Abstract
Plant leaves offer an exclusive windowpane to uncover the changes in organs, tissues, and cells as they advance towards the process of senescence and death. Drought-induced leaf senescence is an intricate process with remarkably coordinated phases of onset, progression, and completion implicated in an extensive reprogramming of gene expression. Advancing leaf senescence remobilizes nutrients to younger leaves thereby contributing to plant fitness. However, numerous mysteries remain unraveled concerning leaf senescence. We are not still able to correlate leaf senescence and drought stress to endogenous and exogenous environments. Furthermore, we need to decipher how molecular mechanisms of the leaf senescence and levels of drought tolerance are advanced and how is the involvement of SAGs in drought tolerance and plant fitness. This review provides the perspicacity indispensable for facilitating our coordinated point of view pertaining to leaf senescence together with inferences on progression of whole plant aging. The main segments discussed in the review include coordination between hormonal signaling, leaf senescence, drought tolerance, and crosstalk between hormones in leaf senescence regulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
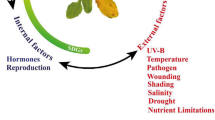
Leaf senescence is thoroughly a coordinated physiological mechanism that advances towards the ultimate stage death. Diverse environmental stresses and plant age are potential determinant factors of senescence. Leaf senescence has a functional significance when plants are exposed to extreme climatic regimes, and it imparts considerable tolerance to plants particularly subjected to drought stress (Jibran et al. 2013). Drought stress provokes varied physiological interruptions leading to senescence which has an imperative function in survival of plants. For example, leaf senescence induced by drought is believed to provide sufficient amounts of nutrients to young leaves and fruits via nutrient remobilization from senescing to young leaves under extreme environmental conditions (Distelfeld et al. 2014). Furthermore, leaf senescence ensures plant survival through nutrient mobilization from vegetative to reproductive tissues which thereby help complete the life cycle of monocarpic plant species in extreme environmental conditions (Gan and Hörtensteiner 2013). Although the leaf senescence induced by drought is a slow process, it is identified by specific microscopic, cellular, biochemical, and molecular alterations (Chen et al. 2015a, b). These changes include leaf depigmentation, convoluted variations in ultra-cellular structures such as condensed nuclear material, thylakoid invaginations, and accretion of plastoglobuli and impairment in metabolic processes leading to protein denaturation, lipid peroxidation, and altered gene expression throughout the process of leaf senescence in plants exposed to drought stress (Wehner et al. 2016). Leaf senescence has three major phases, initiation, re-organization, and terminal phases (Holland et al. 2015). The re-organization phase is characterized by fundamental alterations in cellular metabolism stimulated by a decline in cytokinins and enhanced levels of ABA and ROS (Sarwat et al. 2013). The interplay of phytohormones exclusively cytokinins and ABA is believed to be implicated in controlling leaf senescence in plants grown under water-deficit conditions (Munné-Bosch and Alegre 2004). Furthermore, functional significance of transcriptional factors like NAC, AP2/ERF, WRKY together with ethylene-induced modulation of transcriptional factors cannot be ruled out (Xu et al. 2011). Hence, drought-induced leaf senescence is quite an intricate process; there are still certain loopholes that remain to comprehend the process of leaf senescence completely. There are several other research reports that demonstrate synthesis of cytokinins through enhanced expression of an IPT gene that lead to accretion of cytokinins resulting in higher drought tolerance in transgenic crops like tobacco, cassava, peanut, petunia, and bent grass (Qin et al. 2015). Emerging trends in technology particularly in molecular biology are advancing their tailoring of pathways that regulate drought-induced leaf senescence.
In this review, we will present in-depth synopsis of transcriptional factors NAC, WRKY and other regulatory cascades implicating ethylene-induced modulation of transcriptional factors and their interactions with the phytohormones for commencement of leaf senescence.
Synchronization of hormonal signaling, leaf senescence, and drought tolerance
Identification of leaf senescence is parallel with the knowledge pertaining to understanding of plant responses to prevailing environmental stress. Majority of research illustrates the potential role of phytohormones in drought-induced leaf senescence via nutrient remobilization in economically important crops (Cheng and Guan 2014; Zhang et al. 2015; Burgess and Huang 2016). However, there is a dearth of research reports pertaining to molecular mechanisms involved in controlling gene expression throughout the whole process of leaf senescence particularly in field-grown plants. Involvement of five main groups of phytohormones specifically auxins, gibberellins, cytokinins, ethylene, and ABA is documented in the literature. Moreover, there are also reports pertaining to the potential roles of jasmonic acid, salicylic acid, brassinosteriods, and polyamines play in leaf senescence (Saini et al. 2015; Pandey 2017). Though cytokinins, auxins, and gibberillins are known to hamper leaf senescence, the remaining phytohormones are known to be implicated in the activation of leaf senescence (Zhang et al. 2017a, b); however, the direct implication of other phytohormones in advancing leaf senescence is still lacking (Jajic et al. 2015). For example, Yang et al. (2003) demonstrated that increased ABA levels enhanced macronutrient mobilization within grains from senescing leaves in rice and wheat plants grown under drought stress. Conversely, reduced levels of cytokinins exhibited an inverse association with photosynthetic rate and pigment concentration, while they showed direct relatedness with sucrose phosphate synthase activity, an enzyme known to avert leaf senescence in drought stressed crops (Sami et al. 2016). Cytokinins have a potential function in controlling source to sink reallocation (Thomas 2013; Yu et al. 2015). However, nutrient deficiency itself suppresses cytokinin levels in roots and hence enhances senescence in mature or older leaves, thereby stimulating the transport of nutrients from senescing leaves to younger leaves (Maillard et al. 2015; Have et al. 2016). As nutrients translocate from senescing to young leaves, plants reveal increased anti-oxidative defense during stress advancement until plants thrive under stress conditions and resume their normal growth. This incited an immense debate against the premises that cytokinins function as a main trigger for promoting leaf senescence in case their levels decline below threshold and anticipated that varied sink-source association in transgenic plants may obstruct the regular process of senescence. Hydrogen peroxide generation has been provoked by ABA and salicylic acid, and an involvement of ROS in ABA signaling pathway has been established (Bai et al. 2014; Kumar et al. 2015; Noctor and Foyer 2016). Neill et al. (2003) reported interrelatedness between generation of hydrogen peroxide and nitric oxide that consecutively controls transcription of explicit genes; however, precise process by which it regulates SAG is still unknown. Hoeberichts and Woltering (2002) demonstrated the potential role of ROS in regulation of cell death primarily via ROS modulation in plants, which is quite similar to control of leaf senescence. During recent years, new findings illustrating gene expression under drought stress are widely documented (Wehner et al. 2016; Pandey et al. 2017). These reports clearly depict valuable data to produce inclusive representation of molecular modifications that occur throughout the drought-induced leaf senescence exclusively in case when research is carried out in field conditions displaying varied gene expression.
Crosstalk between hormones in regulating leaf senescence
Several research reports have documented the potential function of phytohormones to control leaf senescence and overall plant fitness under extreme climatic conditions. The time regulation of leaf senescence is not yet elucidated, and disparity in response to varied stresses in inducing senescence has not been demonstrated evidently (Fischer 2012). However, time regulation including commencement and advancement of leaf senescence and stimulatory role of varied stress in inducing leaf senescence is still unclear (Woo et al. 2013, 2016). Several research reports confirm that phytohormones regulate leaf senescence through hermetic response to diverse environmental stresses and varied developmental processes (Woolhouse and Batt 2016; Abdelrahman et al. 2017). Phytohormones impart their effect via modulation of plant adaptability to changing environment through changes in developmental and metabolic processes (Azooz and Ahmad 2016). Sugar and phytohormone signaling play a potential function by regulating communication between source and sink throughout the process of senescence (Thomas and Ougham 2014). Senescence is a program but not programmed, and is an essentially trans-differentiation event subsequent to growth completion. Senescence is not essentially followed by death, and thus, the event is determined by cell viability and explicit gene expression (Thomas 2013). Hence, senescence can be related to age-related changes (ARCs) which are defined as permanent variations that are truly age and development dependent (Jing et al. 2005). The age-related changes are not easily perceptible, and they encompass physical, chemical, and biochemical alterations in developmental processes. All such alterations are contemplated as ARCs (Kim et al. 2016a, b). Environmental stresses and ARCs integrate to lead to leaf senescence except in young leaves wherein the environmental signals cannot provoke senescence mainly due to the absence of ARCs (Sklensky and Davies 2011). As leaves shift from young to mature stage, a multitude of factors along with ARCs, phytohormones, and senescence-associated genes (SAGs) advance leaf senescence, while cytokinins, gibberellins, and auxins are believed to delay senescence (Hu et al. 2017). Several research reports are in corroboration with the anticipation that senescence is controlled by associative and parallel functioning of diverse plant growth regulators (Robert-Seilaniantz et al. 2011; Delatorre et al. 2012). Moreover, leaf senescence induced by ethylene, JA, and ABA is delayed primarily due to defects in the functions of transcriptional factors Ethylene Insensitive 2 (EIN2), F box protein Oresara (ORE9), and transcriptional activators C-repeat-binding factor CBF2/CBF3 (Woo et al. 2013). Thus, the roles of plant growth regulators intersect, and regulation of leaf senescence is carried out through conjoint genetic pathways. However, the role of other potential plant growth regulators such as ABA, ethylene, SA, and JA in modulating senescence depends on how they respond to a stress (Chen et al. 2011). The synergistic and antagonistic functions of hormones with one another have an alarming effect on regulating senescence. Thus, strong relationship among ARCs, hormones, stress, and senescence regulation may determine the timing of senescence.
It is a well-established fact that hormones play a critical function in regulating leaf senescence. Ethylene, jasmonic acid (JA), abscisic acid (ABA), and salicylic acid (SA) act as enhancers of leaf senescence while as cytokinins, gibberellic acid (GA), and auxins delay senescence (Jibran et al. 2013) (Fig. 1). Cytokinins and ethylene are the most significant phytohormones regulating the timing of leaf senescence. Numerous transcription factors are reported to be intermediates of cytokinin signaling comprising class II transcription factor family HAT22 known as positive senescence regulator leading to early chlorophyll degradation (Benner et al. 2012). On contrary, ethylene induces leaf senescence, and an increase in biosynthetic genes of ethylene is observed during senescence. Some of ethylene receptors like EIN2 intercede in ABA and JA signaling paving way towards crosstalk between phytohormones leading to leaf senescence (Jibran et al. 2013). However, ethylene-induced senescence is age dependent, while JA-induced senescence is associated with stress responses indicating JA as an enhancing factor and not an initiator of senescence. Conversely, phytohormones that delay senescence like GA levels decrease with increasing leaf age signifying its negative role in senescence. But, GA is also known to counteract the ABA effects. Along with GA, auxins also function as negative regulators of senescence, but their connotation in senescence is intricate and it is strongly associated to developmental aspects. The implication of brassinosteriods in senescence is expected as indirect possibly because of its impact on leaf development.
Cytokinins
Cytokinins are known to be the first line of hormones that delay senescence and provide better application in advancement of growth in economically important crops (Zwack and Rashotte 2013). Declined levels of cytokinins are often reported to be associated with impairment in nutrient translocation in leaves/roots thereby causing leaf senescence (Paparozzi et al. 2016; Guo et al. 2017). The senescence repressing role of cytokinins has now been well established; however, their declined level being a major cause of senescence is not still experimentally confirmed (Edlund et al. 2017). Cytokinins inhibit senescence via up-regulation of oxidative stress genes to avert chlorophyll degradation and preserve chloroplast structure (Lu et al. 2017). Cytokinins besides their regulatory role in development and growth exert their ameliorative effect on stressed plants (Zwack et al. 2016; Blume et al. 2017). Enhanced levels of cytokinins were reported to confer drought tolerance and delay leaf senescence in creeping bentgrass because of enhanced activity of isopentyl transferase (Merewitz et al. 2016). Declined cytokinin biosynthesis is believed to be often associated with decreased photosynthetic rate (Reguera et al. 2013). However, in transgenic plants thriving in drought stress, photosynthesis is restored by enhancing expression of cytokinin biosynthetic genes, indicating that plants function properly via proper regulation of cytokinin pool (Thu et al. 2017). Ramel et al. (2013) have reported contribution of anti-oxidant pool generated from the methylerythritol phosphate (MEP) pathway. Interestingly, the MEP pathway is intricately related to photosynthetic electron transport which produces a major fraction of ROS in plastids (Dani et al. 2014). The methylerythritol 4-phosphate (MEP) pathway generates isoprene units which conjugate to form diverse sesiqueterpenes like ABA and carotenoids (Nisar et al. 2015). Dani et al. (2016) demonstrated evolution in emission of MEP-derived metabolites following varied selection pressures at intricate biological levels. Numerous research reports document antioxidant potential of isoprenoids and interplay between diverse metabolites generated from the MEP pathway in leaf development. Diverse selection pressures on the MEP pathway induce cytokinin biosynthesis exhibiting advanced functional synergism in impeding plant senescence (Kieber and Schaller 2014). One of the major pressures includes ROS production which leads to plastome integrity, which is vital for proceeding towards leaf senescence and ultimately death. Cytokinins delay senescence by auto-regulation of cytokinin biosynthesis through the isopentenyl transferase gene using a promoter senescence-associated gene 12 (SAG12). In this context, SAG12::IPT gene has been extensively evaluated in diverse species, and all of them exhibited delayed senescence (Xiao et al. 2017).
