Abstract
Germ cell cluster organization and the process of oogenesis in Dactylobiotus parthenogeneticus have been described using transmission electron microscopy and light microscopy. The reproductive system of D. parthenogeneticus is composed of a single, sac-like, meroistic ovary and a single oviduct that opens into the cloaca. Two zones can be distinguished in the ovary: a small germarium that is filled with oogonia and a vitellarium that is filled with germ cell clusters. The germ cell cluster, which has the form of a modified rosette, consists of eight cells that are interconnected by stable cytoplasmic bridges. The cell that has the highest number of stable cytoplasmic bridges (four bridges) finally develops into the oocyte, while the remaining cells become trophocytes. Vitellogenesis of a mixed type occurs in D. parthenogeneticus. One part of the yolk material is produced inside the oocyte (autosynthesis), while the second part is synthesized in the trophocytes and transported to the oocyte through the cytoplasmic bridges. The eggs are covered with two envelopes: a thin vitelline envelope and a three-layered chorion. The surface of the chorion forms small conical processes, the shape of which is characteristic for the species that was examined. In our paper, we present the first report on the rosette type of germ cell clusters in Parachela.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tardigrades (Tardigrada; also called water bears) are minute (approximately 0.5 mm long) invertebrates that are closely related to Onychophora and Arthropoda (Aguinaldo et al. 1997; Philippe et al. 2005; Dunn et al. 2008; Rota-Stabelli et al. 2010, 2013; Campbell et al. 2011; Persson et al. 2012; Mayer et al. 2013a, b). They are widespread in marine, freshwater, and terrestrial habitats throughout the world (Nelson et al. 2010). The phylum Tardigrada is divided into three classes: Heterotardigrada, Eutardigrada, and Mesotardigrada. The class Heterotardigrada contains two orders—Arthrotardigrada (mainly marine species) and Echiniscoidea (principally terrestrial species) and the class Eutardigrada also consists of two orders—Apochela (a limno-terrestrial species) and Parachela (a primarily limno-terrestrial species with several secondary marine taxa), whereas class Mesotardigrada represented by one species has a dubious status (Nelson 2002; Nelson et al. 2010).
According to the literature (Büning 1994; Świątek et al. 2009), two types of oogenesis can be distinguished in animals—panoistic and meroistic. In panoistic oogenesis, all germ cells have the potential to become the egg cell. In meroistic oogenesis, only a few germ cells become egg cells, while the remaining cells differentiate into the nurse cells (trophocytes) that support the oocytes (Büning 1994; Świątek et al. 2009; Jaglarz et al. 2014). Meroistic oogenesis is characterized by the formation of germ cell clusters (germ cell cysts) as a result of the incomplete cytokinesis of germ cells (Büning 1994; Haglund et al. 2011). The germ cells in each cluster are interconnected by stable cytoplasmic bridges (ring canals) that allow the directional transport of nutrients and organelles between the cytoplasm of the adjacent cells (Mazurkiewicz and Kubrakiewicz 2001; Tworzydło and Kisiel 2010; Greenbaum et al. 2011; Haglund et al. 2011). This directional transport promotes the differentiation of one cell into the oocyte and the remaining cells into trophocytes.
Although the first description of the ovaries in Tardigrada was given by Doyère (1840), knowledge about the course of oogenesis in these animals is still poor. The process of oogenesis has been described in detail in four species belonging to order Parachela: Paramacrobiotus richtersi (earlier Macrobiotus richtersi) (Macrobiotidae) (Węglarska 1979, 1982), Macrobiotus polonicus (Macrobiotidae) (Poprawa et al 2014b), Dactylobiotus dispar (Murrayidae) (Poprawa 2005a, b; Poprawa et al. 2014a), and Isohypsibius granulifer granulifer (Isohypsibiidae) (Węglarska 1987; Poprawa and Grzywa 2006; Poprawa and Rost-Roszkowska 2010; Poprawa 2011), while the organization of germ cell clusters has only been examined in Milnesium tardigradum (Apochela, Milnesiidae) (Suzuki 2006). However, nothing is known about germ cell cluster organization in Parachela. Therefore, the main aim of our studies was to describe the organization of germ cell clusters and the process of oogenesis in Dactylobiotus parthenogeneticus (Murrayidae), which is a parthenogenetic species that belongs to Parachela.
Materials and methods
Specimens of the parthenogenetic species D. parthenogeneticus (Eutardigrada, Parachela, Murrayidae) were collected in May and June 2013 from a small pond in the Botanical Garden of Jagiellonian University (Cracow, Poland). Animals were reared in plastic Petri dishes filled with a mixture of distilled water and Żywiec mineral water (8:2) at a temperature of 20 °C and fed with algae (Chlorella sp. and Chlorococcum sp.).
Light and transmission electron microscopy
Forty-six specimens of D. parthenogeneticus were fixed in 2.5 % glutaraldehyde in a 0.1-M phosphate buffer, pH 7.4, and postfixed in 2 % osmium tetroxide in the same buffer. The fixation was carried out in the same manner as in the case of the tardigrades that were previously studied (Rost-Roszkowska et al. 2013a, b). Semi- (700 nm thick) and ultrathin (50 nm) sections were cut on a Leica Ultracut UCT25 ultramicrotome. Six of the 46 specimens (in the stage of middle vitellogenesis) were cut into serial ultrathin sections. Semi-thin sections were stained with 1 % methylene blue in 1 % borax and observed using an Olympus BX60 microscope. Ultrathin sections were mounted on formvar-covered single-hole copper grids, stained with uranyl acetate and lead citrate, and analyzed using a Hitachi H500 transmission electron microscope at 75 kV.
Histochemical methods
Detection of proteins (Bonhag’s method)
Semi-thin sections were treated with a 2 % solution of periodic acid (10 min at room temperature), stained with bromophenol blue (BPB; 24 h at 37 °C). After washing the sections with tap water, the slides were analyzed using an Olympus BX60 light microscope.
