Abstract
The larvae of Bittacidae, a cosmopolitan family in Mecoptera, have an interesting habit of spraying the body surface with soil through the anus after hatching, and each molts. The fine structure of Malpighian tubules, however, remains largely unknown in the larvae of Bittacidae to date. Here, we studied the ultrastructure of the larval Malpighian tubules in the hangingfly Terrobittacus implicatus (Huang & Hua) using scanning and transmission electron microscopy. The larvae of T. implicatus have six elongate Malpighian tubules at the junction of the midgut and hindgut. The tubule comprises a basal lamina, a single-layered epithelium, and a central lumen. The basal plasma membranes of the epithelial cells are conspicuously infolded and generate a labyrinth. The epithelium consists of two types of cells: large principal cells and scattered stellate cells. Mitochondria and cisterns of rough endoplasmic reticulum are numerous in the principal cells but are sparsely distributed in the stellate cells, indicating that the principal cells are active in transport. On the other hand, spherites are only abundant in the principal cells and are likely associated with the soil-spraying habit of the larvae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malpighian tubules are the main excretory and osmoregulatory organs of insects (Maddrell 1978; Chapman 2013; Gullan and Cranston 2014) and are responsible for releasing primary urine, reabsorbing solutes, and maintaining osmotic homeostasis (Bradley 1998; Hazelton et al. 2001). In general, they are ectodermal in origin (Chapman 2013; Yue and Hua 2013) and arise from the junction of the midgut and hindgut. The Malpighian tubules are free in the hemocoel in most insects but connect with the hindgut to form a cryptonephridial system in the meal worm Tenebrio molitor Linnaeus (Coleoptera: Tenebrionidae) (Koefoed 1971). The Malpighian tubules vary in number among different orders (Chapman 2013) and even differ between the larvae and adults in the stingless bees (Hymenoptera: Apidae) (Barbosa-Costa et al. 2012).
The Malpighian tubules are also diverse in morphology among various insect species (Bradley 1998; Chapman 2013). The tubules are in a narrow tubular shape and are divided into two equal branches in the thrips Aeolothrips intermedius Bagnall (Thysanoptera: Aeolothripidae) (Conti et al. 2010) and the larval mosquito Anopheles sinensis (Diptera: Culicidae) (Yu 2003), are often differentiated into several morphologically distinct segments in Hemiptera (Li et al. 2015; Zhong et al. 2015; Özyurt et al. 2017), and are non-segmented and possess a beaded appearance in the adult flesh fly Sarcophaga ruficornis Fabr. (Diptera: Sarcophagidae) (Pal and Kumar 2013). The Malpighian tubule comprises a monolayered epithelium with one or more types of cells and a central lumen in many insects (Martoja and Ballan-Dufrançais 1984; Bradley 1998; Beyenbach et al. 2010). The morphology and histology of Malpighian tubules, however, have only been briefly described in Mecoptera (Grell 1938; Potter 1938a, b; Setty 1940; Liu S and Hua 2009; Liu L and Hua 2017).
Bittacidae is the only cosmopolitan family in Mecoptera (Penny and Byers 1979; Chen et al. 2013; Wang and Hua 2017). The adults are commonly known as hangingflies because they usually hang themselves on the edges of leaves or twigs between flights with their prehensile forelegs in moist shady woodlands (Setty 1940; Byers 2009; Jiang et al. 2015). The larvae of Bittacidae have an interesting habit of spraying soil on their body surface through the anus after the soil passing through the digestive tract (Currie 1932; Setty 1940). The larvae are eruciform with furcated seta-bearing protuberances (Tan and Hua 2008, 2009a, b), which are likely associated with the soil-spraying habit (Jiang et al. 2015). Terrobittacus is a small genus of Bittacidae (Tan and Hua 2009b) and has been studied in detail in the morphology of mouthparts (Ma et al. 2014) and cytology (Miao and Hua 2017). However, the ultrastructure of the Malpighian tubules remains largely unknown in the larval Bittacidae to date.
