Abstract
Embryonic development of reproductive organs in rediae of the digenean Bunocotyle progenetica was studied using transmission electron microscopy. The germinal primordium becomes morphologically distinct in early embryos as a weakly separated cell mass with a forming cavity. It consists of undifferentiated, differentiating, and supporting cells. As embryos develop, the supporting cells form a wall around the enlarging cavity. Other cells of the germinal primordium are incorporated into the wall as solitary cells or as small cell aggregations. Those situated posteriorly give rise to an incipient germinal mass functioning during postembryonic development. Undifferentiated and differentiating cells in the middle and the anterior part of the primordium ensure a considerable growth of the cavity wall, which incorporates solitary germinal cells. In advanced embryonic rediae, these cells mature, cleave, and give rise to germinal balls, which enter the forming brood cavity. In the most mature embryonic rediae, all these early cercarial embryos reside in a brood cavity, which is lined by that time with a syncytium continuous with the supporting tissue of the incipient germinal mass. Based on our results and the literature data, we suggest that the morphogenesis of the reproductive apparatus of the daughter parthenitae in hemiuroid digeneans may be characterized by (1) emergence of an incipient brood cavity within the germinal primordium, (2) formation of the cavity lining from the cells of the germinal primordium, (3) fragmentation and uneven distribution of the germinal material of the germinal primordium around the cavity, and (4) an anticipatory development of some of this germinal material.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
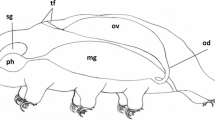
The digenean life cycle involves a successive change of the hermaphroditic and parthenogenetic generations (Dobrovolskij and Ataev 2003; Galaktionov and Dobrovolskij 2003). In hermaphroditic adults, the reproductive function is carried out by the reproductive system, which is always well-developed and represented by the ovary, the testes, the vitelline gland, and complex genital ducts and glands associated with them (see reviews in Smyth and Halton 1983; Galaktionov and Dobrovolskij 2003; Toledo and Fried 2019). In parthenogenetic sporocysts and rediae, the set of reproductive structures is more scanty and less constant. In the most complete variant, which is usually characteristic of the daughter parthenitae, these structures are represented by the germinal mass, the brood cavity, and the birth canal, which opens with the birth pore. At the same time, any of these structures may be absent in some digeneans (see review in Galaktionov and Dobrovolskij 2003).
According to Cort et al. (1948, 1949, 1954) and Van der Woude et al. (1953), related species (representing a family or a superfamily) usually have a fundamentally similar organization of the reproductive structures of the sporocysts and rediae. At the same time, there is evidence that this trend is not pronounced in some digenean taxa. In particular, the literature analysis in a study of the fine structure of the reproductive apparatus in the rediae of Bunocotyle progenetica by Podvyaznaya et al. (2020) revealed a diversity in the organization of the germinal material of the daughter parthenitae in hemiuroid digeneans.
To elucidate the nature of these differences, the data on the early stages of germinal development are needed. This consideration prompted our study of rediae of B. progenetica; the results of which are presented in this paper. Another aim of our study was to fill the gap in the knowledge of the embryonic stages of formation of reproductive structures in daughter parthenitae, which have never been investigated by transmission electron microscopy. Some attention to these issues was given by Ameel et al. (1949, 1951), Cort et al. (1954), and Van der Woude (1954) in a series of papers on germinal development in sporocysts and rediae of trematodes from different taxa. While the detailed descriptions of these researchers are still valid, they need to be verified, as they were performed at the light microscopic level. Besides, the ideas about the reproductive mode of sporocysts and rediae and the nature of the germinal mass and germinal cells have changed since the middle of the twentieth century. Thus, Cort and his co-authors, who were the first to describe the germinal mass, considered it as a “center of multiplication of germinal cells” and thought that the germinal mass consisted only of germinal cells and the adjoining early embryos (reviewed in Cort et al. 1954). They believed that the multiplication of germinal cells was an instance of polyembryony. This hypothesis was later renounced by Clark (1974) and Whitfield and Evans (1983), who suggested that sporocysts and rediae reproduced by budding and that their germinal cells were totipotent stem cells. Subsequent detailed histological (Dobrovolskij et al. 1983; Galaktionov and Dobrovolskij 2003; Dobrovolskij and Ataev 2003; Isakova 2011; Ataev 2017) and electron-microscopic (Podvyaznaya 2007; Podvyaznaya and Galaktionov 2014, 2018; Ataev and Tokmakova 2018; Podvyaznaya et al. 2020) studies of sporocysts and rediae in various digeneans showed that the germinal mass was a fully fledged organ of reproduction, consisting of cells of several types. Germinal cells were shown to have some ultrastructural features indicating that they were germ line cells (Klag et al. 1997; Podvyaznaya 2007; Podvyaznaya and Galaktionov 2014, 2018). The authors of these works consider the reproduction of sporocysts and rediae as apomictic parthenogenesis. At present, this viewpoint coexists with the opinion that germinal cells are stem cells (e.g. Reuter and Kreshchenko 2004; Wang et al. 2013, 2018; Sarfati et al. 2021). We disagree with this opinion and consider that germinal cells emerge in the process of differentiation of stem cells, as discussed in detail in our earlier study (Podvyaznaya and Galaktionov 2014).
