Abstract
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by progressive degeneration of dopaminergic neurons located in the midbrain. The gold-standard therapy for PD is the restoration of dopamine (DA) levels through the chronic administration of the DA precursor levodopa (L-DOPA). Although levodopa therapy is the main therapeutic approach for PD, its use is limited by the development of very disabling dyskinetic movements, mainly due to the fluctuation of DA cerebral content. Experimental animal models of PD identified in DA D1/ERK-signaling pathway aberrant activation, occurring in striatal projection neurons, coupled with structural spines abnormalities, the molecular and neuronal basis of L-DOPA-induced dyskinesia (LIDs) occurrence. Different electrophysiological approaches allowed the identification of the alteration of homeostatic structural and synaptic changes, the neuronal bases of LIDs either in vivo in parkinsonian patients or in vitro in experimental animals. Here, we report the most recent studies showing electrophysiological and morphological evidence of aberrant synaptic plasticity in parkinsonian patients during LIDs in different basal ganglia nuclei and also in cortical transmission, accounting for the complexity of the synaptic changes during dyskinesias. All together, these studies suggest that LIDs are associated with a loss of homeostatic synaptic mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Synaptic plasticity refers to a wide range of synaptic changes occurring in neurons in response to specific physiological events, with the final goal of adaptation to new information. Plasticity represents the set of specific adaptations occurring at synapses and junctions allowing the communication between neurons. The original idea that neurons might communicate each other, and change their synaptic activity in response to an event occurring in a different neuron was proposed in the 1949 by the Canadian psychologist Donald Hebb. Hebb in the “Organization of Behavior. Wiley: New York; 1949”, for the first time, theorized the basis for the concept of Synaptic Plasticity (Hebb 1949); “When an axon of Cell A is near enough to excite a Cell B and repeatedly or persistently takes part in firing it, some growth process or metabolic change takes place in one or both cells such that A’s efficiency, as one of the cells firing B, is increased”.
In 1973, this theory was confirmed by the first report demonstrating the possibility to induce Long-Term Potentiation (LTP) of synaptic transmission in the dentate area of anaesthetized rabbits under perforant path stimulation (Bliss and Lomo 1973). Bliss and colleagues demonstrated both in anaesthetized (Bliss and Lomo 1973) and unanesthetized rabbits (Bliss and Gardner-Medwin 1973) that two different synapses can communicate with different strength and this phenomenon is not static, but rather can change in both short- and long-term manners. The complex range of events that can be included into the definition of Synaptic Plasticity comprises synaptic signal transmission (i.e., Short- and Long-Term Plasticity) as well as structural alterations (i.e., morphological spine density change). These neuronal changes can be the consequence of the physiological maturation during development, the acquisition of new information during learning, or can be induced by brain damage.
Synaptic plasticity changes in Parkinson’s disease
In neurodegenerative disorders, such as Parkinson’s disease (PD), altered synaptic plasticity is the consequence of aberrant cellular and molecular cascades leading to neuronal death, and it is at the same time the cause of behavioral and cognitive dysfunctions. In PD, as well as in other neurodegenerative disorders, synaptic dysfunctions precede the neuronal loss of decades; therefore, their early identification might help to identify the pathological cascade leading to neuronal death and the neuronal basis of early behavioral symptoms, such as non-motor symptoms (Ayala et al. 2017; De Leonibus et al. 2007).
This privileged position makes aberrant synaptic plasticity the target of both disease modifiers and symptomatic rescue strategies, and of their possible side effects. There is no cure for PD, but only symptomatic treatments that help the patients to manage the motor symptomatology. Among the different therapeutic strategies, dopamine (DA) replacement therapy with levodopa (l-DOPA) is still the only pharmacological treatment that has been proved to improve the motor symptoms (Olanow and Schapira 2013). However, chronic treatment with l-DOPA has also major side effects, among which dyskinesia, the occurrence of involuntary movements, is the most invalidating. Therefore, understanding the synaptic mechanisms through which the therapeutic effects of l-DOPA are converted into side effects is crucial to prevent them and to design novel drugs void of dyskinesia effects.
l-DOPA has also other important non-motor side effects, such as impaired cognition and increased risk of psychotic-like episodes (Bastide et al. 2015; Voon et al. 2009, 2017). A range of impulse control disorders including gambling, compulsive shopping, compulsive sexual behaviors, and binge eating, occurs in about 17% of PD patients on dopaminergic medications. In addition, the compulsive use of medication L-DOPA and DA agonists is also associated with an exacerbation of L-DOPA-induced dyskinesias (LIDs) (Voon et al. 2017). Some of these pathological side effects of chronic treatment with l-DOPA might be due to aberrant expression of alpha-synuclein in PD patients. Alpha-synuclein protein accumulation and misfolding in Lewy Body (Spillantini et al. 1997), is the main histological hallmark of a series of late-onset neurodegenerative disorders, including PD, other parkinsonisms such as dementia with Lewy bodies, and multiple system atrophy and other rare diseases (Barker and Williams-Gray 2016). These pathologies are generally referred as synucleinopathies. The strict association between aberrant neuronal alpha-synuclein accumulation and PD is demonstrated by the fact that patients receiving a first diagnosis of dementia with Lewy Body (DLB) have 70% possibility to develop motor PD; viceversa patients with first diagnosis of PD have higher risk of developing dementia if they also have altered beta-amyloid peptides levels in the cerebro spinal fluid (CSF) (Parnetti et al. 2014). The use of l-DOPA to control motor symptoms in DLB patients with PD or in PD patients with dementia is extremely limited as it worsens cognitive symptoms and hallucinations, likely due to its action on cortical regions (Poewe 2005); understanding the aberrant synaptic mechanisms induced by alpha-synuclein and the effects of l-DOPA not only in the striatum, but also in the other major components of the corticolimbic system is fundamental to design personalized therapeutic approaches that takes into account the complex symptomatology associated to PD.
Here, we show the recent scientific contributions in the field of synaptic plasticity alteration in PD and LIDs with particular attention to the structural events underlying these disorders and the related synaptic basal ganglia (BG) network alterations in pre-clinical and clinical studies. Most of the reviewed experimental literature is based on pharmacological animal models of PD, in particular with the use of 6-hydroxydopamine (6-OHDA) and of 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP).