In this study, we investigated the ultrastructure of the Malpighian tubules in the larval stage of the hangingfly Terrobittacus implicatus (Huang & Hua in Cai et al. 2006) using scanning and transmission electron microscopy in an attempt to clarify if the Malpighian tubules have any specialization associated with the soil-spraying habit.
Material and methods
Insect collecting and rearing
Adults of T. implicatus were captured in the Liping National Forest Park (32° 50′ N, 106° 36′ E, elev. 1500–1600 m) in the Michang Mountains, Shaanxi Province, Central China, in early August 2016.
Live female adults of T. implicatus were reared in a nylon gauze cage (40 cm × 40 cm × 60 cm), with wet cotton gauze covered outside to maintain relatively high humidity (Jiang and Hua 2015; Jiang et al. 2015). The adults were provided plant twigs to suspend from and live house flies as food items. Eggs were collected with wet tissue papers at the bottom of the cage and were transferred into plastic jars with soil to overwinter. The larvae were collected in March 2017 and reared to the last (fourth) instar.
Scanning electron microscopy
Live last instar larvae were anesthetized with diethyl ether, and the Malpighian tubules were dissected rapidly. Then, the samples were fixed in a mixture of 2.5% glutaraldehyde and 2.0% paraformaldehyde in phosphate-buffered saline (PBS, 0.1 mol/L, pH 7.2) at 4 °C for 12 h.
For scanning electron microscopy, the samples were rinsed in PBS for six times and dehydrated through a graded ethanol series (30, 50, and 70% for 10 min each, 80% for 15 min, 90% for 20 min, 95% for 25 min, and 100% for 30 min twice). The dehydrated samples were subsequently replaced by tertiary butanol and freeze-dried for 3 h. After being sputter-coated with gold, the samples were examined in a Hitachi S-3400N scanning electron microscope (Hitachi, Tokyo, Japan) at 15 kV.
Transmission electron microscopy
For transmission electron microscopy, the fixed samples were rinsed with PBS for six times and post-fixed in 1% osmium tetroxide (OsO4) in PBS at 4 °C for 1 h (Liu and Hua 2010; Zhang and Hua 2014). The post-fixed samples were rinsed in the same buffer for six times and dehydrated through a graded ethanol series (30, 50, and 70% for 10 min each, 80% for 15 min, 90% for 20 min, and 100% for 30 min twice). The samples were infiltrated in the mixtures of ethanol and LR White resin (3:1 for 2 h, 1:1 for 4 h, and 1:3 for 12 h) and then in pure LR White resin for 24 h twice at 18 °C. The samples were finally embedded in pure LR White resin and polymerized at 55 °C for 48 h.
Ultrathin sections were cut with a diamond knife on a Leica ULTRACUT ultramicrotome (Leica, Nussloch, Germany) and double stained with uranyl acetate and lead citrate. The stained sections were observed in a Tecnai G2 Spirit Bio Twin transmission electron microscope (FEI, Hillsboro, USA) at 80 kV.
Results
Morphology of the Malpighian tubules
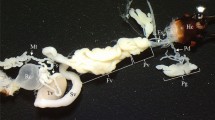
The larvae of T. implicatus have six Malpighian tubules, which are of approximately equal length and extend from the junction of the midgut and hindgut to the body cavity (Fig. 1). The elongate tubules are thin and blindly ended, yellowish at the proximal part. The distal part of tubules is often dark red and wavy. The tubules usually connect with fat bodies or are free in the body cavity. The tubules exhibit a smooth appearance and are unsegmented, with several slender branched tracheoles on the surface (Fig. 2a).
