Abstract
Drought is an important factor limiting plant development and crop production. Dissecting the factors involved in this process is the key for enhancement of plant tolerance to drought stress by genetic approach. Here, we evaluated the regulatory function of a novel rice ethylene response factor (ERF) OsERF109 in drought stress. Expression of OsERF109 was rapidly induced by stress and phytohormones. Subcellular localization and transactivation assay demonstrated that OsERF109 was localized in nucleus and possessed transactivation activity. Transgenic plants overexpressing (OE) and knockdown with RNA interfering (RI) OsERF109 exhibited significantly reduced and improved drought resistance, respectively, indicating that OsERF109 negatively regulates drought resistance in rice. Furthermore, measurement by gas chromatography showed that ethylene contents were less in OE while more in RI lines than these in wild types, supporting the data of drought tolerance and water loss in transgenic lines. Quantitative real-time PCR analysis also proved the regulation of OsERF109 in the expression of OSACS6, OSACO2, and OsERF3, which have been identified to play important roles in ethylene biosynthesis. Based on these results, our data evidence that OsERF109 regulates drought resistance by affecting the ethylene biosynthesis in rice. Overall, our study reveals the negative role of OsERF109 in ethylene biosynthesis and drought tolerance in rice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a primary limitation to plant growth and crop production, drought greatly threatens sustainable agriculture worldwide. To survive from drought stress, plants have developed numbers of physiological and biochemical mechanisms (Shinozaki and Yamaguchi-Shinozaki 2007). For instance, under drought condition, the root of plants will grow deeper and possess lower water potential so as to absorb more water from soil (Wilkinson and Davies 2002). Drought induces the accumulation of endogenous hormones abscisic acid (ABA) and leads to the closure of stoma to reduce the loss of water (Schroeder et al. 2001). The phytohormone ethylene also has been reported to participate in drought response. For instance, overexpression of OsERF3 confers the decrease of ethylene production and the sensitive phenotype to drought, and treatment of ethylene precursors 1-aminocyclopropane-1-carboxylate (ACC) recovered the drought phenotype of transgenic lines, revealing a positive regulatory function of ethylene on drought resistance (Wan et al. 2011; Zhang et al. 2013). The transcription factors Hahb-4 in Helianthus and RAP2.4 in Arabidopsis both regulate the cross-talk between drought stress and ethylene signaling pathway (Lin et al. 2008; Manavella et al. 2006). Rice is a worldwide crop and staple for more than half of the world population. As a semi-aquatic plant, rice can endure a certain degree of salt and acid stress but is very sensitive to drought (Todaka et al. 2012). Thus, it is particularly significant to explore the complex mechanisms of rice drought resistance, further helping the breeding of new rice varieties that possess higher tolerance to drought stress.
Transcriptional regulatory pathway is an effective means for plant to adapt the changeful environments (Bhatnagar-Mathur et al. 2008). Ethylene response factor (ERF) proteins, which belong to the plant specific AP2/ERF transcription factor superfamily, play numbers of roles in plant growth and development, especially in stress response (Boutilier et al. 2002; Joo et al. 2013; Nakano et al. 2006; Onate-Sanchez et al. 2007; Riechmann et al. 2000; Stockinger et al. 1997; Wang et al. 2007). For example, Arabidopsis ERF022 regulates the induction of somatic embryogenesis through ethylene pathway (Nowak et al. 2015). SUB1A-1 improves the tolerant to submergence by suppressing rice shoot elongation (Xu et al. 2006), whereas SNORKEL1 and SNORKEL2 promote the gibberellin acid (GA) biosynthesis to rapidly elongate rice stem so as to avoid submergence (Hattori et al. 2009). Moreover, ERF proteins regulate drought stress in various crops, such as rice, tobacco, sugarcane, and wheat (Oh et al. 2009; Rong et al. 2014; Trujillo et al. 2008; Wu et al. 2008), indicating that ERF family is an abundant source for stress response genes that could be used to improve plant stress resistance by genetic engineering (Umezawa et al. 2006).