Altered synaptic plasticity in experimental LIDs and dyskinetic PD patients
The two main forms of synaptic plasticity expressed in the brain are LTP and Long-Term Depression (LTD) (Malenka and Bear 2004; Calabresi et al. 1992b, c; Lovinger et al. 1993).
Synaptic plasticity alterations, either in experimental animal models or in PD patients, have been widely investigated. It has been consistently reported that the lack of dopaminergic tone in experimental rats lesioned with 6-OHDA causes the loss of synaptic plasticity, i.e., LTD and LTP, in striatal projection neurons (SPNs) (Calabresi et al. 1992a; Centonze et al. 1999; Belujon et al. 2010; Cerovic et al. 2015; Shen et al. 2015). Different stimulation protocols can be used to induce different forms of synaptic plasticity within the striatum in physiological and pathological conditions. In particular, high-frequency stimulation (HFS) protocol consists in three trains at 100 Hz for 3 s, 20 s interval, while spike-timing-dependent plasticity (STDP) is a pairing stimulation in which a synapse is activated by stimulating a pre-synaptic neuron shortly before or after making the post-synaptic neuron fire by injection of a short current pulse. These two different approaches represent the most useful tools to unravel alterations of synaptic and molecular pathways, underlying parkinsonian and dyskinesia conditions (Calabresi et al. 1992a; Centonze et al. 1999; Belujon et al. 2010; Cerovic et al. 2015; Shen et al. 2015; Augustin et al. 2014; Fino et al. 2010; Shen et al. 2008; Surmeier et al. 2007).
The lack of synaptic plasticity in DA denervated animals has been confirmed also in PD patients. The Paired Associative Stimulation (PAS) approach is a useful method to study physiological and aberrant plasticity in the human M1 motor cortex (Stefan et al. 2000, 2004; Wolters et al. 2003). In human studies, the measurement of Motor Evoked Potential (MEP) amplitude is considered a reliable analog of LTP (Stefan et al. 2000). This approach has been used to demonstrate the loss of LTP in the motor cortex of PD patients in OFF medication, compared with the potentiation of PAS parameters measured in control subjects (Morgante et al. 2006; Ueki et al. 2006). The synaptic plasticity in the cortex of PD patients is modulated by dopaminergic tone (Morgante et al. 2006; Ueki et al. 2006; Molina-Luna et al. 2009). In PD patients in ON medication state, chronic l-DOPA treatment restores, in the first phase of the disease, the dopaminergic levels and normalizes cortical M1 LTP (Morgante et al. 2006; Kawashima et al. 2013).
Although, the previous experimental and human studies (Morgante et al. 2006; Ueki et al. 2006; Molina-Luna et al. 2009; Kawashima et al. 2013) have identified a role of DA in synaptic plasticity in the BG input structures and motor cortex, their role in Substantia Nigra pars reticolata (SNr) has been little investigated. Prescott and colleagues extended these observations in a different BG structure of PD patients by the use of Deep Brain Stimulation (DBS) approach (Prescott et al. 2009). In this context, Prescott and Hutchison point out the attention to the possibility to record synaptic plasticity in SNr using extracellular field potentials (fEPs) in PD patients in OFF and ON medication. The application of HFS protocol in the SNr of parkinsonian patients in OFF medication state fails to induce LTP of fEPs amplitude, while in the ON state, this potentiation is present (Prescott et al. 2009).
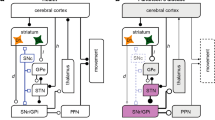
The dysregulated control of BG circuitry and the lack of physiological synaptic plasticity within the corticostriatal terminals ultimately cause a wide range of molecular and synaptic alterations underlying the movement features of PD and l-DOPA-induced (Albin et al. 1989; Calabresi et al. 2016; Wang and Zhang 2016).
The treatment with l-DOPA represents the gold-standard symptomatic PD therapy (Mercuri and Bernardi 2005; Olanow and Schapira 2013). l-DOPA succeeds to counteract the effect of dopaminergic decrease allowing amelioration of motor symptoms during the initial phases of the disease, when not all the dopaminergic terminals are lost (Calabresi et al. 2015). Unfortunately, the so-called “honey moon period” of l-DOPA therapy deteriorates rapidly, after a variable period of time for each patient, and progressively develops in motor fluctuations (Olanow et al. 2004; Calabresi et al. 2015).
The development of LIDs has been associated to impaired bidirectional synaptic plasticity at corticostriatal synapses of SPNs in 6-OHDA parkinsonian rodents (Picconi et al. 2003; Picconi et al. 2008; Cerovic et al. 2015; Belujon et al. 2010). The term “bidirectional plasticity” refers to the possibility to express flexible synaptic plasticity; i.e., the possibility to induce, with the same HFS protocol, a depression or a potentiation of synaptic transmission, depending on the different receptor state or to bring back (“down-scale”) to control levels the potentiated synapse after a HFS protocol. In particular, these pre-clinical studies paved the way to the investigation of the physiological phenomenon of depotentiation of LTP in the striatum, which is lost in hyperkinetic conditions such as LIDs (Picconi et al. 2003; Calabresi et al. 2016; Prescott et al. 2014; Martella et al. 2009; Centonze et al. 2006). Depotentiation is thought be a physiological mechanism to erase unessential information in a previously potentiated synapse and bring it back to basal level to be ready to respond to a next input (Picconi et al. 2003; Calabresi et al. 2016). The induction of dyskinetic movements and the loss of depotentiation are actually two sides of the same coin; parkinsonian condition erases the capability to induce LTP (Centonze et al. 1999; Ueki et al. 2006), while the treatment with l-DOPA either in parkinsonian animals or in PD patients restores LTP induction both in non-dyskinetic and in dyskinetic subjects (Picconi et al. 2003; Picconi et al. 2008; Prescott et al. 2009). However, the LTP in dyskinetic subjects, differently from that of non-dyskinetic, does not depotentiate under the application of a low frequency stimulation (LFS) protocol. In other words, l-DOPA restores synaptic plasticity in denervated animals, but in dyskinetic animals, this form of synaptic plasticity is not “plastic”, as it is not sensitive to homeostatic synaptic inputs. Both pre-clinical and clinical studies demonstrate that the physiological phenomenon of bidirectional or homeostatic plasticity indirectly correlated to LIDs development (Picconi et al. 2003; Picconi et al. 2008; Prescott et al. 2014; Huang et al. 2011). In addition, it has been shown that parkinsonian patients in OFF medication state, independently of their LIDs state, do not express potentiation of MEP amplitude measured with PAS method. Interestingly, l-DOPA treatment restores normal LTP in non-dyskinetic patients, but fails to restore physiological plasticity in dyskinetic subjects (Morgante et al. 2006).
