Abstract
The female reproductive system, the process of oogenesis, and the morphology of the egg capsule of Macrobiotus polonicus were analyzed using transmission and scanning electron microscopy and histochemical methods. The female reproductive system of Macrobiotus polonicus consists of a single ovary and a single oviduct that opens into the cloaca. The seminal receptacle filled with sperm cells is present. The ovary is divided into two parts: a germarium that is filled with oogonia and a vitellarium that is filled with branched clusters of the germ cells. Meroistic oogenesis occurs in the species that was examined. The yolk material is synthesized by the oocyte (autosynthesis) and by the trophocytes and is transported to the oocyte through cytoplasmic bridges. The process of the formation of the egg envelopes starts in the late vitellogenesis. The egg capsule is composed of two envelopes—the vitelline envelope and the three-layered chorion. The vitelline envelope is of the primary type while the chorion is of a secondary type. The surface of the chorion is covered with conical processes that terminate with a strongly indented terminal disc.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oogenesis is a very important biological phenomenon that generates haploid female reproductive cells. Two types of oogenesis can be distinguished depending on the place where the gametes originated—diffuse and localized. The first type, in which gametes form anywhere in the animal’s body, occurs in sponges and some flatworms, while localized oogenesis is characteristic for all other animals. In this type of gametogenesis, the female gametes develop in an ovary (Raven 1961). In both cases, the oocyte can develop independently or can be supported by special cells—trophocytes (derived from germline cells) and/or follicular cells (somatic cells). Localized oogenesis, in which the oocyte is supported entirely by follicular cells is called panoistic oogenesis, while oogenesis in which trophocytes are present together with follicular cells is meroistic (Büning 1994). Meroistic oogenesis is often characterized by the presence of germ cell clusters. In these structures, the germ cells are interconnected by cytoplasmic bridges (Büning 1994; Haglund et al. 2011). These connections are used to transport nutrients from the trophocytes to oocyte (Mazurkiewicz and Kubrakiewicz 2001; Poprawa 2005a; Haglund et al. 2011).
Tardigrades are small (approximately 0.5 mm long), segmented, hydrophilic invertebrates (Kinchin 1994; Nelson et al. 2010). Detailed data about the female reproductive system and the course of oogenesis are poor. Several morphological descriptions of the female reproductive system in tardigrades have been published a few decades ago (Baumann 1964; Renaud-Dobyser 1965; Grimaldi de Zio et al. 1987). However, little is still known about ovary ultrastructure and the process of oogenesis in this group of invertebrates. Information about the pattern of the maturation of the ovary and ovotestis was given by Rebecchi and Bertolani (1994) and Rebecchi and collaborators (2000). A detailed description of oogenesis is known only in Paramacrobiotus richtersi (earlier Macrobiotus richtersi) (Macrobiotidae) (Węglarska 1979, 1982), Dactylobiotus dispar (Murrayidae) (Poprawa 2005a, b; Poprawa et al. 2014), and Isohypsibius granulifer granulifer (Isohypsibiidae) (Węglarska 1987; Poprawa and Grzywa 2006; Poprawa and Rost-Roszkowska 2010; Poprawa 2011). The structure of the germ cell clusters in Milnesium tardigradum (Eutardigrada, Apochela) was described by Suzuki (2006).
Therefore, the main aim of our studies was to describe ovary organization and the process of oogenesis in Macrobiotus polonicus (Macrobiotidae), a species that belongs to the class Eutardigrada, order Parachela (Pilato et al. 2003).
Material and methods
Specimens of Macrobiotus polonicus Pilato, Kaczmarek, Michalczyk & Lisi, 2003 (Eutardigrada, Parachela, Macrobiotidae) were extracted from a moss sample that was collected from a railway embankment on Jeziorna Street in Poznań, Wielkopolska Province, Poland (52° 19′ 09″ N, 16° 48′ 23″ W, 83 m a.s.l.). Animals were reared on Petri dishes with a layer of agarose (2 %) filled with mixture of distilled water and tap water (1:1) at room temperature. Petri dishes were located in a shaded place. The cultures were fed every 3 days with nematodes (Caenorhabditis elegans) and algae (Chlorella sp.).
