Abstract
The herald moths, Scoliopteryx libatrix, overwinter in hypogean habitats. The ultrastructure of their fat body (FB) cells and Malpighian tubule (MT) epithelial cells was studied by light microscopy and transmission electron microscopy, and essential biometric and biochemical measurements were performed. The FB was composed of adipocytes and sparse urocytes. The ultrastructure of both cells did not change considerably during this natural starvation period, except for rough endoplasmic reticulum (rER) which became more abundant in March females. In the cells, the reserve material consisted of numerous lipid droplets, glycogen rosettes, and protein granula. During overwintering, the lipid droplets diminished, and protein granula became laminated. The MTs consisted of a monolayer epithelium and individual muscle cells. The epithelial cells were attached to the basal lamina by numerous hemidesmosomes. The apical plasma membrane was differentiated into numerous microvilli, many of them containing mitochondria. Nuclei were surrounded by an abundant rER. There were numerous spherites in the perinuclear part of the cells. The basal plasma membrane formed infoldings with mitochondria in between. Nuclei were located either in the basal or in the central part of the cells. During overwintering, spherites were gradually exploited, and autophagic structures appeared: autophagosomes, autolysosomes, and residual bodies. There were no statistical differences between the sexes in any measured biometric and biochemical variables in the same time frames. The energy-supplying lipids and glycogen, and spherite stores were gradually spent during overwintering. In March, the augmented rER signified the intensification of synthetic processes prior to the epigean ecophase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During dormancy, insects reduce metabolic activity because of cessation of feeding (Hahn and Denlinger 2011). This natural starvation period represents stressful conditions. The herald moth, Scoliopteryx libatrix (Linnaeus 1758), is a widespread, holarctic member of Erebidae and Noctuoidea (Fibiger and Hacker 2005) that regularly overwinters and estivates in natural and artificial subterranean habitats, like caves, cellars, barns, and adits (Motas et al. 1967; Roeder and Fenton 1973; Bouvet et al. 1974; Bourne and Cherix 1978; Christian and Moog 1982; Novak et al. 2013). In terms of biomass, S. libatrix are, at least in central Europe, among the most important epigean invertebrates overwintering in subterranean habitats (Novak et al. 2012, 2013). There are two, partly overlapping generations; individuals of the first generation hatch between April and May and those of the second one between July and August (Bourne and Cherix 1978; Turquin 1994; Ziegler 2016). After the first autumn frost in November or December, individuals enter hypogean habitats and overwinter in quiescence at the entrance sections of caves. In March, they return to the epigean habitats and die in the late summer or early autumn (Motas et al. 1967; Roeder and Fenton 1973; Bouvet et al. 1974; Bourne and Cherix 1978; Christian and Moog 1982; Novak et al. 2013). Although generally known, the ultrastructure and cell processes in arthropods overwintering in caves have been sparsely studied (e.g., Bourne 1978; Lipovšek et al. 2011).
The fat body (in the following: FB) is the central, specialized organ for storing nutrients, composed mainly of adipocytes and the much less abundant urocytes (Arrese and Soulage, 2010). The adipocytes have storage, detoxification, excretory, and secretory functions (Arrese et al. 2001; Cohen 2003; Šobotnik et al. 2006). The urocytes sequester uric acid and other mineral compounds (Cohen 2003; Paes de Oliveira and Cruz-Landim 2003; Furtado et al., 2013). The FB is of great metabolic importance (Chapman 2008; Cohen 2003; Arrese and Soulages 2010), being where, e.g., the synthesis and utilization of energy-rich compounds (lipids and glycogen), protein synthesis, and nitrogen metabolism take place (Keeley, 1985; Arrese and Soulages 2010).
The Malpighian tubules (in the following: MTs) are distally blind-ended structures extending from the midgut–hindgut junction (Ballan-Dufrançais 2002). The epithelium of the MTs is composed of a single layer of cells (Martoja and Ballan-Dufrançais 1984). The MTs are excretory and osmoregulatory organs (Bradley 1985) that transport organic solutes, break down and remove toxic substances, convert waste metabolites into urine compounds, and maintain water and ionic balance and immune defenses (Bradley 2003; Chapman 2008; Dow 2009; Beyenbach et al. 2010).
Overwintering is a natural starvation period and for this reason is an appropriate ecophase for studying changes in selected organs of non-feeding insects. The FB and the MTs well represent these changes: the FB for its intensive support with energy-supplying compounds and the MTs for their excretion as the final metabolic process.