The cytokinin receptor family has been described in Arabidopsis comprising the genes, Arabidopsis histidine kinases AHK2, AHK3, and AHK4. Any impairment in AHK3 may lead to premature leaf senescence because of phosphoralyting activation of ARR2, type B-ARR. Cytokinins induce a majority of Arabidopsis type A response regulators (ARRs) that up-regulate cytokinin biosynthesis (Cheng and Kieber 2015). Brenner et al. (2012) demonstrated cytokinin involvement in gene regulation of DNA-binding type-A response regulator cytokinin response factors (CRFs) and proteins localized in chloroplasts. The CRFs are cytokinin-regulated APETALA2/ethylene responsive factor (AP2/ERF). The ERF transcription factors have an imperative role in leaf senescence (Zwack and Rashotte 2013). The functional reversibility in cytokinin response factor 6 (CRF6) leads to modulation of leaf sensitivity inhibition and acceleration of leaf senescence in crf6 mutants (Raines et al. 2016). This study confirms a negative regulator of leaf senescence implicated in time-dependent regulation of leaf senescence. Drought stress shows a significant decline in cytokinin pool due to down-regulation of IPT genes (Nishiyama et al. 2011). Alvarez et al. (2008) have illustrated a declined production rate of isoprene-type cytokinins namely tZ and tZ riboside and enhanced levels of cytokinin 6-benzylaminopurine, thereby confirming hermetic response of cytokinin biosynthesis to drought stress. Cytokinins have an intricate relation with drought stress as revealed by Arabidopsis histidine kinases (AHKs) like AHK2, AHK3, and AHK4. These receptors act as negative regulators in ABA and osmotic stress signaling, affirming that cross-talks occur among cytokinins, ABA, and stress signal transduction pathways (Kumar and Verslues 2015). Drought-induced osmotic stress has a distinct effect on AHK cytokinin receptors, AHK2 and AHK4 which were down-regulated in both shoot and roots in Arabidopsis, whereas AHK3 was up-regulated in response to drought stress. This varied set of AHK receptor expression might modulate the final receptor output under drought stress. Keeping in view the ameliorative role of cytokinins in plant survival, Kuppu et al. (2013) illustrated expression of Agrobacterium ipt in cotton from drought-induced promoter (SARK) leading to enhanced tolerance under extreme drought stress. Nishiyama et al. (2011) reported declined cytokinin levels through enhanced levels of CKX3 or CKX4 or via impairment in many IPT genes leading to drought and salt phenotypes because of ABA hypersensitivity and elevated membrane integrity (Kieber and Schaller 2014). Xiao et al. (2017) reported that over-expression of PSAG12:IPT (isopentyl-transferase gene coupled to SAG12) could delay leaf senescence and induce drought tolerance by lowering the levels of ABA and malondialdehyde in transformed eggplants.
Recently, Edlund et al. (2017) confirmed that downstream signal of daylight perception is dubious to entail the decline in CK levels or its signaling. This work presented huge patterns of CK-associated gene expression that are implicated in CK biosynthesis and signaling. Further, it was confirmed that any decline in CK or class of CKs confers no effect on progression or commencement of senescence, indicating no confirmed evidence for CK as a trigger of leaf senescence in aspen plants (Edlund et al. 2017). There was no parallelism between gene expression of CK biosynthesis genes and metabolite levels in senescent leaf tissues. So, it can be conjectured that either level of CK or ratio of two CK-related molecules or rate of metabolic process can be triggered due to leaf senescence. Hence, identification of exact trigger for leaf senescence is still not clear. Leaf senescence can also be assumed as a default leaf developmental process, which requires to be counterfeited with a senescent inhibiting factor leading to impairment in progression of leaf senescence. Hence, so far, there is no distinct evidence that proves CK as upstream or downstream of key trigger in leaf senescence.
Gibberellic acid
Gibberellic acid exhibits inhibitory effect on senescence, but as plant ages, its active form undergoes degradation. For example, during senescence, 18-fold increment in GA deactivating gene GA 2-oxidase signifies degradation of active form of GA in leaf senescence (van der Graaff et al. 2006). Hence, with progression of senescence, availability of active forms or free forms of GA like GA4 and GA7 were reported to be inhibited in lettuce (Li et al. 2012). GA can inhibit leaf senescence by exerting an inhibitory effect on ABA as observed in Paris polyphylla (Yu et al. 2009). Hence, we can conclude that GA is not directly implicated in leaf senescence. Furthermore, regulatory pathways of GA are linked with ABA function in maintaining membrane integrity and opening of flowers as observed in Gladiolus (da Costa and Finger 2016). The functional significance of gibberellins in leaf senescence has been demonstrated through implication of DELLA proteins which are negative regulators of gibberellins. Even though main progress has been made in elucidating the underlying process of GA signaling, but mechanism by which GA alters leaf senescence is still needs to be unraveled. Till now, it has been confirmed that GA interaction with WRKY45 modulates onset and advancement of leaf senescence. However, future efforts are required to identify variation in GA levels during senescence and discover further senescence-associated proteins like DELLAs which could supplement more elucidation of GA-induced leaf senescence.
Auxins
Due to the prime role of auxins in plant growth and development, the significance of auxins in developmental leaf senescence has become an intricate topic (Lim et al. 2007). There is no regular pattern or relations that exist between endogenous levels of auxins and senescence (Rivero et al. 2007). Auxins are known to remarkably decrease the expression of SAG12, thereby delaying senescence (Mueller-Roeber and Balazadeh 2014). Extensive function of GH3 genes which encode for IAA amido synthetase was reported in up-regulation of developmental and dark-induced senescence (van der Graaff et al. 2006). Kim et al. (2011) illustrated over-expression of YUCCA6, a gene which belongs to YUCCA family (flavin monooxygenase proteins that catalyzes a rate-limiting step in de novo auxin biosynthesis) in Arabidopsis thaliana leading to activation of mutants yuc6-1D and 35S:YUC6 that display higher levels of auxins and remarkable longevity. Arabidopsis mutant yuc6-1D displayed declined rate of transcript levels of SAG12, NAC1, and NAC6 compared to wild types in Arabidopsis throughout the process of senescence. Kant et al. (2009) observed higher levels of SAUR39 (small auxin-up RNAs; these belong to a large multigene family involved in early auxin responses) leading to declined IAA levels, due to reduced auxin transport and ultimately resulting in premature senescence. Expression of SAUR39 was more elevated in older leaves than in young ones which show higher auxin levels in meristematic regions. This research report anticipated implication of SAUR39 in auxin signaling and modulation of leaf senescence via auxin biosynthesis and its polar transport in an age-dependent manner. Lim et al. (2010) reported delayed senescence in mutant Oresara14 that encodes ARF2 which represses auxin response genes in Arabidopsis. In another study, it was reported that SAUR36, identical to SAG21, is effectively involved in positively regulating the leaf senescence of Arabidopsis (Narsai et al. 2011). Stamm and Kumar (2013) reported auxin (IAA)-induced SAUR36 expression in 3-day-old seedlings of Arabidopsis thaliana, whereas repression was observed with 1-N-naphthylphthalamic acid. Enhanced thiol reductase activity was observed in Arabidopsis thaliana due to enhanced expression of YUC6 which is essential for ROS detoxification during drought tolerance response. This study confirmed declined ROS accumulation in YUC6-OX during senescence, while YUC6-OXC85S over-expressing mutant YUC6 that lacks thiol reductase activity accumulated ROS. These observations indicate that thiol reductase activity of YUC6 is indispensable for delaying senescence. In a previous study, Cha et al. (2015) have already confirmed dual function of YUC6 in delaying senescence and ROS homeostasis. Feng et al. (2016) illustrated the potential role of AINTEGUMENTA (ANT), a member of AP2/ERF transcription factor family in extending leaf longevity through downstream regulation of ARF2. Moreover, functional significance of auxins in controlling cell division and cell expansion justifies its role in controlling ARCs. Thus, future efforts should be directed towards determination of stage that contributes to the senescence regulating method implicating ORE1 regulon to commence the program. These research endeavors will reveal the potential roles auxins play in leaf senescence.
Ethylene
Ethylene is extensively known as senescence inducing gaseous phytohormone as it speeds up leaf and flower senescence (Jing et al. 2005). In addition to senescence, ethylene controls maturation of fruits, confers stress tolerance, and induces abscission (Skirycz et al. 2011). Transcriptomic analysis has confirmed implication of ethylene-associated process throughout developmentally induced leaf senescence. A direct association has been confirmed between transcript levels related to ethylene signaling genes with pectin esterase and lipid catabolic activity transcripts and an inverse trend in photosynthetic transcript (Breeze et al. 2011). The ethylene signaling can easily unravel the regulatory pathways implicated in progression of leaf senescence. As signal is generated, it is transmitted to the network comprising CTR1, EIN2, and Raf like serine/threonine protein kinase (Berens et al. 2017). In addition to leaf senescence, a significant role of ethylene in inducing flowering in pineapple has been established (Li et al. 2016). This study demonstrates an over-expression of ethylene receptors AcERS1a, AcERS1b, AcETR2a, and AcETR2b in the bract primordia and flower primordia when treated with ethephon. In another study, Li et al. (2016) using a comparative transcriptomic profiling in ethylene-treated juvenile and adult plants of silver vase (Aechmea fasciata) displayed down-regulation of TARGET OF EAT 1(TOE1) and TOE3 associated with AP2-like transcription factors was reported. Dubois et al. (2015) advocated the significance of ethylene response factors ERF5 and ERF6 in Arabidopsis that enhance leaf growth under extreme environmental conditions. The ERF6 gene inhibits cell proliferation, and leaf growth, and this inhibition is driven in association with gibberellins and DELLA signaling (Müller and Munné-Bosch 2015). The ERF6 exists as a connecting link between ethylene forming enzyme 1-aminocyclopropane1-carboxylic acid (ACC) and DELLA signaling within pause-stop model of cell cycle thereby advancing the perception pertaining to reduced leaf primordia proliferation in drought stressed plants (Dubois et al. 2013). Hence, it can be conjectured that ethylene signaling pathway including ETHYLENE RESPONSE1 (ETR1), ETHYLENE INSENSITIVE2 (EIN2), EIN3, and EIN3-LIKE1 (EIL1) transcription factors trigger explicit target gene expression leading to consequent physiological changes. Apart from canonical ethylene signaling pathway, modulation of miRNA164 through EIN2/ORESARA2/3 (ORE2/3) paves a way forward towards trichotomous signaling pathway involved in controlling the expression of a vital transcription factor in senescence-associated gene expression program, NAC2/ANAC092/ORE1 gene products, which lead to activation of SAG12. Although ethylene is not a senescence initiation factor, ethylene-EIN3 signaling functions in execution of senescence progression; therefore, any practical mutilation of EIN3 is pertinent to control plant organ senescence. Thus, it can be concluded that ethylene integrates with transcription factors in the regulation of senescence initiation; however, no clear interaction between ethylene and such TFs is found to trigger leaf senescence.
