Abstract
Rationale
Accumulating evidence in humans demonstrated that visuo-spatial deficits are the most consistently reported cognitive abnormalities in Parkinson disease (PD). These deficits have been generally attributed to cortical dopamine degeneration. However, more recent evidence suggests that dopamine loss in the striatum is responsible for the visuo-spatial abnormalities in PD. Studies based on animal models of PD did not specifically address this question.
Objectives
Thus, the first goal of this study was to analyze the role of dopamine within the dorsal striatum in spatial memory. We tested bilateral 6-OHDA striatal lesioned CD1 mice in an object–place association spatial task. Furthermore, to see whether the effects were selective for spatial information, we measured how the 6-OHDA-lesioned animals responded to a non-spatial change and learned in the one-trial inhibitory avoidance task.
Results
The results demonstrated that bilateral (approximately 75%) dopamine depletion of the striatum impaired spatial change discrimination. On the contrary, no effect of the lesion was observed on non-spatial novelty detection or on passive avoidance learning.
Conclusions
These results confirm that dopamine depletion is accompanied by cognitive deficits and demonstrate that striatal dopamine dysfunction is sufficient to induce spatial information processing deficits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Motor symptoms in Parkinson disease (PD), a neurodegenerative disorder characterized by a progressive dopamine (DA) loss in the nigrostriatal pathway, are often associated with cognitive symptoms. Among them, visuo-spatial deficits are the most consistently reported (Boller et al. 1984; Giraudo et al. 1997).
A conspicuous number of neuroimaging studies on Parkinson patients are being conducted to specifically address the neural basis of their cognitive deficits (Berger et al. 2004; Cools et al. 2002, 2007; Lewis et al. 2003; Owen et al. 1998). These deficits in PD have been generally attributed to the impairment of mesocortical dopaminergic projections (Cools et al. 2002; Crofts et al. 2001). However, it has been recently suggested that they might also be related to the subcortical pathology of the disease (Berger et al. 2004; Collins et al. 2000; Cools et al. 2007; Crofts et al. 2001; Dubois and Pillon 1997). Indeed, the cognitive abnormalities observed in PD patients do not match closely those observed in frontal lobe patients (Owen et al. 1993). Furthermore, they have been reported even at the early stage of the disease when the degeneration of DA neurons is restricted to the striatal projections (Owen et al. 1997; Pillon et al. 1997). Preclinical studies in rodents confirm the involvement of the nigrostriatal DA system in mediating learning and memory processes. For example, bilateral 6-OHDA lesions of the medial forebrain bundle impair visuo-spatial navigation in the Morris water maze; deficits that seem specific to the processing of spatial information rather than to a more general disturbance in motor performance (Courtiere et al. 2005; Miyoshi et al. 2002; Mura and Feldon 2003; Whishaw and Dunnett 1985). However, medial forebrain bundle lesions affect both cortical and subcortical DA projections; thus, they are not a good animal model to study cognitive deficit associated to striatal dopamine loss in PD (Miyoshi et al. 2002). Therefore, we tried to clarify whether DA lesions restricted to the dorsal striatum affect cognitive functions, and, in particular, spatial information processing. For this purpose, we tested the effects of dorsal striatum DA depletion on mice ability to solve a spatial object–place association task (Roullet et al. 2001). The face validity of this task in assessing spatial deficits in an animal model of PD is demonstrated by the fact that PD patients are impaired in a computerized version of the object–place association task (Giraudo et al. 1997; Mollion et al. 2003).
Another interesting point, which is still debated in the human literature, is the nature of the visuo-spatial deficit accompanying the striatal DA loss (Crofts et al. 2001; Mollion et al. 2003; Owen et al. 1997). Indeed, it is not clear whether the deficit observed could be attributed to a general memory impairment, or, alternatively, it is specific for working or long-term memory; a further issue to be clarified is whether it is selective for spatial material (Giraudo et al. 1997; Postle et al. 1997). Unfortunately, none of the studies in rodents available in the literature properly addressed these questions by testing the effects of striatal DA loss on different memory demands in the same task or on different kinds of memory in different tasks.
To help clarify these points, we tested the effects of dorsal striatum 6-OHDA bilateral lesion on spatial information processing using different time intervals to verify the effects on both spatial working and long-term memories. Furthermore, to assess whether the effect of the lesion was limited to the processing of spatial information, we tested the ability of mice to react to a new object (object substitution) and to learn and remember stimulus-aversive reinforcement association in one-trial inhibitory avoidance task.