Materials and methods
Sporocysts of Bunocotyle progenetica containing redial embryos in the brood cavity were obtained from mud snails Peringia ulvae. The snails were collected in summer (August 2016, 2018) and during hydrological winter (March 2017, 2021) in Sukhaya Salma Inlet of the Chupa Bay of the White Sea (the details of “winter” collections are described in Galaktionov and Podvyaznaya (2019)). The molluscs were sampled together with sediment and transported to the laboratory of the White Sea Biological Station of the Zoological Institute of the Russian Academy of Sciences, where they were cleared from the sediment with seawater and dissected under a stereomicroscope. We selected for dissection snails with a shell height of about 4–6 mm (aged 0+ to 1+), since they were most likely to be infected with B. progenetica (see for details Levakin (2008) and Levakin et al. (2013)). The life cycle stages of B. progenetica were identified according to Deblock (1980). The material for transmission electron microscopy was fixed with cold 3% glutaraldehyde solution on 0.1 М cacodylate buffer (pH=7.4) with subsequent (after 8–10 days) post-fixation with 1% osmium tetroxide solution on the same buffer at 4°C. Sucrose was added to the fixators and the wash buffer to reach an osmolarity of 760 mOsm. Then, the samples were dehydrated and embedded into Epon-Araldyte mixture following a standard procedure (Mollenhauer 1964). Series of alternating thin and semi-thin sections of the sporocysts were made with the help of Leica EM UC6rt ultratome. Thin sections were stained with aqueous solution of uranyl acetate and lead citrate and examined under Morgagni 268 transmission electron microscope operating at 80 kV. Semi-thin sections were stained with toluidine blue and viewed under a Leica DMLS light microscope. Living sporocysts containing embryonic rediae were also examined with the help of Olympus CH-40 light microscope equipped with an Olympus XC-30 digital camera.
Germinal primordium and its derivatives were found and described in embryonic rediae from four age groups (I–IV), which represented the successive stages of their development. The degree of maturity of the embryos was determined based on the structure of their covers (at TEM sections), approximate body shape (in living individuals and at the series of sections), the degree of gut development (at TEM sections, in living individuals) and the structure of developing brood cavity (at TEM sections, in living individuals). Thus, in the earliest of the studied stages (age group I), the body of the embryo is slightly elongated, and there is no morphologically distinct primordium of the gut (though excretory canals and excretory pores are already present); an incipient nuclei-containing outer layer of the tegument is surrounded by the primitive epithelium (based on TEM study of two individuals). Slightly more mature embryos (age group II) with a more elongated body have the same covers as the embryos at the previous stage of development, but their gut primordia are already pronounced and a lumen is forming in the midgut (based on TEM study of one individual). Larger embryos with an even longer body (age group III) have well-developed primordial organs of the digestive system, but their fully formed tegument is still covered by the primitive epithelium (based on TEM study of two individuals). Long embryonic rediae of age group IV lack the primitive epithelium and have a fully formed tegument and a brood cavity containing early cercarial embryos (based on TEM study of five individuals). In total, we examined embryos from 12 mother sporocysts obtained from 12 individuals of P. ulvae. Seven of these snails were collected during the cold season, while five snails were collected during the warm season.
Results
Germinal primordium in redial embryos of age group I
In early redial embryos of Bunocotyle progenetica, which do not yet have morphologically distinct primordia of the digestive system (Figs. 1a and 5a), we observed the germinal primordium in the posterior part of the body as a small group of cells, without a clear outer boundary and with a small cavity inside it (Figs. 1b and 5a). The cells of the germinal primordium have a heterogeneous structure. Conspicuous among them are fairly large cells, which have a large nucleus with a prominent nucleolus and small amount of heterochromatin (Fig. 1b). Their cytoplasm has a lower electron density compared to the other cells of the embryo and is mainly filled with free ribosomes and mitochondria. The cell mass of the germinal primordium also contains smaller undifferentiated cells with denser cytoplasm and a greater amount of condensed chromatin in the nucleus (Fig. 1b, c).