Light and transmission electron microscopy
Forty specimens of Macrobiotus polonicus were fixed in 2.5 % glutaraldehyde in a 0.1 M phosphate buffer, pH 7.4 at 4 °C (2 days), rinsed, and postfixed in 2 % osmium tetroxide in the same buffer at room temperature (2 h). Afterward, the material was dehydrated in a series of graded ethanol (50, 70, 90, 95, and 4 × 100 %, each for 15 min) and acetone (15 min) and embedded in epoxy resin (Epoxy Embedding Medium Kit; Sigma). All sections were cut on a Leica Ultracut UCT25 ultramicrotome. Semi-thin sections (700 nm thick) were stained with 1 % methylene blue in 1 % borax and observed using an Olympus BX60 microscope. Ultra-thin sections (50 nm thick) were put on formvar-covered copper grids, stained with uranyl acetate and lead citrate and examined using a Hitachi H500 transmission electron microscope at 75 kV.
Scanning electron microscopy
Ten eggs were fixed in 2.5 % glutaraldehyde, postfixed in 2 % osmium tetroxide, dehydrated in a graded concentration series of ethanol, dried at critical point Pelco CPD2, and coated with gold in a Pelco SC-6 duster. These preparations were examined using a Hitachi UHR FE-SEM SU 8010 scanning electron microscope.
Histochemical methods
After histochemical staining, all of the sections were examined using an Olympus BX60 microscope.
Detection of proteins (Bonhag’s method)
Semi-thin sections were treated with a 2 % solution of periodic acid to remove the osmium (10 min at room temperature), stained with bromophenol blue (BPB) (24 h at 37 °C), and washed in tap water.
Detection of polysaccharides and glycogen (PAS method)
Semi-thin sections were treated with a 2 % solution of periodic acid to remove the osmium (10 min at room temperature), stained with Schiff’s reagent (24 h at 37 °C), and washed in tap water.
Detection of lipids (Sudan black B staining)
Semi-thin sections were stained with Sudan black B at room temperature (15 min) and washed in ethanol and water.
Results
The ovary organization
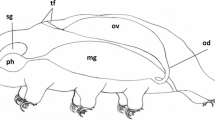
The female reproductive system of Macrobiotus polonicus is composed of a single, dorsally located, sac-like ovary (Figs. 1 and 2a) and a single gonoduct that opens into the cloaca. A seminal receptacle with tightly packed sperm cells is present (Fig. 2b). The ovary is attached to the body wall by terminal filament (Fig. 2c). This filament is composed of two ligaments, which are appendices of the cells of the ovary wall. The somatic ovary wall consists of one layer of flattened epithelial cells and lies on the basal lamina (Figs. 2d, e). Cells of this epithelium have a large nucleus (Figs. 2d, e) with a non-homogenous nucleolus that is surrounded by clumps of heterochromatin (Fig. 2e). The central part of the nucleolus has a lower electron density, while the external part is electron-dense (Fig. 2e). The cytoplasm of these cells is rich in cisterns of the rough endoplasmic reticulum, ribosomes, mitochondria, and Golgi complexes (Fig. 2e). During oogenesis, when the ovary increases in volume, the cells of its wall become more flattened (Fig. 3a, c, f). Two different zones can be distinguished in the Macrobiotus polonicus ovary—the germarium and the vitellarium. The germarium is the small part of the ovary that is filled by non-differentiated cells—oogonia (Fig. 2f). The center of the oogonium is occupied by a large nucleus. Some mitochondria and ribosomes can be observed in the cytoplasm (Fig. 2f). The larger part of the gonad—the vitellarium—is filled with branched clusters of the germ cells (Fig. 2g). The cells in the cluster are connected by cytoplasmic bridges (Figs. 2g and 3a) that have one electron-dense rim. This rim is attached to the plasma membrane (Fig. 3a). The oocytes differentiate in this zone.