In this study, we focused on the ultrastructural changes to the FB and the MT cells because these cells encompass the energy metabolic processes on the whole body level. The natural starvation of S. libatrix during overwintering is an appropriate life cycle period for addressing this question; for this reason, the herald moth was chosen for this study. For this purpose, the ultrastructure of both organs was examined concurrently at the beginning, in the middle, and at the end of overwintering. Light microscopy was applied to localize the FB and the MTs and transmission electron microscopy (TEM) to study the ultrastructural characteristics in different time frames during overwintering. As in other insects, lipids were expected to be the main energy-storing compounds in starved S. libatrix. It was hypothesized that the lipid reserves in FB and the stored compounds in the spherites in MTs would decrease during overwintering.
Material and methods
Material
Specimens of S. libatrix were collected in the Špegličeva jama cave (N 46° 17′ 56″, E 15° 11′ 39″, 400 m a. s. l.) in central Slovenia in three time frames: at the beginning (end of November), in the middle (end of January), and at the end of overwintering (end of March). For each sampling time frame, five specimens of each sex were analyzed.
Methods
Light microscopy was used to localize the FB and the MTs. The ultrastructure of the cells was studied by TEM.
Light microscopy and transmission electron microscopy
The FB and the MTs were dissected into small fragments and fixed in 2.45% glutaraldehyde and 2.45% paraformaldehyde in a 0.1-M sodium cacodylate buffer (pH 7.4) at room temperature for 3 h and at 4 °C for 12 h. The tissue was washed in a 0.1-M sodium cacodylate buffer (pH 7.4) at room temperature for 4 h and postfixed with 2% OsO4 at room temperature for 2 h. They were washed in a 0.1-M sodium cacodylate buffer (pH 7.4) at room temperature for 3 h and dehydrated with a graded series of ethanol (50, 70, 90, 96, and 100%, each for 30 min at room temperature). The tissue pieces were embedded in TAAB embedding resin (Agar Scientific Ltd., Essex, England). For light microscopy, semi-thin sections (5 μm) were stained with 0.5% toluidine blue in aqueous solution. For TEM, ultra-thin sections (70–75 nm) of the tissue were transferred to copper grids, stained with uranyl acetate and lead citrate, and analyzed with a Zeiss EM 902 transmission electron microscope. For each time frame (the beginning, the middle, and the end of overwintering), the ultrastructure of the FB and the epithelial cells of the MTs were analyzed. To estimate the lipid loss, in randomly selected FB cells, the diameter of 100 lipid droplets was measured and the surface of the droplet cross sections was calculated in each time frame for each sex.
Quantification of reserve lipids and glycogen
Specimens were sacrificed by 2-h freezing at −20 °C. The dry mass was determined after 15 days of vacuum desiccation under P2O5. Lipids were extracted from the entire, macerated individuals via diethylether extraction (Kates 1991). After the extraction of lipids, the samples were quantified for glycogen with the anthrone reaction (Plummer 1987). Details are in Lipovšek et al. (2015).
Statistical analysis
Descriptive statistics was used to describe selected biometric and biochemical parameters. The t test was used in testing differences between males and females in each of the three time frames and one-way ANOVA (F test) in testing pairwise differences between the three time frames. The results were evaluated statistically using SPSS 21.0.
Results
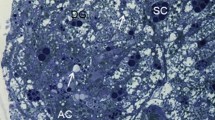
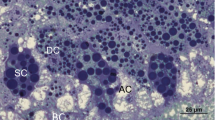
According to the light microscopy, in overwintering S. libatrix, the FB and the MTs were located mostly close to each other in the visceral hemocoel (Fig. 1a). The FB and many extensions of the MTs were seen in the close vicinity of the midgut (Fig. 1a, b). There were no differences between sexes in the structure of the FB and the MTs. The FB was composed of about 40 ribbon-like extensions. In the cross section, the extensions were round or oval, measuring about 400 μm at their longest, and up to 200 μm at their shortest diameter (Figs. 1a, b and 2a, b). The MTs consisted of about 40 oval extensions, measuring up to 350 μm at their longest, and up to 100 μm at their shortest diameter. The height of the epithelial cells measured about 10–45 μm. A brush border was characteristic of the apical part of the MT epithelial cells (Fig. 1a). The general appearance of the FB and the MTs analyzed by light microscopy was comparable for all three time frames: at the beginning (Fig. 1a), in the middle (Fig. 1b), and at the end of overwintering (Fig. 2a, b). The ultrastructural characteristics of the FB and MT cells and their changes are described below.
