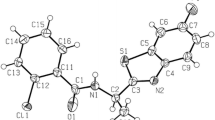
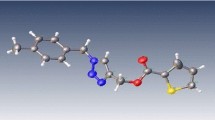
Abstract
A series of 21 amide linked 1,4-disubstituted-1,2,3-triazoles were achieved via one-pot synthesis through Cu(I) catalyzed click reaction between terminal alkynes and 2-azido-N-substituted acetamides. Newly formed triazoles were characterized by various spectroscopic techniques (FT-IR, 1H NMR, 13C NMR spectroscopy, and HRMS) and investigated for in vitro antitubercular evaluation against bacteria, i.e., Mycobacterium tuberculosis and antimicrobial evaluation against Bacillus subtilis, Staphylococcus epidermidis, Escherichia coli, Pseudomonas aeruginosa, Candida albicans, and Aspergillus niger. Some of the synthesized triazole derivatives were found to exhibit moderate inhibitory activity against the tested antitubercular strain, whereas one compound displayed a significant inhibitory activity against most of the tested microbial strains.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tuberculosis is one of the highly contagious and major challenging diseases around the world. Mycobacterium tuberculosis, etiologic agent of tuberculosis, led to the death of large number of people for more than five millennia. Despite availability of useful vaccine bacille Calmette–Guerin (BCG) and effective chemotherapy, still, tuberculosis has become leading cause of mortality. Infectious diseases caused by microorganisms have also been increasing threat to public health. Use of conventional antibiotics has now become ineffective due to increasing resistance in strains against them. Emergence of multi-drug resistant strains against tuberculosis and microbial infections has become an alarming issue that drew attention of medicinal researchers to develop new drug profile for their effective treatment. In this perspective, triazoles have proved to be potent antitubercular and antimicrobial agents due to extensive therapeutic importance [1, 2].
Triazoles, a significant class of nitrogen containing heterocycles, are attractive structural motifs which displayed versatility in diverse fields such as material sciences, synthetic organic chemistry, and drug discovery [3,4,5]. Triazole derivatives have been widely utilized in industries as dyestuffs, agrochemicals, optical brighteners, photostabilizers, corrosion inhibitors [6, 7], etc. In spite of these industrial applications, 1,2,3-triazole scaffolds emerged as imperative pharmacophore owing to their prevalent biological properties like antiviral [8], antimicrobial [9, 10], antiHIV [11], antiproliferative [12], antimalarial [13, 14], anticancer [15], antiallergic [16], anticonvulsant [17], antioxidant [18], antitubercular [19], etc.
In past, plethora of methods have been developed for synthesis of 1,2,3-triazoles for different purposes [20]. Most established strategy for the synthesis of disubstituted 1,2,3-triazoles from azides and terminal alkynes was introduced by Huisgen [21]. This conventional approach affords formation of both 1,4- and 1,5-disubstituted triazoles at elevated temperature. To conquer the problem of poor regioselectivity, Sharpless [22] and Meldal [23] in 2002 invented Cu(I) catalyzed click reaction of terminal alkynes and azides to generate 1,4-disubstituted 1,2,3-triazoles only. However, this experimentally simple and highly regioselective approach appears to possess enormous scope in many other areas like bioconjugation [24], polymer chemistry [25], peptidomimetics [26], and supramolecular chemistry [27].
Prompted by the above considerations and as an extension of our previous work on synthesis of biologically active 1,4-disubstituted 1,2,3-triazoles [28,29,30,31], we, herein, reported the synthesis, and antitubercular and antimicrobial potential of amide linked 1,4-disubstituted 1,2,3-triazoles via Cu(I) catalyzed click reaction between terminal alkynes and 2-azido-N-substituted acetamides. All the synthesized triazoles were well characterized by the spectroscopic techniques FT-IR, 1H NMR, 13C NMR spectroscopy, and HRMS, and also assessed for in vitro antitubercular potential against Mycobacterium tuberculosis; antimicrobial potential against Bacillus subtilis, Staphylococcus epidermidis, Escherichia coli, Pseudomonas aeruginosa, Candida albicans, and Aspergillus niger.
Results and discussion
Chemistry
Synthetic strategy for preparation of N-aryl-2-(4-substituted-1H-1,2,3-triazol-1-yl)acetamides 4a–4u is given in Scheme 1. 2-Bromo-N-substituted acetamides 2a–2g [32] were synthesized by reacting aromatic amines 1a–1g with bromoacetyl bromide in the presence of base potassium carbonate in dichloromethane. Afterwards, target 1,4-disubstituted 1,2,3-triazoles 4a–4u with amide functionality were obtained by one-pot synthesis through click reaction between commercially available terminal alkynes 3a–3c and 2-azido-N-substituted acetamides (which were attained in situ by reaction of 2-bromo-N-substituted acetamides 2a–2g and sodium azide) by utilizing catalytic amount of copper sulfate pentahydrate and sodium ascorbate in N,N-dimethylformamide to lead desired products 4a–4u in good yield.

Structures of newly synthesized triazole derivatives 4a–4u were explicated by different spectral techniques, i.e., FT-IR, 1H NMR, 13C NMR spectroscopy, and HRMS. FT-IR spectra of synthesized compounds confirmed the formation of triazoles due to appearance of absorption bands in the region of 3211–3308 cm−1 (N–H, str., amide), 3123-3185 cm−1 (C–H, str., triazole ring), and 1666–1703 cm−1 (> C=O str. amide). In 1H NMR spectra, singlet resonated in the region at δ = 8.54–8.76 and 10.39–11.18 ppm due to triazolyl proton and N–H proton, respectively. Moreover, in 13C NMR spectra, signals displayed in region at δ = 145.6–146.8, 123.0–124.3, and 164.0–165.8 ppm designated to C4, C5 of triazole ring and carbonyl carbon of amide linkage. Furthermore, the results obtained from high-resolution mass spectral analysis were found in accordance to their calculated values.
Antitubercular activity
All synthesized triazole derivatives 4a–4u were screened for in vitro antitubercular activity against bacterial strain M. tuberculosis H37RV (MTCC 200) by Lowenstein–Jensen (L. J.) slope method. Isoniazid was used as a standard drug. Results were recorded in terms of minimum inhibitory concentration (µmol/cm3). As reflected from Table 1, some of synthesized triazole derivatives were found to display noteworthy antitubercular activity against strain used for experimentation. Compound 4a possessed good antitubercular potential in comparison to the standard drug. It has been deduced that compound 4c (0.1933 µmol/cm3), 4l (0.1530 µmol/cm3), 4q (0.1831 µmol/cm3), and 4t (0.1333 µmol/cm3) also showed moderate inhibitory activity against tested bacterial strain.