The Malpighian tubule of Terrobittacus implicatus. a SEM micrograph of the Malpighian tubule (MT) with branching tracheoles (Tr). b TEM micrograph of the proximal part of the tubule in the cross section, showing the monolayered epithelium with principal cells and a central lumen. c The TEM micrograph of the distal part of the tubule in the cross section, showing the epithelium contained principal and stellate cells. BL basal lamina, Ep epithelium, Lu lumen, Mv microvilli, N nucleus, PC principal cell, SC stellate cell. Scale bars: a 50 μm; b–c 5 μm
Ultrastructure of the Malpighian tubules
The Malpighian tubule comprises a single-layered epithelium surrounded by a non-cellular basal lamina (Fig. 2b, c). The cross section of the tubule shows two types of epithelial cells: large principal cells and small stellate cells. The great majority of epithelial cells are the principal cells, which are present in the whole length of the tubule (Fig. 2b, c). The stellate cells are visible in the distal part and are usually invisible in the proximal part of the tubule (Fig. 2c). Several principal cells and one stellate cell are visible in the cross section of the distal tubule (Fig. 2c). The apical surfaces of the epithelial cells possess numerous microvilli.
The principal cells are characterized by a rectangular shape and amounts of close-packed microvilli (Fig. 3a). The basal plasma membranes of these cells are evidently invaginated and form membranous labyrinths with numerous mitochondria lying in close proximity (Fig. 3b). The adjoining principal and stellate cells are connected loosely in the basal regions due to invaginations formed by the basal plasma membranes (Fig. 3a). Septate junctions are visible between the adjacent cells in the apical regions (Fig. 3c). The rounded nucleus occupies the large space of the cell and contains several patches of heterochromatin (Fig. 2c). The cytoplasm is electron-dense and rich in rough endoplasmic reticulum, mitochondria, and spherites (Fig. 3a–e). Vacuoles are also visible in the cells (Fig. 3a, b). The spherites contain several concentric laminate concretions near the apical membrane (Fig. 3e). The extensive microvilli extend into the central lumen as finger-like projections at the apical surfaces of the cells. The microvilli are swollen and contain mitochondria (Fig. 3f).
TEM micrographs of the principal cells in the cross section of Malpighian tubules in Terrobittacus implicatus. a The principal cells. b The basal region of a principal cell. c Septate junction between two neighboring principal cells. d Apical region of the principal cell with many mitochondria. e Numerous spherites in the apical region of the principal cell. f Closely packed microvilli with mitochondria. BL basal lamina, BM basal plasma membrane, Lu lumen, M mitochondrion, Mv microvilli, PC principal cell, RER rough endoplasmic reticulum, S spherite, SC stellate cell, SJ septate junction, V vacuole. Scale bars: a 2 μm; b–e 1 μm; f 500 nm
The stellate cells are smaller than the principal cells and assume a strip shape in the cross section (Fig. 4a). The stellate cells are scattered among the principal cells and rest on the basal lamina. In the apical region, the neighboring cells are held by septate junctions (Fig. 4b). The septate junctions are scarce between the adjacent epithelial cells in the basal region (Fig. 4c). The basal plasma membrane is conspicuously infolded with a few mitochondria and generates a labyrinth (Fig. 4c). The large oval nucleus with double membranes occupies the central part of the cell (Fig. 4a, f). The cytoplasm is electron-lucent and devoid of vacuole and spherite (Fig. 4). The cells contain rough endoplasmic reticulum and Golgi complex (Fig. 4d, e). The mitochondria of the stellate cells vary in shape and are fewer than those of the principal cells (Fig. 4b, d). The microvilli of the stellate cells are shorter than those of the principal cells and lack mitochondria and are sparsely distributed in the luminal spaces (Fig. 4f).
TEM micrographs of the stellate cells of Malpighian tubules in Terrobittacus implicatus. a A stellate cell with an oval nucleus. b Septate junction between adjacent principal and stellate cells. c Basal region of the stellate cell. d Mitochondria in various shapes in the cytoplasm. e Perinuclear region of the stellate cell. f Short microvilli of the stellate cell. BL basal lamina, BM basal plasma membrane, G Golgi complex, Lu lumen, M mitochondrion, Mv microvilli, N nucleus, PC principal cell, RER rough endoplasmic reticulum, SC stellate cell, SJ septate junction. Scale bars: a 2 μm; b, d–f 500 nm; c 1 μm
Discussion
The larval Malpighian tubules of T. implicatus lack morphologically specialized segments and branches and are similar to those of other mecopterans (Grell 1938; Potter 1938a, b; Setty 1940; Liu S and Hua 2009; Liu L and Hua 2017) and other insects (Bradley 1998), such as the blow fly Calliphora erythrocephala (Meigen) (Diptera: Calliphoridae) (Berridge and Oschman 1969) and the larval mosquito Aedes taeniorhynchus (Wiedemann) (Diptera: Culicidae) (Bradley et al. 1982). The tubule of T. implicatus consists of a monolayered epithelium bounded by a non-cellular basal lamina and a central lumen. The epithelium consists of large principal cells and small stellate cells, the latter of which are unevenly distributed along the length of the tubules. These two types of cells are greatly different in ultrastructure and associated with different functions (Berridge and Oschman 1969).