Detection of polysaccharides and glycogen (PAS method)
Semi-thin sections were treated with a 2 % solution of periodic acid (10 min at room temperature), stained with Schiff’s reagent (24 h at 37 °C), and washed in tap water. The slides were analyzed using an Olympus BX60 light microscope.
Detection of lipids (Sudan black B staining)
Semi-thin sections were stained with Sudan black B at room temperature (15 min), washed in ethanol and water, and analyzed using an Olympus BX60 light microscope.
Results
Gross morphology of the ovary
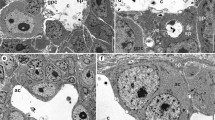
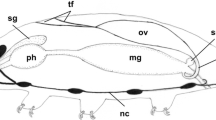
The single, sac-like ovary is located in the dorsal part of the body above the gut (Figs. 1 and 2a). The anterior part of the gonad is suspended by two ligaments (terminal filament) to the body wall, while its posterior end is connected to a single oviduct that opens into the cloaca (Fig. 1). The ovary wall is formed by a single layer of flattened epithelium that lies on the basal lamina (Fig. 2c). The epithelial cells form protrusions (Fig. 2c) that penetrate the ovary among the germ cells. The cytoplasm of these cells is rich in ribosomes, mitochondria, and cisterns of the rough endoplasmic reticulum (Fig. 2c). During oogenesis, the ovary increases in the volume and the epithelial cells of its wall become more flattened (Fig. 5c).
The morphology and ultrastructure of the ovary of D. parthenogeneticus. Cisterns of rough endoplasmic reticulum (RER), cystocytes (c), gut (g), Golgi complex (G), germarium (gr), nucleus (n), nucleolus (nu), mitochondria (m), oocyte (o), ovary (ov), ovary wall (ow), reserve material (y), trophocyte (t), vitellarium (v). a Tardigrade body. LM, bar = 22 μm. b Anterior part of the ovary. LM, bar = 12 μm. c The ovary wall. Protrusions of the gonad wall cells (arrow), basal lamina (arrowhead). TEM, bar = 1.2 μm. d Oogonia in the germarium. TEM, bar = 1.1 μm. e Germ cell cluster in the vitellarium. Stable cytoplasmic bridge (asterisk). Electron-dense rim (arrows). TEM, bar = 0.4 μm. f Stable cytoplasmic bridge (asterisk) connecting cystocytes. TEM, bar = 0.9 μm
The gonad can be divided into two parts—a germarium and a vitellarium (Fig. 2b). The germarium is a small part of the ovary that is located in its anterior region (Fig. 2b). It is filled with oogonia. Oogonia are small, oval cells whose ultrastructure is characteristic for non-differentiated cells (Fig. 2d). The central part of each cell is occupied by a large nucleus. Their cytoplasm contains ribosomes, mitochondria, and a small number of cisterns of the rough endoplasmic reticulum (Fig. 2c). The second region of the ovary is a large vitellarium that is filled with clusters of the germ cells (Fig. 2c, e, f).
Germ cell cluster organization
The number of germ cell clusters in the vitellarium of the species that were examined depended on the age of the specimen and ranged from three to fifteen. Analysis of the serial ultrathin sections of six specimens (26 clusters were analyzed) demonstrated that there are always eight germ line cells in each cluster in the vitellarium (Fig. 3a). The cells that form the cluster are interconnected by stable cytoplasmic bridges (ring canals) (Figs. 2e, f and 3a). These connections are typical cytoplasmic canals that have one electron-dense rim that is suspended to the plasma membrane (Figs. 2e and 3a). The fusome material is not observed. The clusters are asymmetrical—one cell has four cytoplasmic bridges, one cell has three cytoplasmic bridges, one cell has two cytoplasmic bridges, and five cells have only one cytoplasmic bridge (Fig. 3a). During oogenesis, the cell with the largest number of cytoplasmic bridges (four bridges) develops into an oocyte. All alterations of all of the cells that form the germ cell cluster will be described according to the stages of oogenesis.
Vitellarium of D. parthenogeneticus. a Diagrammatic representation of a germ cell cluster: cystocytes (A–E 2 ), cytoplasmic bridges formed during the first (1), second (2), third (3) and fourth (4) division—arrow indicates the future oocyte. TEM and LM images of the germ cells during previtellogenesis and vitellogenesis (b–g). Cisterns of rough endoplasmic reticulum (RER), Golgi complex (G), nucleus (n), nucleolus (nu), mitochondria (m), midgut epithelium (me), oocyte (o), ovary (ov), trophocyte (t), reserve material (y1, y2), and stable cytoplasmic bridge (asterisk). b Previtellogenic cystocyte. TEM, bar = 1.1 μm. c Stable cytoplasmic bridge connecting previtellogenic cystocytes. TEM, bar = 0.5 μm. d Cystocytes during early vitellogenesis. TEM, bar = 1.1 μm. e Cytoplasm of the cystocyte during early vitellogenesis. TEM, bar = 0.6 μm. f The longitudinal section through the body during the stage of middle vitellogenesis. LM, bar = 14.5 μm. g Ultrastructure of the trophocyte. TEM, bar = 1.3 μm
Previtellogenesis
In the previtellogenic vitellarium, germ cells (cystocytes) are oval cells (Fig. 3b) that are connected by stable cytoplasmic bridges (Fig. 3c). Each cytoplasmic bridge has one electron-dense rim (Fig. 3c) and is 0.68–0.75 μm wide (12 bridges were measured). All of the cells in a cluster have a similar ultrastructure. A large nucleus with a spongy nucleolus is located in the central part of the cell (Fig. 3b, c). The cytoplasm of the cystocytes is rich in mitochondria, ribosomes, Golgi complexes, and cisterns of the rough endoplasmic reticulum (Fig. 3b, c).
Vitellogenesis
Early vitellogenesis
At the beginning of vitellogenesis, the cells that form the germ cell clusters are still connected by stable cytoplasmic bridges (0.78–0.91 μm wide (12 bridges were measured)) with one electron-dense rim (Figs. 2e, f and 3d). Each cell starts to grow and gradually accumulates electron-dense material (Figs. 2e, f and 3d, e) and spheres of a medium electron density (Figs. 2f and 3e). The cytoplasm of cystocyte is rich in mitochondria, ribosomes, Golgi complexes, and cisterns of the rough endoplasmic reticulum (Figs. 2c, e, f, and 3d, e).