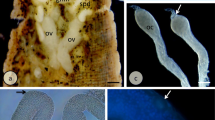
Gonad ultrastructure of the Macrobiotus polonicus. a A longitudinal section through the body of tardigrade: midgut epithelium (me), midgut lumen (ml), ovary (ov), storage cell (sc). LM, bar = 14. μm. b A cross section through the seminal receptacle (sr). TEM, bar = 0.6 μm. c The terminal filament (arrow). TEM, bar = 0.4 μm. d Part of the ovary. Arrow indicates the basal lamina. TEM, bar = 0.5 μm. e A cell of ovary wall. Arrow indicates the basal lamina. TEM, bar = 0.4 μm. f An oogonium in the germarium. TEM, bar = 0.3 μm. g A germ cell cluster in the vitellarium. Arrows indicate the stable cytoplasmic bridges. TEM, bar = 0.7 μm. Cisterns of the rough endoplasmic reticulum (RER), mitochondria (m), nucleus of a cell of a germ cell (n), nucleus of a cell of the ovary wall (n1), nucleolus of a cell of the germ cell (nu), nucleolus of a cell of the ovary wall (nu1), ovary wall (ow)
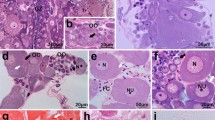
Previtellogenesis and vitellogenesis. a The stable cytoplasmic bridge that connects cystocytes. Arrows indicate the electron-dense rim. TEM, bar = 0.3 μm b Previtellogenic cystocytes. TEM, bar = 0.5 μm. c Part of the gonad during early vitellogenesis. Arrows indicate the spheres of the reserve material. TEM, bar = 1.4 μm. d The stable cytoplasmic bridge (arrows) that connects the cystocytes during early vitellogenesis. The asterisk indicates the precursors of the chorion. TEM, bar = 0.5 μm. e Part of the gonad during middle vitellogenesis. Oocyte (o), trophocyte (t). Arrows indicate stable cytoplasmic bridges. TEM, bar = 0.4 μm. Golgi complex (G), cisterns of the rough endoplasmic reticulum (RER), lipid droplets (l), mitochondria (m), nucleus (n), nucleolus (nu), ovary wall (ow), reserve material (y, y1, y2)
Oogenesis
Previtellogenesis
During previtellogenesis, cystocytes that are interconnected by the stable cytoplasmic bridges have the same shape and size. A large nucleus with large nucleolus is located in the central part of the cell. The nucleolus is non-homogenous and has a spongy structure (Fig. 3b). Its central part, which is of a medium electron density, is surrounded by electron-dense material. The cytoplasm is rich in mitochondria, ribosomes, Golgi complexes, and cisterns of the rough endoplasmic reticulum (Fig. 3b).
Vitellogenesis
Three stages can be distinguished in the process of vitellogenesis—early, middle, and late vitellogenesis.
During early vitellogenesis, the cells of the vitellarium begin to grow and accumulate small spheres of medium electron-dense material (Fig. 3a, c). The center of each of these cells is occupied by a large nucleus with a large non-homogenous nucleolus located close to the nuclear membrane (Fig. 3c, d). The nucleolus has a spongy structure. Its external part has a higher electron density than its internal part. The nucleolus is surrounded by clumps of heterochromatin. A number of free ribosomes, mitochondria, Golgi complexes, and cisterns of the rough endoplasmic reticulum can be observed in the cytoplasm of the cystocyte (Fig. 3c).
During middle vitellogenesis, one cell in each cluster starts to grow intensively and differentiates into the oocyte, while the remaining cells become trophocytes. Lipid droplets and homogenous (Figs. 3e and 4a indicated as y2) and non-homogenous spheres of reserve material (yolk) accumulate in the cytoplasm of the oocyte. These non-homogenous spheres have a mosaic structure (Figs. 3e and 4a indicated as y1). A large nucleus with a large, spongy non-homogenous nucleolus is located in the central part of each cell in a cluster (in both oocyte and trophocytes) (Fig. 3e). The cytoplasm is rich in mitochondria, ribosomes, Golgi complexes, and cisterns of the rough endoplasmic reticulum (Figs. 3e and 4a). Some homogenous spheres of medium electron density can be observed in the cytoplasm of the oocyte and trophocytes (Figs. 3e and 4a). Spheres of a different material and organelles are visible in the cytoplasm of the stable cytoplasmic bridges that connect the cells in cluster (Figs. 2g and 3a, d, e). During late vitellogenesis, the entire oocyte is filled with spheres of yolk. No signs of the pinocytosis were observed. Histochemical analysis showed that the abovementioned spheres are BPB-positive (Fig. 4b, arrows) and PAS-positive (Fig. 4c, arrows). Some spheres are Sudan black B-positive (Fig. 4d, arrows).
Vitellogenesis. a The oocyte during middle vitellogenesis. Cisterns of the rough endoplasmic reticulum (RER), lipid droplets (l), mitochondria (m), reserve material (y1, y2). TEM, bar = 0.5 μm. b–d Histochemical staining of the ovary in the stage of late vitellogenesis. Midgut (mg), ovary (ov); arrows show positive results of staining. b PBP staining, LM, bar = 10 μm. c PAS method, LM, bar = 9 μm. d Sudan black B staining, LM, bar = 8 μm
Choriogenesis
The process of the formation of egg envelopes begins during late vitellogenesis. Small fragments of chorion appear on the surface of the oocyte first (Fig. 5a). The material for the chorion is synthesized and secreted by the epithelial cells of the ovary wall (Figs. 2e and 3d). No signs of participation of the oocyte in chorion formation were observed. Subsequently, the cells of the ovary wall secrete the material that is deposited on the surface of the oocyte. This material connects parts of the chorion and eventually the permanent layer is created (Fig. 5b). After that, the oocyte synthesizes fibrous material (Fig. 5b) that is secreted into the space between the oolemma and the chorion. This material forms the vitelline envelope (Fig. 5c, d), which initially is fragmented and does not form a permanent layer (Fig. 5c).
