Abstract
Thanks to the non-human primate (NHP), we have shown that the pharmacological disturbance of the anterior striatum or of external globus pallidus triggers a set of motivation and movement disorders, depending on the functional subterritory involved. One can, therefore, assume that the aberrant activity of the different subterritories of basal ganglia (BG) could lead to different behavioral disorders in neuropsychiatric disorders as Tourette’s syndrome and Parkinson’s disease. We are now addressing in the NHP the impact of modulating dopamine or serotonin within the BG on behavioral disorders. Indeed, we have shown a prominent role of serotonergic degeneration within the ventral striatum and caudate nucleus in neuropsychiatric symptoms in de novo PD patients. Of note, the serotonergic modulation of these BG regions in the NHP plays also a critical role in the induction or treatment of behavioral disorders. Given that both dopamine and serotonin are targeted to treat neuropsychiatric disorders, we are studying the effects of modulating dopamine and serotonin transporters in the different territories of the striatum, and more particularly within the ventral striatum on decision-making processing at both behavioral and neuronal levels. Finally, we evidence the need to extend the pharmacological approach to the receptors of these two neuromodulator systems as the use of substances targeting receptor subtypes preferentially localized in the associative and limbic territories of BG could be very effective to specifically improve the behavioral disorders in Parkinson’s disease, Gilles de la Tourette syndrome but also in several psychiatric disorders such as depression, anxiety, anorexia, or impulse control disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The non-human primate anatomical investigation and the functional basal ganglia circuits
The basal ganglia (BG) are subcortical structures including the striatum (putamen, caudate nucleus, accumbens or ventral striatum), globus pallidus (external (GPe) and internal (GPi)), subthalamic nucleus (STN) and substantia nigra (pars compacta (SNc) and reticulata (SNr)). The anatomical investigations in the non-human primate (NHP) were very important to demonstrate the functional organization of the BG in distinct parallel circuits connected through the thalamus with different frontal cortical areas. Alexander et al. (1986), were the first to propose a partitioning into five parallel circuits: two sensorimotor loops (motor and oculomotor) involved in the motor control of movement execution, two associative loops (dorsolateral prefrontal and lateral orbitofrontal) involved in the cognitive processes preceding movement executions (preparation of movement) and one limbic loop (anterior cingulate and orbitofrontal) involved in the motivational processes (Alexander et al. 1986, 1990). The cortico-BG loop involving the ventral striatum plays a central role in behavior through the control of motivational processes (Alexander et al. 1986; Graybiel 2005; Haber and Knutson 2010; Tremblay et al. 2015), whereas the cortico-BG loops involving the caudate and the putamen are involved in the processes of selection and execution of the action (Alexander et al. 1986; Middleton and Strick 2000; Tremblay et al. 2009). This architecture in parallel circuits does not exclude the existence of integration in BG circuits. For instance, striatal territories innervated by associated cortical areas show large overlapping zones (Flaherty and Graybiel 1991). The interface existing between the different striatal territories and midbrain DA neurons allows a feed-forward organization from the limbic to the cognitive and motor circuits (Haber 2003). The role of BG in reinforcement and adaptation to accommodate the past in predicting future outcomes is of particular importance, and communication across functionally distinct circuits is required for this to occur. This communication is critical to continually evaluate and adjust to stimuli throughout the development of behaviors.
Consequently, disturbances within particular regions of such cortico-BG-thalamo-cortical loops may induce movement (chorea, dystonia, hemiballism, simple motor TIC) or behavioural (Hyperactivity, Attention deficit, Apathy, depression, Panic) disorders (Tremblay et al. 2015).
First evidences in the non-human primate of the causal link between dysfunctions of the non-motor BG territories and behavioral disorders
Following the anatomical investigations, the neurophysiological approach by neuronal recording in NHP trained to perform delayed response tasks and neuro-imaging studies in humans have largely contributed to delineate these functional domains inside the striatum (see review by Tremblay et al. 2009). Briefly, these studies have shown that (1) the ventral striatum processes the motivational information, the expected outcome and reward (Schultz and Romo 1992; Knutson et al. 2001; O’Doherty et al. 2002; Ernst et al. 2004), (2) the cognitive aspects regarding the selection of conditioned stimuli that guide action towards the goal are treated in the caudate nucleus, with an increasing proportion of neurons expressing anticipatory activity for the conditioned stimuli in the most anterior levels (Hollerman et al. 1998; Lehéricy et al. 2006), (3) movement preparation and execution are processed in the putamen (Alexander and Crutcher 1990; Romo et al. 1992; Schultz et al. 1992; Gerardin et al. 2004).
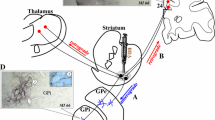
By pharmacological disturbance in different species of male NHP (African green monkeys (n = 5), cynomolgus (n = 9) and rhesus (n = 2) monkeys aged between 3 and 5 years), we have shown that the motor, associative and limbic territories of BG structures are implicated in the expression of movement and behavioural disorders (Grabli et al. 2004; Worbe et al. 2009, 2013; Sgambato-Faure et al. 2016). Indeed, by locally injecting 3 µl of bicuculline (a GABAA antagonist at a concentration of 15 µg/µl) within the anterior part of the striatum, we triggered a set of motivation and movement disorders (Fig. 1). Within the ventral striatum, which corresponds to the limbic territory, we produced three different classes of abnormal behaviors according to the striatal subterritory involved. A first behavioral effect was obtained from the medial part of the anterior striatum and was characterized by sexual manifestations (erection and ejaculation). This effect is associated with an anatomical circuit involving median prefrontal cortex and median parts of BG (Sgambato-Faure et al. 2016). In a normal condition, this medial circuit could be involved in sexual attraction (Bray and O’Doherty 2007) and preference (Ponseti et al. 2006). In contrast, abnormal activity of this medial cortico-striatal circuit could drive hypersexuality, which has been observed in PD patients as a result of DA treatment (Politis et al. 2013).