The principal cells are the primary epithelial cells of the Malpighian tubule in insects (Bradley 1998; Chapman 2013; Gullan and Cranston 2014) and are with minor variation in fine structure among different species (Martoja and Ballan-Dufrançais 1984). In T. implicatus, the principal cells of larval Malpighian tubules are characterized by deeply infolded basal plasma membrane, a lot of mitochondria, rough endoplasmic reticulum, and a large number of closely packed microvilli. These ultrastructural features indicate that they are active in ion and water transport (Pal and Kumar 2013). The water and ion from the hemolymph are transported by an osmotic gradient, which is generated within the tubule cells and the central lumen (Pannabecker 1995; Gullan and Cranston 2014). The transport takes place by a secretory process of tubule cells (Ruiz-Sanchez et al. 2015). The numerous mitochondria lie within microvilli or with the basal plasma membrane and are involved in supplying energy for the transport and secretion of the cells (Bradley 1998). In addition, the principal cells are associated with sequestration of organic or inorganic components as inclusions bounded by membrane (Martoja and Ballan-Dufrançais 1984; Leonard et al. 2009).
The inclusions bounded by membrane such as spherites are universal in the principal cells of Malpighian tubules (Martoja and Ballan-Dufrançais 1984; Bradley 1998) and usually also occur in the midgut epithelium (Pigino et al. 2005; Pinheiro et al. 2008; Santos et al. 2017). Numerous spherites are present in the principal cells of the larval Malpighian tubules in T. implicatus and also occur in the larval midgut of Bittacus planus (Liu L and Hua 2017). The spherites with concentric lamination are formed by mineral accumulation (Pinheiro et al. 2008). The spherites of Malpighian tubules are the vital mineral supply to support the crucial processes of the life cycle in the cave cricket Troglophilus neglectus Krauss (Orthoptera: Rhaphidophoridae) (Lipovšek et al. 2009). In the herald moth Scoliopteryx libatrix Linnaeus (Lepidoptera: Noctuidae), the stored spherites are gradually utilized in the Malpighian tubules during overwintering (Lipovšek et al. 2017). In addition, the accumulation of metals in spherites is also a detoxification mechanism of insects at the cellular level (Ballan-Dufrançais 2002).
In contrast to the principal cells, the stellate cells are thin in the cross section and possess sparser organelles, short microvilli, and a few mitochondria in the larval Malpighian tubules of T. implicatus, as in other insects (Bradley 1998; Chapman 2013). Judged from the ultrastructure, the stellate cells are not actively related to ion transport (Pal and Kumar 2013). The function of stellate cells is involved in sodium resorption in the blow fly C. erythrocephala (Berridge and Oschman 1969) and the larval fruit flies Drosophila hydei Sturtevant and D. melanogaster Meigen (Diptera: Drosophilidae) (Wessing et al. 1999).