Beginning of overwintering (November)
The FB was characterized by many lipid droplets and was composed of numerous adipocytes and sparse urocytes (Fig. 3a–d). Both cells were randomly dispersed in the FB. The adipocytes contained numerous large lipid droplets with diameters up to 10 μm (Fig. 3a–d). Beside the lipid droplets, many protein granula (Fig. 3a) and glycogen rosettes were seen (Fig. 3b–d). The nucleus was in either the central or the basal part of the cell. In most adipocytes, the nucleus was irregularly shaped because of pressure from the lipid droplets and protein granula (Fig. 3a). The urocytes were smaller than the adipocytes and contained vacuoles with electron-dense urate granula, glycogen rosettes, sparse lipid droplets, and some mitochondria (Fig. 3d). The nuclei of the urocytes were irregularly shaped.
The MT consisted of a one-layer epithelium and individual muscle cells (Fig. 1a). On the basal surface of the epithelial cells, a prominent basal lamina was present (Fig. 4a, b). The epithelial cells were attached to it with numerous hemidesmosomes (Fig. 4b). On the basal lamina, tracheoles were seen (Fig. 4a). The basal cell membrane formed many infoldings; abundant mitohondria were present close to the infoldings. The central region of the epithelial cells was characterized by a large round or oval nucleus (Figs. 4a, b and 5a, b), well-developed rough endoplasmic reticulum (rER) (Fig. 4d), and numerous mitochondria and spherites (Figs. 4c and 5c). In the apical part of the epithelial cells, numerous mitochondria and spherites (Figs. 4e and 5d) were seen. Most spherites were composed of many electron-dense and electron-lucent concentric layers (Figs. 4a–d and 5a–c). The apical surface of the cells was differentiated into closely packed microvilli, up to 8 μm long. Many microvilli contained elongated mitochondria (Figs. 4e and 5d) for almost their whole length.
Malpighian tubules in November, male. a, b Basal part of the epithelial cell. BL basal lamina, FB fat body, H hemidesmosomes, M mitochondria, N nucleus, S spherites, T tracheole. Bar 2 μm. c, d Perinuclear part of the cell. N nucleus, rER rough endoplasmatic reticulum, S spherites. Bars c 1 μm; d 500 nm. e Apical part of the cell. M mitochondria, MV microvilli, S spherites. Bar 1 μm
Malpighian tubules in November, female. a, b Basal part of the epithelial cell. BL basal lamina, M mitochondria, N nucleus, S spherites. Bar 2 μm. c Perinuclear part of the cell. M mitochondria, N nucleus, S spherites. Bar 1 μm. Bar 500 nm. d Apical part of the cell. M mitochondria, MV microvilli, S spherites. Bar 500 nm
The middle of overwintering (January)
The FB was morphologically similar to that in the November specimens.
Except for spherites and autophagic structures, no significant changes in the general appearance of the MT epithelial cells were noticed with respect to the beginning of overwintering. Most spherites were composed of reduced number of concentric layers (Fig. 6a−c). In a few spherites, a small amount of flocculent material was present (Fig. 6b), while in others, the material had been completely exploited (Fig. 6a, b). In the cytoplasm of some cells, autophagosomes and residual bodies were seen (Fig. 6d).
Malpighian tubules in January. a, b Male. c, d Female. a, b Basal part of the epithelial cell. BL basal lamina, FB fat body, FM flocculent material, L lipid droplets, M mitochondria, N nucleus, S spherites. Bar 2 μm. c Perinuclear part of the cell. S spherites. Bar 500 nm. d Perinuclear part of the cell. AP autophagosome, M mitochondria, RB residual bodies. Bar 500 nm
The end of overwintering (March)
The adipocytes in the FB were structured as seen in November and January specimens, with a more electron-lucent cytoplasm (Fig. 7a). The amount of lipids diminished (Table. 1), and protein granula changed their appearance; the peripheral electron-dense material was disintegrated and had taken on a lamellar appearance (Fig. 7b). In the cytoplasm extensive rER (Fig. 7c), some mitochondria and glycogen rosettes (Fig. 7b) were present. The urocytes contained some vacuoles with electron-dense urate granula and remnants of the electron-dense material (Fig. 7d).