OsERF109 (LOC_Os09g0309700) can be classified into group VI-L that only contains three members because of the characteristic features with conserved motifs of CMVI-1 and CMVI-2 (Nakano et al. 2006). Even among the group VI-L, OsERF109 is quite different from other factors in terms of the sequence homology (24.93 % identity with OsERF108 and 8.49 % identity with OsERF138). Compared with other ERF proteins, OsERF109 only has 12.85 % identity with OsERF1. Furthermore, OsERF109 is up-regulated by at least 4 abiotic treatments based on microarray analysis (Sharoni et al. 2011). Although OsERF109 is considered as a stress-related transcription factor, the regulatory function of this novel protein is largely unknown. In this study, through analyzing the basic characters of OsERF109, we identified the function of this transcription factor in drought stress, possibly through activating the expression of OsERF3, which further repressing ethylene biosynthesis genes ACS6 and ACO2.
Materials and methods
Plant materials and drought treatment
Two rice cultivars Oryza sativa L. cv. Nipponbare (Nip) and Zhonghua 17 (Zh17) were used in this study. All the rice seeds were germinated at 30 °C for 2 days, then transplanted to the growth chamber at 60 % relative humidity with 16 h white light (50 μmol/m2/s)/8 h dark at 26 °C. For drought assay, 3-week-old seedlings under normal growth conditions were subjected to continuous drought by stopping water supply. The photos were taken when the different drought phenotypes between transgenic plants and wild types were observed (drought treatment for 5–7 days). After all seedling leaves showed certain degrees of rolled and wilted phenotypes (drought treatment for about 9 days), the water supply was recovered and the survival rates were scored at 7 days after the recovery.
Subcellular localization
To confirm the subcellular localization of OsERF109 protein, the coding sequence of OsERF109 was amplified from the cDNA library by PCR with the specific primers (Table S1). Then, the amplification product was fused with GFP in the transient expression vector pAN580-GFP with the control of CaMV 35S promoter (Nelson et al. 2007). The plasmids were transformed into rice protoplasts as previously described (Chen et al. 2006). GFP fluorescence was observed by the confocal fluorescence microscope LSM 700 (Zeiss, Germany).
Transactivation assay
The coding sequence of OsERF109 was amplified and fused in frame to the pGBKT7 (DNA-binding domain vector, Clontech, USA), resulting in pGBKT7-OsERF109. pGBKT7-53 + pGADT7-T and pGBKT7-Lam (Clontech, USA) were used as positive and negative control, respectively. All the plasmids were transformed into yeast strain Y2HGold by using lithium acetate method as described in the manufacturer. The transformed strains were covered on medium without tryptophan (SD/-Trp), and the transactivation activity was evaluated by the activity of α-galactosidase.
β-glucuronidase (GUS) transient assay
The coding sequence of OsERF109 was amplified and fused in frame to the pCAMBIA1307 with the control of CaMV 35S promoter resulting in the effector vector. The CaMV 35S promoter in pBI121 was replaced with the promoter of OsERF3, OsACS6, or OsACO2, respectively, yielding the reporter vector of pOsERF3-GUS, pOsACS6-GUS, and pOsACO2-GUS. GCC box (GCC), and DRE reporter vectors were previously generated (Zhang et al. 2005). The reporter and effector were co-transformed into tobacco leaves by Agrobacterium. GUS activity was evaluated as previously described (Yang et al. 2000). The 35S minimal promoter (−46 bp) from pBI121 was used as negative control.
Generation of transgenic rice
To get the transgenic lines of OsERF109, the coding sequence of OsERF109 was inserted into pCAMBIA1307 and an RNA interference approach was used to knockdown OsERF109. For RNA interference, we used the C-terminus of OsERF109 because this region is less conserved among ERF family proteins (Nakano et al. 2006). The recombinant plasmids were transformed into rice Zh17 or Nip by Agrobacterium. The transgenic lines with overexpression of OsERF109 were indicated as OE, and reduced expression of OsERF109 was indicated as RI, respectively. The numbers were used to denote different transgenic lines.
Leaf water loss assay
The water loss assay was conducted according to previously report (Wang et al. 2012) with minor modifications. The second expanded leaves of 3-week-old seedlings were picked off and soaked in the double-distilled water for 2 h. Then, the detached leaves were placed on plastic plates, abaxial side up, at room temperature. The weight of detached leaves was measured every 30 min. Leaf water loss was indicated as a percentage of the original weight. Fifteen seedlings of each line were chosen randomly and repeated for three times.