Schematic summary of the cortico-striatal circuits underlying motivation (i.e. hypersexuality, anxiety and eating disorders) and movement (hyperactivity and abnormal movements) disorders, induced from pharmacological perturbation of the ventral and dorsal striatum, respectively. Color code is as follows: sexual disorders in green, anxiety in blue, food disorders in orange, hyperactivity state and simple tic in yellow. Abbreviations: CD caudate nucleus, PUT putamen, VS ventral striatum, Ins insula, Temp temporal. Adapted from Worbe et al. (2013) and Sgambato-Faure et al. (2016)
A second behavioral effect was obtained from the central part of the anterior striatum and was characterized by stereotyped behaviors, i.e. compulsive grooming and repeated actions of licking and biting fingers or tail (Fig. 2), probably reflecting an increase of aversive anticipation and anxious state (Saga et al. 2016; Kalin et al. 2005; Grupe and Nitschke 2013). This effect is associated with an anatomical circuit involving the orbitofrontal cortex, the limbic parts of BG and an indirect cortical return to the anterior insula and the amygdala, well known to be involved in aversive encoding and anxiety disorders (Sgambato-Faure et al. 2016; Galineau et al. 2016). In humans, a meta-analysis has clearly shown dysfunctions within this central region in anxiety disorders and particularly in obsessive compulsive disorders (Radua et al. 2010). Furthermore, the role of this central part of the ventral striatum in the processing of anxious states has been evidenced not only in animal studies (Schoenbaum et al. 2003; Yanagimoto and Maeda 2003), but also in human functional imaging studies (Becerra et al. 2001; Jensen et al. 2003; Tom et al. 2007; Delgado et al. 2009. Taken together, these results provide strong evidence that a part of the ventral striatum is engaged in the emotional anticipation of aversive events and their behavioral avoidance. Abnormal activity of this medial cortico-striatal circuit may underlie impulse control disorders observed in PD patients under dopatherapy (Weintraub et al. 2015).
Histograms show the behavioral effects for bicuculline microinjections within the ventral striatum producing anxiety-related behaviors (a) and apathetic state with loss of food motivation (b) (data from 3 monkeys are included for each behavioral effect). The control condition corresponds to a condition without microinjection. Histograms on the right side show the percentage of initiated choices during execution of the simple food retrieval task for monkeys exhibiting anxiety and apathetic-like state. Issued from Sgambato-Faure et al. (2016)
A third and last behavioral effect was obtained from pharmacological disturbance within the lateral part of the anterior striatum and was characterized by a hypoactive state associated with a loss of food motivation (sometimes even evoking vomiting) (Fig. 2). This effect is associated with an anatomical circuit involving the orbital and medial prefrontal cortex, the insula and limbic parts of BG (Sgambato-Faure et al. 2016). Numerous imaging studies in humans have documented that the insula is involved in the feeling of disgust (Wicker et al. 2003), nausea (Napadow et al. 2013) and vomiting (Catenoix et al. 2008). In the monkey, stimulation of the anterior insula triggers food disgust (Caruana et al. 2011; Jezzini et al. 2012). The orbitofrontal has also been linked to the severity of appetite loss in patients with Alzheimer’s disease (Ismail et al. 2004) and a feeling of satiety in healthy controls (Hinton et al. 2004). Overall, our recent anatomical study, therefore, supports the implication of this lateral part of the ventral striatum in a circuit engaged in food intake, as in rodents (Jean et al. 2007, 2012). Dysfunction of this circuit may be involved in eating disorders, such as binge eating, obesity, anorexia or bulimia (Kaye et al. 2009; Tomasi and Volkow 2013; Stefano et al. 2013).
By locally injecting bicuculline within the dorsal part of the anterior striatum (Fig. 1), we also produced hyperactive state with touching and simple motor tics, respectively, associated with prefrontal dorsolateral cortex and associative territories of BG and sensorimotor cortical and BG network (Worbe et al. 2013). These last NHP experiments allowed a better understanding of pathophysiological mechanisms potentially involved in Tourette syndrome (see Tremblay et al. 2015).
The partition of the external pallidum and modulations of non-motor functions
The pallidum is another BG structure from which both motor and behavioral effects could be triggered following bicuculline microinjections according to the subterritory targeted (Fig. 3). For instance, microinjections within the posterior motor part of the GPe triggered dyskinetic movements (Matsumura et al. 1995), while microinjections within the anterior associative-limbic part of GPe induced abnormal behavior (Grabli et al. 2004) (Fig. 3a). Attention deficit with or without hyperactivity, could be obtained from the associative territories (dorsal part) and stereotyped behavior from the limbic territory (ventral part) of the GPe in normal monkeys (Grabli et al. 2004; Fig. 3a). Here again, these results strengthen the idea that a same dysfunction within a structure can impact movement or behavior depending on the subterritory involved. Interestingly, the compulsive behavior triggered within the limbic GPe could be counteracted by deep brain stimulation (DBS) applied into the limbic territory of the STN (Baup et al. 2008), in agreement with the fact that the DBS therapeutic approach is now applied to improve patients with obsessive–compulsive disorders (Mallet et al. 2008; Blomstedt et al. 2013).
a Schematic representation of the functional territories within the GPe (associative in green, limbic in blue and sensorimotor in yellow) and of the effects resulting from dysfunctions of these territories in the normal African green monkey. Issued from Grabli et al. (2004). b Correlation analyses between [18F]DOPA uptake (Ki) in GPe and motor score or recovery time in MPTP-treated cynomolgus monkeys. Issued from Ballanger et al. (2016). c Brain Macaca fascicularis MRI template (in grey) and 11C-DASB PET images (in color) on coronal plane before and after MDMA intoxication at the level of GPe in MPTP-treated cynomolgus monkeys. Colors represent the level of BPND using the cerebellum as the reference region (red indicates high BPND whereas pink indicates low BPND on the scale). Positive correlation found between the repetitive grooming score induced by levodopatherapy (l-DOPA systemically administrated twice daily at increasing doses during 2 months) and 11C-DASB BPND before MDMA within the GPe. Issued from Beaudoin-Gobert et al. 2015. Monkeys were male aged between 3 and 5 years and dopamine-depleted by receiving MPTP injections (0.3–0.5 mg/kg, i.m.) under light anaesthesia on consecutive days (acute protocol in Beaudoin-Gobert et al. 2015) or every 4–5 days (progressive protocol in both cited articles) until the emergence of parkinsonian symptoms. The total MPTP dose given to each monkey varied between 1.1 and 2.2 mg/kg. Abbreviations: AC anterior commissure, BP ND non-displaceable binding potential, Cd caudate nucleus, GPe external globus pallidus, GPi internal globus pallidus, HD/AD hyperactive disorder and attention deficit, Pu Putamen
Besides the impacts of a GABAergic pallidal dysfunction, we have recently shown interesting roles for both dopamine (Ballanger et al. 2016) and serotonin (Beaudoin-Gobert et al. 2015) within the anterior GPe in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) monkey model of Parkinson’s disease (PD). First, we have evidenced an inverse relationship between the pallidal dopamine and the severity of parkinsonian motor symptoms: the lowest pallidal dopamine level assessed using [18F]DOPA uptake, the more severe (the longer recovery time) the animal (Fig. 3b), confirming a strong compensatory role of this pallidal dopamine in the pathophysiological mechanisms of PD, as already suspected in de novo PD patients (Whone et al. 2003; Pavese et al. 2012). Second, we have highlighted a positive correlation between the pallidal serotonin innervation and the severity of repetitive grooming induced by levodopatherapy (Fig. 3c), suggesting that serotonergic fibers within limbic regions can sustain the expression of behavioral disorders via aberrant processing of l-DOPA as it is the case in motor regions for l-DOPA-induced dyskinesias (Politis et al. 2014).