Experimental procedure
Animals
The subjects were CD1 outbred mice obtained from Charles River (Calco, Italy). Upon arrival, mice were housed in groups of 12 in standard breeding cages (46 × 26 × 21.8 cm), placed in a rearing room at a constant temperature (22 ± 1°C) and maintained on a 12-h light/dark cycle with food and water available ad libitum. At the time of surgery, they were 8–10 weeks old.
Dopamine lesion
Mice were anaesthetized with i.p. injection of chloral hydrate (500 mg/kg, Fluka, Milan, Italy) and placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA) with mouse adaptor and lateral ear bars. The head skin was cut longitudinally, and an injector (0.25 mm in diameter), connected with polyethylene tubing to a 2-μl Hamilton syringe, was lowered bilaterally into the dorsal striatum. The following stereotaxic coordinates were used: +0.3 mm anterior to bregma, ±2 mm lateral to midline, −3.0 mm ventral from the skull surface, according to Franklin and Paxinos (1997) mouse brain atlas. Striatal dopamine lesions were performed using 6-hydroxydopamine (6-OHDA) hydrochloride solution (Sigma, Milan, Italy), in a concentration of 1.5 μg/0.1 μl of saline containing Na-metabisulfite (0.1 M; Sigma, Milan, Italy). The injection volume was 0.3 μl per side. The sham lesions consisted of bilateral perforation of the skull at sites corresponding to the structures. To protect noradrenergic terminals, mice were given desipramine (35 mg/kg, i.p.; Sigma). Mice were allowed to recover from the operation for at least 20 days.
Immunohistochemistry
Seven of the lesioned animals and seven sham animals were used to estimate the extent of the lesion using tyrosine hydroxylase (TH) immunolabeling. Animals were deeply anaesthetized with chloral hydrate (500 mg/kg, i.p.) and then perfused with 0.9% saline solution followed by cold 4% formaldehyde solution. Brains were removed and post-fixed for 3 h in a 10% formaldehyde solution at 4°C and then transferred to a 30% sucrose solution at 4°C for cryoprotection. Thirty micrometers coronal sections were collected throughout the rostrocaudal extent of the striatum. Using standard ABC methods (Vector Laboratories, Burlingame, CA, USA), sections were processed for TH immunolabeling using the rabbit anti-TH primary antibody (1:500, AB152, Chemicon International Temecula, CA, USA) and the biotinylated goat anti-rabbit secondary antibody (1:500, Vector Laboratories, Burlingame, CA, USA). Immunoreactivity was visualized with metal-enhanced DAB (Sigma).
Consecutive coronal brain sections of the striatum were taken from each animal between the coordinates +1.18 and −0.10 mm anterior to bregma and analyzed using a microscope in combination with a video camera (Leica) to capture digitized images. The extent of the lesion was analyzed using ImageJ (NIH; Bethesda, MD, USA) to measure optical density in four different striatal subregions distinguished on the coronal plane: MD (mediodorsal), DL (dorsolateral), VM (ventromedial), and VL (ventrolateral). Furthermore, these regions were analyzed over three different levels on the sagittal plane. The three levels were defined as follows: anterior (from AP +1.18 to AP +0.74) medial (from AP +0.62 to AP +0.08) posterior (from AP +0.02 to AP −0.10), according to the mouse atlas of Franklin and Paxinos (1997). Level of gray was measured by measuring the optical density of a constant area (0.298 mm2) applied in the four different subregions at different anteroposterior coordinates.
Behavioral procedures and data collection
For the spatial memory and non-spatial novelty reaction task, the apparatus and behavioral procedure were similar to those previously described (Roullet et al. 2001; Sargolini et al. 1999). In brief, the apparatus was a circular open field. We performed two experiments that differed in terms of the delay between the habituation phase (from session 2 to session 4) and the spatial test session (session 5). In the short-term experiment, the interval imposed was 3 min, while in the long-term experiment, we used a 24-h interval.
For a detailed description of the measures collected, refer to Roullet et al. (2001). In brief, mice were individually submitted to seven consecutive, 6-min sessions. During session 1 (S1), mice were placed into the empty open field. During sessions 2–4 (S2–S4), five objects were present, and mice were placed into the apparatus to allow them to habituate to the apparatus and to the objects configuration (habituation phase). During the 3-min intersession interval, the animals were returned to a waiting cage located inside the test room. During the spatial test session (S5), the objects configuration was changed by moving two objects (displaced objects, DO) and leaving the other three objects in the same position (non-displaced objects, NDO). After the spatial test session in both experiments, animals were submitted to two additional sessions: 6 and 7, with an intersession interval of 3 min. In session 6, the configuration of the objects was kept unchanged as compared to S5. In the last session (session 7), one of the familiar objects was replaced by a new object (substituted object, SO); the other four objects were left unchanged (non-substituted objects, NSO).