Germinal primordium in early redial embryos of Bunocotyle progenetica. a Light microscopic image of a living redial embryo of age group I. Bar 20 μm. b General view of the germinal primordium with an inner cavity in an embryo of age group I. Bar 2 μm. c, d TEM micrographs showing ultrastructural details of supporting cells of the germinal primordium in embryos of age group I, note cell outgrowths (arrowheads) and vesicles in cytoplasm. Bars 1 μm. e Longitudinal-oblique section of the germinal primordium in an embryo of age group II, asterisks mark solitary cells of germinal primordium incorporated into the cavity wall. Bar 2.5 μm. f Micrograph showing a cell aggregation incorporated into the cavity wall, note partition between cells (arrow) formed by supporting cell. Bar 2 μm. g Micrograph showing a solitary cell incorporated into the cavity wall. Bar 1 μm. Abbreviations: ac, aggregation of cells of germinal primordium; c, cavity of germinal primordium; gpc, cells of germinal primordium; re, redial embryo; rm, residual material; sp, supporting cells and their outgrowths; ve, vesicles
The germinal primordium of early embryos also contains several differentiated cells whose perikarya are located near its inner cavity. Their characteristic features are nuclei with a layer of condensed chromatin adjacent to the nuclear envelope, an electron-dense cytoplasm with vesicular structures including residual bodies and long flattened outgrowths (Fig. 1c, d). Some of the outgrowths limit the cavity of the primordium, and small irregularly shaped lamellar projections can be seen on their luminal surface (Fig. 1b–d). Other outgrowths of these supporting cells form thin interlayers between the cells of the primordium described above (Fig. 1c). The cavity inside the primordium always contains some residual material, which looks similar to the contents of the residual bodies (Fig. 1b, d).
Germinal primordium in redial embryos of age group II
In slightly more mature embryos with the gut at an early stage of development (Fig. 5b), the cavity inside the germinal primordium is enlarged, irregular in shape, and still contains residual material (Fig. 1e). At this stage, the cavity has a well-defined wall that separates it and the cells of the germinal primordium from the surrounding embryonic cells, many of which show signs of differentiation. The cavity wall is formed by several differentiated somatic cells (Fig. 1e). Their nuclei are distinguished by a thin layer of heterochromatin adjacent to the nuclear envelope (in addition to small clumps scattered in the nucleoplasm) and a pronounced nucleolus (Fig. 1e, g). The cytoplasm is mainly filled with free ribosomes, mitochondria, and a few vesicular structures. The luminal surface of the cells bears sparse lamellar projections (Fig. 1e, g). The cells forming the cavity wall are at the same time the supporting cells of the germinal primordium. In embryonic rediae of age group II, the cell mass of the germinal primordium is unevenly distributed around the cavity, being divided into several small cell aggregations, as well as several single cells lying separately (Fig. 1e–g). Each of these aggregations and cells is fully or partially surrounded by outgrowths of the cells of the cavity wall (Fig. 1f, g). The cells within the aggregations are often separated by partitions, which are also formed by the cells of the cavity wall (Fig. 1f). Similar to the previous developmental stage, the cells of the germinal primordium have a heterogeneous structure. Solitary cells incorporated into the cavity wall are usually the largest; they have an euchromatic nucleus with a large nucleolus.
Reproductive structures in embryonic rediae of age group III
In older embryos, which have a distinct embryonic gut (Figs. 2a and 5c) but are still covered by the primitive epithelium, the cavity of the germinal primordium is considerably extended and almost reaches the level of anterior end of the midgut (Figs. 2a and 5c). At this stage of development, it can be clearly seen that the cells bordering the cavity form a syncytium (Fig. 2c). In the anterior and the middle part of the cavity, its syncytial wall incorporates developing and mature germinal cells (Fig. 2b, c), which are solitary or arranged in small groups (2–3 cells in a single layer). They have a large euchromatic nucleus with a prominent nucleolus (Fig. 2b). The cytoplasm of these cells often contains electron-dense material of the germ granules in the form of small accumulations often lying near the nuclear envelope.
Embryonic reproductive structures in rediae of age group III of B. progenetica. a Light microscopic image of a living embryonic redia. Bar 50 μm. b, c TEM micrographs showing the cavity wall with incorporated germinal cells, note syncytial structure of supporting tissue (c). Bars 1 μm. d General view of incipient germinal mass. Bar 2 μm. e Micrograph showing a part of “peripheral” supporting syncytium sending outgrowths (arrows) into the depth of the germinal mass. Bar 2 μm. f Micrograph showing an “internal” supporting cell of the germinal mass, note germ granules in neighbouring germinal cell (arrowheads). Bar 2 μm. g “Peripheral” supporting syncytium of the germinal mass continuous with that of the cavity wall. Bar 2 μm. h Micrograph showing a septate junction between elements of supporting tissue. Bar 0.5 μm. Abbreviations: c, cavity; g, gut; ge, germinal cells; gm, primordial germinal mass; n, nucleus; sj, septate junction; sp, elements of supporting tissue
A well-defined primordium of the germinal mass lies in the posterior part of the cavity (Figs. 2d and 5c). It consists of undifferentiated cells, germinal cells at various degrees of maturity (these cells can be identified by the presence of germ granules), and relatively numerous supporting cells (Fig. 2d–f). Those of them that are located at the periphery of the incipient germinal mass form a syncytium, which is continuous with that of the cavity wall (Fig. 2g). They also send out outgrowths into the depth of the germinal mass, which separate its cellular elements (Fig. 2e). Young supporting cells forming inside the germinal mass can be easily distinguished from undifferentiated and germinal cells by a characteristic thin layer of heterochromatin adjacent to the nuclear envelope and multiple differently oriented outgrowths (Fig. 2d, f). These cells and their outgrowths form septate junctions with the outgrowths of the “peripheral” supporting syncytium (Fig. 2h).