Results of antitubercular screening inferred that compounds possessing nitro group on anilide ring found to display a better inhibitory activity in comparison to compounds substituted with methoxy group on anilide ring. Among synthesized compounds substituted with halogen moiety, compound having both fluoro and bromo groups was found to behave as good antitubercular agent.
Antimicrobial activity
All newly synthesized triazole derivatives 4a–4u were assessed for in vitro antimicrobial evaluation against B. subtilis (MTCC 441), S. epidermidis (MTCC 6880) (Gram-positive bacteria), E. coli (MTCC 1652), P. aeruginosa (MTCC 424) (Gram-negative bacteria), and C. albicans (MTCC 183), and A. niger (MTCC 8189) (fungi) by serial dilution technique [33]. Ciprofloxacin and fluconazole were used as standard drugs against bacteria and fungi, respectively. Results were recorded in terms of MIC (minimum inhibitory concentration) which was expressed in µmol/cm3.
It can be revealed from antibacterial screening data (Table 2) that most of the synthesized triazoles exhibited moderate-to-good antibacterial activity against tested bacterial strains. Among all the synthesized triazole derivatives, compound 4r found to exhibit a significant antibacterial activity against all the bacterial strains. Compound 4g (MIC 0.0190 µmol/cm3), 4k (MIC 0.0201 µmol/cm3), and 4r (MIC 0.0199 µmol/cm3) against B. subtilis; 4c (MIC 0.0193 µmol/cm3), 4q (MIC 0.0183 µmol/cm3), 4r (MIC 0.0199 µmol/cm3), and 4t (MIC 0.0167 µmol/cm3) against S. epidermidis; 4d (MIC 0.0211 µmol/cm3), 4f (MIC 0.0175 µmol/cm3), 4q (MIC 0.0183 µmol/cm3), and 4r (MIC 0.0199 µmol/cm3) against E. coli; 4d (MIC 0.0211 µmol/cm3), 4 m (MIC 0.0168 µmol/cm3), and 4r (MIC 0.0199 µmol/cm3) against P. aeruginosa displayed activity comparable to standard drug.
Results clearly illustrated that compounds possessing electron withdrawing nitro group on anilide rings exhibited a better bactericidal activity in comparison to compounds with electron-donating methoxy group. In most of cases, compounds with 4-bromo substituents on anilide ring displayed a better inhibitory activity in comparison to compounds with the other halogen groups.
Results of antifungal screening (Table 3) clearly give a picture that some of triazole derivatives showed promising antifungal activity against tested fungal strains. Among the synthesized triazoles, compounds 4d and 4k exhibited a better antifungal potency against both the fungal strains used. Compounds 4d (MIC 0.0211 µmol/cm3), 4f (MIC 0.0175 µmol/cm3), and 4k (MIC 0.0201 µmol/cm3) against C. albicans; 4d (MIC 0.0211 µmol/cm3), 4j (MIC 0.0185 µmol/cm3), 4k(MIC 0.0201 µmol/cm3), and 4r (MIC 0.0199 µmol/cm3) against A. niger demonstrated appreciable antifungal activity. It is appreciable that some of molecules like 4q, 4t against S. epidermidis; 4f, 4q against E. coli; 4m against P. aeruginosa displayed a better activity in comparison to the standard drug used.
It can be analyzed from antifungal screening that triazoles with electron withdrawing nitro group on anilide ring displayed considerable improvement in antifungal activity as compared to electron-donating methoxy group. In case of A. niger, triazoles with 4-fluorophenyl moiety found to possess a better fungicidal activity than triazole derivatives having other halogens. It is evident from results that compounds 4f, 4k against C. albicans, and 4j, 4k, 4r against A. niger were found to exhibit a better inhibitory activity in comparison to standard drug used.
Conclusion
In summary, synthesis of a series of N-aryl-2-(4-substituted-1H-1,2,3-triazol-1-yl)acetamides 4a–4u were achieved via one-pot Cu(I) catalyzed click reaction between terminal alkynes and 2-azido-N-substituted acetamides. Synthesized triazole derivatives were evaluated for in vitro antitubercular potential against Mycobacterium tuberculosis and antimicrobial potential against Bacillus subtilis, Staphylococcus epidermidis, Escherichia coli, Pseudomonas aeruginosa, Candida albicans, and Aspergillus niger. Compound 4a overall displayed appreciating antitubercular activity against M. tuberculosis. Compounds 4d and 4k emerged as potent antifungal agent than the other triazole derivatives, while compound 4r showed noteworthy microbicidal activity against most of the tested strains used.
Experimental
All reagents and solvents used in the present work were commercially available grade and used as received without further purification. Nutrient broth and Sabouraud dextrose broth used in antimicrobial activity were purchased from Hi-Media, Mumbai. Melting points of the synthesized compounds were recorded on an Electrothermal Melting Point apparatus. The FT-IR absorption spectra were scanned on IR AFFINITY-I FT-IR (SHIMAZDU) spectrometer using potassium bromide (KBr) powder and wave numbers are noted in cm−1. Nuclear magnetic resonance spectra (1H and 13C) were recorded on a 400 MHz BrukerAvance-III spectrometer operating at 400 MHz and 100 MHz, respectively, in DMSO-d6. Chemical shifts (δ) are observed in parts per million (ppm). Coupling constant (J) values were stated in Hertz (Hz). High-resolution mass spectra (HRMS) were scanned on Waters Micromass Q-Tof Micro (ESI) spectrometer. Values were represented in m/z. Ready-made silica gel plates (SIL G/UV254, ALUGRAM) were used for thin-layer chromatography (TLC) and spots were visualized under ultraviolet lamp.
General procedure for the synthesis of N-aryl-2-(4-substituted-1H-1,2,3-triazol-1-yl)acetamides 4a-4u
Synthesis of 2-bromo-N-substituted acetamides 2a–2g [32] was carried out by dissolving aromatic amines (1.0 mmol) 1a–1g in 8–15 cm3 dichloromethane, followed by addition of potassium carbonate (1.5 mmol) as base and stirred the solution. Afterwards, bromoacetyl bromide (1.2 mmol) was added dropwise to above stirred solution at 0–5 °C and continued stirring for 15 min. When reaction was completed, ice cold water was added, and solid product was precipitated, filtered, and dried.