The general appearance of the MT epithelial cells (Figs. 8a−d and 9a−d) did not change with respect to those in the November and January specimens. Near the nucleus, some mitochondria, the exploited spherites (Figs. 8a, b and 9a, b), some cisternae of rER, and residual bodies were seen (Fig. 9c). Autophagic structures included autophagosomes, autolysosomes (Fig. 8d), and residual bodies (Figs. 8b, c and 9c, d). The basal cell membrane formed many infoldings (Fig. 9b); numerous mitohondria were seen in very close vicinity of the infoldings. The cells’ ultrastructural alterations were evident in the spherites. Most spherites were completely or nearly completely exploited; in some, only the spherite membranes could be seen (Figs. 8b, c and 9a, d). At the end of overwintering, the autophagic structures became more abundant compared to the beginning of overwintering.
Malpighian tubules in March, female. a–c Basal part of the epithelial cell. BL basal lamina, H hemidesmosomes, M mitochondria, N nucleus, RB residual body, rER endoplasmatic reticulum, S spherites, T tracheole, TC tracheal cell. d Apical part of the cell. M mitochondria, MV microvilli, S spherites, * residual body. Bars a, c, d 2 μm; b 500 nm
Quantification of reserve lipids and glycogen
The descriptive statistics for body size, fresh and dry mass, lipids per individual and lipids per dry mass, glycogen per individual and glycogen per dry mass, and water content in the three overwintering time frames are presented in Table 1. There were no statistical differences between the sexes in any biometric or biochemical variables in the same time frames; we therefore pooled the results for both sexes in each particular time frame.
In the pairwise comparison of the three overwintering time frames, significant differences were found at the beginning vs. the middle (p = 0.006) and vs. the end (p = 0.011) of overwintering (p = 0.016) in lipids per individual mass (F 2, 27 = 5.47; p = 0.010) and between the beginning and the end of the overwintering in lipids per dry mass (F 2, 27 = 3.44; p = 0.047). The lipid rates did not differ significantly between the middle and the end of overwintering (p > 0.05). During overwintering, lipid droplet cross sections were reduced (Fig. 10), revealing that the droplets were gradually being exploited.
The amounts of glycogen per individual mass at the beginning of overwintering were significantly larger than in the middle and the end of overwintering (F 2, 27 = 16.64; p < 0.001). The amounts of glycogen per dry mass differed significantly between all the three time frames (F 2, 27 = 12.75; p < 0.02).
Water rates augmented significantly from the beginning till the middle of overwintering (F 2, 27 = 3.54 p = 0.043) and diminished at the end.
Discussion
The FB of S. libatrix consisted, as in other insects (Polver et al. 1986; Šobotnik et al. 2006), of adipocytes and sparse urocytes. At the beginning of overwintering, adipocytes showed the ultrastructure of fed cells in the active, epigean ecophase. During overwintering, the amounts for lipids and glycogen were reduced, revealing that after the beginning of the hypogean ecophase, energy-supplying processes take place. Additionally, partly disintegrated protein granula probably supply compounds for the restoration of proteins engaged in the vital processes. An abundant rER, which appeared at the end of overwintering, revealed that intensive protein synthesis had restarted. In the same time frame, abundant rER was also found in the adipocytes of the fat body in the cave cricket Troglophilus neglectus (Lipovšek and Novak 2016). In urocytes, the urate granula were gradually exploited, characterized by a smaller amount of electron-dense compounds during overwintering.
In S. libatrix, at the beginning of the winter dormancy, sparse autophagic structures were observed, while their augmented numbers in the middle and the end of overwintering reveal intensified autophagic activity. During induced starvation, in the adipocytes of the fat body in Drosophila melanogaster, autophagic structures were also found (Scott et al. 2004), indicating that the released compounds were used in maintaining vital processes. The same findings apply to the FB cells in overwintering T. neglectus (Lipovšek and Novak 2016). Comparable processes were described in vertebrates (Yang and Klionsky 2010).