RNA extraction and quantitative real-time PCR (qPCR)
Two-week-old seedlings were removed from soil and washed with water. Then, the seedlings were placed into nutrient solutions with different treatments (200 mM NaCl, 50 μM ACC, and 50 μM ABA) for the indicated time. For the drought treatment, the seedlings were placed in air without water. Total RNA was extracted from the seedling leaves with the indicated treatments by using TRIzol reagent (Invitrogen, CA). The cDNA was reverse transcribed from 5 μg of total RNAs by the M-MLV reverse transcriptase (Promega, WI). Gene expression was measured by qPCR analysis using SYBR Premix (Takara, Japan) in IQ-5 Real-Time system (Bio-Rad, USA). The expression levels were normalized to rice Actin1. The primers used for qPCR are listed in Table S1.
Measurement of ethylene production
Ethylene contents of rice were measured by gas chromatograph (Shimadzu, Japan) as previously report (Zhang et al. 2013). Twenty seeds were surface-sterilized by sodium hypochlorite and sown in 400-ml glass bottles with Murashige and Skoog (MS) medium. After cultured at 25 °C in a growth chamber for 7 days, all the bottles were sealed for 24 h. Then, 1 mL of gas was taken by air-tight syringe to determine the ethylene emission.
Results
The expression of OsERF109 is detected in different tissues and is inducible by diverse treatments
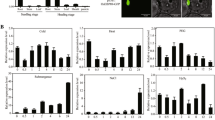
Firstly, we detected the expression of OsERF109 in different rice tissues using qPCR. Under normal growth conditions, our data showed that the transcripts of OsERF109 were highest in leaf no matter at seedling or heading stage among different tissues (Fig. 1a), suggesting that OsERF109 might mainly function in leaf. Then, the expression level of OsERF109 in leaf was further analyzed with the stress and phytohormone treatments. The results showed that the expression of OsERF109 was gradually induced by drought within 1 h and rapidly stimulated by salt stress within 0.5 h (Fig. 1b, c). Similar to the induction of drought stress, treatments of phytohormone ABA and ethylene precursor ACC greatly promoted the expression of OsERF109 within 1 h (Fig. 1d, e). Both the ABA and ethylene are key regulators in the response to abiotic stresses (Wilkinson et al. 2010); thus, the rapid increase of OsERF109 transcripts under stresses and phytohormone treatments indicates that OsERF109 might play a significant role in the response to abiotic stresses.
Expression of OsERF109 is detected in different tissues and is induced by various treatments. The expression of OsERF109 in different tissues of Nipponbare seedling or heading stage (a), in response to drought (b), 200 mM NaCl (c), 50 μM ABA (d), and 50 μM ACC (e). Distribution of stress-related cis-acting regulatory elements in the putative promoter region (f) (∼2000 bp) of OsERF109. Transcripts of OsERF109 are indicated relative to the expression level of the root at seedling stage (a) or of the 0 h in treatments (b–e). Error bars (±SD) are deviated from three independent biological experiments
To further predict the function of OsERF109 in abiotic stresses, we checked its putative promoter sequence (−2000 bp) using plant cis-acting regulatory DNA element database (http://www.dna.affrc.go.jp/PLACE/signalscan.html, Higo et al. 1999). As expected, stress relevant elements, including ABRE (ABA-responsive element), DRE/CRT (dehydration responsive element), GCC box, etc., were found in the putative promoter of OsERF109 (Fig. 1f). These elements can be recognized by the AREB, DREB, ERF, and MYB transcription factors, which play important roles in both phytohormone signaling and abiotic stress resistance (Agarwal et al. 2006; Dubos et al. 2010; Fujita et al. 2005; Lorenzo et al. 2004; Mizoi et al. 2012). Thus, the presence of stress-related elements in OsERF109’s promoter, combined with previous reports (Rashid et al. 2012; Sharoni et al. 2011) and our experimental results, suggests that OsERF109 might have multiple functions in abiotic stresses.