For the synthesis of N-aryl-2-(4-substituted-1H-1,2,3-triazol-1-yl)acetamides 4a–4u, aqueous solution of sodium azide (3.0 mmol) was added to 2-bromo-N-substituted acetamides 2a–2g (1.0 mmol) in 7–14 cm3N,N-dimethylformamide at 25–40 °C and stirred solution for 1 h. Afterwards, terminal alkynes 3a–3c (1.0 mmol) were added to above solution, followed by the addition of copper sulfate pentahydrate (0.1 mmol) and sodium ascorbate (0.4 mmol) and continued stirred the reaction contents at the same temperature for 7–15 h. Progress of reaction was monitored by TLC at regular intervals. As the reaction was completed, ice cold water was added to reaction mixture; solid residues were precipitated, collected by filtration, and washed with ammonia solution. Crude precipitates were then purified by washing with ethyl acetate and dried by applying vacuum to afford target 1,4-disubstituted 1,2,3-triazoles 4a–4u in good yield.
N-Phenyl-2-(4-phenyl-1H-1,2,3-triazol-1-yl)acetamide (4a, C16H14N4O)
White solid; yield: 82%; m.p.: 250–254 °C; FT-IR (KBr): \( \bar{V} \) = 3267 (N–H str.), 3134 (C–H str., triazole ring), 3065 (C–H str., aromatic ring), 2934 (C–H str., aliphatic), 1672 (C=O str., amide), 1599, 1547 (C=C str., aromatic ring) cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 5.42 (s, 2H, NCH2), 7.10 (t, 1H, Ar–H, J = 8.0 Hz), 7.33–7.37 (m, 3H, Ar–H), 7.47 (t, 2H, Ar–H, J = 8.0 Hz), 7.63 (d, 2H, Ar–H, J = 8.0 Hz), 7.90 (d, 2H, Ar–H, J = 8.0 Hz), 8.62 (s, 1H, C–H triazole), 10.55 (s, 1H, N–H amide) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 52.7, 119.7, 123.4 (C5 triazole), 124.2, 125.6, 128.3, 129.4, 131.2, 138.9, 146.7 (C4 triazole), 164.5 (C=O amide) ppm; HRMS: m/z calculated for C16H14N4O ([M + H]+) 279.1201, found 279.1249.
N-(4-Methoxyphenyl)-2-(4-phenyl-1H-1,2,3-triazol-1-yl)acetamide (4b, C17H16N4O2)
White solid; yield: 88%; m.p.: 228–232 °C; FT-IR (KBr): \( \bar{V} \) = 3277 (N–H str.), 3163 (C–H str., triazole ring), 3084 (C–H str., aromatic ring), 2951 (C–H str., aliphatic), 1668 (C=O str., amide), 1605, 1547, 1464 (C=C str., aromatic ring), 1244 (C–O asym. str., ether), 1032 (C–O sym. str., ether) cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 3.73 (s, 3H, OCH3), 5.37 (s, 2H, NCH2), 6.93 (d, 2H, Ar–H, J = 8.0 Hz), 7.35 (t, 1H, Ar–H, J = 8.0 Hz), 7.47 (t, 2H, Ar–H, J = 8.0 Hz), 7.53 (d, 2H, Ar–H, J = 8.0 Hz), 7.89 (d, 2H, Ar–H, J = 8.0 Hz), 8.61 (s, 1H, C–H triazole), 10.40 (s, 1H, N–H amide) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 52.8, 55.3, 114.5, 121.2, 123.4 (C5 triazole), 125.6, 128.3, 129.4, 131.2, 132.0, 146.6 (C4 triazole), 156.1, 164.1 (C=O amide) ppm; HRMS: m/z calculated for C17H16N4O2 ([M + H]+) 309.1307, found 309.1350.
N-(4-Nitrophenyl)-2-(4-phenyl-1H-1,2,3-triazol-1-yl)acetamide (4c, C16H13N5O3)
White solid; yield: 81%; m.p.: 264–268 °C; FT-IR (KBr): \( \bar{V} \) = 3302 (N–H str.), 3160 (C–H str., triazole ring), 3064 (C–H str., aromatic ring), 2935 (C–H str., aliphatic), 1703 (C=O str., amide), 1616, 1599, 1568 (C=C str., aromatic ring), 1504 (N–O asym. str., NO2), 1344 (N–O sym. str., NO2) cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 5.53 (s, 2H, NCH2), 7.39 (t, 1H, Ar–H, J = 8.0 Hz), 7.50 (t, 2H, Ar–H, J = 8.0 Hz), 7.88–7.93 (m, 4H, Ar–H), 8.30 (d, 2H, Ar–H, J = 8.0 Hz), 8.65 (s, 1H, C–H triazole), 11.18 (s, 1H, N–H amide) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 52.8, 119.6, 123.5 (C5 triazole), 125.6, 128.4, 129.3, 131.1, 143.0, 144.9, 146.8 (C4 triazole), 165.8 (C=O amide) ppm; HRMS: m/z calculated for C16H13N5O3 ([M + H]+) 324.1052, found 324.1096.
N-(4-Fluorophenyl)-2-(4-phenyl-1H-1,2,3-triazol-1-yl)acetamide (4d, C16H13FN4O)
White solid; yield: 85%; m.p.: 246–250 °C; FT-IR (KBr): \( \bar{V} \) = 3283 (N–H str.), 3135 (C–H str., triazole ring), 3086 (C–H str., aromatic ring), 2938 (C–H str., aliphatic), 1670 (C=O str., amide), 1616, 1558 (C=C str., aromatic ring) cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 5.40 (s, 2H, NCH2), 7.18–7.22 (m, 2H, Ar–H), 7.35 (t, 1H, Ar–H, J = 8.0 Hz), 7.47 (t, 2H, Ar–H, J = 8.0 Hz), 7.62–7.64 (m, 2H, Ar–H), 7.89 (d, 2H, Ar–H, J = 8.0 Hz), 8.62 (s, 1H, C–H triazole), 10.61 (s, 1H, N–H amide) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 52.8, 116.0 (d, 2C, Ar–C, J = 22.0 Hz), 121.6 (d, 2C, Ar–C, J = 8.0 Hz), 123.5 (C5 triazole), 125.6, 128.3, 129.4, 131.2, 135.3 (d, 2C, Ar–C, J = 3.0 Hz), 146.7 (C4 triazole), 158.7 (d, 1C, Ar–C, J = 239.0 Hz), 164.6 (C=O amide) ppm; HRMS: m/z calculated for C16H13FN4O ([M + H]+) 297.1107, found 297.1152.