As in most other insects (Bradle, 1985; Kalender et al. 2002; Pal and Kumar 2013), the MTs in S. libatrix extend from the digestive tract at the midgut–hindgut junction. The MTs are uniformly shaped along their whole length and consist of a single epithelial cell type, as described for the lepidopteran Agrotis (Kalender et al. 2002). This is in contrast to the cave cricket T. neglectus, with MTs consisting of three MT regions and composed of two epithelial cell types (Lipovšek et al. 2009). In T. neglectus, the middle segment contains numerous type 1 cells and a few type 2 cells (Lipovšek et al. 2009). Two types of epithelial cells in the MTs have also been described in the dipterans Musca (Sohal 1974), Culex, and Anopheles (Yu 1999, 2003) and in the dipluran Campodea (Pigino et al. 2005). The ultrastructure of the epithelial cells in S. libatrix is quite comparable with that in Agrotis, with type 1 cells in T. neglectus, and with the principal epithelial cells in the dipterans. For all these excretive cells, their similar ultrastructure (Hazelton et al. 2001; Yu 1999, 2003; Lipovšek et al. 2009) suggests similar functioning in the species under analysis. Their apical plasma membrane is differentiated into microvilli, and the basal plasma membrane forms infoldings containing mitochondria. In the apical cytoplasm, there are abundant mitochondria, which often intrude into the microvilli. This suggests an important role for these cellular compounds in energy-supplying processes, such as active transport, in the apical plasma membrane.
At the beginning of overwintering, in the MT cells of S. libatrix, there were abundant, fully-formed spherites. These consisted of many tightly packed layers of electron-dense and electron-lucent material. In the middle of overwintering, the spherites were partly or fully exploited, and at the end of overwintering, most of them were completely exploited. A few spherites contained small amounts of remnant material. This indicates their role in supplying relatively intensive cell processes in the excretory cells during overwintering.
In the MTs, the autophagic activity was observed after the middle of overwintering. The autophagic structures observed were autophagosomes and autolysosomes. In a few cells, aggregates of electron-dense material, probably residual bodies, were seen. As in other invertebrates (Mizushima 2007; Mizushima and Klionsky 2007; Klionsky et al. 2012), monomers released from the autolysosomes function as essential compounds supporting cell survival in overwintering S. libatrix.
In accordance with our expectations, lipid and glycogen reserves differed significantly between the beginning and the end of overwintering. Both compounds were intensively depleted from the beginning till the middle of overwintering. Lipids were used up more intensively from the beginning till the middle of overwintering and less intensively from then till the end. From the beginning till the middle of overwintering, glycogen amounts per individual showed the same trend as lipids, while glycogen per dry mass decreased till the middle and was significantly augmented from the middle till the end of overwintering. This reveals that at the end of March, synthesis of glycogen reactivated, as is usual in arthropods at the end of winter dormancy (Hahn and Denlinger 2011; Lipovšek et al. 2015). S. libatrix overwinter in torpor, remaining insensitive to winter weather changes. Therefore, as expected, water content increased from the beginning till the middle of overwintering, mostly or completely owing to metabolic water production, but, unexpectedly, had decreased by the end of overwintering. This is probably due to specific circumstances in the overwintering habitat of the herald moths: cave walls and similar microhabitats in cave entrance sections. At the beginning of winter, these walls are wet, and the moths are often covered with droplets of condensed water; for both, moisture originates from the humid, warm outside air. During winter, the outside air becomes colder, and the relative humidity in the entrance sections often falls below 60% (own unpublished data), which in turn causes wall desiccation and passive loss of water in the animals sojourning on the walls. Animals in places with a winter air current blowing out from the inner, warmer, and more humid cave sections are not affected.
In conclusion, in the FB and the MTs in these starved S. libatrix collected from nature, we found no characteristics distinct from those of other dormant insects (Arrese and Soulages 2010; Lipovšek et al. 2011). As hypothesized, the lipid reserve in the FB and nutrient stores in the spherites in MTs decreased during overwintering. Counter to our expectations, the decreased water content at the end of overwintering is probably caused by the pervasive desiccation of the cave entrance sections during the winter. Lipid droplets, glycogen rosettes, spherites, and autophagic structures most conspicuously demonstrate the ultrastructural changes in dormant herald moths during winter.