Middle and late vitellogenesis
During middle vitellogenesis the cell that has four cytoplasmic bridges in the cluster begins to grow intensively (Fig. 3f). This cell develops into the oocyte, while the remaining cells in the cluster differentiate into nurse cells (trophocytes) (Fig. 3f, g). All of the cells of the cluster (the oocyte and the trophocytes) are still connected by stable cytoplasmic bridges with one electron-dense rim (Fig. 5a) and accumulate spheres of the reserve material (yolk) (Figs. 3g and 5a, b). The cytoplasmic bridges between the trophocytes are 1.21–1.43 μm wide (12 bridges were measured), while the cytoplasmic bridges between the oocytes and trophocytes are 0.80–0.98 μm wide (ten bridges were measured). Organelles and spheres of the reserve material can be observed in the cytoplasm of all of the cytoplasmic bridges (Fig. 5a).
During middle and late vitellogenesis, the oocyte grows intensively and increases its volume. It is connected to four trophocytes by stable cytoplasmic bridges (Fig. 3a). The central part of this cell is occupied by the nucleus (Fig. 5b). A large number of free ribosomes, mitochondria, cisterns of the rough endoplasmic reticulum, and Golgi complexes can be observed in the cytoplasm of the oocyte (Fig. 5b, c). Three different types of sphere reserve material can be distinguished in the cytoplasm of the oocyte: (y1) the spheres, which contain the internal part of medium electron density and an electron-dense external part (Fig. 5b, d) (similar spheres were observed in the cytoplasm of trophocytes (Figs. 3f and 5a)), (y2) heterogenous spheres that have a mosaic structure (Fig. 5b), (y3) homogenous spheres of a medium electron density (Fig. 5b–e). Histochemical analysis showed that spheres of reserve material are BPB positive (Fig. 4a, arrows), PAS positive (Fig. 4b, arrows), and Sudan black B positive (Fig. 4c, arrows).
The trophocytes are still connected to the oocyte and/or other trophocytes by stable cytoplasmic bridges. The large nucleus, which is lobular in shape, is located in the central part of the cell. A large and spongy nucleolus that is surrounded by clumps of heterochromatin can be observed in the nucleoplasm (Fig. 3g). The cytoplasm of the trophocytes is rich in mitochondria, ribosomes, and cisterns of the rough endoplasmic reticulum (Fig. 3g).
Choriogenesis and the morphology of the chorion
The process of the formation of egg envelopes (choriogenesis) begins during the middle vitellogenesis. This process can be divided into three stages: early, middle, and late choriogenesis. During early choriogenesis, the epithelial cells of the ovary wall whose cytoplasm is rich in organelles such as ribosomes, mitochondria, and cisterns of the rough endoplasmic reticulum (Fig. 2c) synthesize and secrete a flocculent material of a medium electron density (Fig. 5c). This material is deposited in the form of clumps on the surface of the oolemma (Fig. 5c, d). Simultaneously, the vesicles with the fibrous, medium electron-dense material can be observed in the cortical cytoplasm of the oocyte (Fig. 5c, d). The membrane of these vesicles fuses with the oolemma (Fig. 5c, d), resulting in a secretion of their content into the ovary lumen. Eventually, this is deposited on the surface of the oocyte to form the parts of the chorion. During the middle choriogenesis, the cells of the ovary wall together with the oocyte secrete material that connects the parts of the chorion into the permanent layer and successive layers of the chorion are deposited. Stable cytoplasmic bridges are still present at this stage of choriogenesis (Fig. 5e). Mitochondria, free ribosomes, and spheres of the yolk can be observed in the cytoplasm of these junctions (Fig. 5e). During late choriogenesis, the cytoplasmic bridges break and the last part of the chorion is secreted by the cells of the ovary wall. When the chorion is completely formed, the oocyte synthesizes and secretes the fibrous, medium electron-dense material, which is deposited between the oolemma and the chorion (Fig. 5f). This material forms the thin vitelline envelope.
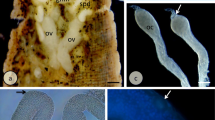
Structure and formation of the egg envelopes in D. parthenogeneticus. Chorion (ch), cisterns of rough endoplasmic reticulum (RER), Golgi complex (G), nucleus (n), nucleolus (nu), mitochondria (m), oocyte (o), ovary wall (ow), trophocyte (t), reserve material (y, y1, y2, y3), inner layer of the chorion (1), labyrinthine layer of the chorion (2), outer layer of the chorion (3). a Stable cytoplasmic bridge (asterisk) connecting vitellogenic trophocytes. TEM, bar = 0.7 μm. b Oocyte during middle vitellogenesis. TEM, bar = 1.5 μm. c Deposition of chorion precursors. Flocculent material secreted by the cells of the ovary wall (asterisk), vesicles filled with chorion precursors (arrows). TEM, bar = 0.6 μm. d Oocyte during early choriogenesis. Flocculent material secreted by the cells of the ovary wall (asterisk), vesicles filled with chorion precursors (arrows), and exocytotic vesicles (arrowhead). TEM, bar = 0.3 μm. e Stable cytoplasmic bridge (asterisk) connecting the oocyte and trophocyte during choriogenesis. TEM, bar = 0.9 μm. f Oocyte during the secretion of the precursors of the vitelline envelope. Exocytotic vesicle (arrows) TEM, bar = 0.4 μm. g–h. Eggs of D. parthenogeneticus. Arrows show conical processes. g LM, bar = 30 μm. h LM, bar = 15.7 μm
The completely formed egg capsule of D. parthenogeneticus is composed of a thin vitelline envelope and a three-layered chorion. The chorion is composed of (1) a medium electron-dense inner layer, which adheres to the vitelline envelope, (2) a labyrinthine layer formed by lamellae connecting the inner and outer layers, and (3) a medium electron-dense outer layer (Fig. 5f).