N-(4-Chlorophenyl)-2-(4-phenyl-1H-1,2,3-triazol-1-yl)acetamide (4e, C16H13ClN4O)
White solid; yield: 86%; m.p.: 258–262 °C; FT-IR (KBr): \( \bar{V} \) = 3263 (N–H str.), 3123 (C–H str., triazole ring), 3076 (C–H str., aromatic ring), 2941 (C–H str., aliphatic), 1674 (C=O str., amide), 1607, 1543, 1493 (C=C str., aromatic ring) cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 5.42 (s, 2H, NCH2), 7.33–7.48 (m, 5H, Ar–H), 7.65 (d, 2H, Ar–H, J = 8.0 Hz), 7.89 (d, 2H, Ar–H, J = 8.0 Hz), 8.62 (s, 1H, C-H triazole), 10.68 (s, 1H, N–H amide) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 52.8, 121.3, 123.5 (C5 triazole), 125.6, 127.9, 128.3, 129.3, 129.4, 131.2, 137.8, 146.7 (C4 triazole), 164.9 (C=O amide) ppm; HRMS: m/z calculated for C16H13ClN4O ([M + H]+) 313.0856 (35Cl), 315.0827 (37Cl), found 313.1152 (35Cl), 315.1083 (35Cl).
N-(4-Bromophenyl)-2-(4-phenyl-1H-1,2,3-triazol-1-yl)acetamide (4f, C16H13BrN4O)
White solid; yield: 78%; m.p.: 268–272 °C; FT-IR (KBr): \( \bar{V} \) = 3211 (N–H str.), 3155 (C–H str., triazole ring), 3007 (C–H str., aromatic ring), 2940 (C–H str., aliphatic), 1676 (C=O str., amide), 1609, 1543, 1489 (C=C str., aromatic ring) cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 5.40 (s, 2H, NCH2), 7.35 (t, 1H, Ar–H, J = 8.0 Hz), 7.47 (t, 2H, Ar–H, J = 8.0 Hz), 7.53–7.60 (m, 4H, Ar–H), 7.88 (d, 2H, Ar–H, J = 8.0 Hz), 8.61 (s, 1H, C–H triazole), 10.67 (s, 1H, N–H amide) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 52.7, 115.9, 121.7, 123.5 (C5 triazole), 125.6, 128.3, 129.4, 131.2, 132.2, 138.2, 146.4 (C4 triazole), 165.0 (C=O amide) ppm; HRMS: m/z calculated for C16H13BrN4O ([M + H]+) 357.0351 (79Br), 359.0331 (81Br), found 357.0350 (79Br), 359.0322 (81Br).
N-(Naphthalen-1-yl)-2-(4-phenyl-1H-1,2,3-triazol-1-yl)acetamide (4g, C20H16N4O)
White solid; yield: 88%; m.p.: 260–264 °C; FT-IR (KBr): \( \bar{V} \) = 3246 (N–H str.), 3132 (C–H str., triazole ring), 3049 (C–H str., aromatic ring), 2934 (C–H str., aliphatic), 1666 (C=O str., amide), 1549, 1470 (C=C str., aromatic ring) cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 5.61 (s, 2H, NCH2), 7.35 (t, 1H, Ar–H, J = 8.0 Hz), 7.45–7.64 (m, 5H, Ar–H), 7.75 (d, 1H, Ar–H, J = 8.0 Hz), 7.82 (d, 1H, Ar–H, J = 8.0 Hz), 7.90 (d, 2H, Ar–H, J = 8.0 Hz), 7.98 (d, 1H, Ar–H, J = 8.0 Hz), 8.20 (d, 1H, Ar–H, J = 8.0 Hz), 8.67 (s, 1H, C–H triazole), 10.49 (s, 1H, N–H amide) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 52.5, 122.1, 123.1, 123.6 (C5 triazole), 125.6, 126.1, 126.2, 126.5, 126.7, 128.0, 128.3, 128.7, 129.4, 131.2, 133.2, 134.2, 146.7 (C4 triazole), 165.6 (C=O amide) ppm; HRMS: m/z calculated for C20H16N4O ([M + H]+) 329.1358, found 329.1410.
N-Phenyl-2-[4-(p-tolyl)-1H-1,2,3-triazol-1-yl]acetamide (4h, C17H16N4O)
White solid; yield: 83%; m.p.: 262–266 °C; FT-IR (KBr): \( \bar{V} \) = 3269 (N–H str.), 3163 (C–H str., triazole ring), 3094 (C–H str., aromatic ring), 2924 (C–H str., aliphatic), 1676 (C=O str., amide), 1599, 1543, 1499 (C=C str., aromatic ring) cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 2.30 (s, 3H, CH3), 5.37 (s, 2H, NCH2), 7.10 (t, 1H, Ar–H, J = 8.0 Hz), 7.27 (d, 2H, Ar–H, J = 8.0 Hz), 7.34 (t, 2H, Ar–H, J = 8.0 Hz), 7.60 (d, 2H, Ar–H, J = 8.0 Hz), 7.77 (d, 2H, Ar–H, J = 8.0 Hz), 8.54 (s, 1H, C-H triazole), 10.52 (s, 1H, N–H amide) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 21.2, 52.8, 119.7, 123.1 (C5 triazole), 124.3, 125.5, 128.3, 129.3, 129.9, 137.6, 138.9, 146.7 (C4 triazole), 164.6 (C=O amide) ppm; HRMS: m/z calculated for C17H16N4O ([M + H]+) 293.1358, found 293.1404.
N-(4-Methoxyphenyl)-2-[4-(p-tolyl)-1H-1,2,3-triazol-1-yl]acetamide (4i, C18H18N4O2)
White solid; yield: 81%; m.p.: 250–254 °C; FT-IR (KBr): \( \bar{V} \) = 3279 (N–H str.), 3166 (C–H str., triazole ring), 3064 (C–H str., aromatic ring), 2912 (C–H str., aliphatic), 1678 (C=O str., amide), 1607, 1543, 1462 (C=C str., aromatic ring), 1244 (C–O asym. str., ether), 1034 (C–O sym. str., ether) cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 2.34 (s, 3H, CH3), 3.73 (s, 3H, OCH3), 5.35 (s, 2H, NCH2), 6.92 (d, 2H, Ar–H, J = 8.0 Hz), 7.27 (d, 2H, Ar–H, J = 8.0 Hz), 7.53 (d, 2H, Ar–H, J = 8.0 Hz), 7.77 (d, 2H, Ar–H, J = 8.0 Hz), 8.54 (s, 1H, C-H triazole), 10.39 (s, 1H, N–H amide) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 21.2, 52.7, 55.6, 114.5, 121.3, 123.0 (C5 triazole), 125.5, 128.4, 129.8, 132.0, 137.5, 146.6 (C4 triazole), 156.1, 164.1 (C=O amide) ppm; HRMS: m/z calculated for C18H18N4O2 ([M + H]+) 323.1463, found 323.1509.