Reproductive structures in embryonic rediae of age group IV
At later stages of their embryonic development, the rediae continue to grow, and the cavity of their germinal primordium enlarges, extending also posteriorly of the incipient germinal mass (Figs. 3a, d and 5d, e). In the cavity wall, individual germinal cells are detected rarely, and tiny early cercarial embryos, which sometimes form small groups, are present instead (Figs. 3b and 5d). Some early germinal balls are also found in the cavity (Figs. 3d and 5d), which can now be termed, in accordance with its main function, the brood cavity. In the most mature embryonic rediae, nearly all the cercarial embryos (with rare exceptions) that have started their development in the cavity wall float freely in the brood cavity (Figs. 3c and 5e), which is lined with a thin nucleated syncytium almost free from germinal cells and embryos.
Embryonic reproductive structures in rediae of age group IV of B. progenetica. a Light microscopic image of a living embryonic redia. Bar 100 μm. b TEM micrograph showing the cavity wall with incorporated early cercarial embryos. Bar 2 μm. c Light microscopic image of a compressed late embryonic redia with a group of germinal balls in the brood cavity. Bar 100 μm. d General view of the embryonic germinal mass with a forming embryo on its edge (arrow). Bar 2 μm. e Micrograph showing a single germinal cell in the brood cavity near embryonic germinal mass. Bar 2 μm. f Pyknotic body within the germinal mass. Bar 1 μm. g Micrograph showing basal surface of the embryonic germinal mass lined by a thin layer of basal matrix (arrowheads). Bar 1 μm. h Micrograph showing a lacuna in the germinal mass, note septate junctions (arrowheads) in supporting tissue, one of them is enlarged in insertion. Bar 1 μm. i Epithelium of incipient birth canal in its proximal part. Bar 2 μm. j Micrograph showing connection (arrow) of epithelial cell with brood cavity lining. Bar 1 μm. k Distal part of birth canal (marked by arrows) lined with tegument. Bar 1 μm. Abbreviations: c, cavity; cl, lining of developing brood cavity; e, cercarial embryos; ep, epithelium of developing birth canal; g, gut; ge, germinal cells; gm, primordial germinal mass; l, lumen of developing birth canal; la, lacuna; pb, pyknotic body; ph, pharynx; rbw, redial body wall; sp, supporting tissue; te, tegument
The size of the germinal mass remains almost the same, and no embryos can be seen inside it (Fig. 3d). Early cleavage stages are found only occasionally in the germinal cells at the border of the mass (Fig. 3d). In advanced embryonic rediae, the basal surface of the germinal mass is lined with a fine layer of dense basal matrix, which has never been noted at the earlier stages of the morphogenesis (Fig. 3g). The incipient germinal mass of late embryonic rediae also contains rare pyknotic bodies and incipient lacunae (Fig. 3f, h), which develop further during the postembryonic period (Podvyaznaya et al. 2020). Septate junctions between the “peripheral” and the “internal” supporting cells of the germinal mass are always seen in the forming lacunae (Fig. 3h). In some late embryonic rediae, we observed solitary germinal cells freely lying in the brood cavity sometimes close to the germinal mass (Fig. 3e). The fate of these cells remains obscure.
An incipient birth canal can be seen in late embryonic rediae (Figs. 3i–k and 5e). Its distal part is represented by an invagination of the tegument near the anterior end of the pharynx (Figs. 3k and 5e). The proximal part of the birth canal looks like a duct with a narrow lumen lined with a cellular epithelium (Fig. 3i, j). The basal surface of this epithelium rests on a fine layer of basal matrix, while the luminal surface is irregularly curved. A septate junction joins the epithelium of the birth canal to the lining of the brood cavity (Fig. 3j). Unfortunately, we failed to identify the site of the transition of the cellular epithelium into the tegument of the distal part of the birth canal. The musculature of the birth canal in embryonic rediae lags behind from the epithelial lining in its development and is feebly expressed.
Seasonal changes in fine morphology of reproductive structures of embryonic rediae
The structure of the germinal primordium in redial embryos collected in the warm and the cold season was fundamentally similar, except for some ultrastructural details of the germinal cells at the middle and the late stages of embryonic development (age groups III–IV). In particular, in more advanced “winter” embryonic rediae, the germinal cells in the incipient germinal mass and, in rare cases, also the cells incorporated into the wall of the developing brood cavity contain clusters, sometimes quite large, of lysosome-like vesicles, including residual bodies (Fig. 4a). Alongside with them, multiple autophagosomes and occasional phagosomes are detected (Fig. 4a, b). We also observed exocytosis of the contents of residual bodies from the germinal cells (Fig. 4c, d). In the germinal cells of the embryos collected in summer, the structures mentioned above were rare.