The Malpighian tubules release primary urine from the lumen towards and into the alimentary canal (Gullan and Cranston 2014) and are in high sensitivity (Giglio and Brandmayr 2017) in altering the epithelial ultrastructure subject to heavy metals (Pigino et al. 2005; Talarico et al. 2014) and insecticides (Sumida et al. 2010; De Almeida Rossi et al. 2013; Decio et al. 2013; Ferreira et al. 2013). After exposure to heavy metals, the larval flesh fly Boettcherisca peregrina Robineau-Desvoidy was found to increase spherites in the midgut and Malpighian tubules, indicating that these are the primary organs to store metals (Wu et al. 2009). In Bittacidae, the larvae swallow soil through the mouthparts and then spray the soil on their body surface through the anus after hatching, and each molts (Currie 1932; Setty 1940). The soil particles pass through the digestive tract and are likely mixed with primary urine before excretion from the larval anus. Spherites are rich in the epithelium of larval Malpighian tubules in T. implicatus and are also abundant in the larval midgut epithelium of the hangingfly B. planus, but are lacking in that of the scorpionfly Neopanorpa longiprocessa (Liu L and Hua 2017). Considering that the larval soil-spraying habit is only present in the larvae of Bittacidae and not in other families of Mecoptera, we suppose that the spherites of bittacid larvae may store heavy metals from the soil particles temporally stored in the alimentary canal.
References
Ballan-Dufrançais C (2002) Localization of metals in cells of pterygote insects. Microsc Res Techn 56(6):403–420. https://doi.org/10.1002/jemt.10041
Barbosa-Costa K, Kerr WE, Carvalho-Zilse GA (2012) Number of Malpighian tubules in larvae and adults of stingless bees (Hymenoptera: Apidae) from Amazonia. Neotrop Entomol 41(1):42–45. https://doi.org/10.1007/s13744-011-0017-5
Berridge MJ, Oschman JL (1969) A structural basis for fluid secretion by Malpighian tubules. Tissue Cell 1(2):247–272. https://doi.org/10.1016/S0040-8166(69)80025-X
Beyenbach KW, Skaer H, Dow JAT (2010) The developmental, molecular, and transport biology of Malpighian tubules. Annu Rev Entomol 55:351–374. https://doi.org/10.1146/annurev-ento-112408-085512
Bradley TJ (1998) Malpighian tubules. In: Harrison FW, Locke M (eds) Microscopic anatomy of invertebrates. Wiley-Liss, New York, p 11B 809–829
Bradley TJ, Stuart AM, Satir P (1982) The ultrastructure of the larval Malpighian tubules of a saline-water mosquito. Tissue Cell 14(4):759–773. https://doi.org/10.1016/0040-8166(82)90064-7
Byers GW (2009) Mecoptera: scorpionflies, hangingflies. In: Resh VH, Cardé RT (Eds.), Encyclopedia of insects, 2nd ed. Academic Press, San Diego, pp 611–614, DOI: https://doi.org/10.1016/B978-0-12-374144-8.00170-3
Cai L-J, Huang P-Y, Hua B-Z (2006) Two new Chinese Bittacus Latreille (Mecoptera: Bittacidae) from Micangshan Mountains. Entomotaxonomia 28:127–130
Chapman RF (2013) The insects: structure and function, 5th edn. Cambridge University Press, Cambridge (UK), pp 546–584
Chen J, Tan J-L, Hua B-Z (2013) Review of the Chinese Bittacus (Mecoptera: Bittacidae) with descriptions of three new species. J Nat Hist 47(21–22):1463–1480. https://doi.org/10.1080/00222933.2012.763065
Conti B, Berti F, Mercati D, Giusti F, Dallai R (2010) The ultrastructure of Malpighian tubules and the chemical composition of the cocoon of Aeolothrips intermedius Bagnall (Thysanoptera). J Morphol 271(2):244–254. https://doi.org/10.1002/jmor.10793