The impact of serotonin lesion in motor and non-motor symptoms of Parkinson’s disease
Idiopathic PD is characterized clinically by cardinal motor symptoms (akinesia/bradykinesia, rigidity, resting tremor) and response to dopamine replacement therapy (Obeso et al. 2000). PD patients also display non-motor symptoms, including frequent and disabling neuropsychiatric symptoms (depression, anxiety, apathy) (Chaudhuri and Schapira 2009). The lesion of the dopaminergic system is involved in the pathophysiology of bradykinesia and rigidity, motor fluctuations, l-DOPA-induced dyskinesia, apathy and depression in PD patients (Politis 2014; Thobois et al. 2010). However, the dysfunction of the serotonergic system has been involved in tremor (Huot et al. 2011a; Politis 2014; Politis and Niccolini 2015), dyskinesia (Rylander et al. 2010; Politis et al. 2014), fatigue and depression (Huot et al. 2011a; Politis 2014; Politis and Niccolini 2015) as well as psychosis (Zahodne and Fernandez 2008). Very recently, we have also brought out a prominent role of serotonergic degeneration in apathy, anxiety and depression in de novo PD patients (Maillet et al. 2016). Specifically the apathetic patients mainly displayed greater serotonergic alteration in the ventral striatum (Fig. 4a), the anterior cingulate cortex, bilaterally, as well as in the right-sided caudate nucleus and the right-sided orbitofrontal cortex. The severity of apathy was, moreover, mainly related to specific serotonergic lesions within the right-sided anterior caudate nucleus and the orbitofrontal cortex, while the degree of both depression and anxiety was primarily linked to serotonergic disruption within the anterior cingulate cortex. These data clearly show that the serotonergic lesion of such BG anterior territories is involved in neuropsychiatric symptoms. Of note, the serotonergic modulation of these BG territories in the monkey also triggers such behavioral symptoms (hyperactivity, stereotypies, apathetic-like state, and anxious-like state) (Neumane et al. 2012; Beaudoin-Gobert et al. 2015, and unpublished data).
a Illustration of the relationships between the severity of apathy (based on the LARS scores) and the reduced binding of serotonin (SERT) tracer ([11C]DASB) within the right-sided orbitofrontal cortex and the ventral part of the right-sided anterior caudate nucleus in de novo parkinsonian patients. Issued from Maillet et al. (2016). b MRI-fusioned PET images on coronal (top) and horizontal (bottom) planes showing the cerebral distribution of 11C-PE2I (for the dopaminergic transporter) on the left before and after MPTP intoxication, and of 11C-DASB (for the serotonergic transporter) on the right before and after MDMA intoxication to illustrate the development of the new monkey model of Parkinson’s disease, exhibiting a double dopaminergic and serotonergic lesion due to the sequential use of MPTP and MDMA. Colors represent the level of non-displaceable binding potential using the cerebellum as the reference region. The time interval between MPTP and MDMA was around 5 months. MPTP-treated monkeys received MDMA injections twice daily for four consecutive days (5 mg/kg, subcutaneously). The extent of the MDMA lesion was assessed both by PET imaging using the serotonergic transporter (SERT) ligand ([11C]-DASB) as shown on this figure and by immunohistochemistry on post-mortem tissue at the end of the protocol. Adapted from Beaudoin-Gobert et al. (2015)
To elucidate the causal role of the serotonergic system in the wide range of parkinsonian motor and non-motor symptoms, we developed a new monkey model of PD (Fig. 4b), presenting a double dopamine/serotonin lesion as the result of sequential use of MPTP and 3,4-methylenedioxy-methamphetamine (MDMA, better known as the recreational drug ecstasy) (Beaudoin-Gobert et al. 2015). Indeed, the MPTP monkey model is the gold standard for toxin-based animal models as it reproduces most of the clinical hallmarks of PD (Porras et al. 2012). And the specific neurotoxic effects of MDMA towards the serotonergic innervating system have been well characterized in the normal monkey (Ricaurte et al. 2000). Despite the limitations of this toxin-based animal model (MPTP being not selective of dopamine neurons and MDMA injuring only serotonergic fibers), the implication of the presynaptic serotonergic system could be addressed for the first time in parkinsonian monkeys, calling the dysfunction of raphe neurons in parkinsonism to mind (Huot et al. 2011a; Del Tredici and Braak 2012; Politis 2014; Politis and Niccolini 2015). In this work, we first established the efficiency of MDMA in inducing a lesion of the serotonergic innervating system in dopamine-depleted monkeys by positron emission tomography (PET) imaging with specific ligands ([11C]PE2I for the dopaminergic transporter (DAT) and [11C]DASB for the SERT) (Fig. 4b) and immunohistochemistry. We then addressed the causal link between the serotonergic fibers alteration driven by MDMA and the parkinsonian symptoms including cardinal motor symptoms (akinesia, rigidity and tremor) and responses to l-DOPA therapy (dyskinesia and neuropsychiatric-like behaviors). As already stated, among the cardinal motor symptoms expressed by parkinsonian patients, only tremor has been clearly linked to serotonergic dysfunction so far (Huot et al. 2011a; Politis 2014; Politis and Niccolini 2015). PET imaging studies have shown that a diminished availability of 5-HT1A receptors in the raphe or of the serotonergic transporter SERT in the thalamus correlates with the severity of resting tremor (Huot et al. 2011a; Politis 2014; Politis and Niccolini 2015). A reduced availability of SERT in the caudate, putamen and raphe correlates with the severity of action and postural but not resting tremor (Loane et al. 2013), suggesting that different pathological mechanisms might be involved in the different forms of tremor. Resting tremor was replaced by action tremor in our MPTP-treated macaques (resting tremor is the only symptom seldom reproduced except in the MPTP-treated African green monkey) (Mounayar et al. 2007; Porras et al. 2012) and not altered by the serotonergic lesion. Bradykinesia was not induced or worsened after MDMA in recovered or symptomatic monkeys, respectively. The striatal blockade of the serotonergic transmission in recovered monkeys does not induce bradykinesia either (Neumane et al. 2012). This is consistent with a human study showing no SERT dysfunction in bradykinetic parkinsonian patients (Loane et al. 2013). The only symptom for which we found an impact after MDMA lesion was rigidity. The expression of rigidity was modified by the serotonergic lesion and this effect strongly depended on the previous serotonergic and dopaminergic innervation state, especially in the posterior putamen and external pallidum (transient appearance mostly in recovered monkeys that have greater compensatory mechanisms and transient disappearance followed by reappearance in symptomatic monkeys that can no longer compensate). Would those preclinical results be strengthened by the observation of a greater serotonergic denervation in the putamen associated with a greater rigidity of parkinsonian patients? This remains to be determined. In any case, such a link, at the preclinical level, between serotonergic dysfunction and rigidity is novel and might have been hidden or undervalued because the scoring of rigidity is seldom in non-human primates (Imbert et al. 2000; Mounayar et al. 2007; Porras et al. 2012) and because akinesia and rigidity are often associated in parkinsonian patients (akinetic-rigid subtype) and related to the lesion of the dopamine system (Jellinger 1999; Politis 2014).