Jasmonic acid
Jasmonic acid is known to induce 3- to 4-fold increase in chlorophyll degradation and plastid protein turnover in Arabidopsis during senescence (Reinbothe 1993a, b). It has also been shown that genetic regulation of miR319-TCP can be altered by miR319-TCP by repressing LOX2 expression thereby modulating JA biosynthetic genes and the endogenous JA levels (Schommer et al. 2008). From this study, it was possible to conclude that TCP2/4/10 positively regulates leaf senescence. This increment in JA levels was later on reported to be due to increase in transcript number of established genetic markers of developmental senescence, e.g., SEN4, ERD1, and senescence-associated gene 21 (SAG21) following application of methyl jasmonate. Several research studies report generation of JA as a by-product of macromolecule breakdown and membrane degradation during senescence (Chini et al. 2016; Hu et al. 2017). In another study, Reinbothe et al. (2009) illustrated a positive correlation between transcript levels of JA biosynthetic genes (AOS, LIPOXYGENASE1 (LOX1), LOX3, LOX4, OPR1, and OPR3) and progression of senescence. Some of studies demonstrated JA as a by-product of macromolecular degradation during progression of senescence. Breeze et al. (2011) also illustrated enhanced transcript levels of AOC1, AOC4, and OPR3 and (MYC2, JAZ1, JAZ6, and JAZ8) during senescence. Recent research reports document potential function of JA biosynthetic genes and JA receptors in time-dependent regulation of senescence (Danisman et al. 2012). However, during recent years, it has been reported that mutation of JA receptors and JA biosynthetic genes remarkably affected time-dependent regulation of senescence in Arabidopsis (Danisman et al. 2012). JA can decline RuBPCase activase (RCA) proteins and transcript levels in COI1 (Coronatine Insensitive 1, the jasmonate receptor)-dependent manner which might explain JA-induced leaf senescence. Some WRKY transcriptional factors are also implicated in JA-induced senescence, e.g., WRKY70 and WRKY53 act as a node of JA- and SA-induced signals and function as regulators of senescence (Xie et al. 2014). Jiang et al. (2014) showed that WRKY 57 represses gene expression of SAGs like SEN4 and SAG12 which play as a junction for JA and auxin-mediated signaling pathway. Recently, Uji et al. (2017) have reported a transcription factor, OSMYC2, as a positive regulator of JA signaling, and its over-expression promotes leaf senescence. JASMONATE ZIM-domain (JAZ) proteins are an imperative group of transcriptional repressors of JA signaling pathway which confer tolerance against varied stresses and induce leaf senescence (Zhang et al. 2015). Previously, JAZ4 and JAZ8 have been reported to interrelate with the WRKY57 TFs which physically regulate inversely JA-induced leaf senescence. Further, JAZ7 was reported to delay dark-induced leaf senescence in contrast to wild-type plants, and the jaz7 mutant exhibited high leaf-senescence phenotype showing more chlorophyll degradation, extra leaf yellowing, and high accretion of hydrogen peroxide in the dark (Yu et al. 2016). This characteristic of dark-induced leaf-senescence expression can be reverted in JAZ7-overexpession lines. Goossens et al. (2016) have reported targets of Jaz receptors like MYC3, and MYC4, three bHLH (basic helix-loop-helix) TFs that control major characteristics of JA signaling pathway. Earlier, Qi et al. (2015) observed that bHLH subgroup IIId TFs (bHLH03, bHLH13, bHLH14, and bHLH17) can ameliorate leaf senescence when MYC2/MYC3/MYC4. MYC2, MYC3, and MYC4 proteins bind to PAO (pheophorbide a oxygenase) promoter stimulating gene expression of enzyme that are significant for chlorophyll degradation during leaf senescence progression (Zhu et al. 2015). A crosstalk between ethylene and jasmonic acid has been observed through temporary activation or constitutive over-expression of EIN2 and EIN3 (Li et al. 2013). Recently, Hu et al. (2017) have reported a mechanistic viewpoint describing the implication of EIN2–EIN3–miR164–NAC2 in leaf senescence regulation through crosstalk linking the JA and ethylene signaling pathways. The regulatory role of JA in senescence is primarily dependent on ethylene signaling to some degree which signifies that at lower levels of JA, EIN2 can be avoided by ethylene signaling (Kim et al. 2013). Although under specific conditions, ethylene signaling can divert EIN2, ethylene perception is believed to be vital for JA response. It can be conjectured that JA can alter leaf senescence via modulation of ethylene biosynthetic pathway as intermediate ACC conjugates with JA leading to biosynthesis of ethylene (Kim et al. 2015). There is an ample scope for tailoring the extensive range of protagonists that arbitrate process of aging and regulate timing of leaf senescence.
Salicylic acid
A significant role of salicylic acid and its signaling pathways in regulation of developmentally induced leaf senescence have been reported in Arabidopsis thaliana (Lim et al. 2007). The mutants developed from Arabidopsis like NahG (naphthalene oxygenase), pad4 (phytolexin deficient 4), and npr1 having dysfunctional SA signaling and altered expression of SAGs lead to delay in leaf senescence (Lu et al. 2016; Huysmans et al. 2017). This study confirmed that SA exhibits a significant role in onset, sequential advancement, and progression of leaf senescence by controlling positive and negative regulators of the pathway. Autophagy is another strategy by which plants regulate leaf senescence through vacuolar dilapidation of cellular components (Xiao et al. 2010). Autophagy and SA integrate to control pathogenic attacks, and the mutants atg5 and atg2 lacking autophagy have elevated SA and ROS levels and hence causing senescence early (Yoshimoto et al. 2009). Xiao et al. (2010) illustrated the potential role of senescence by binding acyl-CoA binding protein 3 (ACBP3) with phosphatidylethanolamine (PE) and its interruption of ATG via hindering conjugation of PE to autophagy-related proteins (ATG8). ACBP3 regulates starvation and development-induced senescence in SA-dependent and JA-independent pathway. Xiao and Chye (2011) also reported over-expression of ACBP3 in Arabidopsis responsible for controlling biotic tolerance against Pseudomonas syringae pv., tomato. This suggests concerned role of SA in regulating stress tolerance during senescence by modulating lipid biosynthesis, ATG, and ROS generation. By summing up different research reports, it can be easily concluded that SA is implicated in the commencement and advancement of leaf senescence via regulation of comparative loads of negative and positive regulators of leaf senescence.
SA and JA are integrated via transcriptional factors, WRKY53, which interact antagonistically with JA-inducible protein EPITHIOSPECIFYING SENESCENCE REGULATOR (ESR/ESP) in response to JA and SA levels, respectively. ESR exhibits dual functionality as positive cytoplasmic epithiospecifier and WRKY53 as negative regulator in the nucleus. Zhang et al. (2017a, b) reported a critical function of mitochondrial AAA-protease gene AtFtSH4 from Arabidopsis in controlling senescence and autophagy by mediating WRKY-dependent accretion of salicylic acid and its signaling. Repression or knock-out of AtFtSH4 leading to ftch4-4 mutant displayed increased senescence, enhanced levels of autophagy ultimately leading to death. Up-regulation of antioxidant defense system and suppression of senescence by counteracting JA-induced leaf senescence via nitric oxide-associated (NOA1)-dependent NO signaling have been reported (Ji et al. 2016). Wang et al. (2016) reported a direct link between non-expresser pathogenesis-related (NPR) namely NPR1, NPR3, and NPR4 and salicylic acid signaling in Arabidopsis thaliana. This study reported enhanced leaf senescence in npr3/npr4 mutants as compared to wild types suggesting NPR3 and NPR4 as negative regulators of leaf senescence. The role of mitogen-activated protein kinases (MAPK) signaling cascades in regulating leaf senescence by modulating SA levels which is imperative in regulating leaf senescence was first reported by Li et al. (2016). In this study, a negative dominant mutant of ZmMEK1 in transgenic lines of Arabidopsis displayed higher salicylic acid accretion which consequently led to salicylic acid-induced leaf senescence. Recently, Deng et al. (2017) have characterized premature leaf senescence mutants yellow leaf and dwarf 1 (yld1) in rice which accumulated higher levels of SA and abnormal starch granules. This study demonstrated new links between leaf senescence, starch metabolism, and salicylic signaling. Though SA biosynthesis is elucidated completely, the catabolic pathways of SA are still poorly understood. The molecular mechanism underlying the functional role of salicylic acid in leaf senescence is known to be mediated through interconnections between leaf senescence and disease response. Even though numerous research has been done for confirming JA as a signaling molecule in disease response, but, there still remains more research to be done in order to elucidate interconnections of SA with positive regulators (ABA, JA, an ET) or negative regulators (CKs and GAs) of leaf senescence. Autophagy acts as both survival strategy as well as alternative cell death mechanism, thereby signifying SA as an inducer of programmed cell death. The optimized SA levels are significant to implement cell death completely.
Abscisic acid
Abscisic acid (ABA) is an important sesquiterpenoid (15- carbon) hormone that controls numerous metabolic and developmental mechanisms including senescence and abscission (Lee et al. 2011). Several studies have reported an important function of abscisic acid in conferring resistance in plants subjected to different abiotic and biotic cues (Lee and Luan 2012). ABA is strongly linked with ARC, so it can be inferred that ABA is a prerequisite for developmental progression of senescence. Lee and Luan (2012) reported stomatal closure against ABA as an adaptation strategy against salinity and drought. Receptor like kinase 1 (RPK1) is a leucine-rich membrane-bound receptor-like kinase that functions as an up-regulator of ABA signaling and its enhanced expression in ABA-dependent during progression of leaf senescence (Lee et al. 2011). While carrying out the functional analysis of RPK1, the authors have shown ABA as a positive regulator in leaf senescence. These findings were further authenticated with rpk1 mutant exhibiting delayed leaf senescence in response to ABA, whereas transgenic line exhibiting over-expression of RPK1 showed increased leaf senescence. Yang et al. (2011) have reported direct association between abiotic stress and leaf longevity. This study demonstrated up-regulation of NAC transcription factor VND-INTERACTING 2 (VNI2) by ABA and salt stress. This transcription factor functions to incorporate ABA-linked abiotic stress signals into developmental senescence program via regulation of COLD REGULATED (COR) and RESPONSIVE TO DEHYDRATION (RD) genes. Over-expression of COR, VNI2, and RD genes led to delayed leaf senescence. ABA-mediated transcript loads of VNI, COR15A, COR15B, RD29A, and RD20 in response to salt stress have been demonstrated in ABA-deficient mutant aba3-1. These studies demonstrate a close association between ABA stress signaling and developmentally induced senescence suggesting increased stress tolerance followed up by increased plant longevity. Zhang and Gan (2012) demonstrated that SAG113 encoding protein phosphatase 2C intervening in ABA metabolism regulates stomatal movement and water loss during leaf senescence. Song et al. (2016) isolated two early Arabidopsis mutants (eas1-1 and eas1-2) which exhibit varied phenotypes like early leaf senescence, larger stomatal aperture, and insensitivity to stress. Numerous SAGs were up-regulated in eas1 mutants. Furthermore, calcium levels as well as calcium channel activity of eas1 mutant guard cells were reported to be lower than those in wild types. These studies confirmed that endogenous levels of ABA were implicated in regulating the commencement of leaf senescence. Zhao et al. (2016) illustrated extensive selection of transgenic plants that exhibit enhanced expression of ABA receptors like pyrabactin resistance 1-like (PYL) family. This study demonstrates that PYL9 induces drought tolerance by reducing transpiration rate leading to senescence in older leaves and declined growth in younger tissues in drought-stressed plants. Song et al. (2016) reported that endogenous levels of ABA promote leaf senescence by increasing cytoplasmic calcium levels in the guard cells. This demonstrates a direct link between ABA levels and Ca2+ signaling. A variation in PSII photo-damage and D1 protein turnover during leaf senescence and association with ABA accumulation in senescing rice mutant psf (premature senescing flag) leaves has been observed (Wang et al. 2016). This mutant displayed a strong association between ABA levels, PSII photo-damage, and D1 protein turnover during leaf senescence. Drought-induced leaf senescence has been reported through contribution of ABA INSENSITIVE GROWTH (AB1G1) transcriptional factor which increases in response to drought (Liu et al. 2016). In this study, mutants such as abig1 exposed to drought and ABA remained green and exhibited lesser senescent leaves. Recently, new early leaf senescence mutants in rice early senescence 3 (est3) have been discovered (Su et al. 2017), which displayed yellowing of leaves and higher accumulation of ABA indicating ABA as a significant positive regulator of leaf senescence. In this study, over-expression of OsNAC2 remarkably promoted leaf senescence in rice by activating chlorophyll-degrading genes OsSGR and OsNYC3. Further, ectopic expression of OsNAC2 is believed to up-regulate ABA biosynthetic genes OsNCED3 and OsZEP1 and down-regulate ABA catabolic gene OsABA8ox1 which leads to build up of high pool of ABA that leads to leaf senescence.