OsERF109 exhibits transcriptional activation
Since OsERF109 might act in the abiotic stresses as a transcription factor, the nuclear localization and transactivation activity should be necessary. To determine the subcellular localization of OsERF109, the GFP vector and GFP-OsERF109 fusion plasmid were transiently transformed into rice protoplasts respectively. Observations with the confocal fluorescence microscope showed that the fluorescence of GFP-OsERF109 was only located in the nucleus, while the signal of GFP distributed in all the protoplast (Fig. 2a), revealing that OsERF109 is localized in nucleus. To confirm the transactivation activity of this nucleus-localized protein, the fusion pGBKT7-OsERF109 and control plasmids were transformed into yeast strain Y2HGold and then covered on SD/-Thr selection media with the supplement of X-α-Gal. By detection of the activity of X-α-Gal with the color change, the colonies of positive control and pGBKT7-OsERF109 presented blue, while those of negative control did not change the color (Fig. 2b), indicating that OsERF109 possesses transactivation activity.
OsERF109 is localized in the nucleus and possesses transactivation activity. a Rice protoplast cell expressing the GFP (upper panel) and OEERF109–GFP (down panel) fusion protein. The same cell was stained by DAPI to indicate the nucleus positions. Bars indicate 5 μm. b Transcriptional activation of OsbERF109 in yeast. pGBKT7-p53 + pGBKT7-T and pGBKT7-Lam were indicated as positive and negative control and the transcriptional activation was evaluated by the activity of α-galactosidase. c Transient assay of the interaction between OsERF109 and cis-element GCC box (GCC) or DRE. Constructs of the reporter and effector are shown in the upper panel. Mini indicates the reporter containing 35S minimal promoter fused with GUS. Agrobacterium containing the effector and reporter were co-infiltrated into tobacco leaves. GUS activity is indicated relative to the control of Mini taken as 1. (−) indicates negative control of effector with empty pCAMBIA1307 vector. Error bars (±SD) are according to three independent biological experiments
Increasing researches reveal that ERF proteins can bind to GCC box and DRE sequence (Zhang et al. 2005; Wu et al. 2008). We then preformed transient assay in tobacco to verify whether OsERF109 interacts with these motifs. The results showed that expression of OsERF109 strongly activated the GUS reporter controlled by GCC box or DRE, which indicated that OsERF109 could interact with the GCC box and DRE sequence in stress-related genes promoter to active their expression and regulate drought tolerance (Fig. 2c).
OsERF109 negatively regulates drought tolerance
Due to the rapid expression of OsERF109 under drought stress (Fig. 1b), the function of OsERF109 in drought tolerance was first examined by using the OsERF109 overexpressors (OE) and RNA interference plants (RI). qPCR analysis showed that the expression level of OsERF109 obviously increased in OE plants and decreased to 30–45 % in RI transgenic lines (Fig. 3a, b). Further detection revealed that RNA interference of OsERF109 in RI lines did not affect the transcripts of its homologue genes, such as OsERF108, OsERF138, Os03g0730400, Os08g0557200, and Os08g0480800 (Fig. S1). In addition, the expressions of OsERF109 in the OE and RI transgenic plants did not obviously affect the growth during seedling and mature stage under normal growth conditions (control in Fig. 3c, d, Fig. S2). However, with the treatment of drought for 5 days, the OE lines showed to be more sensitive to drought stress than the wild-type Zh17, exhibiting a rolled and wilted leaf phenotypes. After the recovery of water, 20–30 % of OE transgenic plants were survived, obviously less than the survival rate in Zh17 (about 65 %, Fig. 3e). Oppositely, the RI lines displayed a drought tolerant phenotype. After the drought treatment for 7 days, most of the wild-type Nip seedlings showed rolled and wilted leaf phenotypes while RI lines still grew well. The survival rates of RI transgenic plants were approximately 2.5-fold higher than the Nip after the recovery of water (Fig. 3f), suggesting that RI transgenic plants were more tolerant to drought stress than the wild type. The drought response of OE and RI transgenic lines indicates that OsERF109 is a negative regulator in drought resistance.