Another major finding of our work was the causal demonstration of serotonergic fibers mediating l-DOPA-induced dyskinesias in symptomatic monkeys by demonstrating the underlying and causal physiopathological mechanism, i.e. the serotonergic hyperinnervation, abolished by MDMA (Beaudoin-Gobert et al. 2015). This strengthens the view that MDMA is effective in reducing l-DOPA-induced dyskinesias in rats (Bishop et al. 2006), monkeys (Iravani et al. 2003; Huot et al. 2011b) and early-onset parkinsonian patients (BBC 2001). In the same vein, the use of 5-HT1A or 5-HT1A/B agonists or antidepressants has been shown to offer an interesting anti-dyskinetic strategy (Carta and Tronci 2014; Politis et al. 2014; Conti et al. 2014; Mazzucchi et al. 2015).
Last, but not least, we found that our l-DOPA treated recovered monkeys (i.e. MPTP-monkeys that no longer express motor symptoms as a function of time after MPTP) did not develop dyskinesias but rather hyperactive and neuropsychiatric-like behaviors (agitation, hallucinatory-like responses, stereotypies and repetitive grooming), which were both reduced or even abolished by subsequent MDMA lesion (Beaudoin-Gobert et al. 2015). These findings demonstrate that serotonergic fibers also play a significant role in the pathophysiology of non-motor symptoms driven by l-DOPA, maybe via aberrant processing of exogenous l-DOPA and release of dopamine as false neurotransmitter in non-motor regions as it is the case in motor regions (putamen) for l-DOPA-induced dyskinesias (Politis et al. 2014). Interestingly, two BG regions were highlighted in those behavioral effects: the posterior ventral putamen, whose serotonergic innervation state positively correlated with either the total neuropsychiatric-like score or the hallucinatory-like score, and the external pallidum, whose serotonergic innervation state positively correlated with the severity of repetitive grooming (Fig. 3c). Again, we have seen that the limbic part of GPe is a region from which stereotyped behaviors (grooming and licking/biting) can be pharmacologically evoked in the monkey (Grabli et al. 2004; Sgambato-Faure et al. 2016; Tremblay et al. 2015). As for the ventral posterior putamen, it is a structure connected to the inferotemporal cortex involved in the visual recognition and discrimination of objects (Baizer et al. 1993; Middleton and Strick 1996) and serotonin dysfunction has already been involved in visual hallucinations in PD (Ballanger et al. 2010; Huot et al. 2010). The slower degeneration of serotonergic (versus dopaminergic) terminals as PD progresses could, therefore, be a risk factor for occurrence of these l-DOPA-induced non-motor symptoms.
The difference obtained between severely lesioned and moderately lesioned monkeys in terms of l-DOPA responses (dyskinesia for the former and hyperactivity with neuropsychiatric-like symptoms for the later) calls the impact of the heterogeneity of the MPTP-induced lesions on symptoms to mind. Indeed, it is well known that at high doses, MPTP is not only selective for dopamine neurons and can affect serotonergic ones (Pifl et al. 1991). These data clearly show that the extent and type of lesions differ between these two groups of monkeys and, therefore, differentially impact the l-DOPA responses. Given the heterogeneity of lesions among PD patients (Kish et al. 1988; Scatton et al. 1983; Gaspar et al. 1991; Gilman et al. 2010; Paulus and Jellinger 1991; Remy et al. 2005), one can assume that the dopamine and/or serotonin lesions in a given subterritory differentially impact the motor and mon-motor symptoms before or under medication. According to the subterritory involved, the threshold of dopamine loss to induce the specific symptoms is different. Indeed, in monkeys with progressive MPTP treatment, the first symptoms to appear are cognitive (Pessiglione et al. 2004) and executive deficits (Pessiglione et al. 2003). Such a deficit is also observed in PD patients during the early stages of the disease before the motor deficit (Marinus et al. 2003; Verbaan et al. 2007). Thus, while the sensorimotor territories are more strongly affected, compared to the associative territories, it is only after stronger dopamine depletion that the deficits in sensorimotor territories result in motor symptoms expression. Another element to take into consideration relates to the hypersensitivity of dopaminergic receptors in response to the dopaminergic lesion (Bezard and Gross 1998). It is likely that with dopatherapy, this hypersensibility induces abnormal movement (dyskinesia), non-adapted actions (punding), or goals (impulsive compulsive behaviours), according to the striatal sub-territory expressing this sensitization to dopamine overstimulation (Voon et al. 2009, 2016). Given our monkey data, dopamine preferential activation of one of the sub-territories within the ventral striatum could result in diverse impulse control disorders in PD patients. In particular, aberrant activity of a medial part of the ventral striatum could lead to hypersexuality, while eating disorder could rather result from the lateral part. Moreover, dysfunction in the central part which is probably involved in the evaluation of the negative cost of decisions, could induce pathological gambling or compulsive shopping.