In all sessions, locomotor activity was recorded by counting the number of sector crossings. From sessions 2 to 7, object exploration was evaluated on the basis of the mean time spent by the animal in contact with the different objects. A contact was defined as the snout of the subject actually touching an object. Habituation to objects exploration was assessed by averaging the duration of contacts with the five objects during sessions 2, 3, and 4 in each group. The animals’ ability to selectively react to the spatial change was analyzed by calculating the spatial re-exploration index (DO [S5] − DO [S4] = DO and NDO [S5] − NDO [S4] = NDO), while the animals’ ability to selectively react to the non-spatial change (novel object) was analyzed by calculating non-spatial re-exploration index (SO [S7] − SO [S6] = SO and NSO [S7] − NSO [S6] = NSO). The effects of the 6-OHDA lesions on the variables measured were analyzed by using two-way analysis of variance (ANOVA) for repeated measure, with treatment (two levels: sham and lesion) as between factor. Tukey honestly significant difference post hoc analysis was used when appropriate. Furthermore, the relationship between the dopamine depletion and spatial novelty reaction was evaluated by means of Pearson’s two-tailed correlation analysis on all animals (sham and lesioned) tested in the spatial task.
In a further experiment, independent groups of sham and 6-OHDA-lesioned animals were tested in the one-trial inhibitory avoidance task. For a detailed description of the apparatus and procedures used, refer to De Leonibus et al. (2003). In brief, the dependent variable measured was the time taken by the animals to completely enter the dark compartment (latency). Training latency refers to the latency measured on the training day. Testing latency refers to the latency measured on the test day performed 24 h after training. A maximum step-through latency of 240 s was imposed. Training and testing latencies were analyzed using a one-way ANOVA, the between-factor always being treatment (two levels: sham and lesion).
The criterion for significance was always set at p < 0.05.
Lesion verification
To assess striatal DA levels in lesioned and sham animals, biochemical analyses were performed ex vivo on tissue samples. All subjects were killed by decapitation, the brain removed and plunged directly into saline (NaCl 9%) contained in a thermic box and left in place for 60 s. The brain was frozen at −10°C before punching. The brain was then fixed on the brain matrix. Punches were obtained from brain slices (frontal sections) no thicker than 300 μm. Stainless steel tubes 1 mm internal diameter were used to punch the dorsal striatum and the nucleus accumbens. Tissue samples were stored in liquid nitrogen. DA was determined by means of reverse high-performance liquid chromatography coupled with electrochemical detection (for a detailed description of the method, refer to Puglisi-Allegra et al. 2000). Tissue levels of DA and norepinephrine (NA) metabolites (pg/mg wet weight) were used for statistical analyses.
Results
Immunohistochemical and biochemical verification of the lesion
Gray values of brain areas stained for TH immunochemistry of lesioned and sham animals were compared dividing the striatum in four different subregions, as shown in Fig. 1. As summarized in Table 1, lesioned animals differed from sham controls in all the subregions considered at all the anteroposterior levels. The only component of the striatum that did not differ between the two groups was the ventromedial. This result shows that the lesion was widespread over the anteroposterior subregions, but only in the dorso-medial and lateral components of the striatum. The three-way ANOVA for repeated measure revealed a significant effect of the lesion (F1/12 = 28.87; p < 0.05), of the striatal subregions (F3/36 = 42.01; p < 0.05) and of the level on the sagittal plane (F2/24 = 4.73; p < 0.05). Furthermore, interactions between lesion and subregions (F3/36 = 21.76; p < 0.05) and subregions for sagittal level (F6/72 = 6.95; p < 0.05) were significant (Table 1).
The biochemical analysis, performed in the sham and the lesioned animals tested in the behavioral experiments, revealed a depletion of tissue levels of DA >60% in 30 animals (out of 40). The final number of animals in the different groups was: 13 in the short-term spatial discrimination experiment (sham n = 8), ten in the long-term spatial discrimination experiment (sham n = 11), seven in the passive avoidance experiment (sham n = 10). Striatal tissue levels of DA in the 6-OHDA-lesioned mice (4,212 ± 303 pg/mg) was significantly reduced (F1/51 = 257.745; p < 0.05) as compared to sham animals (17,938 ± 895 pg/mg tissue). On the contrary, striatal tissue levels of norepinephrine did not differ in lesioned compared with sham animals (6-OHDA lesioned = 179 ± 35 pg/mg tissue; sham = 249 ± 35 pg/mg tissue). DA (sham = 18,388 ± 1,597 pg/mg; 6-OHDA-lesioned = 15,980 ± 1,111 pg/mg) as well as NE (Sham = 546 ± 150 pg/mg; 6-OHDA-lesioned = 481 ± 91 pg/mg) tissue levels in nucleus accumbens were not affected by 6-OHDA injection within the dorsal striatum.