Details of ultrastructure of germinal cells in “winter” advanced embryonic rediae of B. progenetica. a Micrograph showing lysosome-like vesicles and a forming phagosome (arrowhead) in a germinal cell. Bar 0.5 μm. b Germinal cell with multiple autophagosomes (arrowheads). Bar 0.5 μm. c, d Micrographs showing discharge of residual bodies from germinal cells. Bars 0.5 μm. Abbreviations: ge, germinal cells; n, nucleus; sp, supporting tissue of germinal mass
Discussion
In this work, we described the fine morphology of reproductive structures in embryonic rediae using evidence from a hemiuroid digenean Bunocotyle progenetica. We followed the development of the germinal mass and the brood cavity and showed for the first time that in some trematodes, including B. progenetica, the cavity lining is formed as a derivative of the germinal primordium, from which the germinal mass also develops. Based on our data and the information available in the literature, we identified common features characterizing the morphogenesis of the reproductive apparatus of daughter parthenitae of the hemiuroid digeneans as well as the specific features of this process in B. progenetica.
Development of germinal primordium in rediae of B. progenetica
According to our data, in the rediae of B. progenetica, the germinal primordium becomes morphologically distinguishable in early embryos at the initial stages of organogenesis (Fig. 5). Already at this stage of development, an incipient brood cavity is formed within a weakly isolated cell mass of the primordium. The structural variability of the cells of the germinal primordium (their size and shape, nucleus structure, and the set of cell organelles) indicates active processes of growth and differentiation. Somatic supporting cells, which form the cytoplasmic interlayers between the other cells of the primordium and initially limit its incipient inner cavity by their outgrowths, are among the first to differentiate. As the cavity forms, the cytoplasm of these cells seems to degenerate partially, as indicated by the presence of residual bodies in the cells themselves and a similar residual material filling the expanding intercellular space. It is possible that the large cells with euchromatic nuclei and large nucleoli are the first differentiating germinal cells of the primordium. Groups of similar cells have been repeatedly described at the light microscopic level as incipient germinal masses in embryos of parthenitae of other trematodes (Ameel et al. 1949; Cort et al. 1949; Van der Woude et al. 1953; Cort et al. 1954; Isakova 2011).
Diagram summarizing successive stages of development of the reproductive structures (marked in grey) in embryonic rediae of B. progenetica, lateral view. a Germinal primordium in an embryo of age group I. b Germinal primordium in an embryo of age group II. c Incipient reproductive structures in an embryonic redia of age group III. d, e Incipient reproductive structures in embryonic rediae of age group IV. Abbreviations: bc, primordial birth canal; c, developing brood cavity; cw, cavity wall; e, cercarial embryos; g, gut; gm, primordial germinal mass; gp, germinal primordium; ph, pharynx of embryonic rediae
During subsequent embryonic development, the cavity of the germinal primordium enlarges, while the supporting cells of the first generation form a wall around it. Undifferentiated and differentiating cells of the primordium become incorporated in the cavity wall in an irregular manner: as single cells or small cell aggregations. Later, these fragments of the germinal primordium develop differently. The group of cells located closer to the posterior body end gives rise to the primordium of the germinal mass, with its typical cell composition and differentiated supporting tissue, which is continuous with the cavity wall. As the developing rediae increase in length, undifferentiated and differentiating cells situated in the middle and the anterior part of the germinal primordium ensure a considerable growth of the cavity wall, which at this stage of development is represented by a syncytium with inclusions of separate, sometimes closely arranged germinal cells. In advanced embryos, these germinal cells mature, cleave, and give rise to germinal balls (cercarial embryos). The latter gradually move into the nearly formed brood cavity (Fig. 5). In the most mature embryonic rediae, this process is close to completion; as it is being relieved from the germinal cells and the embryos, the syncytium of the cavity wall loses its supporting role and persists only as the lining of the brood cavity.
On the contrary, the germinal cells in the incipient germinal mass of late embryonic rediae mostly remain in the state of relative inactivity. At the same time, the germinal mass itself acquires the features characteristic of its mature state (Galaktionov and Podvyaznaya 2019; Podvyaznaya et al. 2020). The first lacunae and a few pyknotic bodies appear in it, and an incipient layer of the basal matrix arises under its basal surface. Further intensive development of the germinal mass, accompanied by the production of new germinal cells and embryos, begins after the release of the redia from the sporocyst. At the late embryonic stage, the rediae acquire an incipient lining of the birth canal. Unfortunately, we did not manage to ascertain its origin.
To sum up, the germinal development of hemiuroid rediae of B. progenetica is characterized by (1) an early emergence of the incipient brood cavity within the germinal primordium, (2) the formation of the cavity wall by the cells of the primordium, (3) the fragmentation of germinal material of the primordium, and (4) a different localization of these fragments and a different order of their development.