The absence of downscaling mechanism in the striatal SPNs synapse, in pathological conditions such as PD and LIDs, has been associated to dopaminergic D1 receptor downstream pathway hyperactivation. In particular, dyskinetic condition is associated to an abnormal regulation of D1/protein kinase A (PKA)/dopamine- and cAMP-regulated neuronal phosphoprotein 32 kDa (DARPP-32) and Ras–extracellular-signal-regulated kinase (Ras–ERK) signaling pathways (Bido et al. 2015; Cerovic et al. 2015; Fasano et al. 2010; Picconi et al. 2003). An additional confirmation of the relationship between the loss of depotentiation and abnormal DA-related D1 downstream activation has been demonstrated in animals chronically treated with cocaine (Centonze et al. 2006).
Recently, the use of STDP protocol allows to analyze, from a different point of view, the specific role of direct and indirect striatal pathways in experimental parkinsonian and dyskinetic mice, carrying on eGFP targeting D1- and D2-expressing SPNs (Thiele et al. 2014). This study confirmed that bidirectional plasticity (LTD, LTP, and depotentiation) is present in both direct and indirect striatal neurons. The parkinsonian mice lose this form of homeostatic plasticity; i.e. SPNs exhibit only unidirectional plasticity, regardless of the stimulation paradigm (LTD or LTP). After chronic l-DOPA treatment, the mice that do not develop dyskinesia show normal LTP and depotentiation both in D1- and D2-expressing SPNs. Conversely, dyskinetic mice present a restoration of LTD in the D2-expressing neurons (indirect pathway) and of LTP in the D1-expressing neurons (direct pathway) (Thiele et al. 2014).
Other mechanisms might be responsible of LIDs, as demonstrated by recent studies supporting a role of muscarinic M4 receptors in preventing LIDs; in this study the activation of M4R signaling, by a positive allosteric modulator, in parkinsonian mice chronically treated with l-DOPA interferes with the development of dyskinetic movements and blocks bidirectional plasticity in the direct-pathway neurons (Shen et al. 2015).
Although these seminal studies provided the first neuronal and molecular basis underlying LIDs, more recent experimental evidence is adding complexity to the neuroanatomical and neurophysiological micro-circuitry in the BG involved in PD and LIDs (Cerovic et al. 2015; Belujon et al. 2010; Calabresi et al. 2016; Morgante et al. 2006).
Belujon and colleagues (Belujon et al. 2010) suggested a different pattern of plastic changes in parkinsonian rats chronically treated with l-DOPA. In particular, this study measured corticostriatal LTD in vivo through extracellular recordings of striatonigral “direct” pathway versus striatopallidal “indirect” pathway neurons; HFS protocol induces normal LTD in both striatal neuronal pathways of control animals (Belujon et al. 2010). Interestingly, the application of a depotentiation protocol induces a reverse of LTD only in striatonigral neurons, while neuronal activity of striatopallidal cells appears to be less prone to come back to control levels (Belujon et al. 2010). The striatonigral neurons recorded in parkinsonian rats express a normal LTD but the possibility to reverse its expression is lost. Most importantly, long-lasting depression of striatopallidal neurons is fully abolished in parkinsonian condition, accounting for their possible higher vulnerability. Notably, chronic treatment with l-DOPA, in rats not presenting LIDs, restores depotentiation after LFS in striatonigral neurons and induces a shift from LTD to LTP in striatopallidal neurons; the latter is not sensitive to the LFS downscaling protocol. This pattern of activity differs from that of dyskinetic animals exclusively for the effects of LFS downscaling protocol; in animals with LIDs the LFS protocol does not reverse the LTD in striatonigral neurons, but it reverse the LTP in striatopallidal neurons (Belujon et al. 2010).
All together, these in vivo and in vitro data suggest that l-DOPA favors hyperexcitability of the indirect pathway, which if contrasted by flexible downscaled processes in the direct pathway might act with therapeutic result; in contrast, if it is associated to the lack of bidirectional plasticity in the direct pathway, it gives rise to LIDs. Thus, LIDs might be the results of the lack homeostatic mechanisms in the D1 pathway, favored by a hyperactivation of the indirect pathway.
Structural synaptic plasticity in PD and LIDs
Dendritic spines represent the hardware structure on which synaptic plasticity processes run; therefore, they set the limit and the potentiality of the network. The different aspects of spine morphology differently affect synaptic transmission and plasticity, depending on the specific organization of the network.
In the striatum, most if not all, cortical glutamatergic inputs terminate on the head of dendritic spines of SPNs (Deutch et al. 2007; Raju et al. 2006, 2008). Thalamostriatal inputs also reach the same part of SPNs, but they target different types of striatal spines; for instance, the volume as well as the post-synaptic density (PSD) zone of the striatal spines, receiving cortical inputs, are larger as compared to those innervated by thalamic inputs, and they predominantly express the Vesicular Glutamate Transporter 1 (VGluT1), rather than VGluT2. Dopaminergic inputs, as well as cholinergic interneurons contacts, predominantly reach the neck of the spine consistent with a modulator role on the effects of glutamatergic inputs.
Almost complete dopaminergic denervation (> 90% loss) produces long-lasting changes in the morphology of SPNs neurons; this finding has been consistently reported across different species (monkeys, rats, and mice) and by the use of different kinds of toxins (reserpine, 6-OHDA and MPTP). Experimental parkinsonisms lead to reduced spine density, which ranges around 20–50% (Day et al. 2006; Gagnon et al. 2017; Ingham et al. 1993; Shen et al. 2007; Suarez et al. 2014; Toy et al. 2014; Villalba et al. 2009), according to the findings shown in the human putamen of PD patients (McNeill et al. 1988). Partial DA depletion has been shown to lead to reduced spine density in a progressive model of PD in monkeys and mice treated with MPTP (Villalba et al. 2009), but not in partially spared striatal area in an acute model of PD (6-OHDA lesion) (Day et al. 2006). These changes in partial DA denervated model might underlay early behavioral dysfunctions in PD, and need further investigation.