Effects of 6-OHDA bilateral lesions of the dorsal striatum on spatial discrimination and non-spatial novelty reaction
As reported in Table 2, all groups progressively reduced locomotor activity in a similar way over the consecutive sessions. The two-way ANOVA for repeated measures evidenced a significant effect of session [short-term experiment: (F6/114 = 52.442; p < 0.05); long-term experiment: (F6/114 = 79.418; p < 0.05)], but no other significant effect. Whatever the treatment (sham or lesion) or the experimental condition (short-term versus long-term), the animals showed a similar pattern of objects exploration during the habituation phase (from S2 to S4 in Table 2), namely, a high level of exploration in session 2 and a progressive reduction in the following sessions [session effect: short-term (F2/38 = 111.916; p < 0.05); long-term (F2/38 = 122.998; p < 0.05)].
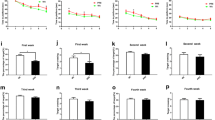
As illustrated in Fig. 2a, in the short-term experiment, sham animals selectively re-explored the displaced objects (DO) as compared to the non-displaced objects (NDO), thus, demonstrating that they were able to discriminate and selectively react to the spatial change. On the contrary, lesioned animals did not show any preference for any of the two object categories re-exploring DO and NDO for a similar amount of time. The statistical analysis revealed a significant effect for all the factors: object category (F1/19 = 9.452; p < 0.05), treatment (F1/19 = 14.9; p < 0.05) and interaction between treatment and object category (F1/19 = 4.471; p < 0.05). Post hoc comparisons confirmed that only the sham-operated animals explored the DO significantly more than the NDO (p < 0.05).
Effects of the 6-OHDA lesion (Les) on animals ability to react to a spatial novelty after a 3-min delay (a) and 24−h delay (b) from S4. The histograms illustrate the mean time (±SEM) spent exploring displaced (DO) and non-displaced (NDO) in S5 minus the time spent exploring the same object category in S4, spatial re-exploration index. *p < 0.05 DO vs NDO within the same group
Similar results were obtained when the animals were tested 24 h after training (Fig. 2b). Indeed, the ability of sham animals to selectively react to DO as compared to NDO was intact 24 h after the last habituation session. On the contrary, 6-OHDA lesions of the dorsal striatum completely abolished the differential exploration of the two object categories (Fig. 2b). Statistical analysis revealed a significant effect of object category (F1/19 = 8.481; p < 0.05) and of the interaction between treatment and object category (F1/19 = 5.744; p < 0.05). Post hoc analysis confirmed that DO was explored more than NDO only in the sham group. Person’s analysis (Fig. 3) revealed that displaced object re-exploration positively correlated with the tissue levels of dopamine within the dorsal striatum (R = 0.416; p < 0.05).
In Fig. 4a and b are represented the re-exploration indexes for the SO and the NSO in the two experiments. 6-OHDA lesions did not affect the ability of the animals to detect and react to the novel object. All groups explored the new object much more than the familiar ones. Statistical analysis revealed only an effect of object category [short-term experiment: (F1/19 = 45.162; p < 0.05); long-term experiment: (F1/19 = 23.397; p < 0.05)].
Effects of the 6-OHDA lesion (Les) on animals ability to react to a non-spatial novelty after a 3-min delay (a) and 24-h delay (b) from S4. The histograms illustrate the mean time (±SEM) spent exploring substituted (SO) and non-substituted (NSO) in S7 minus the time spent exploring the same object category in S6, non spatial re-exploration index. *p < 0.05 SO vs NSO within the same group
Effects of 6-OHDA bilateral lesions of the dorsal striatum on one-trial inhibitory avoidance task
The effects of bilateral striatal 6-OHDA lesions were also assessed in the one-trial inhibitory avoidance task. Lesioned animals did not show any significant difference in the latency to step-through when compared to sham animals on the training (latencies: sham = 10 ± 1.4 s, lesioned = 8 ± 2.3 s) as well as on testing 24 h later (latencies: sham = 146 ± 28 s, lesioned = 126 ± 42 s).
Discussion
In this study, we demonstrate that bilateral 6-OHDA lesions of the striatum impair the ability of mice to perform a spatial discrimination task. Interestingly, we found that this effect was delay independent, thus, providing evidence supporting a deficit in both short- and long-term spatial information processing. Furthermore, the lack of effect of the same lesions on a novel object discrimination paradigm and in an associative learning task demonstrates a selective role of striatal DA in modulating the acquisition and maintenance of spatial information rather than a generic role in memory, independent on the kind of information to be processed.