OsERF109 negatively regulates the drought response in rice. The expression of OsERF109 in OE (a) and RI (b) transgenic lines. Transcripts of OsERF109 are indicated relative to the expression level of the wild type Zh17 or Nip respectively. The phenotype of OE (c) and RI (d) transgenic lines under drought stress. Photos were taken as the “Control” without the drought treatment. The 3-week-old seedlings were subjected to continuous drought by stopping water supply, and photos were taken when obvious different phenotypes between transgenic plants and wild types was observed (fifth day at left panel and seventh day at right panel). Survival rates of the seedlings after water recovery (e, f). The survival rates were indicated by the percentage of recovered seedlings compared to the total treated seedlings. P values were calculated with a Student’s t test (**P < 0.01). Error bars (± SD) are according to three independent biological experiments
OsERF109 affects the water loss and ethylene emission
To explain how OsERF109 regulates drought response, the statistical analysis of water loss was conducted by using dissected leaves of transgenic lines. Our analysis showed that water loss was faster in OE plants (Fig. 4a), but less in RI lines (Fig. 4b), than that in the wild type, indicating that OsERF109 might regulate the leaf water retention ability, consistent with the high expression of OsERF109 in the leaf.
OsERF109 affects water loss in the dissected leaves. Water loss rate in dissected leaves of 3-week-old seedling in OE (a) and RI (b) transgenic lines. Water loss of dissected leaves was indicated as a percentage of the original weight. Error bars (±SD) are according to three independent biological experiments
It has been reported that ERF proteins play important roles in ethylene production (Li et al. 2011; Wan et al. 2011; Zhang et al. 2013); we then detected the ethylene emission in OsERF109 transgenic lines. The results showed that there was less ethylene production in OE lines and more in RI lines compared with the wild types (Fig. 5a, b), supporting the positive regulation of ethylene on drought tolerance in rice (Wan et al. 2011; Zhang et al. 2013). In order to obtain further insights into the mechanism leading to the changed ethylene emission in transgenic plants, we analyzed the expression level of ethylene biosynthesis-related genes OsACS6 and OsACO2 in wild types and transgenic plants. As expected, OsACS6 and OsACO2 were decreased in OE while increased in RI compared with this in wild types, consistent with the ethylene emission in transgenic plants (Fig. 5c, d). The down-regulation of OsACS6 and OsACO2 by the transcriptional activator OsERF109 suggests a complicated relationship. Interestingly, the expression level of OsERF3, a repressor in the expression of OsACS6, OsACO2, and in ethylene biosynthesis (Wan et al. 2011; Zhang et al. 2013), was higher in OE but lower in RI than this in wild types. It appears that OsERF3 fills the gap between OsERF109 and ethylene biosynthesis-related genes. Furthermore, the transient assay proved that OsERF109 could bind to promoter areas of OsERF3, but not those of OsACS6 and OsACO2, to active the reporter (Fig. 5e). These results suggest that OsERF109 negatively regulates ethylene production through activating the repressor of ethylene biosynthesis OsERF3.
OsERF109 restrains the expression of ethylene biosynthesis genes and the production of ethylene. Ethylene emission in OE (a) and RI (b) transgenic lines. P values were calculated with a Student’s t test (*P < 0.05). The expression of OsACS6, OsACO2, and OsERF3 in transgenic lines of OE (c) and RI (d). Transcripts are indicated relative to the expression level of the wild-type Zh17 or Nip respectively. Error bars (±SD) are according to three independent biological experiments. Transient assay of the interaction between OsERF109 and promoter of OsERF3 (−2014 bp), OsACS6 (−2051 bp), or OsACO2 (−2062 bp) (e). Constructs of the reporter and effector are shown in the upper panel. GUS activity is indicated relative to the control taken as 1 (pOsERF3 reporter with empty pCAMBIA1307). Error bars (±SD) are according to three independent biological experiments
Discussion
Drought frequently occurs worldwide and causes severe damage to crop production. To survive from the extreme conditions, plants have evolved complex signaling pathways (Shinozaki and Yamaguchi-Shinozaki 2007). As an important means, plants can promote the expression of many proteins that help them to resist the drought stresses. Among these proteins, transcriptional regulation is one kind of the factors participating in this process. For example, ERF proteins play crucial roles in the regulation of gene expression involved in abiotic stress resistance (Shinozaki et al. 2003; Shinozaki and Yamaguchi-Shinozaki 2007; Zhu 2002). In the present study, we evidenced that the overexpression of OsERF109 decreased, whereas in knockdown this gene promoted the drought tolerance in rice. Interestingly, the ethylene production was oppositely changed in OE and RI transgenic lines compared with this in wild type. Importantly, OsERF109 does not affect the rice normal growth and development. Therefore, OsERF109 might be a potential factor for genetic engineering to improve the tolerance of rice to drought stress.
