crop Sci 204:566–576. https://doi.org/10.1111/jac.12286
Gumerova EA, Akulov AN, Rumyantseva NI (2015) Effect of methyl jasmonate on growth characteristics and accumulation of phenolic compounds in suspension culture of tartary buckwheat. Russ J Plant Physiol 62:195–203. https://doi.org/10.1134/S1021443715020077
Hao H, Li Y, Hu Y, Lin J (2005) Inhibition of RNA and protein synthesis in pollen tube development of Pinus bungeana by actinomycin D and cycloheximide. New Phytol 165:721–730. https://doi.org/10.1111/j.1469-8137.2004.01290.x
Hao H, Chen T, Fan L, Li R, Wang X (2013) 2, 6-dichlorobenzonitrile causes multiple effects on pollen tube growth beyond altering cellulose synthesis in Pinus bungeana Zucc. PLoS One 8:e76660. https://doi.org/10.1371/journal.pone.0076660
Holden MJ, Marty JA, Singh-Cundy A (2003) Pollination-induced ethylene promotes the early phase of pollen tube growth in Petunia inflata. J Plant Physiol 160:261–269. https://doi.org/10.1078/0176-1617-00929
Hudgins JW, Franceschi VR (2004) Methyl jasmonate-induced ethylene production is responsible for conifer phloem defense responses and reprogramming of stem cambial zone for traumatic resin duct formation. Plant Physiol 135:2134–2149. https://doi.org/10.1104/pp.103.037929
Jia H, Yang J, Liesche J, Liu X, Hu Y, Si W, Guo J, Li J (2018) Ethylene promotes pollen tube growth by affecting actin filament organization via the cGMP-dependent pathway in Arabidopsis thaliana. Protoplasma 255:273–284. https://doi.org/10.1007/s00709-017-1158-0
Johri BM (1992) Haustorial role of pollen tubes. Ann Bot 70:471–475. https://doi.org/10.1093/oxfordjournals.aob.a088504
Kondo S, Yamada H, Setha S (2007) Effect of jasmonates differed at fruit ripening stages on 1-aminocyclopropane-1-carboxylate (ACC) synthase and ACC oxidase gene expression in pears. J Am Soc Hortic Sci 132:120–125. https://doi.org/10.21273/JASHS.132.1.120
Kushwah S, Jones AM, Laxmi A (2011) Cytokinin-induced root growth involves actin filament reorganization. Plant Signal Behav 6:1848–1850. https://doi.org/10.4161/psb.6.11.17641
Lazzaro MD (1999) Microtubule organization in germinated pollen of the conifer Picea abies (Norway spruce, Pinaceae). Am J Bot 86:759–766. https://doi.org/10.2307/2656696
Li J, Jia H (2013) cGMP modulates Arabidopsis lateral root formation through regulation of polar auxin transport. Plant Physiol Biochem 66:105–117. https://doi.org/10.1016/j.plaphy.2013.02.014
Li G, Yang L, Chen S, Huang Z, Hu W (2008) Effects of three irradiation methods on pollen germination and pollen tube growth of Pinus thunbergii. Acta Agriculturae Nucleatae Sinica 22:409–432
Li J, Chen S, Wang X, Shi C, Liu H, Yang J, Wei S, Guo J, Jia H (2018a) Hydrogen sulfide disturbs actin polymerization via S-sulfhydration resulting in stunted root hair growth. Plant Physiol 178(2):936–949. https://doi.org/10.1104/pp.18.00838
Li X, Li M, Wang J, Wang L, Han C, Jin P, Zheng Y (2018b) Methyl jasmonate enhances wound-induced phenolic accumulation in pitaya fruit by regulating sugar content and energy status. Postharvest Biol Tech 137:106–112. https://doi.org/10.1016/j.postharvbio.2017.11.016
Lombardo MC, Lamattina L (2018) Abscisic acid and nitric oxide modulate cytoskeleton organization, root hair growth and ectopic hair formation in Arabidopsis. Nitric Oxide 80:89–97. https://doi.org/10.1016/j.niox.2018.09.002
Lora J, Hormaza JI, Herrero M (2016) The diversity of the pollen tube pathway in plants: toward an increasing control by the sporophyte. Front Plant Sci 7:107. https://doi.org/10.3389/fpls.2016.00107
Lovy-Wheeler A, Wilsen KL, Baskin TI, Hepler PK (2005) Enhanced fixation reveals the apical cortical fringe of actin filaments as a consistent feature of the pollen tube. Planta 221:95–104. https://doi.org/10.1007/s00425-004-1423-2
Maksimov N, Evmenyeva A, Breygina M, Yermakov I (2018) The role of reactive oxygen species in pollen germination in Picea pungens (blue spruce). Plant Reprod 31:357–365. https://doi.org/10.1007/s00497-018-0335-4