The significant relation between ABA and senescence has not been elucidated clearly through genetic evaluation. Though pre-existing research reports show that ABA induces senescence via biosynthesis of ethylene; however, recent studies confirmed ethylene-independent ABA signaling (Zhao et al. 2016). ABA regulates both stress-induced responses and leaf senescence through proper adjustment via ARCs. In developing plants, ABA functions as an intrinsic orchestrator which equilibrates the functions that promote morphogenesis and hampers cellular functions. On the contrary, in old plants, ABA acts as a modulator in the aging process. After all, we can put forth assumption that ABA plays an effective role in plant developmental processes including senescence by coordinating the expression of a number of proper genes and induction of a myriad of metabolic changes.
Brassinosteroids
These are polyhydroxysteroids that are reported to influence senescence and confer resistance against different abiotic and biotic stresses (Krishna et al. 2017). Brassinosteroids are positive regulators of leaf senescence. Brassinosteroids were applied exogenously to different plants, and results showed that exogenous application of brassinosteroids promoted senescence, whereas BR-deficient mutants exhibited delay in leaf senescence (Sağlam-Çağ 2007). Progression of leaf senescence in response to higher dosage of exogenous epibrassinolide and delayed senescence following lower BR levels took place in wheat leaves. Increased leaf longevity was reported in parallel to reduced SAGs transcript levels in bri 1 (BR in sensitive 1) null mutants, whereas bri1-EMS suppressor1 showed enhanced senescence because of constitutively active BR response pathway (He et al. 2007). Brassinosteroids induce senescence by altering antioxidant defense, increasing chlorophyll degradation and hampering anthocyanin biosynthesis (Bajguz and Hayat 2009). Plants exhibiting over-expression of P450SU1, encoding CYP105A1 monooxygenase genes, degrade BRs and hence display delayed senescence. Husar et al. (2011) also documented delayed senescence in Arabidopsis against over-expression of UGT73C6 coding UDP-glycosyltransferase which inactivates BRs. BRs hence play a significant role in stress response and developmentally regulate senescence by controlling ARC timing. BRs are significantly implicated in up-regulation of stress-associated genes for instance WRK3, WRKY6, HSP70, and MYB, and their interplay with GA, JA, and SA has not yet been unraveled to decipher their role in stress tolerance (Nawaz et al. 2017). Ye et al. (2017) demonstrated crosstalk between drought tolerance and brassinosteriod signaling via over-expression of BES1-regulated genes. Gene expression studies illustrated interaction of RD26 with Bes1 protein and antagonizing BES1 transcriptional activity of BR-linked genes, and BR signaling can suppress RD26 gene expression and its homologs thereby inhibiting drought responses (Nolan et al. 2017; Yang et al. 2015). A mechanistic approach has been adopted for deciphering association between drought tolerance and BR signaling through BES1/BZR1 family of transcription factors (Ye et al. 2017). This study demonstrated transcription factor RD26 which binds to BES1 and alienates transcriptional activity of BR biosynthetic genes and induce drought tolerance. Enhanced leaf senescence has been reported in response to BR by modulating lipid pool in plants (Fedina et al. 2017). Chen et al. (2017a, b) have reported a significant role of BRASSINOSTERIOD INSENSITIVE1-EMS-SUPPRESSOR 1/BRASSINAZOLE-RESISTANT 1 (BES1/BZR1) transcriptional factors in drought tolerance. This study evaluated three transcriptional factors, WRKY46, WRKY54, and WRKY70, that were positively regulated by BR and negatively by drought stress. There is no direct evidence of BRs in inducing leaf senescence; however, BRs integrate with GA through interaction of (brassinosteroid signaling positive regulator) BZR1 and BES1 with DELLAs and amplify signaling via degradation and inactivation of DELLAs proteins. Moreover, there is no data pertaining to mechanism underlying increase of BR-induced lipid catabolism during leaf senescence.
Transcriptional regulation of plant leaf senescence
In Arabidopsis, genome-wide studies have shown that senescence-associated genes are expressed during different phases of senescence, such as degradation of macromolecules, their remobilization, and transportation, and also during various stress responses (Breeze et al. 2011; Velasco-Arroyo et al. 2017). The vital genes having a prominent role in various processes of senescence have been characterized at the molecular level in the model plant Arabidopsis thaliana, such as Oresara1 (ORE, ANAC092; AtNAC2) (Balazadeh et al. 2010; Rauf et al. 2013), ORE9, Arabidopsis thaliana NAC-like, activated by apetala3/pistillata (AtNAP, ANAC029) (Guo and Gan 2006), WRKY53 (Zentgraf 2007), Lipoxygenase 1 (LOX1, AT1G55020) (Li et al. 2012), senescence-associated gene 101 (SAG101) (He and Gans 2002; Chen et al. 2015a, b), and NAC 124 with Transmembrane Motif1 like 4 (NTL4) (Lee et al. 2012). Among the large number of transcriptional factors (TFs) induced by senescence, NAC and WRKY families of TFs represent an important family having a conspicuous role in leaf senescence (Podzimska-Sroka et al. 2015; Kim et al. 2016a, b; Mao et al. 2017; Shahnejat-Bushehri et al. 2017; Chen et al. 2017a, b).
Regulatory role of NAC transcription factors
On the basis of similarity in the DNA-binding domain, NAC family of transcription factors has been classified in the three major super families namely no apical domain (NAM), arabidopsis transcription activation factor (ATAF), and cup-shaped cotyledon (CUC) (Puranik et al. 2012). Recently, by the availability of whole genome sequences of various plants a large number of NAC genes has been found like 163 NAC genes in Populus spp., 151 NAC genes in Oryza sativa, 117 NAC genes in Arabidopsis thaliana, 152 NAC genes each in Glycine max and Nicotiana tabacum, and 79 NAC genes in Vitis vinifera (Dalman et al. 2017; Huang et al. 2012). The NAC transcription factors play a very diverse role and are involved in the regulation of various developmental processes as well as response to various types of biotic and abiotic stresses (MacMillan et al. 2017; Pimenta et al. 2016). The global transcriptome profiling has identified more than 30 NAC genes in Arabidopsis thaliana that are considerably upregulated in Leaf senescence, hence emphasizing their importance in senescence regulation (Kou et al. 2012; Lindemose et al. 2014). In Arabidopsis thaliana, the regulatory role of various NAC transcriptional factors has been confirmed. Among these transcription factors, the following NAC proteins play important regulatory roles like NAC-like, activated by apetala3/pistillata (AtNAP) (Guo and Gan 2006), Oresara sister1 (ORS1) (Balazadeh et al. 2011), Oresara1 (ORE1; AtNAC2) (Kim et al. 2009), Vascular-related NAC-domain interacting (VNI2) (Yang et al. 2011), Jungbrunnen1 (JUB1) (Wu et al. 2012a, b), Arabidopsis thaliana activating factor1 (ATAF1) (Garapati et al. 2015), and ANAC016 (Kim et al. 2013). However, the over-expression of AtNAP, ORE1, ORS1, ANAC016, and ATAF1 depicted severe senescence phenotype which was deferred to some extent by silencing the function of these genes, thus confirming the positive role of these transcription factors in the regulation of leaf senescence. On the other hand, the experimental evidences have identified JUB1 and VNI2 as repressors of leaf senescence (Shahnejat-Bushehri et al. 2016; Kim et al. 2016a, b; Podzimska-Sroka et al. 2015). Besides, the close homologs of Arabidopsis NAC transcription factors like ANAC019, ANAC055, and RD26 (ANAC072) also show the senescence-induced expression patterns and thus play an important role in senescence-associated regulatory networks (Hickman et al. 2013; Ramaswamy et al. 2017). The characterization of NAC transcription factors in Arabidopsis thaliana has been confirmed long before by Guo and Gan 2006. It has been observed that over-expression of NAP in young leaves of Arabidopsis caused advanced senescence which was further confirmed from the knockout mutant which showed the delayed leaf senescence. The mutant effect was nullified by the introduction of wild-type copy of AtNAP in the mutant plants, thus confirming its positive role in leaf senescence. Later on in 2012, Zhang and Gan have further elucidated that AtNAP directly interacts with the promoter of SAG113, a negative regulator of the abscisic acid (ABA) pathway which inhibits stomatal closure, thus activating leaf senescence.
One of the most important transcription factors that positively regulate leaf senescence in Arabidopsis is ORESARA1 (ORE1) or ANAC092 (Balazadeh et al. 2010). It plays an important regulatory role by controlling the expression of about 170 genes among which altogether 78 genes are SAGs (senescence-associated genes) (Liebsch and Keech 2016). ORE1 acts as crucial transcriptional factor and directly targets the key senescence-associated genes like BIFUNCTIONAL NUCLEASE1 (BFN1), SEVEN IN ABSENTIA (SINA) and SENESCENCE ASSOCIATED GENE 29 (SAG29)/SUGARS WILL EVENTUALLY BE EXPORTED TRANSPORTERS 15 (SWEET15) (Matallana-Ramirez et al. 2013). The expression profile of ORE1 and BFN1 is overlapping and thus together play an important regulatory role in the degradation of nucleic acids during the programmed cell death and senescence (Matallana-Ramirez et al. 2013). SAG29 or SWEET 15 codes for an important sugar transporter gene, and when this gene was overexpressed, the transgenic plants depicted accelerates senescence (Seo et al. 2011), probably by fluctuating sugar transport. Similarly, SINA1 codes a typical E3 ubiquitin ligase and results in the degradation of protein by 26S proteasomal pathway (Zhang et al. 2014; Jensen and Skriver 2014). However, ORE1 is in turn under the tight control of Ethylene Insensitive 2 (EIN2), an ethylene signaling transcription factor at the transcriptional level and microRNA164 (miR164) at the post-transcriptional level. During the commencement of leaf senescence, the expression of ORE1 is induced by EIN2 and is delayed by the regulatory effect of miR164 (Pei et al. 2013). Similarly, Ethylene Insensitive 3 (EIN3) directly binds to the promoters of ORE1 and AtNAP genes and thus induces the transcriptional activity of these genes. Hence, EIN3 indirectly induces leaf senescence by activating the expression of ORE1 and AtNAP (Kim et al. 2014). It has been found that miR164 is negatively regulated by EIN2 and its downstream target EIN3. During leaf senescence, EIN3 directly binds to the promoter of miR164 and downregulates the accumulation of miR164 (Li et al. 2013).
Besides, ORE1 directly interacts with the Golden2-like transcription factors GLK1 and GLK2 which are involved in the proper development and maintenance of chloroplasts (Rauf et al. 2013). It has been found that expression of GLKs is highly induced during early leaf development, which results in the activation of photosynthesis-related genes (Rauf et al. 2013). On the other hand, in the senescing leaves, the expression of ORE1 is augmented, which in turn, seizes GLKs thereby down-regulating their expression (Jensen and Skriver 2014). The interaction of ORE1 with the GLKs doesnot has any effect on the transactivating activity of ORE1 but hampers GLK transcriptional activity (Jensen and Skriver 2014).
Recently, ATAF1 has also been found as activator of leaf senescence by upregulating SAGs along with ABA hormone signaling and repressor of photosynthesis-associated genes (Garapati et al. 2015). ATAF1 directly interacts with the promoters of GLK1 and ORE1 and thus differentially regulates the expression levels of these genes. By balancing the expression profile of ORE1 and GLK1, ATAF1 directly regulates the accumulation of photosynthesis-related genes (REFS). Besides, ATAF1 also elevates the ABA levels by directly interacting with the ABA biosynthetic gene NCED3, and the ABA transporter gene, ABCG40 (Jensen et al. 2013; Garapati et al. 2015). Similarly, ORS1, a subfamily NAC member, was also elucidated as positive regulator of leaf senescence. In 2011, Balazadeh et al. confirmed that the over-expression of ORS1 within the plants promoted, whereas the null mutants delayed senescence. In the same report, it has been shown by the transcriptome analysis that about 76% and 67% of ORS1-regulated genes were elevated by the salinity stress and H2O2 treatment, respectively.