Seasonal changes in the structure of reproductive organs in developing rediae of B. progenetica
In a recent study dealing with the effect of low seasonal temperatures on the structure of reproductive organs in the rediae of B. progenetica (Galaktionov and Podvyaznaya 2019), we recorded a mass degeneration of germinal cells and early embryos in the “winter” parthenitae at the postembryonic stage of their development (1), the filling of the brood cavity with cell debris (2), and the absorption and digestion of degeneration products by the cells of the cavity lining (3). None of these processes was observed in “winter” embryonic rediae, including their late stages with an already formed primordial germinal mass and the first germinal balls in the brood cavity. At the same time, many germinal cells of advanced embryonic stages show clear signs of intensification of autophagous processes, similarly to what was observed in the “winter” mature rediae of B. progenetica (Galaktionov and Podvyaznaya 2019). In both cases, an enhancement of the autophagy is likely to be associated with the lack of exogenous nutrition during the cold season. In embryonic rediae, we observed the “liberation” of germinal cells from the end products of the autophagy, indicating that these processes were reversible and did not lead to cell death.
Shared and specific features in the formation of reproductive structures in the rediae of the Hemiuroidea and the Echinostomatoidea
A developmental type similar to that described above for the rediae of B. progenetica had been described by Ameel et al. (1949) and Cort et al. (1954) for another hemiuroid digenean, Halipegus eccentricus. According to their data, a cavity appears in the germinal primordium (“initial germinal mass”) of the early redial embryos; then the cavity enlarges and the germinal material of the primordium splits into several parts (“individual germinal masses”), which “are attached to the body wall” (for terminology, see Ameel et al. 1949). During further development, one large germinal mass, located in the posterior body part, remains and functions throughout the postembryonic period of ontogenesis of H. eccentricus rediae. The small “individual germinal masses” lying anteriorly (in the case of B. progenetica, they correspond to the closely spaced germinal cells embedded in the cavity wall) gradually disappear: all the germinal cells comprising them give rise to embryos, which are released into the brood cavity. The process of “liberation” of the anterior and middle parts of the cavity wall from the fragmented germinal elements in H. eccentricus, in contrast to B. progenetica, occurs not only at the late stages of embryogenesis but also at the early stage of postembryonic development. It should be noted that although Ameel et al. (1949) and Cort et al. (1954) provided a detailed description of the germinal material of parthenitae of H. eccentricus, they gave almost no information about the somatic supporting structures of the germinal primordium. This is unsurprising, as these delicate structures cannot be reliably detected at total preparations of living rediae, which were the only kind of material studied by Ameel et al. (1949).
An apparent similarity of the early stages of germinal development in the daughter parthenitae of phylogenetically distant hemiuroid digeneans Halipegus and Bunocotyle, which represent different clades within the superfamily, suggests that the abovementioned features of this rather complex development may be characteristic of the entire superfamily Hemiuroidea. At the same time, they may be expressed in different degree in different representatives. For instance, in B. progenetica, relatively few germinal elements develop outside the definitive germinal mass, while in H. eccentricus, these germinal elements are much more numerous and form pronounced clusters (“individual germinal masses”). The temporal framework of some processes may also differ, as evidenced by H. eccentricus and B. progenetica (see above). As a result, daughter parthenitae of hemiuroid digeneans show a marked diversity in the organization of germinal material at the postembryonic stage (Ameel et al. 1949; Dollfus 1950; Matthews 1980, 1982; Stunkard 1980; Kofiadi 1995), as we noted earlier (Podvyaznaya et al. 2020). In this connection, it is important to note that we observed almost all variants of the arrangement of germinal cells and the earliest embryonic stages (in the germinal mass, brood cavity, or cavity wall), described previously in light microscopic studies of hemiuroid parthenitae (Ameel et al. 1949; Dollfus 1950; Stunkard 1980; Kofiadi 1995), in B. progenetica rediae at different stages of their development (Podvyaznaya et al. 2020; this study).
In their comparative studies, Ameel et al. (1949) and Cort et al. (1954) noted certain similarities in the structure of the germinal primordium in early redial embryos and the germinal mass of mature parthenitae between H. eccentricus and those echinostomatoids that they studied in detail (Cort et al. 1948, 1949, 1954). However, they pointed out a significant difference in the patterns of early germinal development of daughter parthenitae in these two trematode taxa. The essence of the difference, as they saw it, was the fact that in hemiuroid digeneans, some of the germinal material of the germinal primordium underwent an anticipatory development, leading to the formation of numerous cercarial embryos and preceding the formation of the definitive germinal mass, while in echinostomatoid digeneans, there was no such stage in the embryonic development of rediae (Cort et al. 1954). Our data on the development of the rediae of B. progenetica and Himasthla elongata (Podvyaznaya and Galaktionov 2014; Galaktionov et al. 2015; this study) confirmed these observations and complemented them with new details. In echinostomatoid digeneans, the entire germinal primordium transforms into the germinal mass, around which a brood cavity lacking its own cell lining is formed during the postembryonic period (Podvyaznaya and Galaktionov 2014; Galaktionov et al. 2015). In hemiuroid digeneans, the development of the cavity precedes that of the germinal mass during the embryogenesis of rediae, and the germinal primordium eventually gives rise not only to the definitive germinal mass but also to the lining of the brood cavity.