The female lays 2–15 eggs (Fig. 5g) freely on the surface of leaves or under the epidermis of leaves. The surface of the chorion forms small conical processes (Fig. 5g, h) that are 4–4.5 μm high. The wide of their base is 3.5–4.2 μm.
Discussion
The germ cell cluster organization
The reproductive system of D. parthenogeneticus, similar to other tardigrades that belong to the order Parachela (Węglarska 1979, 1987; Dewel et al. 1993; Kinchin 1994; Poprawa 2005a; Nelson et al. 2010) is composed of a single, sac-like ovary and a single oviduct that opens into the cloaca. The ovary of tardigrades is of the meroistic type, which means that an oocyte is supported by the nurse cells (trophocytes) that originated from the germ line cells (Büning 1994). In the meroistic ovary mitotic divisions of the germ cells are often followed by incomplete cytokineses, which leads to the formation of germ cell clusters (Świątek et al. 2009, 2014; Greenbaum et al. 2011; Haglund et al. 2011). In such clusters, the germ cells are interconnected by specific cell junctions that are called stable cytoplasmic bridges (Haglund et al. 2011). Germ line clusters have been found in the ovaries of a great number of invertebrates and vertebrates (Robinson and Cooley 1996; de Cuevas et al. 1997; Pepling et al. 1999; Matova and Cooley 2001; Mazurkiewicz and Kubrakiewicz 2001; Świątek et al. 2009; Urbisz et al. 2010; Urbisz and Świątek 2013; Biliński et al. 2014; Jaglarz et al. 2014), and they are supposed to be an early and conservative phase of gametogenesis (Pepling et al. 1999). The literature data show that three major types of germ cell clusters can be distinguished in animal oogenesis: linear germ cell clusters, clusters in the shape of a rosette, and clusters with a central mass of cytoplasm (Świątek et al. 2009). A linear germ cell cluster is the simplest form in which interconnected cells form a chain-like structure. Each cell of the cluster has two cytoplasmic bridges. Only terminal cells are linked to their sister cells by one cytoplasmic bridge. Linear clusters have been found in some polychaetous annelids (Anderson and Huebner 1968), in some crustaceans (Criel 1989; Kubrakiewicz et al. 1991), and in some insects (Biliński 1994). Germ cell clusters in the form of a rosette is the best known type of cluster and is present in the ovary of many insects (i.e., Drosophila melanogaster) (Büning 1994; Lin and Spradling 1995; Kubrakiewicz 1998; Huynh and Johnston 2004). In this type of cluster, the cells might be connected to their neighbor cells by more than two stable cytoplasmic bridges that form “branches.” The last type of germ cell clusters in animal oogenesis are clusters with a central mass of cytoplasm, and this type occurs in clitellate annelids, flat worms, and nematodes (Foor 1968, 1983; Gibert et al. 1984; Świątek 2008; Świątek et al. 2009, 2012). A characteristic feature of this type of cluster is the presence of anucleate, spherical mass of cytoplasm to which each germ cell is connected by only one cytoplasmic bridge. The central cytoplasmic mass in annelids is called a cytophore (Świątek 2008; Świątek et al. 2009, 2012), while in nematodes this mass is called a rachis (Foor 1968, 1983; Gibert et al. 1984).
The germ cell clusters of D. parthenogeneticus consist of eight cells that are connected by stable cytoplasmic bridges. Because some cells have more than two cytoplasmic bridges and because “branches” are formed, this cluster can be classified as a rosette type similar to that described in D. melanogaster and many other insects (Büning 1994; Lin and Spradling 1995; Kubrakiewicz 1998; Huynh and Johnston 2004; Haglund et al. 2011); however, it is a slightly modified rosette. In D. melanogaster, the germ cell clusters consist of sixteen cells and are formed as a result of four incomplete mitotic divisions. During each division all of the cells of the cluster divide in synchrony (Lin and Spradling 1995; Huynh and Johnston 2004). However, the structure of the germ cell clusters of D. parthenogeneticus suggests that this cluster is also formed as a result of four incomplete divisions, but in contrast to D. melanogaster not all of the cells undergo each division (asynchronous divisions). The fusome material that is present in the cytoplasm of cytoplasmic bridges that interconnect germ cells of D. melanogaster provides the synchrony of divisions (Lighthouse et al. 2008; Ong and Tan 2010). Fusome is not observed in D. parthenogeneticus, which may explain the asynchronism of divisions during the formation of germ cell clusters. According to our hypothesis (for a better understanding of the following description see Fig. 3a), the formation of the germ cell clusters of D. parthenogeneticus starts with the mitotic division of the cystoblast (the mother cell of a cluster—cell A on Fig. 3a). As a result, cystocyte B (the sister cell of A) is formed. Both cells A and B divide incompletely during the second division and form cells C1 (the sister cell of A) and C2 (the sister cell of B). All of the sequences proceed in the same manner as in D. melanogaster until the second division (Huynh and Johnston 2004). However, beginning with the third division, differences appear. In D. melanogaster, all of the cells in a cluster divide and form “branches,” while in D. parthenogeneticus only cells A and C2 divide. The ability to divide of the cell B and C1 are temporarily blocked. During this division, cells D1 (the sister cell of A) and D2 (the sister cell of C2) are formed. At this stage, the cell cluster in the species that was examined has an almost linear structure (only one cell makes a “branch”), while it is much more branched in D. melanogaster (Huynh and Johnston 2004). Subsequently, the fourth division occurs in which only cells A and B divide (cell B regains the ability to divide). Eventually, cells E1 (the sister cell of A) and E2 (the sister cell of B) are formed. As a result of the four incomplete mitotic divisions, a germ cell cluster consisting of eight cells that are interconnected by stable cytoplasmic bridges is formed. In D. parthenogeneticus, the directional transport of nutrients and organelles between the cells in a cluster promotes the differentiation of the cell with the highest number of cytoplasmic bridges (cell A) into the oocyte. The remaining cells of the cluster become trophocytes. A similar situation can be observed in a female germ cell cluster of D. melanogaster in which one cell that has the largest number of cytoplasmic bridges (four bridges) differentiates into the oocyte (Lin and Spradling 1995; Huynh and Johnston 2004). The germ cell cluster organization of D. parthenogeneticus is completely different from that described in the tardigrade M. tardigradum, which is a species that belongs to the order Apochela. According to Suzuki (2006), four large multinuclear cells are situated in the center of the M. tardigradum cluster. These multinuclear cells are connected to each other by cytoplasmic bridges and they are considered to be nurse cells (trophocytes). They are surrounded by a large number (104) of mononuclear cells. Each of the mononuclear cells is connected to the trophocyte by one cytoplasmic bridge. The mononuclear cells are oocytes. The germ cell clusters of M. tardigradum can be classified as a modification of the rosette type cluster or as a combination of the rosette cluster and the cluster with a central mass of cytoplasm. Therefore, we present the first report on the rosette type of the germ cell cluster in Parachela.