N-(4-Nitrophenyl)-2-[4-(p-tolyl)-1H-1,2,3-triazol-1-yl]acetamide (4j, C17H15N5O3)
White solid; yield: 87%; m.p.: 276–280 °C; FT-IR (KBr): \( \bar{V} \) = 3277 (N–H str.), 3185 (C–H str., triazole ring), 3064 (C–H str., aromatic ring), 2935 (C–H str., aliphatic), 1701 (C=O str., amide), 1618, 1566, 1468 (C=C str., aromatic ring), 1504 (N–O asym. str., NO2), 1344 (N–O sym. str., NO2) cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 2.34 (s, 3H, CH3), 5.48 (s, 2H, NCH2), 7.28 (d, 2H, Ar–H, J = 8.0 Hz), 7.77 (d, 2H, Ar–H, J = 8.0 Hz), 7.86 (d, 2H, Ar–H, J = 8.0 Hz), 8.27 (d, 2H, Ar–H, J = 8.0 Hz), 8.55 (s, 1H, C–H triazole), 11.14 (s, 1H, N–H amide) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 21.2, 52.8, 119.6, 123.0 (C5 triazole), 125.6, 125.6, 128.2, 123.0, 137.7, 143.1, 145.0, 146.8 (C4 triazole), 165.8 (C=O amide) ppm; HRMS: m/z calculated for C17H15N5O3 ([M + H]+) 338.1208, found 338.1254.
N-(4-Fluorophenyl)-2-[4-(p-tolyl)-1H-1,2,3-triazol-1-yl]acetamide (4k, C17H15FN4O)
White solid; yield: 80%; m.p.: 254–258 °C; FT-IR (KBr): \( \bar{V} \) = 3283 (N–H str.), 3177 (C–H str., triazole ring), 3065 (C–H str., aromatic ring), 2943 (C–H str., aliphatic), 1676 (C=O str., amide), 1614, 1545, 1466 (C=C str., aromatic ring) cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 2.34 (s, 3H, CH3), 5.38 (s, 2H, NCH2), 7.17–7.22 (m, 2H, Ar–H), 7.27 (d, 2H, Ar–H, J = 8.0 Hz), 7.61–7.65 (m, 2H, Ar–H), 7.77 (d, 2H, Ar–H, J = 8.0 Hz), 8.54 (s, 1H, C–H triazole), 10.59 (s, 1H, N–H amide) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 21.2, 52.7, 116.0 (d, 2C, Ar–C, J = 22.0 Hz), 121.5 (d, 2C, Ar–C, J = 8.0 Hz), 123.0 (C5 triazole), 125.4, 128.3, 129.9, 135.3 (d, 2C, Ar–C, J = 3.0 Hz), 137.6, 146.7 (C4 triazole), 158.7 (d, 1C, Ar–C, J = 239.0 Hz), 164.6 (C=O amide) ppm; HRMS: m/z calculated for C17H15FN4O ([M + H]+) 311.1263, found 311.1408.
N-(4-Chlorophenyl)-2-[4-(p-tolyl)-1H-1,2,3-triazol-1-yl]acetamide (4l, C17H15ClN4O)
White solid; yield: 86%; m.p.: 268–272 °C; FT-IR (KBr): \( \bar{V} \) = 3269 (N–H str.), 3128 (C–H str., triazole ring), 3074 (C–H str., aromatic ring), 2914 (C–H str., aliphatic), 1670 (C=O str., amide), 1610, 1547, 1493 (C=C str., aromatic ring) cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 2.34 (s, 3H, CH3), 5.39 (s, 2H, NCH2), 7.27 (d, 2H, Ar–H, J = 8.0 Hz), 7.41 (d, 2H, Ar–H, J = 8.0 Hz), 7.64 (d, 2H, Ar–H, J = 8.0 Hz), 7.77 (d, 2H, Ar–H, J = 8.0 Hz), 8.55 (s, 1H, C–H triazole), 10.67 (s, 1H, N–H amide) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 21.2, 52.5, 121.3, 123.1 (C5 triazole), 125.6, 127.9, 128.4, 129.3, 130.0, 137.6, 137.8, 146.7 (C4 triazole), 164.9 (C=O amide) ppm; HRMS: m/z calculated for C17H15ClN4O ([M + H]+) 327.1013 (35Cl), 329.0983 (37Cl), found 327.1014 (35Cl), 329.0985 (37Cl).
N-(4-Bromophenyl)-2-[4-(p-tolyl)-1H-1,2,3-triazol-1-yl]acetamide (4m, C17H15BrN4O)
White solid; yield: 85%; m.p.: 276–280 °C; FT-IR (KBr): \( \bar{V} \) = 3265 (N–H str.), 3157 (C–H str., triazole ring), 3071 (C–H str., aromatic ring), 2941 (C–H str., aliphatic), 1676 (C=O str., amide), 1607, 1543, 1491 (C=C str., aromatic ring) cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 2.34 (s, 3H, CH3), 5.39 (s, 2H, NCH2), 7.27 (d, 2H, Ar–H, J = 8.0 Hz), 7.52–7.60 (m, 4H, Ar–H), 7.77 (d, 2H, Ar–H, J = 8.0 Hz), 8.54 (s, 1H, C–H triazole), 10.67 (s, 1H, N–H amide) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 21.3, 52.8, 115.9, 121.7, 123.1 (C5 triazole), 125.6, 128.4, 130.0, 132.2, 137.6, 138.3, 146.8 (C4 triazole), 164.7 (C=O amide) ppm; HRMS: m/z calculated for C17H15BrN4O ([M + H]+) 371.0507 (79Br), 373.0487 (81Br), found 371.0509 (79Br), 373.0490 (81Br).
N-(Naphthalen-1-yl)-2-[4-(p-tolyl)-1H-1,2,3-triazol-1-yl]acetamide (4n, C21H18N4O)
White solid; yield: 78%; m.p.: 266–270 °C; FT-IR (KBr): \( \bar{V} \) = 3260 (N–H str.), 3166 (C–H str., triazole ring), 3059 (C–H str., aromatic ring), 2934 (C–H str., aliphatic), 1666 (C=O str., amide), 1549, 1466 (C=C str., aromatic ring) cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 2.34 (s, 3H, CH3), 5.59 (s, 2H, NCH2), 7.28 (d, 2H, Ar–H, J = 8.0 Hz), 7.51–7.64 (m, 3H, Ar–H), 7.74–7.83 (m, 4H, Ar–H), 7.98 (d, 1H, Ar–H, J = 8.0 Hz), 8.20 (d, 1H, Ar–H, J = 8.0 Hz), 8.60 (s, 1H, C–H triazole), 10.48 (s, 1H, N–H amide) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 21.2, 52.5, 122.0, 123.1 (C5 triazole), 123.1, 125.6, 126.0, 126.2, 126.5, 126.7, 128.0, 128.5, 128.7, 129.9, 133.2, 134.2, 137.3, 146.7 (C4 triazole), 165.6 (C=O amide) ppm; HRMS: m/z calculated for C21H18N4O ([M + H]+) 343.1514, found 343.1562.