Reduced spine density has been identified in both types of SPNs expressing D1 or D2 DA receptors in the vast majority of studies (Day et al. 2006; Gagnon et al. 2017; Suarez et al. 2014; Toy et al. 2014; Villalba et al. 2009). Interestingly, DA denervation also reduces spine density in SPNs expressing D1–D2 heterodimers, which represent the 2% of SPNs in the dorsal striatum (Gagnon et al. 2017).
These findings suggest that DA inputs to dendritic spines of SPNs exert a necessary neurotrophic action for their maintenance. Interestingly, a parallelism in the proportion between the number of spines receiving DA inputs and those that are lost after DA denervation supports a possible neurotrophic action of DA on spines growth (Ingham et al. 1998). However, as previously suggested, the neurotrophic action of DA input on dendritic spines might be due to DA containing cells, which also contain other neurotrophic factors such as brain derived neurotrophic factor (BDNF), which is crucial for spine growth and maintenance (Seroogy et al. 1994). Other mechanisms have also been suggested to participate in this process, mainly related to glutamate-dependent excitotoxicity. Although most of the lost spines also contain asymmetric excitatory contacts, ultrastructural changes indicative of increased strength of glutamatergic transmission have been identified. For instance increased spine volume and PSD perforation have been reported after DA denervation in the remaining SPNs spines, which positively correlate with increased neuronal excitability (Villalba et al. 2009).
Interestingly, experimental evidence in pups (17–25 days) suggested a selective pruning of dendritic spines in the indirect pathway, which is mediated by aberrant activity of Cav1.3 Ca2 + channels (Day et al. 2006). Whether this same mechanism applies also to adult animals and DA denervated models showing spine pruning in D1-expressing SPNs, remains to be clarified.
Regardless of the lack of clear evidence on the molecular mechanism leading to synaptic pruning induced by DA denervation, some experimental findings suggest that DA replacement therapy with chronic l-DOPA treatment, as well as other DA agonists, can increase spine density not only in the striatum but also in the primary motor cortex and in the ventral striatum (Funamizu et al. 2017; Suarez et al. 2016; Ueno et al. 2017). These findings are quite surprising considering that they have been obtained also in animals showing dyskinetic movements with almost complete depletion of striatal DA content (Suarez et al. 2014); whether these new spines are fully functional has never been addressed.
In those studies where changes in synaptic density have not been associated to LIDs, consistent alterations in spines morphology have been identified, in particular spine head enlargement, related to increased debrin immunoreactivity (Funamizu et al. 2017; Nishijima et al. 2017; Ueno et al. 2017; Nishijima et al. 2013). Debrin is a F-actin binding protein, almost exclusively expressed in neurons, commonly used as a marker of excitatory input (Nishijima et al. 2013). A still controversial issue is whether these changes in glutamatergic synapses are cellular subtype or afferent subtype specific. Dyskinetic abnormalities seem to be associated to selective abnormal increase in corticostriatal contacts, but not in the thalamostriatal inputs (Zhang et al. 2013), on the indirect pathway (Suarez et al. 2016; Schuster et al. 2009).
Synaptic and structural plasticity in experimental LIDs and dyskinetic PD patients
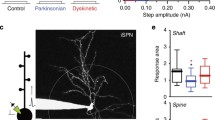
Few studies combined morphological changes with electrophysiological alteration in synaptic activity, which do not allow to establish a direct causal link between the two types of plastic events. Increased head spine and spine density positively correlate with increased amplitude and frequency of miniature excitatory post-synaptic currents (mEPSCs), respectively (Segal 2010). Furthermore, LTP and LTD protocols lead to increased and decreased spine head size, respectively (Matsuzaki et al. 2004; Yasumatsu et al. 2008). Therefore, based on the morphological data, it might be inferred that 6-OHDA lesion should lead to reduced frequency and increased amplitude to excitatory transmission in SPNs neurons. Notably, the lack of a correct dopaminergic tone reflects, in 6-OHDA animals, an increase of striatal glutamatergic transmission (Centonze et al. 2005; Cepeda et al. 2001; Gubellini et al. 2002; Tozzi et al. 2011; Tang et al. 2001; Maccarrone et al. 2003) (Fig. 1).
Structural and synaptic changes in striatal projection neurons. In physiological conditions (a) motor information processing causes a trophic effect on striatal spines, an increase in glutamatergic pre-synaptic vesicles, and a normal synaptic plasticity (b). In Parkinson’s disease (PD), the loss of LTP is accompanied by a significant spine pruning and alterations in glutamatergic receptors expression and glutamate release (c). Chronic levodopa treatment restores LTP by replacing DA release, but do not re-establish depotentiation as a consequence of pre-synaptic mechanisms of DA release regulation and post-synaptic increased spine head size associated to increased glutamatergic activity (d)
l-DOPA treatment leads to initial restoration of synaptic plasticity and behavior likely through some degree of spared mechanism governing synaptic phasic DA release, such as impulse-dependent DA release and proper re-uptake through the dopamine active transported (DAT). However, it could be hypothesized that chronic treatment leads to a shift toward tonic DA release; tonic DA release, under chronic l-DOPA treatment, might induce a shift of DA action on the hyperactive D2 pathway as evidenced in in vivo studies in dyskinetic rats (Belujon et al. 2010) and as evidenced by the shift from LTD to LTP and by increased spine density (Suarez et al. 2016). At the same time, it might lead to a lack of phasic control of DA D1 receptors activation, which is necessary for homeostatic bidirectional synaptic plasticity (Fig. 1).
The results of one recently published study addressing this issue, in some way support this hypothesis. Suarez and colleagues (Suarez et al. 2016) showed that although LIDs lead to increased spines density selectively in the D2-expressing SPNs, the associated hyperexcitability is dependent on D1 receptors activation.