The formation and structure of the egg envelopes. a Oocyte during early choriogenesis. TEM, bar = 0.6 μm. b Permanent layer of the chorion on the surface of the oocyte. Exocytotic vesicles (arrow). TEM, bar = 0.8 μm. c The oocyte during the formation of the vitelline envelope. The vitelline envelope (arrowhead), the conical process (arrow). TEM, bar = 0.8 μm. d The completely developed egg capsule. The vitelline envelope (arrow), cortical granules (arrowhead). TEM, bar = 0.4 μm. e The egg of Macrobiotus polonicus. Arrow indicates a conical process. LM, bar = 13 μm. f The conical processes on the chorion surface. Arrows indicate terminal discs SEM, bar = 2.4 μm. g The structure of the chorion. Arrows indicate the sticks of the labyrinthine layer. SEM, bar = 0.4 μm. h The conical process of the chorion. Arrow indicates an indented terminal disc. SEM, bar = 1.4 μm. i The conical process of the chorion. Arrow indicates the tooth of the terminal disc. SEM, bar = 1.4 μm. Chorion (ch), lipid droplets (l), ovary wall (ow), yolk (y), inner layer of the chorion (1), labyrinthine layer of the chorion (2), outer layer of the chorion (3)
The structure of the egg capsule
The completely developed egg capsule of Macrobiotus polonicus consists of two shells—the vitelline envelope and the chorion (Fig. 5d). The vitelline envelope is a thin (0.06 μm), electron-dense layer that adheres to the oolemma, while the chorion is composed of three layers (Fig. 5d, g): (1) the inner layer, which is medium electron-dense and approximately 0.2 μm thick; (2) the middle, labyrinthine layer, which is composed of a small sticks that connect the inner and outer layers, approximately 0.05 μm thick; and (3) the outer, medium electron-dense layer, approximately 0.09 μm thick. The surface of the chorion is covered with processes in the shape of short truncated cones that terminate with a strongly indented (7–12 teeth on the circumference of the disc) terminal disc (Fig. 5e, f, h, i). The processes are empty inside (Fig. 5i); they are formed by evagination of the outer layer of the chorion. They are 5.1–6.1 μm high, and the width of the base is 5.2–6.7 μm. The diameter of the terminal disc (including the teeth) is 4.9–6.3 μm. The surface of the basic chorion that connects the processes is slightly wrinkled and does not have a network structure (Fig. 5g).
Discussion
The organization of the reproductive system
The present study shows that Macrobiotus polonicus, similar to other tardigrades (Bertolani 1983; Węglarska 1979, 1987; Dewel et al. 1993; Poprawa 2005a; Nelson et al. 2010; Poprawa et al. 2012; Hyra et al. 2014b), has a single ovary in the form of a dorsally located sac. However, during embryogenesis, it develops from paired anlages (Nelson 1982). The anterior part of the ovary is joined to the body wall by a terminal filament, while the posterior part modifies into a single oviduct that opens into the cloaca (Eutardigrada) or on the body surface through the gonopore (Heterotardigrada) (Dewel et al. 1993; Kinchin 1994). In tardigrades that belong to the class Eutardigrada, like the species examined here, the terminal filament is composed of two ligaments that are the protrusions of cells of the ovary wall (Węglarska 1979, 1987; Dewel et al. 1993; Poprawa 2005a). Only one ligament exists that can consist of minute muscle fibers in Heterotardigrada (Dewel et al. 1993). Marcus (1929) reported the presence of a seminal receptacle that terminates in a duct in the rectum of female specimens of Macrobiotus and Hypsibius, while Bertolani (1983) revealed the existence of this structure in only two species—Macrobiotus hufelandi and Macrobiotus pallarii. This structure is used to store the sperm cells after copulation. Our studies show that a seminal receptacle is present in Macrobiotus polonicus, and it is filled with sperm cells that are stuck together.