2-[4-(3-Fluorophenyl)-1H-1,2,3-triazol-1-yl]-N-phenylacetamide (4o, C16H13FN4O)
White solid; yield: 80%; m.p.: 258–262 °C; FT-IR (KBr): \( \bar{V} \) = 3263 (N–H str.), 3132 (C–H str., triazole ring), 3069 (C–H str., aromatic ring), 2937 (C–H str., aliphatic), 1668 (C=O str., amide), 1593, 1549, 1485 (C=C str., aromatic ring) cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 5.42 (s, 2H, NCH2), 7.10 (t, 1H, Ar–H, J = 8.0 Hz), 7.17–7.21 (m, 1H, Ar–H), 7.35 (t, 2H, Ar–H, J = 8.0 Hz), 7.49–7.55 (m, 1H, Ar–H), 7.61 (d, 2H, Ar–H, J = 8.0 Hz), 7.70–7.76 (m, 2H, Ar–H), 8.70 (s, 1H, C–H triazole), 10.54 (s, 1H, N–H amide) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 52.8, 112.2 (d, 1C, Ar–C, J = 23.0 Hz), 115.0 (d, 1C, Ar–C, J = 21.0 Hz), 119.6, 121.6 (d, 1C, Ar–C, J = 3.0 Hz), 124.2 (C5 triazole), 124.3, 129.4, 131.5 (d, 1C, Ar–C, J = 9.0 Hz), 133.6 (d, 1C, Ar–C, J = 9.0 Hz), 138.8, 145.6 (d, 1C, Ar–C, J = 3.0 Hz, C4 triazole), 163.1 (d, 1C, Ar–C, J = 241.0 Hz), 164.5 (C=O amide) ppm; HRMS: m/z calculated for C16H13FN4O ([M + H]+) 297.1107, found 297.1155.
2-[4-(3-Fluorophenyl)-1H-1,2,3-triazol-1-yl]-N-(4-methoxyphenyl)acetamide (4p, C17H15FN4O2)
White solid; yield: 77%; m.p.: 276-280 °C; FT-IR (KBr): \( \bar{V} \) = 3265 (N–H str.), 3155 (C–H str., triazole ring), 3007 (C–H str., aromatic ring), 2912 (C–H str., aliphatic), 1672 (C=O str., amide), 1608, 1543, 1489 (C=C str., aromatic ring), 1229 (C–O asym. str., ether), 1076 (C–O sym. str., ether) cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 3.73 (s, 3H, OCH3), 5.38 (s, 2H, NCH2), 6.92 (d, 2H, Ar–H, J = 8.0 Hz), 7.16–7.20 (m, 1H, Ar–H), 7.49–7.54 (m, 3H, Ar–H), 7.69–7.76 (m, 2H, Ar–H), 8.69 (s, 1H, C-H triazole), 10.41 (s, 1H, N–H amide) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 53.0, 55.6, 112.2 (d, 1C, Ar–C, J = 23.0 Hz), 114.5, 115.0 (d, 1C, Ar–C, J = 21.0 Hz), 121.3, 121.6 (d, 1C, Ar–C, J = 2.0 Hz), 124.2 (C5 triazole), 124.3, 131.5 (d, 1C, Ar–C, J = 8.0 Hz), 131.9, 133.6 (d, 1C, Ar–C, J = 8.0 Hz), 145.6 (d, 1C, Ar–C, J = 3.0 Hz, C4 triazole), 156.1, 163.1 (d, 1C, Ar–C, J = 242.0 Hz), 164.0 (C=O amide) ppm; HRMS: m/z calculated for C17H15FN4O2 ([M + H]+) 327.1213, found 327.1258.
2-[4-(3-Fluorophenyl)-1H-1,2,3-triazol-1-yl]-N-(4-nitrophenyl)acetamide (4q, C16H12FN5O3)
White solid; yield: 88%; m.p.: 240–244 °C; FT-IR (KBr): \( \bar{V} \) = 3308 (N–H str.), 3148 (C–H str., triazole ring), 3084 (C–H str., aromatic ring), 2957 (C–H str., aliphatic), 1701 (C=O str., amide), 1616, 1564 (C=C str., aromatic ring), 1501 (N–O asym. str., NO2), 1342 (N–O sym. str., NO2) cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 5.51 (s, 2H, NCH2), 7.16–7.20 (m, 1H, Ar–H), 7.49–7.54 (m, 1H, Ar–H), 7.69–7.75 (m, 2H, Ar–H), 7.86 (d, 2H, Ar–H, J = 8.0 Hz), 8.26 (d, 2H, Ar–H, J = 8.0 Hz), 8.70 (s, 1H, C-H triazole), 11.15 (s, 1H, N–H amide) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 52.7, 112.2 (d, 1C, Ar–C, J = 23.0 Hz), 115.0 (d, 1C, Ar–C, J = 21.0 Hz), 119.6, 121.7 (d, 1C, Ar–C, J = 3.0 Hz), 124.2 (C5 triazole), 125.6, 131.5 (d, 1C, Ar–C, J = 9.0 Hz), 133.5 (d, 1C, Ar–C, J = 9.0 Hz), 143.1, 144.9, 145.7 (d, 1C, Ar–C, J = 3.0 Hz, C4 triazole), 163.1 (d, 1C, Ar–C, J = 242.0 Hz), 165.7 (C=O amide) ppm; HRMS: m/z calculated for C16H12FN5O3 ([M + H]+) 342.0958, found 342.1000.