The non-human primate model allows to study the BG involvement in the therapeutic effects of pharmacological modulations in neuropsychiatric disorders
We began to address the specific influence of dopamine and serotonin in the BG and more specifically in the ventral striatum on decision-making by using an approach and avoidance behavioral task. Although the ventral striatum has traditionally been associated with the approaching behavior (reward seeking), it could also play a role in the avoidance of aversive stimuli or act as an interaction site with regions encoding aversive stimuli such as the amygdala, the insula, or the lateral orbitofrontal cortex (Tremblay et al. 2009; Saga and Tremblay 2016). An investigation of the role of the ventral striatum in aversive processing would improve our understanding of the physiopathology of neuropsychiatric disorders (OCD, panic disorder, anorexia, depression), for which decision-making processes are affected. The role of the ventral striatum in processing reward value, motivation, and decision making is now generally accepted, based on a broad range of neuroimaging studies in humans and neuronal recording or pharmacological perturbation studies in animals. The recording of neuronal activity in monkeys engaged in classic delayed response and reward tasks has been an important approach in characterizing functional contributions of the ventral striatum at the single neuron level (Tremblay et al. 2009). The ventral striatum would be mainly involved in tracking the motivational value of primary and conditioned stimuli and in the expectation of this value during goal-directed actions in different contexts. The ventral striatum is one of the main targets of the dopaminergic and serotonergic projections originating from the SNc and the raphe nuclei, respectively (Lavoie and Parent 1990; Szabo et al. 2002; Parent et al. 1983). By PET imaging using specific radioligands targeting the DAT ([11C]PE2I) and the SERT ([11C]DASB) transporters, we have seen that the SERT has a preferential distribution within the ventral part of the anterior striatum while the DAT is uniformly distributed (Fig. 5). Human neuroimaging and primate electrophysiology studies have shown that activity of dopaminergic neurons reflect the value of stimuli predicting both immediate and future rewards (Schultz et al. 1997, 1998; Tobler et al. 2005; Gregorios-Pippas et al. 2009). Furthermore, elevated dopamine levels have been linked to changes in striatal activity and to increases in impulsive-compulsive behavior and risk-taking choices (St Onge and Floresco 2009; Pine et al. 2010; O’Sullivan et al. 2011; Fineberg et al. 2010), suggesting more specific involvement of dopamine in appetitive reward value and in the learning of approach towards these appetitive stimuli without taking possible aversive outcomes into account. Serotonin signaling also seems to play an important role in decision making (Bromberg-Martin et al. 2010; McCabe et al. 2010; Tanaka et al. 2007), and the clinical improvement of anxiety disorders (Buoli et al. 2013) and depression (Stark and Hardison 1985) by selective serotonin reuptake inhibitor (SSRI) treatment could be mediated in part by the effects on the processing of appetitive and aversive rewarding stimuli inside the ventral striatum (McCabe et al. 2010).
a Illustration of the anatomo-functional organization of the cortico-BG circuits involved in goal-directed behavior (from motivation to action) and of the dopaminergic and serotonergic innervations of BG issued from the substantia nigra pars compacta and the raphe, respectively. BG appear divided into three large functional territories in close relation to different territories of the frontal cortex: sensorimotor (in yellow), associative (in green), and limbic (in blue), which are respectively involved in the selection and execution of movement, the selection of action, and the motivational processes upstream of all these functions. The cortico-BG circuit illustrated represents the circuit of motivation. Microinjections will be performed within the ventral striatum to determine the impacts of pharmacological perturbation of the subterritories involved in anxiety (central part of VS) and food (lateral part of VS) disorders on decision making while monkeys will be performing a delayed reward task. We will study the modulatory effect of both dopamine (with methylphenidate) and serotonin (with fluoxetine) on value-based decision making. b MRI-fusioned PET images on coronal planes showing the cerebral distribution of 11C-PE2I (top) and of 11C-DASB (bottom) at baseline at the level of the anterior striatum in the normal monkey. Colors represent the level of BPND using the cerebellum as the reference region (red indicates high BPND whereas pink indicates low BPND on the scale). Abbreviations: GPe external globus pallidus, GPi internal globus pallidus, STN subthalamic nucleus, SNc substantia nigra pars compacta, SNr substantia nigra pars reticulata, DAT dopaminergic transporter, SERT serotonergic transporter
These two neuromodulators dopamine and serotonin are targeted to treat neuropsychiatric disorders, for which anticipation, detection and avoidance of aversive events appear affected. Neuroleptics and antidepressants are used in clinical practice for example for schizophrenia (Leucht et al. 2013), anxiety (Buoli et al. 2013) and depression (Cipriani et al. 2009). The serotonergic therapeutic agents may have preferential action on the limbic territory in the processing of emotions and motivations, while the dopaminergic therapeutic agents may act indifferently on all functional to process motivation, attention, action and movement selection. Chronic fluoxetine administration decreases stereotypies and self-biting behavior in rhesus monkeys (Fontenot et al. 2009). Furthermore, a recent ethological study (Camus et al. 2013a, b) identified cynomolgus macaques with anxiety-like symptoms, reminiscent of generalized anxiety disorders, or depressive symptoms, reminiscent of depressive disorders but the effects of SSRIs on those have not yet been addressed. It would, therefore, be very interesting to study the effects of modulating dopamine (with methylphenidate blocking the DAT) and serotonin (with fluoxetine, an SSRI blocking the SERT) in the different territories of the striatum, and more particularly within the ventral striatum on decision-making processing at both behavioral and neuronal levels (Fig. 5). This would elucidate whether or not the therapeutic effects of such drugs (methylphenidate better known as Ritalin and fluoxetine better known as Prozac) are mediated by BG and more specifically by the ventral striatum. Furthermore, as serotonergic and dopaminergic neurons project to the cerebral cortex, limbic cortical regions may also be involved in mediating the beneficial effects of such pharmacological agents.