It has been evidenced that OsERF3 represses the expression of ethylene biosynthesis gene OsACS6 and OsACO2, downstream of OsDERF1 (Wan et al. 2011; Zhang et al. 2013). Similar to this regulation, we find in this study that OsERF109 directly binds to the promoter of OsERF3 that contains 6 GCC-box and 2 DRE. Thus, it is possible that OsERF109 directly activates the OsERF3 transcription, which further suppresses the expression of ethylene biosynthesis related genes OsACS6 and OsACO2.
OsERF109 is classified as a member of the group VI-L ERF proteins in rice (Nakano et al. 2006). Although OsERF109 contains an imperfect AP2/ERF domain, it possesses typical transcription factor characteristics, i.e., nuclear localization and transactivation activity. In addition, the expression of OsERF109 was quickly induced by drought, NaCl, ACC, and ABA treatment. It should be possible that OsERF109 might participate in multiple abiotic stress responses at transcriptional level. Further researches with transgenic plants overexpressing or interfering OsERF109 demonstrate that OsERF109 negatively regulates plant drought resistance, through repression of ethylene emission, consistent with the positive regulatory role of ethylene in drought resistance (Wan et al. 2011; Zhang et al. 2013). Thus, combined with the transcriptional modulation of OsERF109 in the expression of ethylene biosynthesis-related genes, and the inducible expression to ethylene precursor ACC, OsERF109 may act as a switch during the drought tolerance to avoid excessive ethylene production.
Overall, this study reveals the function of a novel ERF protein OsERF109 in rice, mediating negative regulation in rice ethylene production and drought tolerance. It is worth noting that the expression of OsERF109 was induced by different stress conditions. There is a probability that the OsERF109 possesses more broad function in the abiotic stress response. It also suggests that OsERF109 regulates the water-retention ability of leaves because of the different water loss rate observed between the transgenic plants and wild type, but the detailed molecular mechanism is unclear. Nevertheless, the fact that RI lines of OsERF109 significantly improved the tolerance to drought indicates the potential application of OsERF109 to increase rice tolerance to abiotic stress.
Abbreviations
- ABA:
-
Abscisic acid
- ABRE:
-
ABA-responsive elements
- ACC:
-
1-Aminocyclopropane-1-carboxylic acid
- AP2:
-
APETALA2
- CaMV:
-
Cauliflower mosaic virus
- cDNA:
-
DNA complementary to RNA
- CRT:
-
C-repeat
- DRE:
-
Dehydration responsive element
- DREB:
-
Dehydration responsive element binding factor
- ERF:
-
Ethylene response factor
- GA:
-
Gibberellic acid
- GFP:
-
Green fluorescent protein
- GUS:
-
β-Glucuronidase
- MS:
-
Murashige and Skoog
- MYBRS:
-
MYC recognition site
- MYCRS:
-
MYC recognition site
- qPCR:
-
Quantitative real-time polymerase chain reaction
- Trp:
-
Tryptophan
References
Agarwal PK, Agarwal P, Reddy MK, Sopory SK (2006) Role of DREB transcription factors in abiotic and biotic stress tolerance in plants. Plant Cell Rep 25:1263–1274
Bhatnagar-Mathur P, Vadez V, Sharma KK (2008) Transgenic approaches for abiotic stress tolerance in plants: retrospect and prospects. Plant Cell Rep 27:411–424
Boutilier K, Offringa R, Sharma VK et al (2002) Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell 14:1737–1749
Chen S, Tao L, Zeng L, VEGA-SANCHEZ ME, Umemura K, WANG GL (2006) A highly efficient transient protoplast system for analyzing defence gene expression and protein–protein interactions in rice. Mol Plant Pathol 7:417–427
Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L (2010) MYB transcription factors in Arabidopsis. Trends Plant Sci 15:573–581
Fujita Y, Fujita M, Satoh R et al (2005) AREB1 is a transcription activator of novel ABRE-dependent ABA signaling that enhances drought stress tolerance in Arabidopsis. Plant Cell 17:3470–3488