Conclusion
DA innervation in the striatum supports many important behavioral and cognitive functions such as movement control, procedural learning and working memory (De Leonibus et al. 2007; Yin et al. 2009; Cools et al. 2008). These behavioral functions require not only synaptic and structural plasticity but also metaplasticity (Giordano et al. 2018), which is a set of homeostatic mechanisms that confer to the synaptic matrix the necessary degree of flexibility to adjust to the dynamic changes in incoming inputs.
The findings we reviewed here show that DA denervation leads to a loss of synaptic and structural plasticity in corticostriatal pathway. DA restoration with l-DOPA has beneficial effects on behavior as long as its action can be modulated by spared pre-synaptic mechanisms of DA re-uptake and impulse-dependent release and by flexible dynamic regulation of direct and indirect pathways.
References
Albin RL, Young AB, Penney JB (1989) The functional anatomy of basal ganglia disorders. Trends Neurosci 12(10):366–375
Augustin SM, Beeler JA, McGehee DS, Zhuang X (2014) Cyclic AMP and afferent activity govern bidirectional synaptic plasticity in striatopallidal neurons. J Neurosci 34(19):6692–6699. https://doi.org/10.1523/JNEUROSCI.3906-13.2014
Ayala A, Trivino-Juarez JM, Forjaz MJ, Rodriguez-Blazquez C, Rojo-Abuin JM, Martinez-Martin P (2017) Parkinson’s disease severity at 3 years can be predicted from non-motor symptoms at baseline. Front Neurol 8:551. https://doi.org/10.3389/fneur.2017.00551
Barker RA, Williams-Gray CH (2016) Review: the spectrum of clinical features seen with alpha synuclein pathology. Neuropathol Appl Neurobiol 42(1):6–19. https://doi.org/10.1111/nan.12303
Bastide MF, Meissner WG, Picconi B, Fasano S, Fernagut PO, Feyder M, Francardo V, Alcacer C, Ding Y, Brambilla R, Fisone G, Jon Stoessl A, Bourdenx M, Engeln M, Navailles S, De Deurwaerdere P, Ko WK, Simola N, Morelli M, Groc L, Rodriguez MC, Gurevich EV, Quik M, Morari M, Mellone M, Gardoni F, Tronci E, Guehl D, Tison F, Crossman AR, Kang UJ, Steece-Collier K, Fox S, Carta M, Angela Cenci M, Bezard E (2015) Pathophysiology of l-DOPA-induced motor and non-motor complications in Parkinson’s disease. Prog Neurobiol 132:96–168. https://doi.org/10.1016/j.pneurobio.2015.07.002
Belujon P, Lodge DJ, Grace AA (2010) Aberrant striatal plasticity is specifically associated with dyskinesia following levodopa treatment. Mov Disord 25(11):1568–1576. https://doi.org/10.1002/mds.23245
Bido S, Solari N, Indrigo M, D’Antoni A, Brambilla R, Morari M, Fasano S (2015) Differential involvement of Ras-GRF1 and Ras-GRF2 in l-DOPA-induced dyskinesia. Ann Clin Transl Neurol 2(6):662–678. https://doi.org/10.1002/acn3.202
Bliss TV, Gardner-Medwin AR (1973) Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. J Physiol 232(2):357–374
Bliss TV, Lomo T (1973) Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol 232(2):331–356
Calabresi P, Ghiglieri V, Mazzocchetti P, Corbelli I, Picconi B (2015) Levodopa-induced plasticity: a double-edged sword in Parkinson’s disease? Philos Trans R Soc Lond B Biol Sci 370(1672):20140184
Calabresi P, Maj R, Mercuri NB, Bernardi G (1992a) Coactivation of D1 and D2 dopamine receptors is required for long-term synaptic depression in the striatum. Neurosci Lett 142(1):95–99
Calabresi P, Maj R, Pisani A, Mercuri NB, Bernardi G (1992b) Long-term synaptic depression in the striatum: physiological and pharmacological characterization. J Neurosci 12(11):4224–4233
Calabresi P, Pisani A, Mercuri NB, Bernardi G (1992c) Long-term potentiation in the striatum is unmasked by removing the voltage-dependent magnesium block of NMDA receptor channels. Eur J Neurosci 4(10):929–935
Calabresi P, Pisani A, Rothwell J, Ghiglieri V, Obeso JA, Picconi B (2016) Hyperkinetic disorders and loss of synaptic downscaling. Nat Neurosci 19(7):868–875. https://doi.org/10.1038/nn.4306
Centonze D, Costa C, Rossi S, Prosperetti C, Pisani A, Usiello A, Bernardi G, Mercuri NB, Calabresi P (2006) Chronic cocaine prevents depotentiation at corticostriatal synapses. Biol Psychiatry 60(5):436–443
Centonze D, Gubellini P, Picconi B, Calabresi P, Giacomini P, Bernardi G (1999) Unilateral dopamine denervation blocks corticostriatal LTP. J Neurophysiol 82(6):3575–3579
Centonze D, Gubellini P, Rossi S, Picconi B, Pisani A, Bernardi G, Calabresi P, Baunez C (2005) Subthalamic nucleus lesion reverses motor abnormalities and striatal glutamatergic overactivity in experimental parkinsonism. Neuroscience 133(3):831–840
Cepeda C, Hurst RS, Altemus KL, Flores-Hernandez J, Calvert CR, Jokel ES, Grandy DK, Low MJ, Rubinstein M, Ariano MA, Levine MS (2001) Facilitated glutamatergic transmission in the striatum of D2 dopamine receptor-deficient mice. J Neurophysiol 85(2):659–670
Cerovic M, Bagetta V, Pendolino V, Ghiglieri V, Fasano S, Morella I, Hardingham N, Heuer A, Papale A, Marchisella F, Giampa C, Calabresi P, Picconi B, Brambilla R (2015) Derangement of Ras-guanine nucleotide-releasing factor 1 (Ras-GRF1) and extracellular signal-regulated kinase (ERK) dependent striatal plasticity in l-DOPA-induced dyskinesia. Biol Psychiatry 77(2):106–115. https://doi.org/10.1016/j.biopsych.2014.04.002