Research perspectives opened with the non-human primate on the serotonin modulations inside BG in neuropsychiatric disorders
There is a real challenge to extend the pharmacological approach to the receptors of these two neuromodulator systems. Indeed, the use of substances targeting receptor subtypes preferentially localized in the associative and limbic territories of BG could be very effective to specifically improve the behavioral disorders in PD, GTS or psychiatric disorders. We know for example that dopaminergic D3 receptor subtypes are primarily located inside the pallidum and the ventral striatum (Graff-Guerrero et al. 2008; Morissette et al. 1998) and may be involved in impulse control disorders in PD patients (Moore et al. 2014; Boileau et al. 2013, 2014; Payer et al. 2015; Seeman 2015; Lee et al. 2009). The D3 receptor subtypes would also explain the beneficial effects of dopaminergic agonists on apathy in PD patients (Thobois et al. 2013). The 5-HT1B serotonin receptor subtype, which is also preferentially located inside the pallidum and the ventral striatum (Varnäs et al. 2011), is downregulated in those regions in major depressive disorder (Murrough et al. 2011) and may be a potential site of SSRI therapeutic action in major depression (Heller et al. 2013). It is known that the beneficial effect of SSRI takes several weeks to be accomplished (Berton and Nestler 2006) for two major reasons: first, because there is a desensitization of 5-HT1A autoreceptors (Schatzberg and Nemeroff 2009), and second because of the proposed de novo SERT expression in noradrenergic neurons (Baudry et al. 2010). But the precise cerebral sites from which SSRI could play their therapeutic effects on anxiety disorders and depression should still be determined. Given the dense serotonergic innervation of BG structures and the diversity of serotonergic subtypes, one can address whether the therapeutic action of serotonergic drugs can take place in the non-motor territories of the BG. By PET imaging, we have used and compared four different serotonergic radioligands ([11C]DASB, [18F]MPPF, [11C]SB207145, [18F]2FNQ1P), respectively, allowing to target the SERT and the 5-HT1A, 5-HT4 and 5-HT6 receptor subtypes (Fig. 6).
PET imaging of the serotonergic system using four radioligands at baseline in monkeys (n = 4). The tracers are: [11C]DASB for the SERT transporter, [18F]MPPF for 5-HT1A receptors, [11C]SB207145 for 5-HT4 receptors and [18F]2FNQ1P for 5-HT6 receptors. The spatially normalized mean parametric images of each radiotracer were collected at baseline and superimposed on our brain Macaca fascicularis MRI template. The parametric images are shown in color, whereas the MRIs are shown in grayscale. Colors represent the level of BPND using the cerebellum as a nonspecific reference for each radioligand. Red represents high BPND, whereas blue denotes low BPND. For each radiotracer, three coronal (first three rows) and one horizontal (fourth row) planes are shown. Abbreviations: BP ND non-displaceable binding potential, ACC anterior cingulate cortex, CD caudate nucleus, PUT putamen, PreMot premotor cortex, VS ventral striatum, GPe external globus pallidus, Amyg amygdala, Mot motor cortex, Hippo hippocampus, OFC orbitofrontal cortex, STR striatum, Thal thalamus
The [18F]2FNQ1P ligand is a recently developed tracer, that binds with high affinity and specificity to 5-HT6Rs in several species, including macaques (Colomb et al. 2014; Becker et al. 2014). From these PET imaging data, it is very interesting to note the complementarity of the binding obtained with the radioligands [11C]DASB and [18F]MPPF, and with the radioligands [18F]MPPF, [11C]SB207145 and [18F]2FNQ1P. Indeed, while [18F]MPPF labels essentially cortical areas, the other radioligands, except [18F]2FNQ1P which also reveals some cortical binding, preferentially label subcortical regions such as the striatum. Even at the striatal level, we can notice some specificity of binding for each tracer (more or less binding in the caudate, putamen and ventral striatum). In any case, targeting those striatum-enriched receptor subtypes, besides the SERT, would bring new insights regarding their potential involvement in mediating behavioral disorders. Evidence from human studies suggests that the SERT is important in regulating feeding behaviors. Administration of SSRIs is used clinically to treat bulimia nervosa (Walsh et al. 2004) and to prevent relapse in anorexia nervosa (Kaye et al. 2001). However, minimal or no effect of SSRI medication has been evidenced in adolescent suffering from anorexia nervosa (Holtkamp et al. 2005). Patients with anorexia exhibit an increased 5-HT1A binding (notably in the prefrontal cortex and dorsal raphe nucleus) (Bailer et al. 2007), which can reduce the firing rate of serotonergic neurons and decrease extracellular serotonin (Nichols and Nichols 2008). This upregulation of 5-HT1A receptors may be a compensatory mechanism as serotonin inhibits food intake. Thus, mice models exhibiting a decreased extracellular serotonin rate have been developed such as mice overexpressing SERT. Surprisingly, food intake and satiety are not impaired by SERT overexpression in mice (Pringle et al. 2008), which suggest that adaptive mechanisms install progressively to circumvent the absence of SERT to modulate satiety.
The role of 5-HT4 receptors in the regulation of food intake has been evidenced by Compan and collaborators. Indeed, the 5-HT4 receptor knock-out mice displayed attenuated feeding and locomotor behavior in response to stress (Compan et al. 2004), suggesting that those receptors are involved in stress-induced anorexia. Local microinjection of 5-HT4 agonist (BIMU8) in the nucleus accumbens of fed or food-deprived mice reduced food intake while accumbal blockade of 5-HT4 receptors by an antagonist (RS39604) or siRNA-mediated 5-HT4 knockdown evoke a hyperphagia in fed mice (Jean et al. 2007). Moreover, overexpression of 5-HT4 in the nucleus accumbens triggers anorexia-like behavior characterized by a decreased food intake and an increased motor activity (Jean et al. 2012). Our preliminary data obtained in the monkey confirm that 5-HT4, whose expression has been well characterized in monkeys (Caillé et al. 2013; Tavares et al. 2014), are involved in the regulation of food intake, as a loss of food motivation (accompanied by a hypoactive state) is induced following microinjection of BIMU8 within the lateral part of the ventral striatum (unpublished data; V. Sgambato-Faure). In obese subjects, the body mass index is positively correlated with the 5-HT4R density in the reward circuit (Haahr et al. 2012, 2014), confirming an involvement of 5-HT4 in eating disorders. Given that anorexia nervosa often coexist with anxiety (Godart et al. 2000), depression (Casper 1998), motor hyperactivity (Casper 2006; Davis 1997), it will be important in the near future to investigate the impact of 5-HT4 modulation within the different striatal subterritories on both movement and behavioral disorders in NHPs. Regarding the 5-HT6 receptor subtype, its exclusive brain localization (Woolley et al. 2004) and its presence in the pharmacological spectrums of several psychotropic drugs have led to the suggestion that it may have therapeutic utility in the treatment of various psychiatric disorders (Arnt and Olsen 2011). More recently, it appeared that the 5-HT6R is also involved in memory (Meneses 2014) and cognitive processes (Benhamú et al. 2014) and in the regulation of food intake (Heal et al. 2011), reinforcing its status as an emerging target in anti-dementia and anti-obesity therapeutic agents. Given that 5-HT6 are highly enriched in the striatum, we will test their potential involvement in the different domains of motivation (food, safety, sex) and for their opposite values (appetitive and aversive).