In the first two experiments, we assessed the effects of striatal DA depletion on the ability of the animals to react to a spatial change. The results clearly demonstrate that 6-OHDA-lesioned animals were impaired in spatial novelty detection and reaction. This deficit was independent of the delays. Indeed, lesioned mice re-explored the two object categories for the same amount of time independently on whether they were tested 3 min or 24 h after the last session of habituation, thus, indicating that 6-OHDA bilateral lesion of the striatum impaired both short- and long-term spatial memory. This conclusion is further confirmed by the positive correlation (Fig. 3) found between tissue levels of dopamine within the dorsal striatum and the re-exploration of the displaced objects, indicating that the less the dopamine in the dorsal striatum the worst was the performance in the spatial discrimination task.
The nigrostriatal dopamine system has been involved in the control of motor, and motivational processes (De Leonibus et al. 2006; Keitz et al. 2003; Robbins and Everitt 1996); hence, the impairment we observed in mice ability to react to the spatial change might be interpreted as secondary to motivational or motor deficits. However, a deeper analysis of the results suggests that this interpretation is unlikely. For example, objects exploration and locomotor activity during the habituation phase was comparable in sham and lesioned animals, which rules out any deficit in the motor activity of the lesioned animals. Furthermore, the discrimination and the reactivity to a novel object was not affected by the lesion, thus, suggesting that the ability to react to a novelty was intact. These results are in agreement with the occurrence of visuo-spatial deficits in medial forebrain bundle 6-OHDA-lesioned animals (Mura and Feldon 2003). Further, they demonstrate that dopamine depletion of the striatum is sufficient to induce cognitive deficits, suggesting that the animal model we present has a high face validity to mimic the cognitive deficits associated to striatal dopamine loss in PD. Indeed, our data are perfectly consistent with those reporting a spatial but not object working memory deficit in mild PD patients (Boller et al. 1984; Giraudo et al. 1997; Owen et al. 1997; Postle et al. 1997).
Regarding the relative contribution of different DA terminal regions in processing the same kind of spatial information, previous studies reported the effects of D1 and D2 receptor antagonists administration within the prefrontal cortex and the ventral striatum in the same spatial task (Coccurello et al. 2000; Rinaldi et al. 2007). Similar to the present findings, DA receptors blockade within the prefrontal cortex impairs short-term spatial memory, and does not affect novel object discrimination (Rinaldi et al. 2007). However, the same pharmacological manipulations within the ventral striatum produce receptors subtype-dependent effect; in particular, D2 dopamine receptor blockade affects both spatial and non-spatial novelty discrimination (Coccurello et al. 2000; Rinaldi et al. 2007). Taking into account that a direct comparison between these studies is limited by the use of different pharmacological manipulations of DA system, all together, these data, on the one hand, provide support to the idea that normal transmission of information through frontostriatal circuitries is necessary for normal visuo-spatial information processing (Cools et al. 2002; Lewis et al. 2003; Owen et al. 1998; Pillon et al. 1996). On the other hand, they confirm the functional specialization of the dopamine terminal regions in processing different kinds of information (Bracs et al. 1984; Cools et al. 2007; De Leonibus et al. 2006; Smith-Roe et al. 1999).
Interestingly, bilateral 6-OHDA lesions of the dorsal striatum do not affect mice ability to perform a passive avoidance task when animals are tested 24 h after training. Very few studies have assessed the role of the striatal complex and in particular of DA system within this region in aversive learning (Bracs et al. 1984; Lorenzini et al. 1995; Reyes Vazquez et al. 1978; White and Salinas 2003). However, even if the results relative to dorsal component of the striatum are somewhat contradictory in the literature, thus, not allowing us to draw definite conclusions, experimental evidence suggests that it is the ventral rather than the dorsal striatum involved in mediating the response in this task (Bracs et al. 1984; Lorenzini et al. 1995; Reyes Vazquez et al. 1978). The present findings are in line with this suggestion, demonstrating that normal DA activity within the dorsal striatum does not seem to be involved in mediating this response. In fact, the TH immunostaining and tissue levels measures of DA and NE demonstrated that the extent of the lesion was limited to the dorsal and lateral components of the striatum spearing the ventromedial component (i.e., the nucleus accumbens).