Cools R, Gibbs SE, Miyakawa A, Jagust W, D’Esposito M (2008) Working memory capacity predicts dopamine synthesis capacity in the human striatum. J Neurosci 28(5):1208–1212. https://doi.org/10.1523/JNEUROSCI.4475-07.2008
Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, Wokosin D, Ilijic E, Sun Z, Sampson AR, Mugnaini E, Deutch AY, Sesack SR, Arbuthnott GW, Surmeier DJ (2006) Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci 9(2):251–259. https://doi.org/10.1038/nn1632
De Leonibus E, Pascucci T, Lopez S, Oliverio A, Amalric M, Mele A (2007) Spatial deficits in a mouse model of Parkinson disease. Psychopharmacology 194(4):517–525. https://doi.org/10.1007/s00213-007-0862-4
Deutch AY, Colbran RJ, Winder DJ (2007) Striatal plasticity and medium spiny neuron dendritic remodeling in parkinsonism. Parkinsonism Relat Disord 13(Suppl 3):S251–258. https://doi.org/10.1016/S1353-8020(08)70012-9
Fasano S, Bezard E, D’Antoni A, Francardo V, Indrigo M, Qin L, Dovero S, Cerovic M, Cenci MA, Brambilla R (2010) Inhibition of Ras-guanine nucleotide-releasing factor 1 (Ras-GRF1) signaling in the striatum reverts motor symptoms associated with l-DOPA-induced dyskinesia. Proc Natl Acad Sci USA 107(50):21824–21829. https://doi.org/10.1073/pnas.1012071107
Fino E, Paille V, Cui Y, Morera-Herreras T, Deniau JM, Venance L (2010) Distinct coincidence detectors govern the corticostriatal spike timing-dependent plasticity. J Physiol 588(Pt 16):3045–3062. https://doi.org/10.1113/jphysiol.2010.188466
Funamizu Y, Nishijima H, Ueno T, Ueno S, Mizukami H, Yagihashi S, Tomiyama M (2017) Morphological dendritic spine changes of medium spiny neurons in the nucleus accumbens in 6-hydroxydopamine-lesioned rats treated with levodopa. Neurosci Res 121:49–53. https://doi.org/10.1016/j.neures.2017.03.010
Gagnon D, Petryszyn S, Sanchez MG, Bories C, Beaulieu JM, De Koninck Y, Parent A, Parent M (2017) Striatal neurons expressing D1 and D2 receptors are morphologically distinct and differently affected by dopamine denervation in mice. Sci Rep 7:41432. https://doi.org/10.1038/srep41432
Giordano N, Iemolo A, Mancini M, Cacace F, De Risi M, Claudio Latagliata EC, Ghiglieri V, Bellenchi GC, Puglisi-Allegra S, Calabresi P, Picconi B, De Leonibus E (2018) Motor learning and metaplasticity in striatal neurons: relevance for Parkinson’s disease. Brain 141(2):505–520. https://doi.org/10.1093/brain/awx351
Gubellini P, Picconi B, Bari M, Battista N, Calabresi P, Centonze D, Bernardi G, Finazzi-Agro A, Maccarrone M (2002) Experimental parkinsonism alters endocannabinoid degradation: implications for striatal glutamatergic transmission. J Neurosci 22(16):6900–6907
Hebb DO (1949) The organization of behavior. Wiley, New York
Huang YZ, Rothwell JC, Lu CS, Chuang WL, Chen RS (2011) Abnormal bidirectional plasticity-like effects in Parkinson’s disease. Brain 134(Pt 8):2312–2320. https://doi.org/10.1093/brain/awr158
Ingham CA, Hood SH, Taggart P, Arbuthnott GW (1998) Plasticity of synapses in the rat neostriatum after unilateral lesion of the nigrostriatal dopaminergic pathway. J Neurosci 18(12):4732–4743
Ingham CA, Hood SH, van Maldegem B, Weenink A, Arbuthnott GW (1993) Morphological changes in the rat neostriatum after unilateral 6-hydroxydopamine injections into the nigrostriatal pathway. Exp Brain Res 93(1):17–27
Kawashima S, Ueki Y, Mima T, Fukuyama H, Ojika K, Matsukawa N (2013) Differences in dopaminergic modulation to motor cortical plasticity between Parkinson’s disease and multiple system atrophy. PLoS One 8(5):e62515. https://doi.org/10.1371/journal.pone.0062515
Lovinger DM, Tyler EC, Merritt A (1993) Short- and long-term synaptic depression in rat neostriatum. J Neurophysiol 70(5):1937–1949
Maccarrone M, Gubellini P, Bari M, Picconi B, Battista N, Centonze D, Bernardi G, Finazzi-Agro A, Calabresi P (2003) Levodopa treatment reverses endocannabinoid system abnormalities in experimental parkinsonism. J Neurochem 85(4):1018–1025
Malenka RC, Bear MF (2004) LTP and LTD: an embarrassment of riches. Neuron 44(1):5–21. https://doi.org/10.1016/j.neuron.2004.09.012
Martella G, Tassone A, Sciamanna G, Platania P, Cuomo D, Viscomi MT, Bonsi P, Cacci E, Biagioni S, Usiello A, Bernardi G, Sharma N, Standaert DG, Pisani A (2009) Impairment of bidirectional synaptic plasticity in the striatum of a mouse model of DYT1 dystonia: role of endogenous acetylcholine. Brain 132(Pt 9):2336–2349. https://doi.org/10.1093/brain/awp194
Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H (2004) Structural basis of long-term potentiation in single dendritic spines. Nature 429(6993):761–766. https://doi.org/10.1038/nature02617
McNeill TH, Brown SA, Rafols JA, Shoulson I (1988) Atrophy of medium spiny I striatal dendrites in advanced Parkinson’s disease. Brain Res 455(1):148–152
Mercuri NB, Bernardi G (2005) The ‘magic’ of l-DOPA: why is it the gold standard Parkinson’s disease therapy? Trends Pharmacol Sci 26(7):341–344. https://doi.org/10.1016/j.tips.2005.05.002
Molina-Luna K, Pekanovic A, Rohrich S, Hertler B, Schubring-Giese M, Rioult-Pedotti MS, Luft AR (2009) Dopamine in motor cortex is necessary for skill learning and synaptic plasticity. PLoS One 4(9):e7082. https://doi.org/10.1371/journal.pone.0007082