Conclusion
The use of the non-human primate has been crucial to improve our understanding of both movement and behavioral disorders. There is still a need to address on the NHP the impact of modulating neurotransmitter systems to dissect the pathophysiological mechanisms involved in Parkinson’s disease, Gilles de la Tourette syndrome or neuropsychiatric disorders. Indeed, the medication used to counteract the symptoms that are present in those pathologies is quite limited and is not always efficient. Finding and validating new potential therapeutic targets in the NHP may open new avenues to improve quality of life of these patients.
References
Alexander GE, Crutcher MD (1990) Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci 13:266–271
Alexander GE, DeLong MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9:357–381
Alexander GE, Crutcher MD, DeLong MR (1990) Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res 85:119–146
Arnt J, Olsen CK (2011) 5-HT6 receptor ligands and their antipsychotic potential. Int Rev Neurobiol 96:141–161
Bailer UF, Frank GK, Henry SE, Price JC, Meltzer CC, Mathis CA, Wagner A, Thornton L, Hoge J, Ziolko SK, Becker CR, McConaha CW, Kaye WH (2007) Exaggerated 5-HT1A but normal 5-HT2A receptor activity in individuals ill with anorexia nervosa. Biol Psychiatry 61(9):1090–1099
Baizer JS, Desimone R, Ungerleider LG (1993) Comparison of subcortical connections of inferior temporal and posterior parietal cortex in monkeys. Vis Neurosci 10:59–72
Ballanger B, Strafella AP, van Eimeren T, Zurowski M, Rusjan PM, Houle S, Miyasaki JM, Zurowski M, Lang AE, Strafella AP (2010) Serotonin 2A receptors and visual hallucinations in Parkinson disease. Arch Neurol 67:416–421
Ballanger B, Beaudoin-Gobert M, Neumane S, Epinat J, Metereau E, Duperrier S, Broussolle E, Thobois S, Bonnefoi F, Tourvielle C, Lavenne F, Costes N, Lebars D, Zimmer L, Sgambato-Faure V, Tremblay L (2016) Imaging dopamine and serotonin systems on MPTP Monkeys: a longitudinal PET investigation of compensatory mechanisms. J Neurosci 36(5):1577–1589
Baudry A, Mouillet-Richard S, Schneider B, Launay JM, Kellermann O (2010) miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science 329(5998):1537–1541
Baup N, Grabli D, Karachi C, Mounayar S, François C, Yelnik J, Féger J, Tremblay L (2008) High-frequency stimulation of the anterior subthalamic nucleus reduces stereotyped behaviors in primates. J Neurosci 28(35):8785–8788
BBC (2001) Ecstasy & Agony. http://www.bbc.co.uk/science/horizon/2000/ecstasyagony.shtml
Beaudoin-Gobert M, Epinat J, Météreau E, Duperrier S, Neumane S, Ballanger B, Lavenne F, Liger F, Tourvielle C, Bonnefoi F, Costes N, Bars DL, Broussolle E, Thobois S, Tremblay L, Sgambato-Faure V (2015) Behavioural impact of a double dopaminergic and serotonergic lesion in the non-human primate. Brain 138(Pt 9):2632–2647
Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D (2001) Reward circuitry activation by noxious thermal stimuli. Neuron 32(5):927–946
Becker G, Colomb J, Sgambato-Faure V, Tremblay L, Billard T, Zimmer L (2014) Preclinical evaluation of [18F]2FNQ1P as the first fluorinated serotonin 5-HT6 radioligand for PET imaging. Eur J Nucl Med Mol Imaging 42:495–502
Benhamú B, Martín-Fontecha M, Vázquez-Villa H, Pardo L, López-Rodríguez ML (2014) Serotonin 5-HT6 receptor antagonists for the treatment of cognitive deficiency in Alzheimer’s disease. J Med Chem 57(17):7160–7181
Berton O, Nestler EJ (2006) New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci 7(2):137–151
Bezard E, Gross CE (1998) Compensatory mechanisms in experimental and human parkinsonism: towards a dynamic approach. Prog Neurobiol 55:93–116
Bishop C, Taylor JL, Kuhn DM, Eskow KL, Park JY, Walker PD (2006) MDMA and fenfluramine reduce l-DOPA-induced dyskinesia via indirect 5-HT1A receptor stimulation. Eur J Neurosci 23:2669–2676
Blomstedt P, Sjöberg RL, Hansson M, Bodlund O, Hariz MI (2013) Deep brain stimulation in the treatment of obsessive-compulsive disorder. World Neurosurg 80(6):e245–e253
Boileau I, Payer D, Chugani B, Lobo D, Behzadi A, Rusjan PM, Houle S, Wilson AA, Warsh J, Kish SJ, Zack M (2013) The D2/3 dopamine receptor in pathological gambling: a positron emission tomography study with [11C]-(+)-propyl-hexahydro-naphtho-oxazin and [11C]raclopride. Addiction. 108(5):953–963
Boileau I, Payer D, Chugani B, Lobo DS, Houle S, Wilson AA, Warsh J, Kish SJ, Zack M (2014) In vivo evidence for greater amphetamine-induced dopamine release in pathological gambling: a positron emission tomography study with [(11)C]-(+)-PHNO. Mol Psychiatry 19(12):1305–1313
Bray S, O’Doherty J (2007) Neural coding of reward-prediction error signals during classical conditioning with attractive faces. J Neurophysiol 97(4):3036–3045
Bromberg-Martin ES, Hikosaka O, Nakamura K (2010) Coding of task reward value in the dorsal raphe nucleus. J Neurosci 30:6262–6272
Buoli M, Caldiroli A, Caletti E, Paoli RA, Altamura AC (2013) New approaches to the pharmacological management of generalized anxiety disorder. Expert Opin Pharmacother 14(2):175–184