The sac-like ovary of Macrobiotus polonicus, similar to other tardigrades that belong to the order Parachela (Węglarska 1979, 1987; Poprawa 2005a), is divided into two zones—a germarium that is filled with oogonia and a vitellarium that is filled with branched clusters of the germ cells. The cells in the cluster are interconnected by stable cytoplasmic bridges that have one electron-dense rim that is connected to the plasma membrane. A similar structure of the cytoplasmic bridge was observed in the female germ cell clusters of Dactylobiotus parthenogeneticus and Dactylobiotus dispar (Poprawa 2005a; Poprawa et al. 2014), while two rims were observed in the cytoplasmic bridges in the female clusters of Isohypsibius granulifer granulifer— an electron-dense outer rim and a less electron-dense inner rim (Poprawa et al. 2014).
The ovary wall of tardigrades is formed by a simple squamous epithelium (Bertolani 1983; Węglarska 1979, 1987; Poprawa 2005a). During oogenesis, when the volume of the ovary increases, the cells of epithelium become more flattened (Poprawa 2005a). The apical part of this epithelium is turned into the lumen of the ovary. A similar orientation has been described in the cells of the follicular epithelium that surrounds the oocytes in arthropods (Büning 1994; Poprawa et al. 2002; Jędrzejowska et al. 2013, 2014; Jaglarz et al. 2014; Liu et al. 2014). Moreover, the epithelial cells have an ultrastructure that reveals their secretory activity in both cases. Their cytoplasm is rich in mitochondria, ribosomes, and cisterns of the rough endoplasmic reticulum. Additionally, the somatic cells of the ovary wall in tardigrades play a similar function as the follicular cells in arthropods. They synthesize and secret the precursors of the egg envelope (chorion) (Węglarska 1982; Poprawa 2005b, 2011).
Oogenesis
Previtellogenesis
Similar to other tardigrades (Węglarska 1979, 1987; Poprawa 2005a; Hyra et al. 2014b), meroistic oogenesis occurs in Macrobiotus polonicus. This means that the oocyte is accompanied by nurse cells (trophocytes) that are connected to the oocyte by stable cytoplasmic bridges. The process of animal previtellogenesis is characterized by the synthesis and accumulation of macromolecules (mRNAs) and the accumulation of ribosomes and other organelles in the cytoplasm of cystocytes (Büning 1994; Kubrakiewicz 2002). In tardigrades, all cystocytes accumulate macromolecules and organelles and eventually transport them to the future oocyte by cytoplasmic bridges during previtellogenesis (Węglarska 1979; Poprawa 2005a). This directional transport, which is similar to Drosophila melanogaster (Lin and Spradling 1995; Huynh and Johnston 2004), promotes the differentiation of one cell in the cluster into the oocyte.
Vitellogenesis
Vitellogenesis is the process of the synthesis and accumulation of the yolk in the oocyte. This material will be used by the developing embryo. According to Biliński (1979) and Eckelbarger (1994), two major types of vitellogenesis can be distinguished—autosynthesis and heterosynthesis. In the first case, the yolk is synthesized by the oocyte, while in the second type, it is synthesized outside the oocyte and sometimes outside the gonad (Biliński 1979; Eckelbarger 1994; Poprawa 2005a). In tardigrades, as well as in Macrobiotus polonicus that was studied, at the beginning of the vitellogenesis, all cystocytes in the cluster (including the future oocyte) start to synthesize the reserve material (yolk) (Węglarska 1979, 1987; Poprawa 2005a). This means that the process of autosynthesis occurs. However, during vitellogenesis, the yolk that is synthesized in the trophocytes is transported to the future oocyte (Poprawa 2005a). Moreover, in tardigrades, the yolk precursor can be synthesized in the storage cells (Szymańska 1994; Poprawa 2006; Hyra et al. 2014a) and the cells of the midgut epithelium (Rost-Roszkowska et al. 2011). Nothing is known about the participation of these structures in the synthesis of the yolk precursors in Macrobiotus polonicus. Given these data, we can conclude that a mixed vitellogenesis occurs in tardigrades. This type of vitellogenesis is common among animals (e.g., it has been described in annelids, some insects, crustaceans) (Biliński 1976, 1979; Siekierska 2003). Histochemical research showed that the reserve material accumulated in the oocytes of Macrobiotus polonicus contains a large amount of proteins and polysaccharides and a small amount of lipids.