Mogami N, Nakamura S, Nakamura N (1999) Immunolocalization of the cell wall components in Pinus densiflora pollen. Protoplasma 206:1–10. https://doi.org/10.1007/BF01279247
Mukkun L, Singh Z (2009) Methyl jasmonate plays a role in fruit ripening of ‘Pajaro’ strawberry through stimulation of ethylene biosynthesis. Sci Hort 123:5–10. https://doi.org/10.1016/j.scienta.2009.07.006
Muradoğlu F, Yıldız K, Balta F (2010) Methyl jasmonate influences of pollen germination and pollen tube growth of apricot (Prunus armeniaca L.). Yüzüncü Yıl University J Agric Sci 20:183–188
Owens JN, Bennett J, Hirondelle SL (2005) Pollination and cone morphology affect cone and seed production in lodgepole pine seed orchards. Can J For Res 35:383–400. https://doi.org/10.1139/X04-176
Ozturk B, Yıldız K, Ozkan Y (2015) Effects of pre-harvest methyl jasmonate treatments on bioactive compounds and peel color development of “Fuji” apples. Int J Food Prop 18:954–962. https://doi.org/10.1080/10942912.2014.911312
Parrotta L, Faleri C, Cresti M, Cai G (2016) Heat stress affects the cytoskeleton and the delivery of sucrose synthase in tobacco pollen tubes. Planta 243:43–63. https://doi.org/10.1007/s00425-015-2394-1
Parrotta L, Faleri C, Guerriero G, Cai G (2019) Cold stress affects cell wall deposition and growth pattern in tobacco pollen tubes. Plant Sci 283:329–342. https://doi.org/10.1016/j.plantsci.2019.03.010
Perez AG, Sanz C, Olias R, Olias JM (1997) Effect of methyl jasmonate on in vitro strawberry ripening. J Agric Food Chem 45:3733–3737. https://doi.org/10.1021/jf9703563
Qu X, Jiang Y, Chang M, Liu X, Zhang R, Huang S (2015) Organization and regulation of the actin cytoskeleton in the pollen tube. Front Plant Sci 5:786. https://doi.org/10.3389/fpls.2014.00786
Sheng X, Zhang S, Jiang L, Li K, Gao Y, Li X (2012) Lead stress disrupts the cytoskeleton organization and cell wall construction during Picea wilsonii pollen germination and tube growth. Biol Trace Elem Res 146:86–93. https://doi.org/10.1007/s12011-011-9212-9
Stone LM, Seaton KA, Kuo J, McComb JA (2004) Fast pollen tube growth in Conospermum species. Ann Bot 93:369–378. https://doi.org/10.1093/aob/mch050
Terasaka O, Niitsu T (1994) Differential roles of microtubule and actin-myosin cytoskeleton in the growth of Pinus pollen tubes. Sex Plant Reprod 7:264–272. https://doi.org/10.1007/BF00227708
Tilquin J, De Brouwer K, Mathieu A, Calier M (1983) Haustorial pollen tubes in Fuchsia boliviana. Ann Bot 52:425–428. https://doi.org/10.1093/oxfordjournals.aob.a088504
Wang Y, Chen T, Zhang C, Hao H, Liu P, Zheng M, Baluska F, Samaj J, Lin J (2009) Nitric oxide modulates the influx of extracellular Ca2+ and actin filament organization during cell wall construction in Pinus bungeana pollen tubes. New Phytol 182:851–862. https://doi.org/10.1111/j.1469-8137.2009.02820.x
Wu YY, Liu XF, Fu BL, Zhang QY, Tong Y, Wang J, Wang W, Grierson D, Yin XR (2020) Methyl jasmonate enhances ethylene synthesis in kiwifruit by inducing NAC genes that activate ACS1. J Agric Food Chem 68:3267–3276. https://doi.org/10.1021/acs.jafc.9b07379
Yildiz K, Yilmaz H (2002) Effect of jasmonic acid, ACC and ethephon on pollen germination in strawberry. Plant Growth Regul 38:145–148. https://doi.org/10.1023/A:1021229131579
Zhang R, Qu X, Zhang M, Jiang Y, Dai A, Zhao W, Cao D, Lan Y, Yu R, Wang H, Huang S (2019) The balance between actin-bundling factors controls actin architecture in pollen tubes. iScience 16:162–176. https://doi.org/10.1016/j.isci.2019.05.026
Acknowledgments
We thank Prof. Meral Unal and Prof. Giampiero Cai for their supports.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Handling Editor: Benedikt Kost
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Çetinbaş-Genç, A., Vardar, F. Effect of methyl jasmonate on in-vitro pollen germination and tube elongation of Pinus nigra. Protoplasma 257, 1655–1665 (2020). https://doi.org/10.1007/s00709-020-01539-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-020-01539-4