The structure of the stable cytoplasmic bridges
The cytoplasmic bridges that connect the cells in the germ cell clusters of D. parthenogeneticus form the standard ring canals that have one electron-dense rim that is linked to the plasma membrane. A similar structure of the cytoplasmic bridges was observed in the female germ cell clusters of the tardigrades D. dispar and M. polonicus (Poprawa 2005a; Poprawa et al. 2014ab) and the male germ cell clusters of Xerobiotus pseudohufelandi and I. granulifer granulifer (Rebecchi 1997; Świątek et al. 2014), while in the female clusters of I. granulifer granulifer, the cytoplasmic bridge has two rims: an electron-dense outer rim that is linked to the plasma membrane and a less electron-dense inner rim (Poprawa et al. 2014a). The structure of this connection is similar to the female ring canals that were described for D. melanogaster (Robinson et al. 1994; Ong and Tan 2010). The presence of stable cytoplasmic bridges in the oogenesis of tardigrades that belong to the order Parachela plays a similar role to that described for other animals (Mazurkiewicz and Kubrakiewicz 2001; Tworzydło and Kisiel 2010; Urbisz et al. 2010; Greenbaum et al. 2011; Haglund et al. 2011). Because of these connections, the cytoplasm, organelles, macromolecules (mostly mRNAs), and the yolk can be transported from the trophocytes to the oocyte. However, the cooperation of cells in a germ cell cluster of Apochela remains unknown.
Vitellogenesis
The process of oogenesis in tardigrades has a meroistic character (Węglarska 1979, 1987; Poprawa 2005a; Hyra et al. 2014b). As was mentioned above, the oocyte is supported by the trophocytes. According to the literature (Biliński 1979; Eckelbarger 1994), two types of vitellogenesis can be distinguished in animals. The participation of the oocyte in the process of yolk formation in the oogenesis of animals is called autosynthesis (Biliński 1979; Eckelbarger 1994). The precursors of a yolk can be synthesized in other structures such as the trophocytes (Adiyodi and Subramoniam 1983; Siekierska 2003; Urbisz et al. 2010; Urbisz and Świątek 2013; Jaglarz et al. 2014), the epithelial cells of the midgut (Rost-Roszkowska et al. 2011), the storage cells (Szymańska 1994; Poprawa 2006; Hyra et al. 2014a), and the fat body (Raikhel and Dhadiala 1992) and then transported to the oocyte by pinocytosis or by cytoplasmic bridges. This type of yolk formation is called heterosynthesis. Sometimes, a mixed vitellogenesis is distinguished. In this case, both autosynthesis and heterosynthesis occur during oogenesis (Biliński 1979; Eckelbarger 1994).
In D. parthenogeneticus, similar to other tardigrades (Węglarska 1979, 1987; Poprawa 2005a; Poprawa et al. 2014a), a mixed vitellogenesis takes place. Both the oocytes and trophocytes participate in the formation of the yolk. At the beginning of vitellogenesis, electron-dense granules of the reserve material begin to be synthesized in the cytoplasm of all of the cystocytes in the germ cluster. This material is synthesized by the cisterns of the rough endoplasmic reticulum, modified in Golgi complexes, and then accumulated in vacuoles. Sometimes the reserve material is also formed in the mitochondria (Węglarska 1979; Poprawa 2005a). The yolk material that is synthesized in the cytoplasm of the trophocytes is transported to the oocyte by stable cytoplasmic bridges (Węglarska 1979, 1987; Poprawa 2005a). The participation of the storage cells in the process of yolk formation was described in P. richtersi (Szymańska 1994), D. dispar (Poprawa 2006), and I. granulifer granulifer (Hyra et al. 2014a). Moreover, the synthesis of the yolk precursors in the cells of the midgut epithelium of I. granulifer granulifer was described by Rost-Roszkowska et al. (2011). Histochemical analysis showed that the reserve material accumulated in the oocytes of D. parthenogeneticus contains a large amount of proteins, polysaccharides, and lipids.
Choriogenesis
In tardigrades, the process of choriogenesis starts during middle vitellogenesis (Węglarska 1982; Poprawa 2005b, 2011). This is the process of the formation of the egg envelopes, the structures that protect the oocyte and embryo against any harmful effects of the environment and provide optimal conditions for development (Margaritis 1985; Poprawa and Rost 2004). All of the egg envelopes that covered the egg cell form an egg capsule. The precursors for the egg envelopes can be synthesized by the oocyte, by the follicular cells, by the cells of various parts of the reproductive system (Margaritis 1985; Poprawa et al. 2002; Poprawa and Rost 2004; Norman and Tait 2008), or outside the reproductive system, for example, in the liver of fish (Wallace 1985). The egg capsule of tardigrades is composed of two envelopes—a thin vitelline envelope and a three-layered chorion (Węglarska 1982; Poprawa 2005b, 2011). In D. parthenogeneticus, similar to other tardigrades (Węglarska 1982; Poprawa 2005b, 2011), precursors of the chorion are synthesized and secreted by the oocyte and by the cells of the ovary wall, while the precursors of the vitelline envelope are synthesized and secreted by only the oocyte. The classification of egg envelopes that is based on the way and the place of the formation of the egg envelope precursors that is most often applied was established by Ludwig in 1874 (Węglarska 1982). Ludwig distinguished three types of egg envelopes: primary, secondary, and tertiary. The primary egg envelopes are produced by the oocyte, the secondary are secreted in the ovary but not by the oocyte (they are secreted by follicular cells or by the cells of the ovary wall), and the tertiary are secreted outside the ovary. According to this classification, the vitelline envelope of D. parthenogeneticus and other tardigrades (Węglarska 1982; Poprawa 2005b, 2011) is of the primary type while the chorion is of a mixed type—primary (secreted by the oocyte) and secondary (secreted by the cells of ovary wall).