Currie GA (1932) Some notes on the biology and morphology of the immature stages of Harpobittacus tillyardi (Order Mecoptera). Proc Linn Soc NSW 57:116–122
De Almeida RC, Roat TC, Tavares DA, Cintra-Socolowski P, Malaspina O (2013) Effects of sublethal doses of imidacloprid in Malpighian tubules of africanized Apis mellifera (Hymenoptera, Apidae). Microsc Res Techn 76:552–558. https://doi.org/10.1002/jemt.22199
Decio P, Silva-Zacarin ECM, Bueno FC, Bueno OC (2013) Toxicological and histopathological effects of hydramethylnon on Atta sexdens rubropilosa (Hymenoptera: Formicidae) workers. Micron 45:22–31. https://doi.org/10.1016/j.micron.2012.10.008
Ferreira RAC, Silva Zacarin ECM, Malaspina O, Bueno OC, Tomotake MEM, Pereira AM (2013) Cellular responses in the Malpighian tubules of Scaptotrigona postica (Latreille, 1807) exposed to low doses of fipronil and boric acid. Micron 46:57–65. https://doi.org/10.1016/j.micron.2012.12.008
Giglio A, Brandmayr P (2017) Structural and functional alterations in Malpighian tubules as biomarkers of environmental pollution: synopsis and prospective. J Appl Toxicol 37(8):889–894. https://doi.org/10.1002/jat.3454
Grell KG (1938) Der Darmtraktus von Panorpa communis L. und seine Anhänge bei Larve und Imago. Zool Jb Anat 64:1–86
Gullan PJ, Cranston PS (2014) The insects: an outline of entomology, 5th edn. Blackwell Publishing, Oxford, pp 74–84
Hazelton SR, Felgenhauer BE, Spring JH (2001) Ultrastructural changes in the Malpighian tubules of the house cricket, Acheta domesticus, at the onset of diuresis: a time study. J Morphol 247(1):80–92. https://doi.org/10.1002/1097-4687(200101)247:1<80::AID-JMOR1004>3.0.CO;2-X
Jiang L, Hua B-Z (2015) Functional morphology of the larval mouthparts of Panorpodidae compared with Bittacidae and Panorpidae (Insecta: Mecoptera). Org Divers Evol 15(4):671–679. https://doi.org/10.1007/s13127-015-0225-7
Jiang L, Gao Q-H, Hua B-Z (2015) Larval morphology of the hanging-fly Bittacus trapezoideus Huang & Hua (Insecta: Mecoptera: Bittacidae). Zootaxa 3957(3):324–333. https://doi.org/10.11646/zootaxa.3957.3.5
Koefoed BM (1971) Ultrastructure of the cryptonephridial system in the meal worm Tenebrio molitor. Cell Tissue Res 116(4):487–501. https://doi.org/10.1007/BF00335054
Leonard EM, Pierce LM, Gillis PL, Wood CM, O’Donnell MJ (2009) Cadmium transport by the gut and Malpighian tubules of Chironomus riparius. Aquat Toxicol 92(3):179–186. https://doi.org/10.1016/j.aquatox.2009.01.011
Li Q-L, Zhong H-Y, Zhang Y-L, Wei C (2015) Comparative morphology of the distal segments of Malpighian tubules in cicadas and spittlebugs, with reference to their functions and evolutionary indications to Cicadomorpha (Hemiptera: Auchenorrhyncha). Zool Anz 258:54–68. https://doi.org/10.1016/j.jcz.2015.07.002
Lipovšek S, Letofsky-Papst I, Novak T, Hofer F, Pabst MA (2009) Structure of the Malpighian tubule cells and annual changes in the structure and chemical composition of their spherites in the cave cricket Troglophilus neglectus Krauss, 1878 (Rhaphidophoridae, Saltatoria). Arthropod Struct Dev 38(4):315–327. https://doi.org/10.1016/j.asd.2009.02.001
Lipovšek S, Janžekovič F, Novak T (2017) Ultrastructure of fat body cells and Malpighian tubule cells in overwintering Scoliopteryx libatrix (Noctuoidea). Protoplasma 254(6):2189–2199. https://doi.org/10.1007/s00709-017-1110-3
Liu S-Y, Hua B-Z (2009) Morphology and histology of the alimentary canal in scorpionfly Panorpa obtusa (Mecoptera: Panorpidae). Acta Entomol Sin 52:808–813. http://www.insect.org.cn/CN/Y2009/V52/I7/808