In contrary to positive regulators of leaf senescence, another NAC transcription factor JUNGBRUNNEN1 (JUB1) delays leaf senescence by decreasing cellular H2O2 levels and thus acts as negative regulators of leaf senescence. It has been found that JUB1 is induced by H2O2 and stimulates the expression of DREB2A and several ROS-responsive genes (Wu et al. 2012a, b). Subsequently, it was elucidated that in Arabidopsis, AtJUB1 imparts important crosstalk with the gibberellin (GA) and brassinosteroid (BR) biosynthetic pathways (Shahnejat-Bushehri et al. 2016). AtJUB1 suppresses the expression of two important genes of GA and BR biosynthetic pathways by directly binding to the promoters of GA3ox1 and DWF4, respectively (REFS). Furthermore, AtJUB1 triggers the DELLA encoding genes by increasing the expression levels of GAI and RGL1 (Thirumalaikumar et al. 2017). Thus, suppression of GA and BR biosynthetic pathway genes, GA3ox1 and DWF4, and up-regulation of DELLA genes, i.e., GAI and RGL1, result in the amassing of DELLA proteins in AtJUB1 over-expression lines. Thus, over-expression lines AtJUB1 display the typical phenotypic characteristic of low-GA and low-BR mutants (Shahnejat-Bushehri et al. 2016). Similarly, in tomato, the over-expression of AtJUB1 resulted in GA and BR deficiency phenotypes similar to those observed in Arabidopsis AtJUB1 over-expression transgenic lines (Bushehri et al. 2017). Recently, it has been found that AtJUB1 inhibits cell elongation by directly suppressing the expression of PIF4 (Shahnejat-Bushehri et al. 2016). The proteolysis of plasma membrane during ABA-mediated drought stress results in the release of membrane-bound NTL4 (Lee et al. 2012). The increased expression of NTL4 directly elevates the NADPH oxidases (Atrboh A, C, and E genes) which in turn are involved in the production of ROS and ultimately results in the programmed cell death during drought-induced leaf senescence (Lee et al. 2012).
Although the NAC transcription factors has been fully characterized in Arabidopsis, but recently, the regulatory role of these factors in senescence has been validated in many economically important crop plants to a large extent. The NAC transcription factors in crop plants like rice OSNAC2, barley HvNAC005, litchi LcNAC1, and in soybean GmNAC81 were characterized as positive regulators of age-related leaf senescence (Mao et al. 2017; Christiansen et al. 2016; Jiang et al. 2017; Pimenta et al. 2016). Thus, the NAC transcription factors play an important regulatory role in leaf senescence and act as key players in integrating external environmental signals with the overall development of the plant. Although an intricate mechanism regarding the regulation of NAC transcription factors has been proposed, still more research is required to link the regulatory mechanism of NAC transcription factors with the onset of leaf senescence within plants.
Regulatory role of WRKY transcription factors
The involvement of WRKY transcription factors in regulating leaf senescence has been studied rigorously. The functional genomics approach has provided considerable confirmation that WRKY proteins besides playing an important role in various types of abiotic stresses like drought are important regulators of leaf senescence in plants (Qin et al. 2015; He et al. 2016). The first confirmation about the probable role of WRKY transcription factors in senescence came from the studies of Arabidopsis thaliana AtWRKY6 (Besseau et al. 2012.). It has been shown that during senescence, AtWRKY6 strongly induces the expression of AtSIRK (Arabidopsis thaliana senescence-induced receptor-like kinase). In the over-expression transgenic lines of WRKY6, the transcript levels of AtSIRK were evidently elevated, and in the wrky6 knockout mutants, the expression level was drastically reduced (Ciolkowski et al. 2008). Another WRKY transcription factor WRKY53 was characterized as a positive regulator of leaf senescence, and it was reported that it targets various SAGs, stress-related genes, and other transcription factors (Miao et al. 2004). Also, it has been reported that WRKY53 directly interacts with EPITHIOSPECIFYING SENESCENCE REGULATOR (ESR), which inhibits the DNA-binding activity of WRKY53 and thus represses leaf senescence (Miao and Zentgraf 2007). The single mutants of WRKY54 show an obvious senescence phenotype, whereas that of WRKY70 depicts the weak and premature senescence phenotype. On the other hand, the double mutants of the wrky54 and wrky70 revealed an early-senescence phenotype as compared to the weak and premature senescence phenotype of WRKY70 (Besseau et al. 2012). This showed that WRKY70 and WRKY54 play redundant functions in controlling leaf senescence. The protein-protein interactions have shown that WRKY30, having a major role in ROS signaling, interacts individually with WRKY53, WRKY54, and WRKY70 (Scarpeci et al. 2013). Thus, WRKY30 acts as an important mediator for regulating the positive and negative transcription factors of senescence in a concerted manner. It has been shown that WRKY22 also positively regulates dark-induced leaf senescence and acts downstream of WRKY53. Like all other transcription factors, the over-expression of WRKY22 induces leaf senescence, whereas the knock-out mutants show a delay in leaf senescence (Zhou et al. 2011). Recently, Zhang et al. (2016) have identified 116 WRKY transcription factors from wheat (Triticum aestivum L.) genome database, of which 13 TaWRKYs were found to be senescence-associated genes. Out of the 13 TaWRKYs, TaWRKY7 was fully characterized and its expression was shown to be induced during leaf senescence. Similarly, Fan et al. (2017) have characterized WRKY65 from the economically important leafy vegetable, Brassica rapa L. It has been found that expression of BrWRKY65 was highly induced during post-harvest senescence. Moreover, DNA-binding interactions have shown that BrWRKY65 directly interacts with various promoters of senescence-associated genes like BrNYC1 and BrSGR1. Altogether, these interpretations demonstrate that WRKY transcription factors play an important role in regulating the complex transcriptional networks of leaf senescence not only in model plants but also in some other economically important crops.
Regulatory role of other transcription factors
Besides the well-characterized NAC and WRKY transcription factors, the other transcription factors also play a significant role in the regulatory mechanism of leaf senescence. For example, in Arabidopsis thaliana, B3-domain type transcription factor, related to ABA-insensitive3 (ABI3)/Viviparous1 (AtRAV1), positively regulates leaf senescence (Woo et al. 2010). On the other hand, C-repeat/dehydration-responsive element binding factor 2 (CBF2) negatively regulates leaf senescence (Sharabi-Schwager et al. 2010). Further, Zhang et al. (2011) reported that an Arabidopsis R-R-type MYB-type transcription factor, MYBL, acts as a positive regulator of leaf senescence. Recently, phytochrome-interacting factors (PIFs), a family of bHLH transcription factors, has been found as positive regulators of dark-induced leaf senescence (Song et al. 2014; Liebsch and Keech 2016). On the basis of phenotypic evaluation of over-expression and mutant lines, it has been demonstrated that PIF3, 4, and 5 induce both dark-induced and age-triggered leaf senescence. For example, PIF4 positively induces the expression of chlorophyll degradation genes and down-regulates the genes responsible for the suppression of chlorophyll biosynthesis and chloroplast activity (Song et al. 2014). Also, PIF4 stimulated ROS generation systems to promote senescence (Song et al. 2014). Thus, altogether, these studies provide strong evidence that PIF3, 4, and 5 are novel-positive regulators of leaf senescence (Song et al. 2014). Moreover, numerous transcription factors that directly mediate hormone signaling pathways and are indirectly involved in leaf senescence have been already discussed in the hormone section. Further, Auxin Response Factor2 (ARF2) plays a significant role in regulating leaf senescence augmented by auxin signaling (Lim et al. 2010). The Signal Responsive 1 (SR1), a calmodulin-binding transcription factor, regulates ethylene-induced leaf senescence by directly binding to the EIN3 promoter, a positive regulator of ethylene signaling pathway (Nie et al. 2012; Sarwat 2017). It has been found that these transcriptional factors play a significant role in the diverse growth and developmental processes of plants. There is a complex interaction among these transcriptional factors, and it will be advisable to identify their specific downstream effectors which are responsible for controlled cellular and biochemical regulation during senescence.
Conclusions and future prospects
Aging and consequent death is a significant stage in the plant life cycle. In this review, we have discussed intricate processes that operate within plants experiencing particularly drought and senescence. Though leaf senescence has been investigated, all molecular mechanisms and signaling pathways have been highlighted only at senescent phases. How environmental signals coordinate with endogenous hormonal levels to promote senescence has not been fully elucidated. Both molecular and genetic strategies can be employed to screen sequential progression of young leaves towards senescence. Research studies that integrate hormonal signals and transcriptional factors associated with drought tolerance need to be elucidated. Though genetic and molecular approaches are employed to decipher the role of phytohormones in regulation of leaf senescence, so far, there is no direct link or involvement of environmental cues for triggering leaf senescence. Research done so far presents an orchestra of events interwoven intricately within the frame of metabolic and molecular networks regulating together towards progression of senescence. Researchers still lack lucidity to pinpoint a common junction which prompts the plants towards the onset of senescence.
References
Abdelrahman M, El-Sayed M, Jogaiah S, Burritt DJ, Tran LSP (2017) The “STAY-GREEN” trait and phytohormone signaling networks in plants under heat stress. Plant Cell Rep 1–17
Alvarez S, Marsh EL, Schroeder SG, Schachtman DP (2008) Metabolomic and proteomic changes in the xylem sap of maize under drought. Plant Cell Environ 31:325–340
Azooz MM, Ahmad P (2016) Plant-environment interaction: responses and approaches to mitigate stress. John Wiley & Sons
Bai L, Wang P, Song CP (2014) Reactive Oxygen Species (ROS) and ABA Signalling. In: Abscisic Acid: Metabolism, Transport and Signaling. Springer, Netherlands, p 191–223
Bajguz A, Hayat S (2009) Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol Biochem 47:1–8
Balazadeh S, Kwasniewski M, Caldana C, Mehrnia M, Zanor MI, Xue GP, Mueller-Roeber B (2011) ORS1, an H2O2-responsive NAC transcription factor, controls senescence in Arabidopsis thaliana. Mol Plant 4(2):346–360
Balazadeh S, Wu A, Mueller-Roeber B (2010) Salt-triggered expression of the ANAC092-dependent senescence regulon in Arabidopsis thaliana. Plant Signal Behav 5(6):733–735