Light microscopic data available on the reproduction of the parthenitae (see reviews in Cort et al. 1954; Galaktionov and Dobrovolskij 2003) suggest that besides the above two patterns of early germinal development in embryos and young rediae, there are other, yet poorly known ways of specialization of the germinal primordium. Our study highlighted the necessity of taking into account the structure and the transformations of the somatic supporting elements of the germinal primordium and their derivatives during investigation and analysis of these processes. So far, TEM remains the only technique allowing a reliable study of the formation of these elusive structures.
References
Ameel DJ, Cort WW, Van der Woude A (1949) Germinal development in the mother sporocyst and redia of Halipegus eccentricus Thomas, 1939. J Parasitol 35(6):569–578. https://doi.org/10.2307/3273635
Ameel DJ, Cort WW, Van der Woude A (1951) Development of the mother sporocyst and rediae of Paragonimus kellicotti Ward, 1908. J Parasitol 37:395–404. https://doi.org/10.2307/3273575
Ataev GL (2017) Reproduction of trematode parthenitae: review of main theories. Nauka, Saint Petersburg (In Russian)
Ataev GL, Tokmakova AS (2018) Reproduction of Echinostoma caproni mother sporocysts (Trematoda). Parasitol Res 117:2419–2426. https://doi.org/10.1007/s00436-018-5930-7
Clark RB (1974) Interpretation of life history pattern in Digenea. Int J Parasitol 4:115–123. https://doi.org/10.1016/0020-7519(74)90093-9
Cort WW, Ameel DJ, Van der Woude A (1948) Studies on germinal development in rediae of the trematode order Fasciolatoidea Szidat, 1936. J Parasitol 34:428–451. https://doi.org/10.2307/3273607
Cort WW, Ameel DJ, Van der Woude A (1949) Germinal masses in redial embryos of an echinostome and a psilostome. J Parasitol 35(6):579–582. https://doi.org/10.2307/3273636
Cort WW, Ameel DJ, Van der Woude A (1954) Germinal development in the sporocysts and rediae of the digenetic trematodes. Exp Parasitol 3:185–255. https://doi.org/10.1016/0014-4894(54)90008-9
Deblock S (1980) Inventaire des trématodes larvaires parasites des mollusques Hydrobia (Prosobranches) des côtes de France. Parassitologia 22:1–105
Dobrovolskij AA, Galaktionov KV, Muhamedov GK, Sinha BK, Tihomirov IA (1983) Parthenogenetic generations of trematodes. Tr Leningr Obshestva Estestvoispytatelei 82:1–108 (In Russian)
Dobrovolskij AA, Ataev GL (2003) The nature of reproduction of trematodes rediae and sporocysts. In: Combes C, Jourdane J (eds) Taxonomy ecology and evolution of metazoan parasites, vol I. PUP, Perpignan, pp 249–272
Dollfus RP (1950) Hôtes et distribution géographique des cercaires cystophores. Ann Parasitol Hum Comp 25:276–296
Galaktionov KV, Dobrovolskij AA (2003) The biology and evolution of trematodes. Kluwer Academic Publishers, Dordrecht, London
Galaktionov KV, Podvyaznaya IM, Nikolaev KE, Levakin IA (2015) Self-sustaining infrapopulation or colony? Redial clonal groups of Himasthla elongata (Trematoda: Echinostomatidae) in Littorina littorea (Gastropoda: Littorinidae) do not support the concept of eusocial colonies in trematodes. Folia Parasitol 62:067. https://doi.org/10.14411/fp.2015.067
Galaktionov KV, Podvyaznaya IM (2019) Reproductive organs of trematode parthenitae during the cold season: an ultrastructural analysis using evidence from rediae of Bunocotyle progenetica (Markowski, 1936) (Digenea, Hemiuroidea). Invertebr Zool 16(4):329–341. https://doi.org/10.15298/invertzool.16.4.02
Isakova NP (2011) Germinal mass of the rediae of Trematoda. Parazitologiya 45:358–366 (In Russian)
Klag J, Niewiadomska K, Czubaj A (1997) Ultrastructural studies on the sporocyst wall of Diplostomum pseudospathaceum Niewiadomska, 1984 (Digenea, Diplostomidae). Int J Parasitol 27:919–929. https://doi.org/10.1016/S0020-7519(97)00063-5
Kofiadi AK (1995) An organisation of germinal masses and some questions of the dynamics of daughter sporocysts’ development in Hemiuridae gen. sp. Parazitologiya 29:404–416 (In Russian)
Levakin IA (2008) Implementation of the monoxenous life cycle of Bunocotyle progenetica (Trematoda: Hemiuroidea,Bunocotylinae) under conditions of the White Sea intertidal. Dissertation, Zoological Institute RAS, St. Petersburg (In Russian)
Levakin IA, Nikolaev KE, Galaktionov KV (2013) Long-term variation in trematode (Trematoda, Digenea) component communities associated with intertidal gastropods is linked to abundance of final hosts. Hydrobiologia 706:103–118. https://doi.org/10.1007/s10750-012-1267-x