The lack of effect observed after striatal DA depletion on the performance of one-trial inhibitory avoidance task is also of interest because it helps to interpret the nature of spatial deficit. Indeed, it confirms that the deficit found in the spatial task at 24-h delay is specific for spatial information, and it is not due to an aspecific memory impairment. It is worth noting, in this respect, that to solve the one-trial inhibitory avoidance task are necessary intact abilities not only to form conditional association but also to processes contextual (spatial) information. This allegation is supported by the impairment induced by hippocampal pharmacological manipulations on passive avoidance memory (Roesler et al. 2006), an effect that has been suggested to depend upon the inability to discriminate between the shocked and the safe compartment. Indeed, the impairment induced by the hippocampal lesion in aversive conditioning disappears if the shock is coupled with a discrete conditional stimulus (White and Salinas 2003). All together, this suggests that the spatial deficit observed in the object–place association task after striatal dopamine depletion is selective for a particular kind of spatial information, which is necessary to discriminate between displaced and non-displaced objects, but irrelevant to distinguish between contexts (De Leonibus et al. 2005).
Another possible interpretation of the present results is that the striatum is involved in memory processes only when visuo-spatial information must be actively organized to guide behavior (Berger et al. 2004; Buytenhuijs et al. 1994; Giraudo et al. 1997; Pillon et al. 1996). Such a hypothesis has been frequently used to explain why PD patients are most consistently found to be impaired in free recall tasks (which require active organization of the material), but not in familiarity recognition task (which requires stimulus-driven cognitive processes; Breen 1993; Farina et al. 2000; Weingartner et al. 1984). The results of our study fit well with this explanation. In fact, striatal DA-depleted mice were selectively impaired in the spatial novelty task, which requires comparing an internal representation of the previous disposition (which was not available during the discrimination) with the current position of the objects to guide selective re-exploration of the displaced objects.
The last interesting point raised by the results of our study is whether the lack of discrimination observed could be due to an attentional or a mnemonic visuo-spatial deficit. It has been reported that both processes are affected by the degeneration of the dopaminergic system (Collins et al. 2000; Crofts et al. 2001; Lewis et al. 2004). The fact that the spatial deficit was not selective for working or long-term memory seems to suggest either that it was due to an encoding or to an attentional deficit. Consistently, studies on PD patients often report that the visuo-spatial deficits are not delay-dependent (Pillon et al. 1996; Sahakian et al. 1988), but that they seem to worsen if the “task used exceeds the normal limit of working memory capacity” or when the memory load is increased (Owen et al. 1997; Sahakian et al. 1988). However, we cannot exclude a differential interaction between the two different processes at the two different delays. On the one hand, indeed, the deficit observed at the 3-min delay might be due to an attentional deficit, namely, to focus on the perceived spatial change. On the other hand, at 24-h delay, the spatial attentional deficit might add to a deficit to consolidate and/or retrieve spatial memory.
In conclusion, in this study, we confirmed a role of the nigrostriatal dopamine system in cognitive functions. Further we demonstrated for the first time in rodents that the cognitive deficit induced by striatal DA depletion is selective for visuo-spatial information processing rather than caused by a generalized memory deficit or a motivational impairment. The similarity of the results of the present study with those obtained in patients at the early stages of PD demonstrates that some of cognitive deficits in PD might not be a consequence of frontal dysfunction per se, but rather due to impaired DA activity in the striatum (Owen et al. 1992, 1998; Postle et al. 1997; Pillon et al. 1997). The high face validity of the spatial task used prompts the use of this model to better assess the cognitive deficits in PD and to discover new pharmacological treatments. Finally, a mice model of cognitive deficit in PD might be extremely useful to test knockout mice, which, nowadays, are the most frequent tool used to address possible genetic cause of PD.