Choriogenesis
The eggs of tardigrades, like those of insects (Margaritis 1985; Poprawa et al. 2002; Poprawa and Rost 2004) and onychophorans (Norman and Tait 2008), are covered with an egg capsule that is composed of two egg envelopes—the vitelline envelope and the chorion (Węglarska 1982; Poprawa 2005b, 2011). The egg capsule provides the optimal conditions for the developing embryo and protects the oocyte and embryo against the harmful effects of the environment. In Macrobiotus polonicus the process of the formation of the egg envelopes starts in late vitellogenesis; this is a little later than in Paramacrobiotus richtersi, Dactylobiotus dispar, and Isohypsibius granulifer granulifer in which choriogenesis starts during middle vitellogenesis (Węglarska 1982; Poprawa 2005b, 2011). Although the eggs of insects, onychophorans, and tardigrades are covered by similar envelopes, the sequence of events during choriogenesis is different. In insects and onychophorans, the vitelline envelope is produced first, followed by the appearance of the chorion (Poprawa et al. 2002; Norman and Tait 2008), while in tardigrades, this sequence of events is reversed. The chorion is formed first, after which the precursors of the vitelline envelope are secreted by the oocyte and deposited in the space between the oolemma and the chorion (Węglarska 1982; Poprawa 2005b, 2011). A similar order of the formation of the egg envelopes was described in Tetrodontophora bielanensis, which is an insect species that belongs to Collembola (Krzysztofowicz and Kisiel 1989).
According to the widely accepted classification of Ludwig (1874) (Węglarska 1982), the vitelline envelope of previously studied tardigrades (Węglarska 1982; Poprawa 2005b, 2011) is of the primary type, while the chorion is regarded as a mixed type—primary (secreted by the oocyte) and secondary (produced by the cells of the gonad/ovary wall). In Macrobiotus polonicus the vitelline envelope, similar to other tardigrades, is of a primary type, but the chorion is the secondary envelope, whose precursors are synthesized and secreted by the cells of the ovary wall.
Conclusions
Our studies show that (1) a seminal receptacle is present in Macrobiotus polonicus; (2) the cells of the ovary wall play the same function as the follicular cells in arthropods—they synthesize and secrete precursors of the egg envelope; (3) meroistic oogenesis occurs in species that was studied; (4) the cells in the germ cell clusters are connected by stable cytoplasmic bridges that have one electron-dense rim; (5) mixed vitellogenesis occurs; and (6) the vitelline envelope is of a primary type (secreted by the oocyte), while the chorion is of a secondary type (produced by the cells of the ovary wall).
References
Baumann H (1964) Über den Lebenslauf und die Lebensweise von Milnesium tardigradum Doyère (Tardigrada). Veroff Uberseemus Bremen 3:161–171
Bertolani R (1983) Tardigrada. In: Adiyodi KG, Adiyodi RG (eds) Reproductive biology of invertebrates. Volume I. Oogenesis, oviposition and oosorption. Wiley, Chichester, pp 431–441
Biliński S (1976) Ultrastructural studies on the vitellogenesis of Tetrodontophora bielanensis (Wega) (Collembola). Cell Tissue Res 168:399–410
Biliński S (1979) Powstawanie żółtka w oogenezie bezkręgowców. Post Biol Kom 6(4):249–266
Büning J (1994) The insect ovary. Ultrastructure previtellogenic growth and evolution. Chapman and Hall, London
Dewel RA, Nelson DR, Dewel WC (1993) Tardigrada. In: Harrison FW, Rice ME (eds) Microscopic anatomy of invertebrates, volume 12. Onychophora chilopoda and lesser protostomata. Wiley-Liss, New York, pp 143–183
Eckelbarger KJ (1994) Diversity of metazoan ovaries and vitellogenic mechanisms—implication for life history theory. Proc Biol Soc Wash 107:193–208
Grimaldi de Zio S, D’Addabbo Gallo M, Morone de Lucia MR, D’Addabbo L (1987) Marine Arthrotardigrade and Echiniscoidea (Tardigrada, Heterotardigrada) from the Indian Ocean. Boll Zool 4:347–357
Haglund K, Nezis IP, Stenmark H (2011) Structure and functions of stable intercellular bridges formed by incomplete cytokinesis during development. Commun Integr Biol 4:1–9