Matthews BF (1980) Cercaria vaullegeardi Pelseneer, 1906 (Digenea: Hemiuridae); the daughter sporocyst and emergence of the cercaria. Parasitology 81:61–69. https://doi.org/10.1017/S0031182000055049
Matthews BF (1982) The biology of British marine hemiuridae. PhD Thesis, University of Plymouth. http://hdl.handle.net/10026.1/2238
Mollenhauer HH (1964) Plastic embedding mixtures for use in electron microscopy. Stain Technol 39:111–114. https://doi.org/10.3109/10520296409061216
Podvyaznaya IM (2007) An ultrastructural study of reproduction in the sporocysts of Prosorhynchoides gracilescens (Digenea: Bucephalidae). Parasitol Res 101:35–42. https://doi.org/10.1007/s00436-006-0443-1
Podvyaznaya IM, Galaktionov KV (2014) Trematode reproduction in the molluscan host: an ultrastructural study of the germinal mass in the rediae of Himasthla elongata (Mehlis, 1831) (Digenea: Echinostomatidae). Parasitol Res 113:1215–1224. https://doi.org/10.1007/s00436-014-3760-9
Podvyaznaya IM, Galaktionov KV (2018) Reproduction of trematodes in the molluscan host: an ultrastructural study of the germinal mass and brood cavity in daughter rediae of Tristriata anatis Belopolskaia, 1953 (Digenea: Notocotylidae). Parasitol Res 117:2643–2652. https://doi.org/10.1007/s00436-018-5956-x
Podvyaznaya IM, Petrov AA, Galaktionov KV (2020) The fine structure of the germinal mass, brood cavity and birth canal of the rediae of the monoxenous hemiuroid digenean Bunocotyle progenetica Chabaud & Buttner, 1959. J Helminthol 94(e85):1–10. https://doi.org/10.1017/S0022149X19000816
Reuter M, Kreshchenko N (2004) Flatworm asexual multiplication implicates stem cells and regeneration. Can J Zool 82:334–356. https://doi.org/10.1139/z03-219
Sarfati DN, Li P, Tarashansky AJ, Wang B (2021) Single-cell deconstruction of stem-cell-driven schistosome development. Trends Parasitol 37(9):790–802. https://doi.org/10.1016/j.pt.2021.03.005
Smyth JD, Halton DW (1983) The Physiology of Trematodes. 2ndedition, CUP, Cambridge, New York, Melbourne
Stunkard HW (1980) The morphology, life history, and systematic relations of Tubulovesicula pinguis (Linton, 1940) Manter, 1947 (Trematoda: Hemiuridae). Biol Bull 159:737–751. https://doi.org/10.2307/1540838
Toledo R, Fried B (2019) Digenetic Trematodes. (AEMB, 1154) Springer, Cham
Van der Woude A (1954) Germ cell cycle of Megalodiscus temperatus (Stafford, 1905) Harwood, 1932 (Paramphistomidae: Trematoda). Am Midl Nat 51(1):172–202. https://doi.org/10.2307/2422218
Van der Woude A, Cort WW, Ameel DJ (1953) The early development of the daughter sporocysts of Strigeoidea (Trematoda). J Parasitol 39:38–44. https://doi.org/10.2307/3274057
Wang B, Collins JJ, Newmark PA (2013) Functional genomic characterization of neoblast-like stem cells in larval Schistosoma mansoni. eLife. https://doi.org/10.7554/eLife.00768
Wang B, Lee J, Li P, Saberi A, Yang H, Liu C, Zhao M, Newmark PA (2018) Stem cell heterogeneity drives the parasitic life cycle of Schistosoma mansoni. eLife. https://doi.org/10.7554/eLife.35449.001
Whitfield PJ, Evans NA (1983) Parthenogenesis and asexual multiplication among parasitic platyhelminths. Parasitology 86:121–160. https://doi.org/10.1017/S0031182000050873
Acknowledgements
The authors are grateful to the White Sea Biological Station of the Zoological Institute of the Russian Academy of Sciences (ZIN RAS) for providing fieldwork infrastructure. We would also like to thank Natalia Lentsman for her help with the preparation of the English version of the manuscript. The research was completed using equipment of the Core Facilities Centre “Taxon” (http://www.ckp-rf.ru/ckp/3038/?sphrase_id=8879024) at the Zoological Institute of the Russian Academy of Sciences (St. Petersburg, Russia).
Funding
The fieldwork at the White Sea Biological Station and at the “Taxon” Research Resource Center was partly financed by the research programme of Zoological Institute RAS, project number AAAA-A19-119020690109-2. The treatment and analysis of the accumulated data were supported by the Russian Science Foundation (Grant No. 18-14-00170).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Section Editor: David Bruce Conn
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Podvyaznaya, I.M., Galaktionov, K.V. Germinal development in embryonic rediae of the hemiuroid digenean Bunocotyle progenetica: an ultrastructural study. Parasitol Res 120, 4001–4012 (2021). https://doi.org/10.1007/s00436-021-07349-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-021-07349-8