Conclusions
Our studies show that (1) the germ cell clusters consist of eight cells that are connected by stable cytoplasmic bridges; (2) the germ cell clusters have the form of a modified rosette; (3) the cell that has the largest number of stable cytoplasmic bridges (four bridges) develops into the oocyte, while the remaining cells become trophocytes; (4) in D. parthenogeneticus, meroistic oogenesis takes place; (5) a mixed vitellogenesis occurs in the species that was studied; and (6) the vitelline envelope is of the primary type, while the chorion is of a mixed type—primary (secreted by the oocyte) and secondary (produced by the cells of the gonad wall).
References
Adiyodi RG, Subramoniam T (1983) Arthropoda—crustacea. In: Adiyodi KG, Adiyodi RG (eds) Reproductive biology of invertebrates, vol I, Oogenesis, oviposition and oosorption. John Wiley & Sons Ltd., Chichester, pp 443–495
Aguinaldo A, Turbeville J, Linford L, Rivera M, Garey J, Raff R, Lake J (1997) Evidence for a clade of nematodes, arthropods, and other moulting animals. Nature 387:489–493
Anderson E, Huebner E (1968) Development of the oocyte and its accessory cells of the polychaete, Diopatra cuprea (Bosc). J Morphol 126:163–198
Biliński S (1979) Powstawanie żółtka w oogenezie bezkręgowców. Post Biol Kom 6(4):249–266
Biliński S (1994) The ovary of Enthognata. In: Büning J (ed) The insect ovary. Ultrastructure, previtellogenic growth and evolution. Chapman & Hall, London, pp pp 7–pp 30
Biliński SM, Kocarek P, Jankowska W, Kisiel E, Tworzydło W (2014) Ovaries and phylogeny of dermapterans once more: ovarian characters support paraphyly of spongiphoridae. Zool Anz 253:321–326
Büning J (1994) The insect ovary. Ultrastructure previtellogenic growth and evolution. Chapman and Hall, London
Campbell LI, Rota- Stabelli O, Edgecombe GD, Marchioro T, Longhorn SJ, Telford MJ, Philippe H, Rebecchi L, Peterson KJ, Pisani D (2011) MicroRNAs and phylogenomics resolve the phylogenetic relationships of the Tardigrada and suggest the velvet worms as the sister group of Arthropoda. Proc Natl Acad Sci U S A 108:15920–15924
Criel GRJ (1989) Morphological study of the ovary of Artemia. In: Warner AH, MacRae TH, Bagshaw JC (eds) Cell and molecular biology of Artemia development. Plenum, New York, pp 99–129
de Cuevas M, Lilly MA, Spradling AC (1997) Germline cyst formation in Drosophila. Annu Rev Genet 31:405–428
Dewel RA, Nelson DR, Dewel WC (1993) Tardigrada. In: Harrison FW, Rice ME (eds) Microscopic anatomy of invertebrates. Volume 12. Onychophora Chilopoda and Lesser Protostomata. Wiley-Liss, Inc, pp 143–183
Doyère PM (1840) Mémoire sur les Tardigrades. Annales des Sciences Naturelles, series 2, vol. 14
Dunn CW, Hejno A, Matus DQ, Pang K, Browne WE, Smith SA, Seaver E, Rouse GW, Obst M, Edgecombe GD, Sørensen MV, Haddock SHD, Schmidt-Rhaesa A, Okusu A, Kristensen RM, Wheeler WC, Martindale MQ, Giribet G (2008) Broad phylogenomic sampling improves resolution of the animal tree of life. Nature 452:745–750
Eckelbarger KJ (1994) Diversity of metazoan ovaries and vitellogenic mechanisms—implication for life history theory. Proc Biol Soc Wash 107:193–208
Foor WE (1968) Cytoplasmic bridges in the ovary of Ascaris lumbricoides. Bull Tulane Univ Med Fac 27:23–30
Foor WE (1983) Nematoda. In: Adiyodi KG, Adiyodi RG (eds) Reproductive biology of invertebrates, vol I, Oogenesis, oviposition and oosorption. John Wiley & Sons Ltd., Chichester, pp 223–256
Gibert MA, Starck J, Beguet B (1984) Role of the gonad cytoplasmic core during oogenesis of the nematode Caenorhabditis elegans. Biol Cell 50:77–85
Greenbaum MP, Iwamori T, Buchold GM, Matzuk MM (2011) Germ cell intercellular bridges. Cold Spring Harb Perspect Biol 2011 3(8):a005850. doi:10.1101/cshperspect.a005850
Haglund K, Nezis IP, Stenmark H (2011) Structure and functions of stable intercellular bridges formed by incomplete cytokinesis during development. Commun Integr Biol 4:1–9
Huynh J-R, Johnston DS (2004) The origin of asymmetry: early polarisation of the Drosophila germline cyst and oocyte. Curr Biol 14:R438–R449
Hyra M, Deperas M, Kszuk-Jendrysik M, Włodarczyk A, Sonakowska L, Rost-Roszkowska M, Poprawa I (2014a) Relationship between the storage bodies and the oogenesis in Isohypsibius granulifer granulifer (Tardigrada: Eutardigrada). Acta Biol Crac 56(suppl 1):61
Hyra M, Kszuk-Jendrysik M, Rost-Roszkowska M, Poprawa I (2014b) Oogenesis in Dactylobiotus parthenogeneticus Bertolani, 1982 (Tardigrada, Eutardigrada, Murrayidae). Acta Biol Crac 56(suppl 1):31
Jaglarz MK, Kubrakiewicz J, Jędrzejowska I, Gołdyn B, Biliński SM (2014) Ultrastructural analysis of the ovary and oogenesis in Spinicaudata and Laevicaudata (Branchiopoda) and its phylogenetic implications. Zoology 117:207–215