N-(4-Fluorophenyl)-2-[4-(3-fluorophenyl)-1H-1,2,3-triazol-1-yl]acetamide (4r, C16H12F2N4O)
White solid; yield: 79%; m.p.: 246–250 °C; FT-IR (KBr): \( \bar{V} \) = 3260 (N–H str.), 3136 (C–H str., triazole ring), 3067 (C–H str., aromatic ring), 2937 (C–H str., aliphatic), 1672 (C=O str., amide), 1614, 1549, 1481 (C=C str., aromatic ring) cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 5.40 (s, 2H, NCH2), 7.17–7.22 (m, 3H, Ar–H), 7.49–7.55 (m, 1H, Ar–H), 7.61–7.75 (m, 4H, Ar–H), 8.69 (s, 1H, C–H triazole), 10.61 (s, 1H, N–H amide) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 52.8, 112.8 (d, 1C, Ar–C, J = 23.0 Hz), 115.0 (d, 1C, Ar–C, J = 21.0 Hz), 116.0 (d, 2C, Ar–C, J = 22.0 Hz), 121.6 (d, 1C, Ar–C, J = 8.0 Hz), 121.6 (d, 1C, Ar–C, J = 3.0 Hz), 124.2 (C5 triazole), 131.5 (d, 1C, Ar–C, J = 8.0 Hz), 133.5 (d, 1C, Ar–C, J = 9.0 Hz), 135.2 (d, 1C, Ar–C, J = 3.0 Hz), 145.6 (d, 1C, Ar–C, J = 2.0 Hz, C4 triazole), 158.7 (d, 1C, Ar–C, J = 239.0 Hz), 163.1 (d, 1C, Ar–C, J = 242.0 Hz), 164.5 (C=O amide) ppm; HRMS: m/z calculated for C16H12F2N4O ([M + H]+) 315.1013, found 315.1063.
N-(4-Chlorophenyl)-2-[4-(3-fluorophenyl)-1H-1,2,3-triazol-1-yl]acetamide (4s, C16H12ClFN4O)
White solid; yield: 75%; m.p.: 264–268 °C; FT-IR (KBr): \( \bar{V} \) = 3267 (N–H str.), 3159 (C–H str., triazole ring), 3078 (C–H str., aromatic ring), 2995 (C–H str., aliphatic), 1676 (C=O str., amide), 1610, 1549, 1489 (C=C str., aromatic ring) cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 5.42 (s, 2H, NCH2), 7.16–7.21 (m, 1H, Ar–H), 7.41 (d, 2H, Ar–H, J = 8.0 Hz), 7.49–7.54 (m, 1H, Ar–H), 7.64 (d, 2H, Ar–H, J = 8.0 Hz), 7.69–7.75 (m, 2H, Ar–H), 8.69 (s, 1H, C–H triazole), 10.69 (s, 1H, N–H amide) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 52.5, 112.2 (d, 1C, Ar–C, J = 23.0 Hz), 115.0 (d, 1C, Ar–C, J = 21.0 Hz), 121.3, 121.6 (d, 1C, Ar–C, J = 3.0 Hz), 124.2 (C5 triazole), 127.9, 129.3, 131.5 (d, 1C, Ar–C, J = 9.0 Hz), 133.5 (d, 1C, Ar–C, J = 8.0 Hz), 137.8, 145.6 (d, 1C, Ar–C, J = 2.0 Hz, C4 triazole), 163.0 (d, 1C, Ar–C, J = 247.0 Hz), 164.7 (C=O amide) ppm; HRMS: m/z calculated for C16H12ClFN4O ([M + H]+) 331.0762 (35Cl), 333.0732 (37Cl), found 331.0766 (35Cl), 333.0736 (37Cl).
N-(4-Bromophenyl)-2-[4-(3-fluorophenyl)-1H-1,2,3-triazol-1-yl]acetamide (4t, C16H12BrFN4O)
White solid; yield: 85%; m.p.: 250–254 °C; FT-IR (KBr): \( \bar{V} \) = 3254 (N–H str.), 3132 (C–H str., triazole ring), 3063 (C–H str., aromatic ring), 2941 (C–H str., aliphatic), 1668 (C=O str., amide), 1618, 1549, 1479 (C=C str., aromatic ring) cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 5.42 (s, 2H, NCH2), 7.16–7.21 (m, 1H, Ar–H), 7.49–7.60 (m, 5H, Ar–H), 7.69–7.75 (m, 2H, Ar–H), 8.69 (s, 1H, C–H triazole), 10.69 (s, 1H, N–H amide) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 52.7, 112.2 (d, 1C, Ar–C, J = 23.0 Hz), 115.0 (d, 1C, Ar–C, J = 21.0 Hz), 115.9, 121.7 (d, 1C, Ar–C, J = 4.0 Hz), 121.7, 124.2 (C5 triazole), 131.5 (d, 1C, Ar–C, J = 8.0 Hz), 132.2, 133.5 (d, 1C, Ar–C, J = 8.0 Hz), 138.2, 145.6 (d, 1C, Ar–C, J = 3.0 Hz, C4 triazole), 163.1 (d, 1C, Ar–C, J = 242.0 Hz), 164.8 (C=O amide) ppm; HRMS: m/z calculated for C16H12BrFN4O ([M + H]+) 375.0257 (79Br), 377.0236 (81Br), found 375.0252 (79Br), 377.0232 (81Br).
2-[4-(3-Fluorophenyl)-1H-1,2,3-triazol-1-yl]-N-(naphthalen-1-yl)acetamide (4u, C20H15FN4O)
White solid; yield: 87%; m.p.: 258–262 °C; FT-IR (KBr): \( \bar{V} \) = 3258 (N–H str.), 3152 (C–H str., triazole ring), 3051 (C–H str., aromatic ring), 2937 (C–H str., aliphatic), 1670 (C=O str., amide), 1553, 1481 (C=C str., aromatic ring) cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 5.63 (s, 2H, NCH2), 7.16–7.21 (m, 1H, Ar–H), 7.49–7.64 (m, 4H, Ar–H), 7.71–7.83 (m, 4H, Ar–H), 7.97 (d, 1H, Ar–H, J = 8.0 Hz), 8.21 (d, 1H, Ar–H, J = 8.0 Hz), 8.76 (s, 1H, C–H triazole), 10.55 (s, 1H, N–H amide) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 52.6, 112.2 (d, 1C, Ar–C, J = 23.0 Hz), 115.0 (d, 1C, Ar–C, J = 21.0 Hz), 121.7 (d, 1C, Ar–C, J = 3.0 Hz), 122.1, 123.1, 124.3 (C5 triazole), 126.0, 126.2, 126.5, 126.7, 128.0, 128.7, 131.5 (d, 1C, Ar–C, J = 8.0 Hz), 133.2, 133.6 (d, 1C, Ar–C, J = 9.0 Hz), 134.2, 145.6 (d, 1C, Ar–C, J = 3.0 Hz, C4 triazole), 163.1 (d, 1C, Ar–C, J = 242.0 Hz), 165.5 (C=O amide) ppm; HRMS: m/z calculated for C20H15FN4O ([M + H]+) 347.1213, found 347.1307.