References
Berger HJ, Cools AR, Horstink MW, Oyen WJ, Verhoeven EW, van der Werf SP (2004) Striatal dopamine and learning strategy-an (123)I-FP-CIT SPECT study. Neuropsychologia 42:1071–1078
Boller F, Passafiume D, Keefe NC, Rogers K, Morrow L, Kim Y (1984) Visuospatial impairment in Parkinson’s disease. Role of perceptual and motor factors. Arch Neurol 41:485–490
Bracs PU, Gregory P, Jackson DM (1984) Passive avoidance in rats: disruption by dopamine applied to the nucleus accumbens. Psychopharmacology (Berl) 83:70–75
Breen EK (1993) Recall and recognition memory in Parkinson’s disease. Cortex 29:91–102
Buytenhuijs EL, Berger HJ, Van Spaendonck KP, Horstink MW, Borm GF, Cools AR (1994) Memory and learning strategies in patients with Parkinson’s disease. Neuropsychologia 32(3):335–342
Coccurello R, Adriani W, Oliverio A, Mele A (2000) Effect of intra-accumbens dopamine receptor agents on reactivity to spatial and non-spatial changes in mice. Psychopharmacology 152(2):189–199
Collins P, Wilkinson LS, Everitt BJ, Robbins TW, Roberts AC (2000) The effect of dopamine depletion from the caudate nucleus of the common marmoset (Callithrix jacchus) on tests of prefrontal cognitive function. Behav Neurosci 114:3–17
Cools R, Stefanova E, Barker RA, Robbins TW, Owen AM (2002) Dopaminergic modulation of high-level cognition in Parkinson’s disease: the role of the prefrontal cortex revealed by PET. Brain 125(Pt 3):584–594
Cools R, Lewis SJ, Clark L, Barker RA, Robbins TW (2007) l-DOPA disrupts activity in the nucleus accumbens during reversal learning in Parkinson’s disease. Neuropsychopharmacology 32(1):180–189
Courtiere A, Hardouin J, Locatelli V, Turle-Lorenzo N, Amalric M, Vidal F, Hasbroucq T (2005) Selective effects of partial striatal 6-OHDA lesions on information processing in the rat. Eur J Neurosci 21:1973–1983
Crofts HS, Dalley JW, Collins P, Van Denderen JC, Everitt BJ, Robbins TW, Roberts AC (2001) Differential effects of 6-OHDA lesions of the frontal cortex and caudate nucleus on the ability to acquire an attentional set. Cereb Cortex 11:1015–1026
De Leonibus E, Costantini VJ, Castellano C, Ferretti V, Oliverio A, Mele A (2003) Distinct roles of the different ionotropic glutamate receptors within the nucleus accumbens in passive-avoidance learning and memory in mice. Eur J Neurosci 18:2365–2373
De Leonibus E, Oliverio A, Mele A (2005) A study on the role of the dorsal striatum and the nucleus accumbens in allocentric and egocentric spatial memory consolidation. Learn Memory 12:491–503
De Leonibus E, Verheij MM, Mele A, Cools A (2006) Distinct kinds of novelty processing differentially increase extracellular dopamine in different brain regions. Eur J Neurosci 23:1332–1340
Dubois B, Pillon B (1997) Cognitive deficits in Parkinson’s disease. J Neurol 244:2–8
Farina E, Gattellaro G, Pomati S, Magni E, Perretti A, Cannata AP, Nichelli P, Mariani C (2000) Researching a differential impairment of frontal functions and explicit memory in early Parkinson’s disease. Eur J Neurol 7:259–267
Franklin BJ, Paxinos G (1997) The mouse brain in stereotaxic coordinates. Academic, San Diego, CA
Giraudo MD, Gayraud D, Habib M (1997) Visuospatial ability of parkinsonians and elderly adults in location memory tasks. Brain Cogn 34:259–273
Keitz M, Martin-Soelch C, Leenders KL (2003) Reward processing in the brain: a prerequisite for movement preparation? Neural Plast 10:121–128
Lewis SJ, Cools R, Robbins TW, Dove A, Barker RA, Owen AM (2003) Using executive heterogeneity to explore the nature of working memory deficits in Parkinson’s disease. Neuropsychologia 41(6):645–654
Lewis SJ, Slabosz A, Robbins TW, Barker RA, Owen AM (2004) Dopaminergic basis for deficits in working memory but not attentional set-shifting in Parkinson’s disease. Neuropsychologia 43:823–832
Lorenzini CA, Baldi E, Bucherelli C, Tassoni G (1995) Time-dependent deficits of rat’s memory consolidation induced by tetrodotoxin injections into the caudate-putamen, nucleus accumbens, and globus pallidus. Neurobiol Learn Mem 63:87–93
Miyoshi E, Wietzikoski S, Camplessei M, Silveira R, Takahashi RN, Da Cunha C (2002) Impaired learning in a spatial working memory version and in a cued version of the water maze in rats with MPTP-induced mesencephalic dopaminergic lesions. Brain Res Bull 58:41–47
Mollion H, Ventre-Dominey J, Dominey PF, Broussolle E (2003) Dissociable effects of dopaminergic therapy on spatial versus non-spatial working memory in Parkinson’s disease. Neuropsychologia 41:1442–1451