References
Arrese EL, Soulages JL (2010) Insect fat body: energy, metabolism, and regulation. Annu Rev Entomol 55:207–225
Arrese EL, Canavoso LE, Jouni ZE, Pennington JE, Tsuchida K, Wells MA (2001) Lipid storage and mobilization in insects: current status and future directions. Insect Biochem Mol Biol 31:7–17
Ballan-Dufrancais C (2002) Localization of metals in cells of pterygote insects. Microsc Res Techniq 56:403–420
Beyenbach KW, Skaer H, Dow JAT (2010) The developmental, molecular, and transport biology of Malpighian tubules. Annu Rev Entomol 55:351–374
Bourne JD, Cherix D (1978) Note sur l'écophase souterraine de Triphosa dubitata L.(Lep., Geometridae) et Scoliopteryx libatrix L. (Lep., Noctuiidae). Bull Soc Vaudoise Sc Nat 354(74):147–156
Bouvet Y, Turquin M-J, Bornard C, Desvignes S, Notteghem P (1974) Étude des déclencheurs visuels intervenant lors de la pénétration souterraine de Scoliopteryx libatrix L. et Triphosa dubitata L. (Lépidoptères trogloxènes). Ann Spéléol 29(2):229–236
Bradle TJ (1985) The excretory system: structure and physiology. In: Kerkut GA, Chopard L (eds) La biologie des Orthoptères. Lechevalier, Paris, p 541
Bradley TJ (2003) Excretion. In: Resh VH, Cardé RT (eds) Encyclopaedia of insects. Academic Press, Elsevier Science, San Diego, pp 380–386
Chapman RF (2008) The insects: structure and function. Cambridge University Press, New York, pp 478–508
Christian E, Moog O (1982) Zur Frage der ökologischen Klassifikazion der Kavernikolen am Beispiel der Höhlen-Schmetterlinge Österreichs. Zool Anz 5(6):382–392
Cohen E (2003) Fat body. In: Resh VH, Cardé RT (eds) Encyclopedia of insects. Academic, Amsterdam, pp 407–409
Dow JAT (2009) Insights into the Malpighian tubule from functional genomics. J Exp Biol 212:435–445
Fibiger M, Hacker HH (2005) Systematic list of Noctuoidea of Europe. Esperiana Schwanfeld Bucherei zur Entomologie 11:93–205
Furtado WCA, Azevedo DO, Martins GF, Zanuncio JC (2013) Histochemistry and ultrastructure of urocytes in the pupae of the stingless bee Melipona quadrifasciata (Hymenoptera: Meliponini). Microsc Microanal 19:1502–1510
Hahn DA, Denlinger DL (2011) Energetics of insect diapause. Annu Rev Entomol 56:103–121
Hazelton SR, Felgenhauer BE, Spring JH (2001) Ultrastructural changes in the Malpighian tubules of the house cricket, Acheta domesticus, at the onset of diuresis: a time study. J Morphol 247:80–92
Kalender Y, Kalender S, Candan S (2002) Fine structure of Malpighian tubules in the Agrotis segetum (Lepidoptera: Noctuidae) pupae. Acta Zool Bulg 54:87–96
Kates M (1991) Techniques in Lipidology. Elsevier, Amsterdam
Keeley LL (1985) Physiology and biochemistry of the fat body. Structure of the fat body. In: Kerkut, GA, Gilbert LI (Eds.), Comprehensive insect physiology and pharmacology. Integument, respiration and circulation 3. Pergamon Press, Oxford, pp. 211–248
Klionsky DJ, Abdalla FC, Zuckerbraun B (2012) Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 8(4):445–544
Lipovšek S, Novak (2016) Autophagy in the fat body cells of the cave cricket Troglophilus neglectus Krauss, 1878 (Rhaphidophoridae, Saltatoria) during overwintering. Protoplasma 253:457–466
Lipovšek S, Letofsky-Pabst I, Novak T, Hofer F, Pabst MA (2009) Structure of the Malpighian tubule cells and annual changes in the structure and chemical composition of their spherites in the cave cricket Troglophilus neglectus Krauss, 1878 (Rhaphidophoridae, Saltatoria). Arthropod Struct Dev 38:315–327. doi:10.1016/j.asd.2009.02.001
Lipovšek S, Novak T, Janžekovič F, Pabst MA (2011) Role of the fat body in the cave crickets Troglophilus cavicola and Troglophilus neglectus (Rhaphidophoridae, Saltatoria) during overwintering. Arthropod Struct Dev 40(1):54–63