Berens ML, Berry HM, Mine A, Argueso CT, Tsuda K (2017) Evolution of hormone signaling networks in plant defense. Ann Rev Phytopath
Besseau S, Li J, Palva ET (2012) WRKY54 and WRKY70 co-operate as negative regulators of leaf senescence in Arabidopsis thaliana. J Exp Bot 63(7):2667–2679
Blume YB, Krasylenko Y A, Yemets, A I (2017) The role of the plant cytoskeleton in phytohormone signaling under abiotic and biotic stresses. Mechanism of plant hormone signaling under stress, p 127–185
Breeze E, Harrison E, McHattie S, Hughes L, Hickman R, Hill C, Zhang C (2011) High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. Plant Cell 23(3):873–894
Brenner WG, Ramireddy E Heyl A, Schmülling T (2012) Gene regulation by cytokinin in Arabidopsis. Fron Plant Sci 3:8
Burgess P, Huang B (2016) Mechanisms of hormone regulation for drought tolerance in plants. In: Drought stress tolerance in plants, vol 1. Springer International Publishing, p 45–75
Cha JY, Kim WY, Kang SB, Kim JI, Baek D, Jung IJ (2015) A novel thiol-reductase activity of Arabidopsis YUC6 confers drought tolerance independently of auxin biosynthesis. Nat Commun 6:8041
Chen MK, Hsu WH, Lee PF, Thiruvengadam M, Chen HI, Yang CH (2011) The MADS box gene, FOREVER YOUNG FLOWER, acts as a repressor controlling floral organ senescence and abscission in Arabidopsis. Plant J 68(1):168–185
Chen J, Nolan T, Ye H, Zhang M, Tong H, Xin P, Yin Y (2017b) Arabidopsis WRKY46, WRKY54 and WRKY70 transcription factors are involved in brassinosteroid-regulated plant growth and drought response. Plant Cell 29(6):1425–1439
Chen H, Ruiz PD, McKimpson WM, Novikov L, Kitsis RN, Gamble MJ (2015a) MacroH2A1 and ATM play opposing roles in paracrine senescence and the senescence-associated secretory phenotype. Mol Cell 59(5):719–731
Chen QF, Xu L, Tan WJ, Chen L, Qi H, Xie LJ, Chen MX, Liu BY, Yu LJ, Yao N, Zhang JH (2015b) Disruption of the Arabidopsis defense regulator genes SAG101, EDS1, and PAD4 confers enhanced freezing tolerance. Mol Plant 8(10):1536–1549
Chen J, Zhu X, Ren J, Qiu K, Li Z, Xie Z, Kuai B (2017a) Suppressor of overexpression of CO 1 negatively regulates dark-induced leaf Degreening and senescence by directly repressing Pheophytinase and other senescence-associated genes in Arabidopsis. Plant Physiol, pp-01457 173:1881–1891
Cheng Y, Guan J (2014) Involvement of pheophytinase in ethylene-mediated chlorophyll degradation in the peel of harvested ‘Yali’pear. J Plant Growth Regul 33(2):364–372
Cheng CY, Kieber JJ (2015) Signaling: cytokinin signaling. Mol Biol 1–19
Chini A, Gimenez-Ibanez S, Goossens A, Solano R (2016) Redundancy and specificity in jasmonate signalling. Curr Opin Plant Biol 33:147–156
Christiansen MW, Matthewman C, Podzimska-Sroka D, O’Shea C, Lindemose S, Møllegaard NE, Holme IB, Hebelstrup K, Skriver K, Gregersen PL (2016) Barley plants over-expressing the NAC transcription factor gene HvNAC005 show stunting and delay in development combined with early senescence. J Exp Bot 67(17):5259–5273
Ciolkowski I, Wanke D, Birkenbihl RP, Somssich IE (2008) Studies on DNA-binding selectivity of WRKY transcription factors lend structural clues into WRKY-domain function. Plant Mol Biol 68(1-2):81–92
da Costa LC, Finger FL (2016) Flower opening and vase life of gladiolus cultivars: the sensitivity to ethylene and the carbohydrate content. Ornam Hortic 22(2):147–153
Dalman K, Wind JJ, Nemesio-Gorriz M, Hammerbacher A, Lundén K, Ezcurra I, Elfstrand M (2017) Overexpression of PaNAC03, a stress induced NAC gene family transcription factor in Norway spruce leads to reduced flavonol biosynthesis and aberrant embryo development. BMC Plant Biol 17(1):6
Dani KGS, Fineschi S, Michelozzi M, Loreto F (2016) Do cytokinins, volatile isoprenoids and carotenoids synergically delay leaf senescence? Plant Cell Environ
Dani KGS, Jamie IM, Prentice IC, Atwell BJ (2014) Evolution of isoprene emission capacity in plants. Trends Plant Sci 19:439–446
Danisman S, Van der Wal F, Dhondt S, Waites R, de Folter S, Bimbo A, Angenent GC (2012) Arabidopsis class I and class II TCP transcription factors regulate jasmonic acid metabolism and leaf development antagonistically. Plant Physiol 159(4):1511–1523
Delatorre CA, Cohen Y, Liu L, Peleg Z, Blumwald E (2012) The regulation of the SARK promoter activity by hormones and environmental signals. Plant Sci 193:39–47
Deng L, Qin P, Liu Z, Wang G, Chen W, Tong J, He H (2017) Characterization and fine-mapping of a novel premature leaf senescence mutant yellow leaf and dwarf 1 in rice. Plant Physiol Biochem 111:50–58
Distelfeld A, Avni R, Fischer AM (2014) Senescence, nutrient remobilization, and yield in wheat and barley. J Exp Bot 65(14):3783–3798
Dubois M, Skirycz A, Claeys H, Maleux K, Dhondt S, De Bodt S, Inzé D (2013) ETHYLENE RESPONSE FACTOR6 acts as a central regulator of leaf growth under water-limiting conditions in Arabidopsis. Plant Physiol 162(1):319–332
Dubois M, Van den Broeck L, Claeys H, Van Vlierberghe K, Matsui M, Inzé D (2015) The ETHYLENE RESPONSE FACTORs ERF6 and ERF11 antagonistically regulate mannitol-induced growth inhibition in Arabidopsis. Plant Physiol, pp-00335
Edlund E, Novak O, Karady M, Ljung K, Jansson S (2017) Contrasting patterns of cytokinins between years in senescing aspen leaves. Plant Cell Environ 40(5):622–634
Fan ZQ, Tan XL, Shan W, Kuang JF, Lu WJ, Chen JY (2017) BrWRKY65, a WRKY transcription factor, is involved in regulating three leaf senescence-associated genes in Chinese flowering cabbage. Int J Mol Sci 18(6):1228
Fedina E, Yarin A, Mukhitova F, Blufard A, Chechetkin I (2017) Brassinosteroid-induced changes of lipid composition in leaves of Pisum sativum L. during senescence. Steroids 117:25–28
Feng G, Xu Q, Wang Z, Zhuoma Q (2016) AINTEGUMENTA negatively regulates age-dependent leaf senescence downstream of AUXIN RESPONSE FACTOR 2 in Arabidopsis thaliana. Plant Biotechnol 33(2):71–76
Fischer AM (2012) The complex regulation of senescence. Crit Rev Plant Sci 31(2):124–147
Gan SS, Hörtensteiner S (2013) Frontiers in plant senescence research: from bench to bank. Plant Mol Biol 82:503–504
Garapati P, Xue GP, Munné-Bosch S, Balazadeh S (2015) Transcription factor ATAF1 in Arabidopsis promotes senescence by direct regulation of key chloroplast maintenance and senescence transcriptional cascades. Plant Physiol 168(3):1122–1139
Guo Y, Gan S (2006) AtNAP, a NAC family transcription factor, has an important role in leaf senescence. Plant J 46(4):601–612
Guo P, Li Z, Huang P, Li B, Fang S, Chu J, Guo H (2017) A tripartite amplification loop involving the transcription factor WRKY75, salicylic acid, and reactive oxygen species accelerates leaf senescence. Plant Cell, tpc-00438
Goossens J, Fernández-Calvo P, Schweizer F, Goossens A (2016) Jasmonates: signal transduction components and their roles in environmental stress responses. Plant Mol Biol 91:673–689
Have M, Marmagne A, Chardon F, Masclaux-Daubresse C (2016) Nitrogen remobilisation during leaf senescence: lessons from Arabidopsis to crops. J Exper Bot 68(10):2513–2529
He Y, Gans S (2002) A gene encoding an acyl hydrolase is involved in leaf senescence in Arabidopsis. Plant Cell 14:805–815
He K, Gou X, Yuan T, Lin H, Asami T, Yoshida S, Russell SD, Li J (2007) BAK1 and BKK1 regulate brassinosteroid-dependent growth and brassinosteroid-independent cell-death pathways. Curr Biol 17:1109–1115
He GH, Xu JY, Wang YX, Liu JM, Li PS, Chen M (2016) Drought-responsive WRKY transcription factor genes TaWRKY1 and TaWRKY33 from wheat confer drought and/or heat resistance in Arabidopsis. BMC Plant Biol 16:116
Hickman R, Hill C, Penfold CA, Breeze E, Bowden L, Moore JD, Zhang P, Jackson A, Cooke E, Bewicke-Copley F, Mead A (2013) A local regulatory network around three NAC transcription factors in stress responses and senescence in Arabidopsis leaves. Plant J 75(1):26–39
Hoeberichts FA, Woltering EJ (2002) Multiple mediators of plant programmed cell death: interplay of conserved cell death mechanisms and plant-specific regulators. BioEss 25:47–57
Holland V, Koller S, Lukas S, Brüggemann W (2015) Drought-and frost-induced accumulation of soluble carbohydrates during accelerated senescence in Quercus pubescens. Trees 1–12
Hu Y, Jiang Y, Han X, Wang H, Pan J, Yu D (2017) Jasmonate regulates leaf senescence and tolerance to cold stress: crosstalk with other phytohormones. J Exp Bot 68(6):1361–1369
Huang H, Wang Y, Wang S, Wu X, Yang K, Niu Y, Dai S (2012) Transcriptome-wide survey and expression analysis of stress-responsive NAC genes in Chrysanthemum lavandulifolium. Plant Sci 193:18–27
Husar S, Berthiller F, Fujioka S, Rozhon W, Khan M, Kalaivanan F, Elias L, Higgins GS, Li Y, Schuhmacher R, Krska R, Seto H, Vaistij FE, Bowles D, Poppenberger B (2011) Overexpression of the UGT73C6 alters brassinosteroid glucoside formation in Arabidopsis thaliana. BMC Plant Biol 11:51
Huysmans M, Lema S, Coll NS, Nowack MK (2017) Dying two deaths—programmed cell death regulation in development and disease. Curr Opin Plant Biol 35:37–44
Jajic I, Sarna T, Strzalka K (2015) Senescence, stress, and reactive oxygen species. Plan 4(3):393–411
Jensen MK, Lindemose S, De Masi F, Reimer JJ, Nielsen M, Perera V et al (2013) ATAF1 transcription factor directly regulates abscisic acid biosynthetic gene NCED3 in Arabidopsis thaliana. FEBS Open Bio 3(1):321–327
Jensen MK, Skriver K (2014) NAC transcription factor gene regulatory and protein–protein interaction networks in plant stress responses and senescence. IUBMB Life 66(3):156–166
Ji Y, Liu J, Xing D (2016) Low concentrations of salicylic acid delay methyl jasmonate-induced leaf senescence by up-regulating nitric oxide synthase activity. J Exper Bot 67(17):5233–5245
Jing HC, Schippers JH, Hille J, Dijkwel PP (2005) Ethylene-induced leaf senescence depends on age-related changes and OLD genes in Arabidopsis. J Exp Bot 56(421):2915–2923
Jiang Y, Liang G, Yang S, Yu D (2014) Arabidopsis WRKY57 functions as a node of convergence for jasmonic acid–and auxin-mediated signaling in jasmonic acid–induced leaf senescence. Plant Cell 26(1):230–245
Jiang G, Yan H, Wu F, Zhang D, Zeng W, Qu H, Chen F, Tan L, Duan X, Jiang Y (2017) Litchi fruit LcNAC1 is a target of LcMYC2 and regulator of fruit senescence through its interaction with LcWRKY1. Plant Cell Physiol 58(6):1075–1089
Jibran R, Hunter DA, Dijkwel PP (2013) Hormonal regulation of leaf senescence through integration of developmental and stress signals. Plant Mol Biol 82(6):547–561
Kant S, Bi YM, Zhu T, Rothstein SJ (2009) SAUR39, a small auxinup RNA gene, acts as a negative regulator of auxin synthesis and transport in rice. Plant Physiol 151:691–701
Kieber JJ, Schaller GE (2014) Cytokinins. Arabidopsis Book 12:e0168
Kim HJ, Hong SH, Kim YW, Lee IH, Jun JH, Phee BK, Rupak T, Jeong H, Lee Y, Hong BS, Nam HG (2014) Gene regulatory cascade of senescence-associated NAC transcription factors activated by ETHYLENE-INSENSITIVE2-mediated leaf senescence signaling in Arabidopsis. J Exp Bot 65(14):4023–4036
Kim H, Kim Y, Yeom M, Lim J, Nam HG (2016a) Age-associated circadian period changes in Arabidopsis leaves. J Exp Bot 67(9):2665–2673
Kim HJ, Nam HG, Lim PO (2016b) Regulatory network of NAC transcription factors in leaf senescence. Curr Opin Plant Biol 33:48–56