Kinchin IM (1994) The biology of tardigrades. Portland Press, London
Kubrakiewicz J (1998) Struktura i funkcja zespołów komórek płciowych w politroficznych owariolach sieciarek (Insecta: Neuroptera). Rozprawa habilitacyjna. Wydaw, Uniwersytetu Wrocławskiego, Wrocław
Kubrakiewicz J, Adamski RT, Biliński SM (1991) Ultrastructural studies on accessory nuclei in developing oocytes of the crustacean, Siphonophanes grubei. Tissue Cell 23:903–907
Lighthouse DV, Buszczak M, Spradling AC (2008) New components of the Drosophila fusome suggest it plays novel roles in signaling and transport. Dev Biol 317:59–71
Lin H, Spradling AC (1995) Fusome asymmetry and oocyte determination in Drosophila. Dev Genet 16:6–12
Margaritis LH (1985) Structure and physiology of the eggshell. In: Kerkut GA, Gilbert LJ (eds) Comprehensive insect physiology, biochemistry and pharmacology, vol 1, Embryogenesis and reproduction. Pergamon, Oxford, pp 153–230
Matova N, Cooley L (2001) Comparative aspects of animal oogenesis. Dev Biol 231(2):291–320
Mayer G, Kauschke S, Rüdiger J, Stevenson PA (2013a) Neural markers reveal a one-segmented head in tardigrades (water bears). PLoS One 8(3):e59090
Mayer G, Martin C, Rüdiger J, Kauschke S, Stevenson PA, Poprawa I, Hohberg K, Schill RO, Pflüger HJ, Schlegel M (2013b) Selective neuronal staining in tardigrades and onychophorans provides insights into the evolution of segmental ganglia in panarthropods. BMC Evol Biol 13:230
Mazurkiewicz M, Kubrakiewicz J (2001) Intercellular cytoplasm transport during oogenesis of the moth midge Tinearia alternata Say (Diptera: Psychodidae). Folia Biol 49(3/4):205–213
Nelson DR (2002) Current status of Tardigrada: evolution and ecology. Integr Comp Biol 42:652–659
Nelson DR, Guidetti R, Rebecchi L (2010) Tardigrada. In: Thorp JH, Covich AP (eds) Ecology and classification of North American freshwater invertebrates, vol 14, Academic., pp 455–484
Norman JM, Tait NN (2008) Ultrastructure of the eggshell and its formation in Planipapillus mundus (Onychophora: Peripatopsidae). J Morphol 269:1263–1275
Ong S, Tan C (2010) Germline cyst formation and incomplete cytokinesis during Drosophila melanogaster oogenesis. Dev Biol 337:84–98
Pepling ME, de Cuveas M, Spradling AC (1999) Germline cysts: a conserved phase of germ cell development. Trends Cell Biol 9:257–262
Persson DK, Halberg KA, Jørgensen A, Møbjerg N, Kristensen RM (2012) Neuroanatomy of Halobiotus crispae (Eutardigrada: Hypsibiidae): tardigrade brain structure supports the clade Panarthropoda. J Morphol 273:1227–1245
Philippe H, Lartillot N, Brinkmann H (2005) Multigene analyses of bilaterian animals corroborate the monophyly of Ecdysozoa, Lophotrochozoa, and Protostomia. Mol Biol Evol 22:1246–1253
Poprawa I (2005a) The ovary structure, previtellogenic and vitellogenic stages in parthenogenetic species Dactylobiotus dispar (Murray, 1097) (Tardigrada: Eutardigrada). Tissue Cell 37:385–392
Poprawa I (2005b) The structure and the formation of egg shells in parthenogenetic species Dactylobiotus dispar Murray, 1097 (Tardigrada: Eutardigrada). Folia Biol (Cracow) 53:173–177
Poprawa I (2006) Ultrastructural changes of the storage cells during oogenesis in Dactylobiotus dispar (Murray, 1907) (Tardigrada: Eutardigrada). Zool Pol 51:13–18
Poprawa I (2011) Ultrastructural studies of the formation of the egg capsule in hermaphroditic species Isohypsibius granulifer granulifer Thulin, 1928 (Tardigrada: Eutardigrada). Zool Sci 28(1):37–40
Poprawa I, Grzywa K (2006) Ultrastructure of the young gonad of the hermaphroditic species Isohypsibius granulifer Thulin, 1928 (Tardigrada: Eutardigrada). Acta Biol Crac 48(suppl 1):62
Poprawa I, Rost MM (2004) Structure and ultrastructure of the egg capsule of Thermobia domestica (Packard) (Insecta: Zygentoma). Folia Biol (Cracow) 52:185–190
Poprawa I, Rost-Roszkowska M (2010) Curriculum vitae of trophocytes during oogenesis in Isohypsibius granulifer (Tardigrada: Eutardigrada). Acta Biol Crac 52(suppl 1):35
Poprawa I, Baran A, Rościszewska E (2002) Structure of ovaries and formation of egg envelopes in the stonefly, Leuctra autumnalis Aubert, 1948 (Plecoptera: Leuctridae). Ultrastructural studies. Folia Biol (Cracow) 50:29–38
Poprawa I, Hyra M, Kszuk-Jendrysik M, Rost-Roszkowska M (2014a) Intercellular bridges in the oogenesis of Parachela (Tardigrada, Eutardigrada). Acta Biol Crac 56(suppl 1):75
Poprawa I, Schlechte-Wełnicz W, Hyra M (2014b) Ovary organization and oogenesis in the tardigrade Macrobiotus polonicus Pilato, Kaczmarek, Michalczyk & Lisi, 2003 (Eutardigrada, Macrobiotidae): ultrastructural and histochemical analysis. Protoplasma. doi:10.1007/s00709-014-0725-x
Raikhel AS, Dhadiala TS (1992) Accumulation of yolk proteins in insect oocytes. Annu Rev Entomol 37:217–251