Hattori Y, Nagai K, Furukawa S et al (2009) The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature 460:1026–1030
Higo K, Ugawa Y, Iwamoto M, Korenaga T (1999) Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res 27:297–300
Joo J, Choi HJ, Lee YH, Kim YK, Song SI (2013) A transcriptional repressor of the ERF family confers drought tolerance to rice and regulates genes preferentially located on chromosome 11. Planta 238:155–170
Li Z, Zhang L, Yu Y, Quan R, Zhang Z, Zhang H, Huang R (2011) The ethylene response factor AtERF11 that is transcriptionally modulated by the bZIP transcription factor HY5 is a crucial repressor for ethylene biosynthesis in Arabidopsis. Plant J 68:88–99
Lin R, Park H, Wang H (2008) Role of Arabidopsis RAP2.4 in regulating light-and ethylene-mediated developmental processes and drought stress tolerance. Mol Plant 1:42–57
Lorenzo O, Chico JM, Sanchez-Serrano JJ, Solano R (2004) JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell 16:1938–1950
Manavella PA, Arce AL, Dezar CA, Bitton F, Renou JP, Crespi M, Chan RL (2006) Cross-talk between ethylene and drought signalling pathways is mediated by the sunflower Hahb-4 transcription factor. Plant J 48:125–137
Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K (2012) AP2/ERF family transcription factors in plant abiotic stress responses. Biochim Biophys Acta 1819:86–96
Nakano T, Suzuki K, Fujimura T, Shinshi H (2006) Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol 140:411–432
Nelson BK, Cai X, Nebenführ A (2007) A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J 51:1126–1136
Nowak K, Wojcikowska B, Gaj MD (2015) ERF022 impacts the induction of somatic embryogenesis in Arabidopsis through the ethylene-related pathway. Planta 241:967–985
Oh SJ, Kim YS, Kwon CW, Park HK, Jeong JS, Kim JK (2009) Overexpression of the transcription factor AP37 in rice improves grain yield under drought conditions. Plant Physiol 150:1368–1379
Onate-Sanchez L, Anderson JP, Young J, Singh KB (2007) AtERF14, a member of the ERF family of transcription factors, plays a nonredundant role in plant defense. Plant Physiol 143:400–409
Rashid M, Guangyuan H, Guangxiao Y, Hussain J, Xu Y (2012) AP2/ERF transcription factor in rice: genome-wide canvas and syntenic relationships between monocots and eudicots. Evol Bioinform 8:321
Riechmann JL, Heard J, Martin G et al (2000) Arabidopsis transcription factors: genome-wide comparative analysis among eukaryotes. Science 290:2105–2110
Rong W, Qi L, Wang A et al (2014) The ERF transcription factor TaERF3 promotes tolerance to salt and drought stresses in wheat. Plant Biotechnol J 12:468–479
Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D (2001) Guard cell signal transduction. Annu Rev Plant Biol 52:627–658
Sharoni AM, Nuruzzaman M, Satoh K et al (2011) Gene structures, classification and expression models of the AP2/EREBP transcription factor family in rice. Plant Cell Physiol 52:344–360
Shinozaki K, Yamaguchi-Shinozaki K (2007) Gene networks involved in drought stress response and tolerance. J Exp Bot 58:221–227
Shinozaki K, Yamaguchi-Shinozaki K, Seki M (2003) Regulatory network of gene expression in the drought and cold stress responses. Curr Opin Plant Biol 6:410–417
Stockinger EJ, Gilmour SJ, Thomashow MF (1997) Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc Natl Acad Sci U S A 94:1035–1040
Todaka D, Nakashima K, Shinozaki K, Yamaguchi-Shinozaki K (2012) Toward understanding transcriptional regulatory networks in abiotic stress responses and tolerance in rice. Rice 5:1–9
Trujillo LE, Sotolongo M, Menendez C et al (2008) SodERF3, a novel sugarcane ethylene responsive factor (ERF), enhances salt and drought tolerance when overexpressed in tobacco plants. Plant Cell Physiol 49:512–525