Liu S-Y, Hua B-Z (2010) Histology and ultrastructure of the salivary glands and salivary pumps in the scorpionfly Panorpa obtusa (Mecoptera: Panorpidae). Acta Zool 91(4):457–465. https://doi.org/10.1111/j.1463-6395.2009.00436.x
Liu L, Hua B-Z (2017) Ultrastructure of the larval midgut of Bittacus planus (Mecoptera: Bittacidae) and Neopanorpa longiprocessa (Mecoptera: Panorpidae). Tissue Cell 49(5):622–631. https://doi.org/10.1016/j.tice.2017.08.001
Ma N, Huang J, Hua B-Z (2014) Fine structure and functional morphology of the mouthparts of Bittacus planus and Terrobittacus implicatus (Insecta: Mecoptera: Bittacidae). Zool Anz 253(6):441–448. https://doi.org/10.1016/j.jcz.2014.05.001
Maddrell SHP (1978) Physiological discontinuity in an epithelium with an apparently uniform structure. J Exp Biol 75:133–145. http://jeb.biologists.org/content/75/1/133
Martoja R, Ballan-Dufrançais C (1984) The ultrastructure of the digestive and excretory organs. In: King RC, Akai H (Eds.), Insect ultrastructure. Plenum Press, New York, 2: 199–268
Miao Y, Hua B-Z (2017) Cytogenetic comparison between Terrobittacus implicatus and Bittacus planus (Mecoptera: Bittacidae) with some phylogenetic implications. Arthropod Syst Phylogeny 75:175–183. http://www.senckenberg.de/files/content/forschung/publikationen/arthropodsystematics/asp_75_2/01_asp_75_2_miao_175-183.pdf
Özyurt N, Amutkan D, Polat I, Kocamaz T, Candan S, Suludere Z (2017) Structural and ultrastructural features of the Malpighian tubules of Dolycoris baccarum (Linnaeus 1758), (Heteroptera: Pentatomidae). Microsc Res Techn 80(4):357–363. https://doi.org/10.1002/jemt.22802
Pal R, Kumar K (2013) Malpighian tubules of adult flesh fly, Sarcophaga ruficornis Fab. (Diptera: Sarcophagidae): an ultrastructural study. Tissue Cell 45(5):312–317. https://doi.org/10.1016/j.tice.2013.04.002
Pannabecker T (1995) Physiology of the Malpighian tubule. Annu Rev Entomol 40:493–510. https://doi.org/10.1146/annurev.en.40.010195.002425
Penny ND, Byers GW (1979) A check-list of the Mecoptera of the world. Acta Amazon 9(2):365–388. https://doi.org/10.1590/1809-43921979092365
Pigino G, Migliorini M, Paccagnini E, Bernini F, Leonzio C (2005) Fine structure of the midgut and Malpighian papillae in Campodea (Monocampa) quilisi Silvestri, 1932 (Hexapoda, Diplura) with special reference to the metal composition and physiological significance of midgut intracellular electron-dense granules. Tissue Cell 37(3):223–232. https://doi.org/10.1016/j.tice.2005.02.001
Pinheiro DO, Conte H, Gregório EA (2008) Spherites in the midgut epithelial cells of the sugarcane borer parasitized by Cotesia flavipes. Biocell 32:61–67
Potter E (1938a) The internal anatomy of the order Mecoptera. Trans R Entomol Soc Lond 87(20):467–501. https://doi.org/10.1111/j.1365-2311.1938.tb00726.x
Potter E (1938b) The internal anatomy of the larvae of Panorpa and Boreus (Mecoptera). Proc R Entomol Soc Lond A 13(7–9):117–130. https://doi.org/10.1111/j.1365-3032.1938.tb00445.x
Ruiz-Sanchez E, O’Donnell MJ, Donini A (2015) Secretion of Na+, K+ and fluid by the Malpighian (renal) tubule of the larval cabbage looper Trichoplusia ni (Lepidoptera: Noctuidae). J Insect Physiol 82:92–98. https://doi.org/10.1016/j.jinsphys.2015.09.007
Santos HP, Rost-Roszkowska M, Vilimova J, Serrão JE (2017) Ultrastructure of the midgut in Heteroptera (Hemiptera) with different feeding habits. Protoplasma 254(4):1743–1753. https://doi.org/10.1007/s00709-016-1051-2