Lipovšek S, Novak T, Janžekovič F, Leitinger G (2015) Changes in the midgut diverticula in the harvestmen Amilenus aurantiacus (Phalangiidae, Opiliones) during winter diapause. Arthropod Struct Dev. doi:10.1016/j.asd.2014.12.002
Martoja R, Ballan-Dufrançais C (1984) The ultrastructure of the digestive and excretory organs. In: King RC, Akai H (eds) Insect ultrastructure. Plenum, New York, pp 199–268
Mizushima N (2007) Autophagy: process and function. Genes Dev 21:2861–2873
Mizushima N, Klionsky DJ (2007) Protein turnover via autophagy: implications for metabolism. Annu Rev Nutr 27:19–40
Motas C, Decou V, Burghele A (1967) Sur l’association pariétale des grottes d’Oltenie (Roumanie). Ann Spéléol 22(3):475–522
Novak T, Perc M, Lipovšek S, Janžekovič F (2012) Duality of terrestrial subterranean fauna. Int J Speleol 41(2):181–188
Novak T, Janžekovič F, Lipovšek S (2013) Contribution of non-troglobiotic terrestrial invertebrates to carbon input in hypogean habitats. Acta Carsologica 42:301–309
Paes de Oliveira VT, Cruz-Landim C (2003) Morphology and function of insect fat body cells: a review. Biociências, Porto Alegre 11:195–205
Pal R, Kumar K (2013) Malpighian tubules of adult flesh fly, Sarcophaga ruficornis fab. (Diptera: Sarcophagidae): an ultrastructural study. Tissue Cell 45(5):312–317
Pigino G, Migliorini M, Paccagnini E, Bernini F, Leonzio C (2005) Fine structure of the midgut and Malpighian papillae in Campodea (Monocampa) quilisi Silvestri, 1932 (Hexapoda, Diplura) with special reference to the metal composition and physiological significance of midgut intracellular electron-dense granules. Tissue Cell 37:223–232
Plummer DT (1987) An introduction to practical biochemistry. McGraw-Hill, London
Polver PD, Sacchi L, Grigolo A, Laudani U (1986) Fine structure of the fat body and its bacteroids in Blattella germanica (Blattodea). Acta Zool 67:63–71
Roeder KD, Fenton MB (1973) Acoustic responsiveness of Scoliopteryx libatrix (Lepidoptera, Noctuidae), a moth that shares hibernacula with some insectivorous bats. Canad J Zool 51(7):681–685
Scott RC, Schuldiner O, Neufeld TP (2004) Role and regulation of starvation-induced autophagy in the Drosophila fat body. Develop Cell 7:167–178
Šobotnik J, Weyda F, Hanus R, Cvačka J, Nebesáŕová J (2006) Fat body of Prorhinotermes simplex (Isoptera: Rhinotermitidae): ultrastructure, inter-caste differences and lipid composition. Micron 37:648–656
Sohal RS (1974) Fine structure of the Malpighian tubules in the housefly, Musca domestica. Tissue Cell 6:719–728
Turqiun M-J (1994) Lepidoptera. In: Encyclopaedia biospeologica I. Société de Biospéologie. Moulis (CNRS) and Bucarest (Academie Roumaine), pp. 333–339
Yang Z, Klionsky DJ (2010) Eaten alive: a history of macroautophagy. Nature Cell Biol 12(9):814–822
Yu CH (1999) Ultrastructure of the Malpighian tubule cells in the mosquito larvae, Culex pipiens pallens. Korean J Entomol 29:141–147
Yu CH (2003) Ultrastructure of the Malpighian tubule cells in the mosquito larvae, Anopheles sinesis. Korean J Entomol 33:151–159
Ziegler H (2016) Scoliopteryx libatrix (Linnaeus, 1767). In: Ziegler. H., Schmetterlinge der Paläarktischen Region. http://www.euroleps.ch/index.php Accessed 27.11.2016
Acknowledgements
We would like to thank Elisabeth Bock und Rudi Schmied (Medical University Graz) for their excellent technical assistance. Michelle Gadpaille valuably improved the English of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Handling Editor: Douglas Chandler
Rights and permissions
About this article
Cite this article
Lipovšek, S., Janžekovič, F. & Novak, T. Ultrastructure of fat body cells and Malpighian tubule cells in overwintering Scoliopteryx libatrix (Noctuoidea). Protoplasma 254, 2189–2199 (2017). https://doi.org/10.1007/s00709-017-1110-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-017-1110-3