Kim YS, Sakuraba Y, Han SH, Yoo SC, Paek NC (2013) Mutation of the Arabidopsis NAC016 transcription factor delays leaf senescence. Plant Cell Physiol 54(10):1660–1672
Kim J, Chang C, Tucker ML (2015) To grow old: regulatory role of ethylene and jasmonic acid in senescence. Front Plant Sci 6
Kim JH, Chung KM, Woo HR (2011) Three positive regulators of leaf senescence in Arabidopsis, ORE1, ORE3 and ORE9, play roles in crosstalk among multiple hormone-mediated senescence pathways. Genes Genomics 33:373–381
Kim JH, Woo HR, Kim J, Lim PO, Lee IC, Choi SH, Hwang D, Nam HG (2009) Trifurcate feed-forward regulation of age-dependent cell death involving miR164 in Arabidopsis. Science 323:1053–1057
Kou X, Watkins CB, Gan SS (2012) Arabidopsis AtNAP regulates fruit senescence. J Exp Bot 63(17):6139–6147
Krishna P, Prasad BD, Rahman T (2017) Brassinosteroid action in plant abiotic stress tolerance. Brassinosteroids: Meth Prot, p 193–202
Kumar MN, Verslues PE (2015) Stress physiology functions of the Arabidopsis histidine kinase cytokinin receptors. Physiol Plant 154(3):369–380
Kumar D, Haq I, Chapagai D, Tripathi D, Donald D, Hossain M, Devaiah S (2015) Hormone signaling: current perspectives on the roles of salicylic acid and its derivatives in plants. In: The formation, structure and activity of phytochemicals. Springer International Publishing, p 115–136
Kuppu S, Mishra N, Hu R, Sun L, Zhu X, Shen G et al (2013) Water-deficit inducible expression of a cytokinin biosynthetic gene IPT improves drought tolerance in cotton. PLoS One 8(5):e64190
Lee IC, Hong SW, Whang SS, Lim PO, Nam HG, Koo JC (2011) Age-dependent action of an ABAinducible receptor kinase, RPK1, as a positive regulator of senescence in Arabidopsis leaves. Plant Cell Physiol 52:651–662
Lee SC, Luan S (2012) ABA signal transduction at the crossroad of biotic and abiotic stress responses. Plant Cell Environ 35:53–60
Lee S, Seo PJ, Lee HJ, Park CM (2012) A NAC transcription factor NTL4 promotes reactive oxygen species production during drought-induced leaf senescence in Arabidopsis. Plant J 70(5):831–844
Li Y, Chang Y, Zhao C, Yang H, Ren D (2016) Expression of the inactive ZmMEK1 induces salicylic acid accumulation and salicylic acid-dependent leaf senescence. J Integr Plant Biol 6(134):32–42
Li Z, Wang J, Zhang X, Lei M, Fu Y, Zhang J et al (2016) Transcriptome sequencing determined flowering pathway genes in Aechmea fasciata treated with ethylene. J Plant Growth Regul 35(2):316–329
Li Z, Peng J, Wen X, Guo H (2013) ETHYLENE-INSENSITIVE3 is a senescence-associated gene that accelerates age-dependent leaf senescence by directly repressing miR164 transcription in Arabidopsis. Plant Cell 25:3311–3328
Li QF, Wang C, Jiang L, Li S, Sun SS, He JX (2012) An interaction between BZR1 and DELLAs mediates direct signaling crosstalk between brassinosteroids and gibberellins in Arabidopsis. Sci Signal 5(244):72–72
Liebsch D, Keech O (2016) Dark-induced leaf senescence: new insights into a complex light-dependent regulatory pathway. New Phytol 212(3):563–570
Lim PO, Kim HJ, Gil Nam H (2007) Leaf senescence. Annu Rev Plant Biol 58:115–136
Lim PO, Lee IC, Kim J, Kim HJ, Ryu JS, Woo HR, Nam HG (2010) Auxin response factor 2 (ARF2) plays a major role in regulating auxin-mediated leaf longevity. J Exp Bot 61:1419–1430
Lindemose S, Jensen MK, de Velde JV, O'shea C, Heyndrickx KS, Workman CT, Masi FD (2014) A DNA-binding-site landscape and regulatory network analysis for NAC transcription factors in Arabidopsis thaliana. Nucleic Acids Res 42(12):7681–7693
Liu T, Longhurst AD, Talavera-Rauh F, Hokin SA, Barton MK (2016) The Arabidopsis transcription factor ABIG1 relays ABA signaled growth inhibition and drought induced senescence. eLife 5:e13768
Lu G, Casaretto JA, Ying S, Mahmood K, Liu F, Bi YM, Rothstein SJ (2017) Overexpression of OsGATA12 regulates chlorophyll content, delays plant senescence and improves rice yield under high density planting. Plant Mol Biol 94(1–2):215–227
Lu H, Greenberg JT, Holuigue L (2016) Salicylic acid signaling networks. Fron Plant Sci 7
MacMillan CP, Birke H, Chuah A, Brill E, Tsuji Y, Ralph J, Pettolino FA (2017) Tissue and cell-specific transcriptomes in cotton reveal the subtleties of gene regulation underlying the diversity of plant secondary cell walls. BMC Genet 18(1):539
Maillard A, Diquélou S, Billard V, Laîné P, Garnica M, Prudent M, Ourry A (2015) Leaf mineral nutrient remobilization during leaf senescence and modulation by nutrient deficiency. Front Plant Sci 6
Mao C, Lu S, Lv B, Zhang B, Shen J, He J, Luo L, Xi D, Chen X, Ming F (2017) A rice NAC transcription factor promotes leaf senescence via ABA biosynthesis. Plant Physiol 174(3):1747–1763
Matallana-Ramirez LP, Rauf M, Farage-Barhom S, Dortay H, Xue GP, Dröge-Laser W, Mueller-Roeber B (2013) NAC transcription factor ORE1 and senescence-induced BIFUNCTIONAL NUCLEASE1 (BFN1) constitute a regulatory cascade in Arabidopsis. Mol Plant 6(5):1438–1452
Merewitz E, Xu Y, Huang B (2016) Differentially expressed genes associated with improved drought tolerance in creeping Bentgrass overexpressing a gene for cytokinin biosynthesis. PLoS One 11(11):e0166676
Miao Y, Laun T, Zimmermann P, Zentgraf U (2004) Targets of the WRKY53 transcription factor and its role during leaf senescence in Arabidopsis. Plant Mol Biol 55(6):853–867
Miao Y, Zentgraf U (2007) The antagonist function of Arabidopsis WRKY53 and ESR/ESP in leaf senescence is modulated by the jasmonic and salicylic acid equilibrium. Plant Cell 19(3):819–830
Mueller-Roeber B, Balazadeh S (2014) Auxin and its role in plant senescence. J Plant Growth Regul 33(1):21–33
Müller M, Munné-Bosch S (2015) Ethylene response factors: a key regulatory hub in hormone and stress signaling. Plant Physiol 169(1):32–41
Munné-Bosch S, Alegre L (2004) Die and let live: leaf senescence contributes to plant survival under drought stress. Funct Plant Biol 31(3):203–216
Narsai R, Law SR, Carrie C, Xu L, Whelan J (2011) In-depth temporal transcriptome profiling reveals a crucial developmental switch with roles for RNA processing and organelle metabolism that are essential for germination in Arabidopsis. Plant Physiol 157(3):1342–1362
Nawaz F, Naeem M, Zulfiqar B, Akram A, Ashraf MY, Raheel M et al (2017) Understanding brassinosteroid-regulated mechanisms to improve stress tolerance in plants: a critical review. Environ Sci Pollut Res 24(19):15959–15975
Nie H, Zhao C, Wu G, Wu Y, Chen Y, Tang D (2012) SR1, a calmodulin-binding transcription factor, modulates plant defense and ethylene-induced senescence by directly regulating NDR1 and EIN3. Plant Physiol 158(4):1847–1859
Neill SJ, Desikan R, Hancock JT (2003) Nitric oxide signalling in plants. New Phytol 159:11–35
Nisar N, Li L, Lu S, Khin NC, Pogson BJ (2015) Carotenoid metabolism in plants. Mol Plant 8(1):68–82
Nishiyama R, Watanabe Y, Fujita Y, Le DT, Kojima M, Werner T, Vankova R, Yamaguchi-Shinozaki K, Shinozaki K, Kakimoto T, Sakakibara H, Schmülling T, Tran LS (2011) Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell 23:2169–2183
Noctor G, Foyer CH (2016) Intracellular redox compartmentation and ROS-related communication in regulation and signaling. Plant Physiol 171(3):1581–1592
Nolan TM, Brennan B, Yang M, Chen J, Zhang M, Li Z, Yin Y (2017) Selective autophagy of BES1 mediated by DSK2 balances plant growth and survival. Dev Cell 41(1):33–46
Pandey GK (ed) (2017) Mechanism of Plant Hormone Signaling Under Stress, 2 Volume Set (Vol. 1). John Wiley & Sons
Pandey N, Iqbal Z, Pandey BK, Sawant SV (2017) Phytohormones and drought stress: plant responses to transcriptional regulation. Mechanism of Plant Hormone Signaling under Stress, p 477–504
Paparozzi ET, Chahal JK, Dobrev P, Claassen EA, Stroup WW, Vankova R (2016) Cytokinin dynamics during the response to nitrogen in two contrasting plectranthus genotypes. J Am Soc Hortic Sci 141(3):264–274
Pei H, Ma N, Tian J, Luo J, Chen J, Li J, Zheng Y, Chen X, Fei Z, Gao J (2013) An NAC transcription factor controls ethylene-regulated cell expansion in flower petals. Plant Physiol 163(2):775–791
Pimenta MR, Silva PA, Mendes GC, Alves JR, Caetano HDN, Machado JPB, Rosado GL (2016) The stress-induced soybean NAC transcription factor GmNAC81 plays a positive role in developmentally programmed leaf senescence. Plant Cell Physiol 57(5):1098–1114
Podzimska-Sroka D, O'Shea C, Gregersen PL, Skriver K (2015) NAC transcription factors in senescence: from molecular structure to function in crops. Plants 4(3):412–448
Puranik S, Sahu PP, Srivastava PS, Prasad M (2012) NAC proteins: regulation and role in stress tolerance. Tren Plant Sci 17(6):369–381
Qi T, Huang H, Song S, Xie D (2015) Regulation of jasmonate-mediated stamen development and seed production by a bHLH-MYB complex in Arabidopsis. Plant Cell 27:1620–1633
Qin Y, Tian Y, Liu X (2015) A wheat salinity-induced WRKY transcription factor TaWRKY93 confers multiple abiotic stress tolerance in Arabidopsis thaliana. Biochem Biophys Res Commun 464:428–433
Raines T, Shanks C, Cheng CY, McPherson D, Argueso CT, Kim HJ, Schaller GE (2016) The cytokinin response factors modulate root and shoot growth and promote leaf senescence in Arabidopsis. Plant J 85(1):134–147
Ramaswamy M, Narayanan J, Manickavachagam G, Athiappan S, Arun M, Gomathi R, Ram B (2017) Genome wide analysis of NAC gene family ‘sequences’ in sugarcane and its comparative phylogenetic relationship with rice, sorghum, maize and Arabidopsis for prediction of stress associated NAC genes. Agri Gene 3:1–11
Ramel F, Ksas B, Havaux M (2013) Jasmonate: a decision maker between cell death and acclimation in the response of plants to singlet oxygen. Plant Signal Behav 8(12):e26655
Rauf M, Arif M, Dortay H, Matallana-Ramírez LP, Waters MT, Nam HG, Lim PO, Mueller-Roeber B, Balazadeh S (2013) ORE1 balances leaf senescence against maintenance by antagonizing G2-like-mediated transcription. EMBO Rep 14(4):382–388
Reguera M, Peleg Z, Abdel-Tawab YM, Tumimbang EB, Delatorre CA, Blumwald E (2013) Stress-induced cytokinin synthesis increases drought tolerance through the coordinated regulation of carbon and nitrogen assimilation in rice. Plant Physiol 163(4):1609–1622
Reinbothe C, Springer A, Samol I, Reinbothe S (2009) Plant oxylipins: role of jasmonic acid during programmed cell death, defence and leaf senescence. FEBS J 276(17):4666–4681
Reinbothe S, Reinbothe C, Heintzen C, Seidenbecher C, Parthier B (1993a) A methyl jasmonate-induced shift in the length of the 5′ untranslated region impairs translation of the plastid rbcL transcript in barley. EMBO J 12(4):1505–1512
Reinbothe S, Reinbothe C, Parthier B (1993b) Methyl jasmonate represses translation initiation of a specific set of mRNAs in barley. Plant J 4(3):459–467
Rivero RM, Kojima M, Gepstein A, Sakakibara H, Mittler R, Gepstein S, Blumwald E (2007) Delayed leaf senescence induces extreme drought tolerance in a flowering plant. Proc Natl Acad Sci 104(49):19631–19636
Robert-Seilaniantz A, Grant M, Jones JD (2011) Hormone crosstalk in plant disease and defense: more than just jasmonate-salicylate antagonism. Annu Rev Phytopathol 49:317–343
Sağlam-Çağ S (2007) The effect of epibrassinolide on senescence in wheat leaves. Biotechnol Biotechnol Equip 21(1):63–65