Rebecchi L (1997) Ultrastructural study of spermiogenesis and the testicular and spermathecal spermatozoon of the gonochoristic tardigrade Xerobiotus pseudohufelandi (Eutardigrada, Macrobiotidae). J Morphol 234:11–24
Robinson DN, Cooley L (1996) Stable intercellular bridges in development: the cytoskeleton lining the tunnel. Trends Cell Biol 6:474–479
Robinson DN, Cant K, Cooley L (1994) Morphogenesis of Drosophila ovarian ring canals. Development 120:2015–2025
Rost-Roszkowska MM, Poprawa I, Wójtowicz M, Kaczmarek Ł (2011) Ultrastructural changes of the midgut epithelium in Isohypsibius granulifer granulifer Thulin, 1928 (Tardigrada: Eutardigrada) during oogenesis. Protoplasma 248(2):405–414
Rost-Roszkowska MM, Poprawa I, Hyra M, Marek-Swędzioł M, Kaczmarek Ł (2013a) The fine structure of the midgut epithelium in Xerobiotus pseudohufelandi (Iharos, 1966) (Tardigrada, Eutardigrada, Macrobiotidae). J Limnol 72(s1):54–61
Rost-Roszkowska MM, Poprawa I, Kaczmarek Ł (2013b) Autophagy as the cell survival in response to a microsporidian infection of the midgut epithelium of Isohypsibius granulifer granulifer (Eutardigrada: Hypsibiidae). Acta Zool (Stockholm) 94:273–279
Rota-Stabelli O, Kayal E, Gleeson D, Daub J, Boore JL, Telford MJ, Pisani D, Blaxter M, Lavrov DV (2010) Ecdysozoan mitogenomics: evidence for a common origin of the legged invertebrates, the Panarthropoda. Gen Biol Evol 2:425–440
Rota-Stabelli O, Daley AC, Pisani D (2013) Molecular timetrees reveal a Cambrian colonization of land and a new scenario for ecdysozoan evolution. Curr Biol 23:392–398
Siekierska E (2003) The structure of the ovary and oogenesis in the earthworm, Dendrobaena veneta (Annelida Clitellata). Tissue Cell 35:252–259
Suzuki A (2006) Ovarian structure in Milnesium tardigradum (Tardigrada: Milnesiidae) during early vitellogenesis. Hydrobiologia 558:61–66
Świątek P (2008) Ovary cord structure and oogenesis in Hirudo medicinalis and Haemopis sanguisuga (Clitellata, Annelida): remarks on different ovaries organization in Hirudinea. Zoomorphology 127:213–226
Świątek P, Kubrakiewicz J, Klag J (2009) Formation of germ-line cyst with a central cytoplasmic core is accompanied by specific orientation of mitotic spindles and partitioning of existing intercellular bridges. Cell Tissue Res 337:137–148
Świątek P, Urbisz AZ, Strużyński W, Płachno BJ, Bielecki A, Cios S, Salonen E, Klag J (2012) Ovary architecture of two branchiobdellid species and Acanthobdella peledina (Annelida, Clitellata). Zool Anz 251:71–82
Świątek P, Małota K, Hyra M, Gorgoń S, Poprawa I (2014) Stabilne mostki międzykomórkowe–kanały komunikacji międzykomórkowej. Post Biol Komórki 41:507–532
Szymańska B (1994) Independence between storage bodies and egg developmental stages in Macrobiotus richtersi Murray, 1911 (Tardigrada). Acta Biol Crac Ser Zool 36:41–50
Tworzydło W, Kisiel E (2010) Structure of ovaries and oogenesis in dermapterans. II. The nurse cells, nuage aggregates and sponge bodies. Folia Biol (Cracow) 58:67–72
Urbisz AZ, Świątek P (2013) Ovary organization and oogenesis in two species of Lumbriculida (Annelida, Clitellata). Zoology 116:118–128
Urbisz AZ, Krodkiewska M, Świątek P (2010) Ovaries of Tubificinae (Clitellata, Naididae) are meroistic and resemble ovary cords found in leeches (Clitellata, Hirudinea). Zoomorphology 129:235–247
Wallace RA (1985) Vitellogenesis and oocyte growth in nonmammalian vertebrates. In: Browder LW (ed) Developmental biology. Plenum Publisher, pp 127–177
Węglarska B (1979) Electron microscope study of previtellogenesis and vitellogenesis in Macrobiotus richtersi. J Murr (Eutardigrada) Zesz Nauk Uniw Jagiellon Pr Zool 25:169–189
Węglarska B (1982) Ultrastructural study of the formation of egg envelops in Macrobiotus richtersi (Eutardigrada). In: Nelson DR (ed) Proceedings of the third international symposium on Tardigrada. East Tennessee State University Press, pp 115–128
Węglarska B (1987) Yolk formation in Isohypsibius (Eutardigrada). Zoomorphology 107:287–292
Acknowledgments
We would like to express our gratitude to Professor Jerzy Klag (University of Silesia, Katowice, Poland) for reading the manuscript critically and Professor Józef Mitka (Botanical Garden, Jagiellonian University, Cracow, Poland) for his help in collecting the material.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Lucy M Collinson
Rights and permissions
About this article
Cite this article
Poprawa, I., Hyra, M. & Rost-Roszkowska, M.M. Germ cell cluster organization and oogenesis in the tardigrade Dactylobiotus parthenogeneticus Bertolani, 1982 (Eutardigrada, Murrayidae). Protoplasma 252, 1019–1029 (2015). https://doi.org/10.1007/s00709-014-0737-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-014-0737-6