Setty LR (1940) Biology and morphology of some North American Bittacidae (Order Mecoptera). Am Midl Nat 23(2):257–353. https://doi.org/10.2307/2420667
Sumida S, Silva-Zacarin ECM, Decio P, Malaspina O, Bueno FC, Bueno OC (2010) Toxicological and histopathological effects of boric acid on Atta sexdens rubropilosa (Hymenoptera: Formicidae) workers. J Econ Entomol 103(3):676–690. https://doi.org/10.1603/EC09159
Talarico F, Brandmayr P, Giulianini PG, Ietto F, Naccarato A, Perrotta E, Tagarelli A, Giglio A (2014) Effects of metal pollution on survival and physiological responses in Carabus (Chaetocarabus) lefebvrei (Coleoptera, Carabidae). Eur J Soil Biol 61:80–89. https://doi.org/10.1016/j.ejsobi.2014.02.003
Tan J-L, Hua B-Z (2008) Morphology of immature stages of Bittacus choui (Mecoptera: Bittacidae) with notes on its biology. J Nat Hist 42(31–32):2127–2142. https://doi.org/10.1080/00222930802209775
Tan J-L, Hua B-Z (2009a) Description of the immature stages of Bittacus planus Cheng (Mecoptera: Bittacidae) with notes on its biology. Proc Entomol Soc Wash 111(1):111–121. https://doi.org/10.4289/0013-8797-111.1.111
Tan J-L, Hua B-Z (2009b) Terrobittacus, a new genus of the Chinese Bittacidae (Mecoptera) with descriptions of two new species. J Nat Hist 43(47–48):2937–2954. https://doi.org/10.1080/00222930903359628
Wang J-S, Hua B-Z (2017) An annotated checklist of the Chinese Mecoptera with description of male Panorpa guttata Navás, 1908. Entomotaxonomia 39(1):24–42
Wessing A, Zierold K, Polenz A (1999) Stellate cells in the Malpighian tubules of Drosophila hydei and D. melanogaster larvae (Insecta, Diptera). Zoomorphology 119(2):63–71. https://doi.org/10.1007/s004350050081
Wu G-X, Gao X, Ye G-Y, Li K, Hu C, Cheng J-A (2009) Ultrastructural alterations in midgut and Malpighian tubules of Boettcherisca peregrina exposure to cadmium and copper. Ecotoxicol Environ Saf 72(4):1137–1147. https://doi.org/10.1016/j.ecoenv.2008.02.017
Yu C-H (2003) Ultrastructure of the Malpighian tubule cells in the mosquito larvae, Anopheles sinensis. Entomol Res 33(3):151–159. https://doi.org/10.1111/j.1748-5967.2003.tb00064.x
Yue C, Hua B-Z (2013) Embryonic development of the alimentary canal of the scorpionfly Panorpa obtusa Cheng (Mecoptera: Panorpidae). Microsc Res Techn 76(5):457–466. https://doi.org/10.1002/jemt.22187
Zhang G-W, Hua B-Z (2014) Fine structure of the midgut of Sinopanorpa tincta (Navás) (Mecoptera: Panorpidae). Tissue Cell 46(5):388–396. https://doi.org/10.1016/j.tice.2014.07.002
Zhong H-Y, Zhang Y-L, Wei C (2015) Morphology and ultrastructure of the Malpighian tubules in Kolla paulula (Hemiptera: Cicadellidae). Zool Anz 257:22–28. https://doi.org/10.1016/j.jcz.2015.04.003
Acknowledgments
We thank Wei Du for the assistance in specimen collection, Ying Miao for the assistance in taking care of the egg and larval stages, and Qi-Hui Lyu and Ke-Rang Huang for the assistance in TEM.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 31372186).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This article does not contain any studies with animals and human participants performed by any of the authors.
Additional information
Handling Editor: Douglas Chandler
Rights and permissions
About this article
Cite this article
Liu, L., Hua, BZ. Ultrastructure of the larval Malpighian tubules in Terrobittacus implicatus (Mecoptera: Bittacidae). Protoplasma 255, 1121–1128 (2018). https://doi.org/10.1007/s00709-018-1221-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-018-1221-5