Huynh J-R, Johnston DS (2004) The origin of asymmetry: early polarisation of the drosophila germline cyst and oocyte. Curr Biol 14:R438–R449
Hyra M, Deperas M, Kszuk-Jendrysik M, Włodarczyk A, Sonakowska L, Rost-Roszkowska M, Poprawa I (2014a) Relationship between the storage bodies and the oogenesis in Isohypsibius granulifer granulifer (Tardigrada: Eutardigrada). Acta Biol Crac 56(suppl 1):61
Hyra M, Kszuk-Jendrysik M, Rost-Roszkowska M, Poprawa I (2014b) Oogenesis in Dactylobiotus parthenogeneticus Bertolani, 1982 (Tardigrada, Eutardigrada, Murrayidae). Acta Biol Crac 56(suppl 1):31
Jaglarz MK, Kubrakiewicz J, Jędrzejowska I, Gołdyn B, Biliński SM (2014) Ultrastructural analysis of the ovary and oogenesis in Spinicaudata and Laevicaudata (Branchiopoda) and its phylogenetic implications. Zoology 117:207–215
Jędrzejowska I, Mazurkiewicz-Kania M, Garbiec A, Kubrakiewicz J (2013) Differentiation and function of the ovarian somatic cells in the pseudoscorpion, Chelifer cancroides (Linnaeus, 1761) (Chelicerata: Arachnida: Pseudoscorpionida). Arthropod Struct Dev 42(1):27–36
Jędrzejowska I, Szymusiak K, Mazurkiewicz-Kania M, Garbiec A (2014) Differentiation of somatic cells in the ovariuteri of the apoikogenic scorpion Euscorpius italicus (Chelicerata, Scorpiones, Euscorpiidae). Arthropod Struct Dev 43(4):361–370
Kinchin IM (1994) The biology of tardigrades. Portland Press, London
Krzysztofowicz A, Kisiel E (1989) Further studies on the morphogenesis of the first and second egg envelopes of Tetrodontophora bielanensis (Wega) (Collembola). In: Dallai R (ed) 3rd international seminar on apterygota. University of Siena, Siena, pp 221–228
Kubrakiewicz J (2002) Extrachromosomal rDNA amplification in the oocyte of Polystoechotes punctatus (Fabricius) (Insecta-Neuroptera-Polystoechotidae). Arthropod Struct Dev 31:23–31
Lin H, Spradling AC (1995) Fusome asymmetry and oocyte determination in Drosophila. Dev Genet 16:6–12
Liu W, Xie Y, Dong J, Xue J, Tian F, Wu J (2014) Ultra- and microstructure of the female reproductive systemof Matsucoccus matsumurae. Arthropod Struct Dev 43(3):243–253
Ludwig H (1874) Über die Eibildung in Thierreiche. Arb Physiol Lab Wuertzburg 1:287–510
Marcus E (1929) Tardigrada. In: Bronn HG (ed) Klassen und Ordungen des Tierreichs, vol. 5, Section 4, Part 3, pp 1–608
Margaritis LH (1985) Structure and physiology of the eggshell. In: Kerkut GA, Gilbert LJ (eds) Comprehensive insect physiology, biochemistry and pharmacology, vol 1, Embryogenesis and reproduction. Pergamon, Oxford, pp 153–230
Mazurkiewicz M, Kubrakiewicz J (2001) Intercellular cytoplasm transport during oogenesis of the moth midge Tinearia alternata Say (Diptera: psychodidae). Folia Biol 49(3/4):205–213
Nelson DR (1982) Tardigrada. In: Hurlbert SH, Villalobos-Figueroa A (eds) Aquatic biota of Mexico, Central America and the West Indies. San Diego State University Press, San Diego, pp 154–158
Nelson DR, Guidetti R, Rebecchi L (2010) Tardigrada. In: Thorp JH, Covich AP (eds) Ecology and classification of North American freshwater invertebrates. Academic Press (Elsevier), San Diego, pp 455–484
Norman JM, Tait NN (2008) Ultrastructure of the eggshell and its formation in Planipapillus mundus (Onychophora: Peripatopsidae). J Morphol 269:1263–1275
Pilato G, Kaczmarek Ł, Michalczyk Ł, Lisi O (2003) Macrobiotus polonicus, a new species of Tardigrada from Poland (Eutardigrada, Macrobiotidae, ‘hufelandi grup’). Zootaxa 258:1–8
Poprawa I (2005a) The ovary structure, previtellogenic and vitellogenic stages in parthenogenetic species Dactylobiotus dispar (Murray, 1097) (Tardigrada: Eutardigrada). Tissue Cell 37:385–392
Poprawa I (2005b) The structure and the formation of egg shells in parthenogenetic species Dactylobiotus dispar Murray, 1097 (Tardigrada: Eutardigrada). Folia Biol (Cracow) 53:173–177
Poprawa I (2006) Ultrastructural changes of the storage cells during oogenesis in Dactylobiotus dispar (Murray, 1907) (Tardigrada: Eutardigrada). Zool Pol 51:13–18