Morgante F, Espay AJ, Gunraj C, Lang AE, Chen R (2006) Motor cortex plasticity in Parkinson’s disease and levodopa-induced dyskinesias. Brain 129(Pt 4):1059–1069. https://doi.org/10.1093/brain/awl031
Nishijima H, Arai A, Kimura T, Mori F, Yamada J, Migita K, Wakabayashi K, Baba M, Ueno S, Tomiyama M (2013) Drebrin immunoreactivity in the striatum of a rat model of levodopa-induced dyskinesia. Neuropathology 33(4):391–396. https://doi.org/10.1111/neup.12009
Nishijima H, Ueno T, Ueno S, Mori F, Miki Y, Tomiyama M (2017) Levodopa-induced morphologic changes of prefrontal pyramidal tract-type neurons in a rat model of Parkinson’s disease. Neurosci Res 115:54–58. https://doi.org/10.1016/j.neures.2016.10.001
Olanow CW, Agid Y, Mizuno Y, Albanese A, Bonuccelli U, Damier P, De Yebenes J, Gershanik O, Guttman M, Grandas F, Hallett M, Hornykiewicz O, Jenner P, Katzenschlager R, Langston WJ, LeWitt P, Melamed E, Mena MA, Michel PP, Mytilineou C, Obeso JA, Poewe W, Quinn N, Raisman-Vozari R, Rajput AH, Rascol O, Sampaio C, Stocchi F (2004) Levodopa in the treatment of Parkinson’s disease: current controversies. Mov Disord 19(9):997–1005. https://doi.org/10.1002/mds.20243
Olanow CW, Schapira AH (2013) Therapeutic prospects for Parkinson disease. Ann Neurol 74(3):337–347. https://doi.org/10.1002/ana.24011
Parnetti L, Farotti L, Eusebi P, Chiasserini D, De Carlo C, Giannandrea D, Salvadori N, Lisetti V, Tambasco N, Rossi A, Majbour NK, El-Agnaf O, Calabresi P (2014) Differential role of CSF alpha-synuclein species, tau, and Abeta42 in Parkinson’s Disease. Front Aging Neurosci 6:53. https://doi.org/10.3389/fnagi.2014.00053
Picconi B, Centonze D, Hakansson K, Bernardi G, Greengard P, Fisone G, Cenci MA, Calabresi P (2003) Loss of bidirectional striatal synaptic plasticity in l-DOPA-induced dyskinesia. Nat Neurosci 6(5):501–506
Picconi B, Paille V, Ghiglieri V, Bagetta V, Barone I, Lindgren HS, Bernardi G, Angela Cenci M, Calabresi P (2008) l-DOPA dosage is critically involved in dyskinesia via loss of synaptic depotentiation. Neurobiol Dis 29(2):327–335
Poewe W (2005) Treatment of dementia with Lewy bodies and Parkinson’s disease dementia. Mov Disord 20(Suppl 12):S77–82. https://doi.org/10.1002/mds.20544
Prescott IA, Dostrovsky JO, Moro E, Hodaie M, Lozano AM, Hutchison WD (2009) Levodopa enhances synaptic plasticity in the substantia nigra pars reticulata of Parkinson’s disease patients. Brain 132(Pt 2):309–318. https://doi.org/10.1093/brain/awn322
Prescott IA, Liu LD, Dostrovsky JO, Hodaie M, Lozano AM, Hutchison WD (2014) Lack of depotentiation at basal ganglia output neurons in PD patients with levodopa-induced dyskinesia. Neurobiol Dis 71:24–33. https://doi.org/10.1016/j.nbd.2014.08.002
Raju DV, Ahern TH, Shah DJ, Wright TM, Standaert DG, Hall RA, Smith Y (2008) Differential synaptic plasticity of the corticostriatal and thalamostriatal systems in an MPTP-treated monkey model of parkinsonism. Eur J Neurosci 27(7):1647–1658. https://doi.org/10.1111/j.1460-9568.2008.06136.x
Raju DV, Shah DJ, Wright TM, Hall RA, Smith Y (2006) Differential synaptology of vGluT2-containing thalamostriatal afferents between the patch and matrix compartments in rats. J Comp Neurol 499(2):231–243. https://doi.org/10.1002/cne.21099
Schuster S, Doudnikoff E, Rylander D, Berthet A, Aubert I, Ittrich C, Bloch B, Cenci MA, Surmeier DJ, Hengerer B, Bezard E (2009) Antagonizing L-type Ca2 + channel reduces development of abnormal involuntary movement in the rat model of L-3,4-dihydroxyphenylalanine-induced dyskinesia. Biol Psychiatry 65(6):518–526. https://doi.org/10.1016/j.biopsych.2008.09.008
Segal M (2010) Dendritic spines, synaptic plasticity and neuronal survival: activity shapes dendritic spines to enhance neuronal viability. Eur J Neurosci 31(12):2178–2184. https://doi.org/10.1111/j.1460-9568.2010.07270.x
Seroogy KB, Lundgren KH, Tran TM, Guthrie KM, Isackson PJ, Gall CM (1994) Dopaminergic neurons in rat ventral midbrain express brain-derived neurotrophic factor and neurotrophin-3 mRNAs. J Comp Neurol 342(3):321–334. https://doi.org/10.1002/cne.903420302
Shen W, Flajolet M, Greengard P, Surmeier DJ (2008) Dichotomous dopaminergic control of striatal synaptic plasticity. Science 321(5890):848–851. https://doi.org/10.1126/science.1160575
Shen W, Plotkin JL, Francardo V, Ko WK, Xie Z, Li Q, Fieblinger T, Wess J, Neubig RR, Lindsley CW, Conn PJ, Greengard P, Bezard E, Cenci MA, Surmeier DJ (2015) M4 muscarinic receptor signaling ameliorates striatal plasticity deficits in models of l-DOPA-induced dyskinesia. Neuron 88(4):762–773. https://doi.org/10.1016/j.neuron.2015.10.039
Shen W, Tian X, Day M, Ulrich S, Tkatch T, Nathanson NM, Surmeier DJ (2007) Cholinergic modulation of Kir2 channels selectively elevates dendritic excitability in striatopallidal neurons. Nat Neurosci 10(11):1458–1466. https://doi.org/10.1038/nn1972
Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (1997) Alpha-synuclein in Lewy bodies. Nature 388(6645):839–840. https://doi.org/10.1038/42166
Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J (2000) Induction of plasticity in the human motor cortex by paired associative stimulation. Brain 123(Pt 3):572–584