Umezawa T, Fujita M, Fujita Y, Yamaguchi-Shinozaki K, Shinozaki K (2006) Engineering drought tolerance in plants: discovering and tailoring genes to unlock the future. Curr Opin Biotechnol 17:113–122
Wan L, Zhang J, Zhang H, Zhang Z, Quan R, Zhou S, Huang R (2011) Transcriptional activation of OsDERF1 in OsERF3 and OsAP2-39 negatively modulates ethylene synthesis and drought tolerance in rice. PLoS One 6, e25216
Wang A, Tan D, Takahashi A, Li TZ, Harada T (2007) MdERFs, two ethylene-response factors involved in apple fruit ripening. J Exp Bot 58:3743–3748
Wang Y, Wan L, Zhang L et al (2012) An ethylene response factor OsWR1 responsive to drought stress transcriptionally activates wax synthesis related genes and increases wax production in rice. Plant Mol Biol 78:275–288
Wilkinson S, Davies WJ (2002) ABA-based chemical signalling: the co-ordination of responses to stress in plants. Plant Cell Environ 25:195–210
Wilkinson S, Davies WJ (2010) Drought, ozone, ABA and ethylene: new insights from cell to plant to community. Plant Cell Environ 33:510–525
Wu L, Zhang Z, Zhang H, Wang XC, Huang R (2008) Transcriptional modulation of ethylene response factor protein JERF3 in the oxidative stress response enhances tolerance of tobacco seedlings to salt, drought, and freezing. Plant Physiol 148:1953–1963
Xu K, Xu X, Fukao T et al (2006) Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature 442:705–708
Yang Y, Li R, Qi M (2000) In vivo analysis of plant promoters and transcription factors by agroinfiltration of tobacco leaves. Plant J 22:543–551
Zhang X, Zhang Z, Chen J et al (2005) Expressing TERF1 in tobacco enhances drought tolerance and abscisic acid sensitivity during seedling development. Planta 222:494–501
Zhang H, Zhang J, Quan R, Pan X, Wan L, Huang R (2013) EAR motif mutation of rice OsERF3 alters the regulation of ethylene biosynthesis and drought tolerance. Planta 237:1443–1451
Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247
Acknowledgments
This research was supported by the Major Special Foundation of Transgenic Plants in China (2014ZX08009-15B and 2013ZX08001003) and by National Natural Science Foundation of China (31171465).
Author Contribution
RH, JD, and DY conceived and designed the research. YY and DY conducted the experiments. RH, JD, JG, SZ and FW analyzed and discussed the data. YY wrote the manuscript. All authors read and approved the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Bhumi Nath Tripathi
Yanwen Yu and Dexin Yang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
The knockdown of OsERF109 in RI lines did not interfere the expression of its homologous genes. (a) The comparison of the C terminus with its homologous genes by DNAMAN software, which was used to the interference of OsERF109 transcription. (b) The expression of OsERF109 and homologous genes in RI transgenic lines. Transcripts are indicated relative to the expression level of the wild type Nip and error bars (± SD) are according to three independent biological experiments. (PDF 414 kb)
Fig. S2
Transgenic lines overexpression or knockdown of OsERF109 did not affect the growth at mature stage. Transgenic lines of 4-month-old rice grew in paddy field did not show obvious different phenotypes with the corresponding wild types. The OE-95 and RI-20 were selected from the paddy field to take photos with the corresponding wild type Zh17 and Nip, respectively. (PDF 165 kb)
Table S1
The primers used in this study. (PDF 173 kb)
Rights and permissions
About this article
Cite this article
Yu, Y., Yang, D., Zhou, S. et al. The ethylene response factor OsERF109 negatively affects ethylene biosynthesis and drought tolerance in rice. Protoplasma 254, 401–408 (2017). https://doi.org/10.1007/s00709-016-0960-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-016-0960-4