Poprawa I (2011) Ultrastructural studies of the formation of the egg capsule in hermaphroditic species Isohypsibius granulifer granulifer Thulin, 1928 (Tardigrada: Eutardigrada). Zool Sci 28(1):37–40
Poprawa I, Grzywa K (2006) Ultrastructure of the young gonad of the hermaphroditic species Isohypsibius granulifer Thulin, 1928 (Tardigrada: Eutardigrada). Acta Biol Crac 48(suppl 1):62
Poprawa I, Rost MM (2004) Structure and ultrastructure of the egg capsule of Thermobia domestica (Packard) (Insecta: Zygentoma). Folia Biol (Cracow) 52:185–190
Poprawa I, Rost-Roszkowska M (2010) Curriculum vitae of trophocytes during oogenesis in Isohypsibius granulifer (Tardigrada: Eutardigrada). Acta Biol Crac 52(suppl 1):35
Poprawa I, Baran A, Rościszewska E (2002) Structure of ovaries and formation of egg envelopes in the stonefly, Leuctra autumnalis Aubert, 1948 (Plecoptera: Leuctridae) Ultrastructural studies. Folia Biol (Cracow) 50:29–38
Poprawa I, Rost-Roszkowska MM, Świeczka M, Kaczmarek Ł, Wełnicz W (2012) The oogenesis of Macrobiotus polonicus (Tardigrada, Eutardigrada, Macrobiotidae). Acta Biol Crac Ser Bot 54(suppl 1):39
Poprawa I, Hyra M, Kszuk-Jendrysik M, Rost-Roszkowska M (2014) Intercellular bridges in the oogenesis of Parachela (Tardigrada, Eutardigrada). Acta Biol Crac 56(suppl 1):75
Raven CP (1961) Oogenesis: the storage of developmental information. Pergamon, Oxford
Rebecchi L, Bertolani R (1994) Maturative pattern of ovary and testis in eutardigrades of freshwater and terrestrial habitats. Invertebr Reprod Dev 26:107–118
Rebecchi L, Guidi A, Bertolani R (2000) Mutarotative patern of the ovotestis in two hermaphrodite species of eutardigrada. Invertebr Reprod Dev 37:25–34
Renaud-Dobyser J (1965) Parastygarctus higginsi n.g., n.sp., Tardigrade marin interstitial de Madagascar. C R de l’Acad des Sci (Paris) 260:955–957
Rost-Roszkowska MM, Poprawa I, Wójtowicz M, Kaczmarek Ł (2011) Ultrastructural changes of the midgut epithelium in Isohypsibius granulifer granulifer Thulin, 1928 (Tardigrada: Eutardigrada) during oogenesis. Protoplasma 248(2):405–414
Siekierska E (2003) The structure of the ovary and oogenesis in the earthworm, Dendrobaena veneta (Annelida Clitellata). Tissue Cell 35:252–259
Suzuki A (2006) Ovarian structure in Milnesium tardigradum (Tardigrada: Milnesiidae) during early vitellogenesis. Hydrobiologia 558:61–66
Szymańska B (1994) Independence between storage bodies and egg developmental stages in Macrobiotus richtersi Murray, 1911 (Tardigrada). Acta Biol Crac Ser Zool 36:41–50
Węglarska B (1979) Electron microscope study of previtellogenesis and vitellogenesis in Macrobiotus richtersi. J Murr. (Eutardigrada). Zesz Nauk Uniw Jagiellon Pr Zool 25:169–189
Węglarska B (1982) Ultrastructural study of the formation of egg envelops in Macrobiotus richtersi (Eutardigrada). In: Nelson DR (ed) Proceedings of the Third international symposium on Tardigrada. East Tennessee State University Press, pp 115–128
Węglarska B (1987) Yolk formation in Isohypsibius (Eutardigrada). Zoomorphology 107:287–292
Acknowledgments
We would like to express our gratitude to Dr. Łukasz Kaczmarek (A. Mickiewicz University, Poznań, Poland) for collecting and determining the species taxonomically, Dr. Magdalena Rost-Roszkowska (University of Silesia, Katowice, Poland) for reading the manuscript critically, Dr. Jagna Karcz (University of Silesia, Katowice, Poland), MSc Justyna Płoszaj-Pyrek (University of Silesia, Katowice, Poland), and MSc Malwina Marona (University of Silesia, Katowice, Poland) for their technical assistance.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Pavel Dráber
Rights and permissions
About this article
Cite this article
Poprawa, I., Schlechte-Wełnicz, W. & Hyra, M. Ovary organization and oogenesis in the tardigrade Macrobiotus polonicus Pilato, Kaczmarek, Michalczyk & Lisi, 2003 (Eutardigrada, Macrobiotidae): ultrastructural and histochemical analysis. Protoplasma 252, 857–865 (2015). https://doi.org/10.1007/s00709-014-0725-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-014-0725-x