Stefan K, Wycislo M, Classen J (2004) Modulation of associative human motor cortical plasticity by attention. J Neurophysiol 92(1):66–72. https://doi.org/10.1152/jn.00383.2003
Suarez LM, Solis O, Aguado C, Lujan R, Moratalla R (2016) l-DOPA oppositely regulates synaptic strength and spine morphology in D1 and D2 striatal projection neurons in dyskinesia. Cereb Cortex 26(11):4253–4264. https://doi.org/10.1093/cercor/bhw263
Suarez LM, Solis O, Carames JM, Taravini IR, Solis JM, Murer MG, Moratalla R (2014) l-DOPA treatment selectively restores spine density in dopamine receptor D2-expressing projection neurons in dyskinetic mice. Biol Psychiatry 75(9):711–722. https://doi.org/10.1016/j.biopsych.2013.05.006
Surmeier DJ, Ding J, Day M, Wang Z, Shen W (2007) D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci 30(5):228–235. https://doi.org/10.1016/j.tins.2007.03.008
Tang K, Low MJ, Grandy DK, Lovinger DM (2001) Dopamine-dependent synaptic plasticity in striatum during in vivo development. Proc Natl Acad Sci USA 98(3):1255–1260. https://doi.org/10.1073/pnas.031374698
Thiele SL, Chen B, Lo C, Gertler TS, Warre R, Surmeier JD, Brotchie JM, Nash JE (2014) Selective loss of bi-directional synaptic plasticity in the direct and indirect striatal output pathways accompanies generation of parkinsonism and l-DOPA induced dyskinesia in mouse models. Neurobiol Dis 71:334–344. https://doi.org/10.1016/j.nbd.2014.08.006
Toy WA, Petzinger GM, Leyshon BJ, Akopian GK, Walsh JP, Hoffman MV, Vuckovic MG, Jakowec MW (2014) Treadmill exercise reverses dendritic spine loss in direct and indirect striatal medium spiny neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson’s disease. Neurobiol Dis 63:201–209. https://doi.org/10.1016/j.nbd.2013.11.017
Tozzi A, de Iure A, Di Filippo M, Tantucci M, Costa C, Borsini F, Ghiglieri V, Giampa C, Fusco FR, Picconi B, Calabresi P (2011) The distinct role of medium spiny neurons and cholinergic interneurons in the D(2)/A(2)A receptor interaction in the striatum: implications for Parkinson’s disease. J Neurosci 31(5):1850–1862. https://doi.org/10.1523/JNEUROSCI.4082-10.2011
Ueki Y, Mima T, Kotb MA, Sawada H, Saiki H, Ikeda A, Begum T, Reza F, Nagamine T, Fukuyama H (2006) Altered plasticity of the human motor cortex in Parkinson’s disease. Ann Neurol 59(1):60–71. https://doi.org/10.1002/ana.20692
Ueno T, Nishijima H, Ueno S, Tomiyama M (2017) Spine Enlargement of pyramidal tract-type neurons in the motor cortex of a rat model of levodopa-induced dyskinesia. Front Neurosci 11:206. https://doi.org/10.3389/fnins.2017.00206
Villalba RM, Lee H, Smith Y (2009) Dopaminergic denervation and spine loss in the striatum of MPTP-treated monkeys. Exp Neurol 215(2):220–227. https://doi.org/10.1016/j.expneurol.2008.09.025
Voon V, Fernagut PO, Wickens J, Baunez C, Rodriguez M, Pavon N, Juncos JL, Obeso JA, Bezard E (2009) Chronic dopaminergic stimulation in Parkinson’s disease: from dyskinesias to impulse control disorders. Lancet Neurol 8(12):1140–1149. https://doi.org/10.1016/S1474-4422(09)70287-X
Voon V, Napier TC, Frank MJ, Sgambato-Faure V, Grace AA, Rodriguez-Oroz M, Obeso J, Bezard E, Fernagut PO (2017) Impulse control disorders and levodopa-induced dyskinesias in Parkinson’s disease: an update. Lancet Neurol 16(3):238–250. https://doi.org/10.1016/S1474-4422(17)30004-2
Wang Q, Zhang W (2016) Maladaptive synaptic plasticity in l-DOPA-induced dyskinesia. Front Neural Circuits 10:105. https://doi.org/10.3389/fncir.2016.00105
Wolters A, Sandbrink F, Schlottmann A, Kunesch E, Stefan K, Cohen LG, Benecke R, Classen J (2003) A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J Neurophysiol 89(5):2339–2345. https://doi.org/10.1152/jn.00900.2002
Yasumatsu N, Matsuzaki M, Miyazaki T, Noguchi J, Kasai H (2008) Principles of long-term dynamics of dendritic spines. J Neurosci 28(50):13592–13608. https://doi.org/10.1523/JNEUROSCI.0603-08.2008
Yin HH, Mulcare SP, Hilario MR, Clouse E, Holloway T, Davis MI, Hansson AC, Lovinger DM, Costa RM (2009) Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci 12(3):333–341. https://doi.org/10.1038/nn.2261
Zhang Y, Meredith GE, Mendoza-Elias N, Rademacher DJ, Tseng KY, Steece-Collier K (2013) Aberrant restoration of spines and their synapses in L-DOPA-induced dyskinesia: involvement of corticostriatal but not thalamostriatal synapses. J Neurosci 33(28):11655–11667. https://doi.org/10.1523/JNEUROSCI.0288-13.2013
Funding
This work was supported by grants from Ministry of University of education and research, Progetto di Ricerca di Interesse Nazionale 2015 (2015FNWP34) (to E.D.L. and P.C.), from the Italian Ministry of Health, Ricerca Finalizzata (RF-2013-02357386 to B.P. and RF-2013-02356215 to P.C.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors reported no biomedical financial interests or potential conflicts of interest.
Rights and permissions
About this article
Cite this article
Picconi, B., De Leonibus, E. & Calabresi, P. Synaptic plasticity and levodopa-induced dyskinesia: electrophysiological and structural abnormalities. J Neural Transm 125, 1263–1271 (2018). https://doi.org/10.1007/s00702-018-1864-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-018-1864-6