General procedure for in vitro antitubercular activity
All synthesized triazole derivatives 4a–4u were evaluated for in vitro antitubercular activity against bacterial strain M. tuberculosis H37RV (MTCC 200) by Lowenstein–Jensen (L. J.) slope method in the Microcare Laboratory and TRC, Surat, Gujrat.
Minimum inhibition concentration was used to evaluate the antitubercular activity. Results were expressed in terms of µmol/cm3 and isoniazid was used as reference drug. Lowenstein–Jensen (L. J.) was used as nutrient medium to grow and dilute the suspension of compounds for the test. Inoculum size for test strain was adjusted to 1 mg/cm3. DMSO was used as diluent/vehicle to get desired concentration of synthesized compounds to test upon the standard bacterial strain. Each synthesized drug was diluted obtaining 2000 µg/cm3 concentration, as a stock solution.
Following steps were taken to precede antitubercular activity
Primary screen: in primary screening, 500 µg/cm3, 250 µg/cm3, and 125 µg/cm3 concentrations of the synthesized compounds were taken. The active synthesized compounds found in this primary screening were further tested in a second set of dilution against the tested strain.
Secondary screen: the compounds found active in primary screening were similarly diluted to obtain 100 µg/cm3, 50 µg/cm3, 25 µg/cm3, 12.5 µg/cm3, 6.250 µg/cm3, 3.125 µg/cm3, and 1.562 µg/cm3 concentrations.
Reading result: the highest dilution showing at least 99% inhibition is taken as MIC. The result of this is much affected by the size of the inoculum. The test mixture should contain 108 organism/cm3.
The standard drugs: the Standard strain M. tuberculosis, H37RV, was tested with each new batch of medium. The recommended drug concentration was 0.2 mg/cm3 for isoniazid.
General procedure for in vitro antimicrobial activity
All the synthesized triazole derivatives 4a–4u were examined for their in vitro antimicrobial activity against two Gram-positive bacterial strains, i.e., B. subtilis (MTCC 441) and S. epidermidis (MTCC 6880), two Gram-negative bacterial strains, i.e., E. coli (MTCC 1652) and P. aeruginosa (MTCC 424), and two fungal strains, i.e., C. albicans (MTCC 183) and A. niger (MTCC 8189) by the serial dilution technique [33]. Ciprofloxacin and fluconazole were used as reference drugs against bacteria and fungi, respectively.
References
Kaushik CP, Kumar K, Singh SK, Singh D, Saini S (2016) Arab J Chem 9:865
Anand A, Kulkarni MV, Joshi SD, Dixit SR (2016) Bioorg Med Chem Lett 26:4709
Jurıcek M, Kouwer PHJ, Rowan AE (2011) Chem Commun 47:8740
Rostovtsev VV, Green LG, Fokin VV, Sharpless KB (2002) Angew Chem Int Ed 41:2596
Li H, Aneja R, Chaiken I (2013) Molecules 18:9797
Duan T, Fan K, Fu Y, Zhong C, Chen X, Peng T, Qin J (2012) Dyes Pigm 94:28
Zhang T, Cao S, Quan H, Huang Z, Xu S (2015) Res Chem Intermed 41:2709
Zhou L, Amer A, Korn M, Burda R, Balzarini J, Clercq ED, Kern ER, Torrence PF (2005) Antivir Chem Chemother 16:375
Kaushik CP, Kumar K, Lal K, Singh SK (2014) Chem Biol Interface 4:341
Kaushik CP, Luxmi R, Singh D, Kumar A (2017) Mol Divers 21:137
Whiting M, Tripp JC, Lin YC, Lindstrom W, Olson AJ, Elder JH, Sharpless KB, Fokin VV (2006) J Med Chem 49:7697
Nagesh HN, Suresh N, Prakash GVSB, Gupta S, Rao JV, Sekhar KVGC (2015) Med Chem Res 24:523
Guantai EM, Ncokazi K, Egan TJ, Gut J, Rosenthal PJ, Smith PJ, Chibale K (2010) Bioorg Med Chem 18:8243
Manohar S, Khan SI, Rawat DS (2011) Chem Biol Drug Des 78:124
Panathur N, Gokhale N, Dalimba U, Koushik PV, Yogeeswari P, Sriram D (2016) Med Chem Res 25:135
Buckle DR, Rockell CJM, Smith H, Spicer BA (1986) J Med Chem 29:262
Karakurt A, Aytemir MD, Stables JP, Ozalp M, Kaynak FB, Ozbey S, Dalkara S (2006) Arch Pharm Chem Life Sci 339:513
Shaikh MH, Subhedar DD, Khan FAK, Sangshetti JN, Shingate BB (2016) Chin Chem Lett 27:295
Anand A, Naik RJ, Revankar HM, Kulkarni MV, Dixit SR, Joshi SD (2015) Eur J Med Chem 105:194
Quan XJ, Ren ZH, Wang YY, Guan ZH (2014) Org Lett 16:5728
Huisgen R, Szeimies G, Moebius L (1967) Chem Ber 100:2494
Kolb HC, Finn MG, Sharpless KB (2001) Angew Chem Int Ed 40:2004
Tornфe CW, Christensen C, Meldal M (2002) J Org Chem 67:3057
Cheng J, Gu Z, He C, Jin J, Wang L, Li G, Sun B, Wang H, Bai J (2015) Carbohyd Res 414:72
Dijk MV, Mustafa K, Dechesne AC, Nostrum CFV, Hennink WE, Rijkers DTS, Liskamp RMJ (2007) Biomacromol 8:327
Mascarin A, Valverdea IE, Mindt TL (2016) Med Chem Commun 7:1640
Ghosh K, Panja A, Panja S (2016) New J Chem 40:3476
Kaushik CP, Pahwa A (2017) Asian J Chem 29:2171
Kaushik CP, Kumar K, Narasimhan B, Singh D, Kumar P, Pahwa A (2017) Monatsh Chem 148:765
Kaushik CP, Pahwa A, Kumar A, Singh D, Kumar K (2017) Synth Commun 47:1485
Kaushik CP, Pahwa A (2018) Med Chem Res 27:458
Kaushik CP, Pahwa A, Thakur R, Kaur P (2017) Synth Commun 47:368
Kaushik CP, Kumar K, Singh D, Singh SK, Jindal DK, Luxmi R (2015) Synth Commun 45:1977
Acknowledgements
Authors are highly thankful to University Grants Commission, New Delhi, for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kaushik, C.P., Pahwa, A., Singh, D. et al. Efficient synthesis, antitubercular and antimicrobial evaluation of 1,4-disubstituted 1,2,3-triazoles with amide functionality. Monatsh Chem 150, 1127–1136 (2019). https://doi.org/10.1007/s00706-019-2361-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-019-2361-9