Mura A, Feldon J (2003) Spatial learning in rats is impaired after degeneration of the nigrostriatal dopaminergic system. Mov Disord 18:860–871
Owen AM, James M, Leigh PN, Summers BA, Marsden CD, Quinn NP, Lance KW, Robbins TW(1992) Fronto-striatal cognitive deficits at different stages of Parkinson’s disease. Brain 115:1727–1751
Owen AM, Roberts AC, Hodges JR, Summers BA, Polkey CE, Robbins TW (1993) Contrasting mechanisms of impaired attentional set-shifting in patients with frontal lobe damage or Parkinson’s disease. Brain 116:1159–1175
Owen AM, Iddon JL, Hodges JR, Summers BA, Robbins TW (1997) Spatial and non-spatial working memory at different stages of Parkinson’s disease. Neuropsychologia 35:519–532
Owen AM, Doyon J, Dagher A, Sadikot A, Evans AC (1998) Abnormal basal ganglia outflow in Parkinson’s disease identified with PET. Implications for higher cortical functions. Brain 121(Pt 5):949–965
Pillon B, Ertle S, Deweer B, Sarazin M, Agid Y, Dubois B (1996) Memory for spatial location is affected in Parkinson’s disease. Neuropsychologia 34:77–85
Pillon B, Ertle S, Deweer B, Bonnet AM, Vidailhet M, Dubois B (1997) Memory for spatial location in ‘de novo’ parkinsonian patients. Neuropsychologia 35:221–228
Postle BR, Locascio JJ, Corkin S, Growdon JH (1997) The time course of spatial and object learning in Parkinson’s disease. Neuropsychologia 35(10):1413–1422
Puglisi-Allegra S, Cabib S, Pascucci T, Ventura R, Cali F, Romano V (2000) Dramatic brain aminergic deficit in a genetic mouse model of phenylketonuria. Neuroreport 27:1361–1364
Reyes Vazquez C, Zarco-Coronado I, Brust-Carmona H (1978) Effects of intracaudate microinjections of 6-hydroxydopamine upon the suppression of lever pressing and upon passive avoidance conditioning in cats. Pharmacol Biochem Behav 9:747–751
Rinaldi A, Mandillo S, Oliverio A, Mele A (2007) D1 and D2 receptor antagonist injections in the prefrontal cortex selectively impair spatial learning in mice. Neuropsychopharmacology 32(2):309–319
Robbins TW, Everitt BJ (1996) Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobio 6:228–236
Roesler R, Vianna MR, Schroder N, Ferreira MB, Quevedo J (2006) Aversive learning under different training conditions: effects of NMDA receptor blockade in area CA1 of the hippocampus. Neurochem Res 31:679–683
Roullet P, Sargolini F, Oliverio A, Mele A (2001) NMDA and AMPA antagonist infusions into the ventral striatum impair different steps of spatial information processing in a nonassociative task in mice. J Neurosci 21:2143–2149
Sahakian BJ, Morris RG, Evenden JL, Heald A, Levy R, Philpot M (1988) A comparative study of visuospatial memory and learning in Alzheimer-type dementia and Parkinson’s disease. Brain 111:695–718
Sargolini F, Roullet P, Oliverio A, Mele A (1999) Effects of lesions to the glutamatergic afferents to the nucleus accumbens in the modulation of reactivity to spatial and non-spatial novelty in mice. Neuroscience 93:855–867
Smith-Roe SL, Sadeghian K, Kelley AE (1999) Spatial learning and performance in the radial maze is impaired after N-methyl-d-aspartate (NMDA) receptor blockade in striatal subregions. Behav Neurosci 113:703–717
Weingartner H, Burns S, Diebel R, LeWitt PA (1984) Cognitive impairments in Parkinson’s disease: distinguishing between effort-demanding and automatic cognitive processes. Psychiatry Res 11:223–235
Whishaw IQ, Dunnett SB (1985) Dopamine depletion, stimulation or blockade in the rat disrupts spatial navigation and locomotion dependent upon beacon or distal cues. Behav Brain Res 18:11–29
White NM, Salinas JA (2003) Mnemonic functions of dorsal striatum and hippocampus in aversive conditioning. Behav Brain Res 142:99–107
Acknowledgments
The authors would like to thank Dr. Arianna Rinaldi for her assistance with the TH staining. The present study has been supported by a Galileo grant (to A.M. and M.A.), P.R.I.N. and F.I.R.B. grants from M.I.U.R. to A.O. to A.M. and a D.C.M.C grant from A.S.I. to A.O. and A.M. Every possible effort was made to minimize animal suffering, and all procedures were in strict accordance with the European Communities Council directives (86/609/EEC) and regulations on the use of animals in research and NIH guidelines on animal care.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Leonibus, E., Pascucci, T., Lopez, S. et al. Spatial deficits in a mouse model of Parkinson disease. Psychopharmacology 194, 517–525 (2007). https://doi.org/10.1007/s00213-007-0862-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-007-0862-4