Saini S, Sharma I, Pati PK (2015) Versatile roles of brassinosteroid in plants in the context of its homoeostasis, signaling and crosstalks. Front Plant Sci 6
Sami F, Yusuf M, Faizan M, Faraz A, Hayat S (2016) Role of sugars under abiotic stress. Plant Physiol Biochem 109:54–61
Sarwat M 2017 Leaf senescence in plants: nutrient remobilization and gene regulation. In: stress signaling in plants: genomics and proteomics perspective, volume 2. Springer International Publishing, p 301-316
Sarwat M, Naqvi AR, Ahmad P, Ashraf M, Akram NA (2013) Phytohormones and microRNAs as sensors and regulators of leaf senescence: assigning macro roles to small molecules. Biotechnol Adv 31(8):1153–1171
Scarpeci TE, Zanor MI, Mueller-Roeber B, Valle EM (2013) Overexpression of AtWRKY30 enhances abiotic stress tolerance during early growth stages in Arabidopsis thaliana. Plant Mol Biol 83(3):265–277
Schommer C, Palatnik JF, Aggarwal P, Chételat A, Cubas P, Farmer EE et al (2008) Control of jasmonate biosynthesis and senescence by miR319 targets. PLoS Biol 6(9):e230
Seo PJ, Park JM, Kang SK, Kim SG, Park CM (2011) An Arabidopsis senescence-associated protein SAG29 regulates cell viability under high salinity. Planta 233(1):189–200
Shahnejat-Bushehri S, Allu AD, Mehterov N, Thirumalaikumar VP, Alseekh S, Fernie AR, Balazadeh S (2017) Arabidopsis NAC transcription factor JUNGBRUNNEN1 exerts conserved control over gibberellin and brassinosteroid metabolism and signaling genes in tomato. Front Plant Sci 8:214
Shahnejat-Bushehri S, Tarkowska D, Sakuraba Y, Balazadeh S (2016) Arabidopsis NAC transcription factor JUB1 regulates GA/BR metabolism and signalling. Nat Plants 2:16013
Sharabi-Schwager M, Lers A, Samach A, Guy CL, Porat R (2010) Overexpression of the CBF2 transcriptional activator in Arabidopsis delays leaf senescence and extends plant longevity. J Exp Bot 61:261–273
Skirycz A, Claeys H, De Bodt S, Oikawa A, Shinoda S, Andriankaja M, Maleux K, Eloy NB, Coppens F, Yoo SD, Saito K, Inze D (2011) Pause-and-stop: the effects of osmotic stress on cell proliferation during early leaf development in Arabidopsis and a role for ethylene signaling in cell cycle arrest. Plant Cell 23:1876–1888
Sklensky DE, Davies PJ (2011) Resource partitioning to male and female flowers of Spinacia oleracea L. in relation to whole-plant monocarpic senescence. J Exp Bot 62(12):4323–4336
Song Y, Xiang F, Zhang G, Miao Y, Miao C, Song CP (2016) Abscisic acid as an internal integrator of multiple physiological processes modulates leaf senescence onset in Arabidopsis thaliana. Front Plant Sci 7:181
Song Y, Yang C, Gao S, Zhang W, Li L, Kuai B (2014) Age-triggered and dark-induced leaf senescence require the bHLH transcription factors PIF3, 4, and 5. Mol Plant 7(12):1776–1787
Stamm P, Kumar PP (2013) Auxin and gibberellin responsive Arabidopsis SMALL AUXIN UP RNA36 regulates hypocotyl elongation in the light. Plant Cell Rep 32(6):759–769
Su Y, Hu S, Zhang B, Ye W, Niu Y, Guo L, Qian Q (2017) Characterization and fine mapping of a new early leaf senescence mutant es3 (t) in rice. Plant Growth Regul 81(3):419–431
Thirumalaikumar VP, Devkar V, Mehterov N, Ali S, Ozgur R, Turkan I, Balazadeh S 2017 NAC transcription factor JUNGBRUNNEN1 enhances drought tolerance in tomato. Plant Biotechnol J
Thomas H (2013) Senescence, ageing and death of the whole plant. New Phytol 197(3):696–711
Thomas H, Ougham H (2014) The stay-green trait. J Exp Bot 65(14):3889–3900
Thu NBA, Hoang XLT, Truc MT, Sulieman S, Thao NP, Tran LSP (2017) Cytokinin signaling in plant response to abiotic stresses. Mechanism of Plant Hormone Signaling under Stress, 71–100
Uji Y, Akimitsu K, Gomi K (2017) Identification of OsMYC2-regulated senescence-associated genes in rice. Planta 245(6):1241–1246
van der Graaff E, Schwacke R, Schneider A, Desimone M, Flugge UI, Kunze R (2006) Transcription analysis of arabidopsis membrane transporters and hormone pathways during developmental and induced leaf senescence. Plant Physiol 141:776–792
Velasco-Arroyo, B., Diaz-Mendoza, M., Santamaria, M. E., Gonzalez-Melendi, P., Gomez-Sanchez, A., Arnaiz, A., Diaz, I., 2017. Senescence-associated genes in response to abiotic/biotic stresses. p 1–21
Wang F, Liu J, Chen M, Zhou L, Li Z, Zhao Q, Cheng F (2016) Involvement of abscisic acid in PSII photodamage and D1 protein turnover for light-induced premature senescence of rice flag leaves. PLoS One 11(8):e0161203
Wehner G, Balko C, Humbeck K, Zyprian E, Ordon F (2016) Expression profiling of genes involved in drought stress and leaf senescence in juvenile barley. BMC Plant Biol 16(1):3
Woo HR, Kim JH, Kim J, Kim J, Lee U, Song IJ, Lim PO (2010) The RAV1 transcription factor positively regulates leaf senescence in Arabidopsis. J Exp Bot 61(14):3947–3957
Woo HR, Kim HJ, Nam HG, Lim PO (2013) Plant leaf senescence and death–regulation by multiple layers of control and implications for aging in general. J Cell Sci 126(21):4823–4833
Woolhouse HW, Batt T (2016) The nature and regulation of senescence in plastids. Perspec Exp Biol 2:163–175
Woo HR, Koo HJ, Kim J, Jeong H, Yang JO, Lee IH et al (2016) Programming of plant leaf senescence with temporal and inter-organellar coordination of transcriptome in Arabidopsis. Plant Physiol 171:452–467. https://doi.org/10.1104/pp.15.01929
Wu A, Allu AD, Garapati P, Siddiqui H, Dortay H, Zanor MI, Asensi-Fabado MA, Munné-Bosch S, Antonio C, Tohge T, Fernie AR (2012b) JUNGBRUNNEN1, a reactive oxygen species–responsive NAC transcription factor, regulates longevity in Arabidopsis. Plant Cell 24(2):482–506
Wu XY, Kuai BK, Jia JZ, Jing HC (2012a) Regulation of leaf senescence and crop genetic improvement. J Int Plant Biol 54(12):936–952
Xiao S, Gao W, Chen QF, Chan SW, Zheng SX, Ma J, Wang M, Welti R, Chye ML (2010) Overexpression of Arabidopsis AcylCoA binding protein ACBP3 promotes starvation-induced and agedependent leaf senescence. Plant Cell 22:1463–1482
Xiao S, Chye ML (2011) Overexpression of Arabidopsis Acyl-CoA-Binding Protein 3 Enhances NPR1-Dependent Plant Resistance to Pseudomonas syringe pv. tomato DC3000. Plant Physiol, pp-111
Xiao XO, Zeng YM, Cao BH, Lei JJ, Chen QH, Meng CM, Cheng YJ (2017) PSAG12-IPT overexpression in eggplant delays leaf senescence and induces abiotic stress tolerance. The J Horticul Sci. Biotech 92(4):349–357
Xie Y, Huhn K, Brandt R, Potschin M, Bieker S, Straub D, Wenkel S (2014) REVOLUTA and WRKY53 connect early and late leaf development in Arabidopsis. Development, dev-117689
Xu ZS, Chen M, Li LC, Ma YZ (2011) Functions and application of the AP2/ERF transcription factor family in crop improvementF. J Int Plant Biol 53(7):570–585
Yang JC, Zhang JH, Wang ZQ, Zhu QS, Liu LJ (2003) Involvement of abscisic acid and cytokinins in the senescence and remobilization of carbon reserves in wheat subjected to water stress during grain filling. Plant Cell Environ 26(10):1621–1631
Yang SD, Seo PJ, Yoon HK, Park CM (2011) The Arabidopsis NAC transcription factor VNI2 integrates abscisic acid signals into leaf senescence via the COR/RD genes. Plant Cell 23(6):2155–2168
Yang X, Wang X, Ji L, Yi Z, Fu C, Ran J et al (2015) Overexpression of a Miscanthus lutarioriparius NAC gene MlNAC5 confers enhanced drought and cold tolerance in Arabidopsis. Plant Cell Rep 34(6):943–958
Ye H, Liu S, Tang B, Chen J, Xie Z, Nolan TM, Wang Y (2017) RD26 mediates crosstalk between drought and brassinosteroid signalling pathways. Nat Commun 8:14573
Yoshimoto K, Jikumaru Y, Kamiya Y, Kusano M, Consonni C, Panstruga R, Ohsumi Y, Shirasu K (2009) Autophagy negatively regulates cell death by controlling NPR1-dependent salicylic acid signaling during senescence and the innate immune response in Arabidopsis. Plant Cell 21:2914–2927
Yu SM, Lo SF, Ho THD (2015) Source–sink communication: regulated by hormone, nutrient, and stress cross-signaling. Trends Plant Sci 20(12):844–857
Yu K, Wei J, Ma Q, Yu D, Li J (2009) Senescence of aerial parts is impeded by exogenous gibberellic acid in herbaceous perennial Paris polyphylla. J Plant Physiol 166(8):819–830
Yu J, Zhang Y, Di C (2016) JAZ7 negatively regulates dark-induced leaf senescence in Arabidopsis. J Exp Bot 67:751–762
Zentgraf U (2007) Oxidative stress and leaf senescence. In: Gan S (ed) Annual plant reviews: senescence processes in plants. Blackwell Publishing Ltd, Oxford, UK, p 26
Zhang K, Gan SS (2012) An abscisic acid-AtNAP transcription factor-SAG113 protein phosphatase 2C regulatory chain for controlling dehydration in senescing Arabidopsis leaves. Plant Physiol 158(2):961–969
Zhang X, Ju HW, Chung MS, Huang P, Ahn SJ, Kim CS (2011) The RR-type MYB-like transcription factor, AtMYBL, is involved in promoting leaf senescence and modulates an abiotic stress response in Arabidopsis. Plant Cell Physiol 52(1):138–148
Zhang J, Shi Y, Zhang X, Du H, Xu B, Huang B (2017a) Melatonin suppression of heat-induced leaf senescence involves changes in abscisic acid and cytokinin biosynthesis and signaling pathways in perennial ryegrass (Lolium perenne L.). Environ Exp Bot 138:36–45
Zhang WY, Xu YC, Li WL, Yang L, Yue X, Zhang XS, Zhao XY (2014) Transcriptional analyses of natural leaf senescence in maize. PLoS One 9(12):e115617
Zhang XM, Yu HJ, Sun C, Deng J, Zhang X, Liu P, Jiang WJ (2017b) Genome-wide characterization and expression profiling of the NAC genes under abiotic stresses in Cucumis sativus. Plant Physiol Biochem 113:98–109
Zhang J, Yu G, Wen W, Ma X, Xu B, Huang B (2015) Functional characterization and hormonal regulation of the PHEOPHYTINASE gene LpPPH controlling leaf senescence in perennial ryegrass. J Exp Bot 67(3):935–945
Zhang H, Zhao M, Song Q, Zhao L, Wang G, Zhou C (2016) Identification and function analyses of senescence-associated WRKYs in wheat. Biochem Biophys Res Commun 474(4):761–767
Zhao Y, Chan Z, Gao J, Xing L, Cao M, Yu C, Gong Y (2016) ABA receptor PYL9 promotes drought resistance and leaf senescence. Proc Nat Acad Sci 113(7):1949–1954
Zhou X, Jiang Y, Yu D (2011) WRKY22 transcription factor mediates dark-induced leaf senescence in Arabidopsis. Mol Cells 31(4):303–313
Zhu X, Chen J, Xie Z, Gao J, Ren G, Gao S et al (2015) Jasmonic acid promotes degreening via MYC 2/3/4-and ANAC 019/055/072-mediated regulation of major chlorophyll catabolic genes. Plant J 84(3):597–610
Zwack PJ, De Clercq I, Howton TC, Hallmark HT, Hurny A, Keshishian EA, Rashotte AM (2016) Cytokinin response factor 6 represses cytokinin-associated genes during oxidative stress. Plant Physiol 172(2):1249–1258
Zwack PJ, Rashotte AM (2013) Cytokinin inhibition of leaf senescence. Plant Signal Behav 8(7):e24737
Funding
This work was supported by the Deanship of Scientific Research at King Saud University for funding this research group no. RG-1438-039.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Bhumi Nath Tripathi
Rights and permissions
About this article
Cite this article
Jan, S., Abbas, N., Ashraf, M. et al. Roles of potential plant hormones and transcription factors in controlling leaf senescence and drought tolerance. Protoplasma 256, 313–329 (2019). https://doi.org/10.1007/s00709-018-1310-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-018-1310-5