Caillé F, Morley TJ, Tavares AA, Papin C, Twardy NM, Alagille D, Lee HS, Baldwin RM, Seibyl JP, Barret O, Tamagnan GD (2013) Synthesis and biological evaluation of positron emission tomography radiotracers targeting serotonin 4 receptors in brain: [18F]MNI-698 and [18F]MNI-699. Bioorg Med Chem Lett 23(23):6243–6247
Camus SM, Blois-Heulin C, Li Q, Hausberger M, Bezard E (2013a) Behavioural profiles in captive-bred cynomolgus macaques: towards monkey models of mental disorders? PLoS ONE 8:e62141
Camus SM, Rochais C, Blois-Heulin C, Li Q, Hausberger M, Bezard E (2013b) Birth origin differentially affects depressive-like behaviours: are captive-born cynomolgus monkeys more vulnerable to depression than their wild-born counterparts? PLoS ONE 8(7):e67711
Carta M, Tronci E (2014) Serotonin system implication in l-DOPA-induced dyskinesia: from animal models to clinical investigations. Front Neurol 5:78
Caruana F, Jezzini A, Sbriscia-Fioretti B, Rizzolatti G, Gallese V (2011) Emotional and social behaviors elicited by electrical stimulation of the insula in the macaque monkey. Curr Biol 21(3):195–199
Casper RC (1998) Depression and eating disorders. Depress. Anxiety 8(Suppl. 1):96e104
Casper RC (2006) The “drive for activity” and “restlessness” in anorexia nervosa: potential pathways. J Affect Disord 92:99e107
Catenoix H, Isnard J, Guénot M, Petit J, Remy C, Mauguière F (2008) The role of the anterior insular cortex in ictal vomiting: a stereotactic electroencephalography study. Epilepsy Behav 13(3):560–563
Chaudhuri KR, Schapira AHV (2009) Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol 8:464–474
Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, Watanabe N, Nakagawa A, Omori IM, McGuire H, Tansella M, Barbui C (2009) Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet 373(9665):746–758
Colomb J, Becker G, Fieux S, Zimmer L, Billard T (2014) Syntheses, Radiolabeling, and in vitro evaluations of fluorinated PET radioligands of 5-HT6 serotoninergic receptors. J Med Chem 57:3884–3890
Compan V, Zhou M, Grailhe R, Gazzara RA, Martin R, Gingrich J, Dumuis A, Brunner D, Bockaert J, Hen R (2004) Attenuated response to stress and novelty and hypersensitivity to seizures in 5-HT4 receptor knockout mice. J Neurosci 24(2):412–419
Conti MM, Ostock CY, Lindenbach D, Goldenberg AA, Kampton E, Dell’isola R, Katzman AC, Bishop C (2014) Effects of prolonged selective serotonin reuptake inhibition on the development and expression of l-DOPA-induced dyskinesia in hemi-parkinsonian rats. Neuropharmacology 77:1–8
Davis C (1997) Eating disorders and hyperactivity: a psychobiological perspective. Can J Psychiatry Rev Can Psychiatr 42:168–175
Del Tredici K, Braak H (2012) Lewy pathology and neurodegeneration in premotor Parkinson’s disease. Mov Disord 27:597–607
Delgado MR, Jou RL, LeDoux JE, Phelps EA (2009) Avoiding negative outcomes: tracking the mechanisms of avoidance learning in humans during fear conditioning. Front Behav Neurosci 3:33
Ernst M, Nelson EE, McClure EB, Monk CS, Munson S, Eshel N, Zarahn E, Leibenluft E, Zametkin A, Towbin K, Blair J, Charney D, Pine DS (2004) Choice selection and reward anticipation: an fMRI study. Neuropsychologia 42(12):1585–1597
Fineberg NA, Potenza MN, Chamberlain SR, Berlin HA, Menzies L, Bechara A, Sahakian BJ, Robbins TW, Bullmore ET, Hollander E (2010) Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology 35(3):591–604
Flaherty AW, Graybiel AM (1991) Corticostriatal transformations in the primate somatosensory system. Projections from physiologically mapped body-part representations. J Neurophysiol 66:1249–1263
Fontenot MB, Musso MW, McFatter RM, Anderson GM (2009) Dose-finding study of fluoxetine and venlafaxine for the treatment of self-injurious and stereotypic behavior in rhesus macaques (Macaca mulatta). J Am Assoc Lab Anim Sci 48:176–184
Galineau L, Kas A, Worbe Y, Chaigneau M, Herard A-S, Guillermier S, Delzescaux T, Féger J, Hantraye P, Tremblay L (2016) Cortical areas involved in behavioral expression of external pallidum dysfunctions: A PET imaging study in non-human primates. Neuroimage (in press)
Gaspar P, Duyckaerts C, Alvarez C, Javoy-Agid F, Berger B (1991) Alterations of dopaminergic and noradrenergic innervations in motor cortex in Parkinson’s disease. Ann Neurol 30:365–374
Gerardin E, Pochon JB, Poline JB, Tremblay L, Van de Moortele PF, Levy R, Dubois B, Le Bihan D, Lehéricy S (2004) Distinct striatal regions support movement selection, preparation and execution. NeuroReport 15(15):2327–2331
Gilman S, Koeppe RA, Nan B, Wang CN, Wang X, Junck L, Chervin RD, Consens F, Bhaumik A (2010) Cerebral cortical and subcortical cholinergic deficits in parkinsonian syndromes. Neurology 74(18):1416–1423
Godart NT, Flament MF, Lecrubier Y, Jeammet P (2000) Anxiety disorders in anorexia nervosa and bulimia nervosa: co-morbidity and chronology of appearance. Eur Psychiatry 15:38–45
Grabli D, McCairn K, Hirsch EC, Agid Y, Féger J, François C, Tremblay L (2004) Behavioural disorders induced by external globus pallidus dysfunction in primates: I. Behavioural study. Brain 127:2039–2054
Graff-Guerrero A, Willeit M, Ginovart N, Mamo D, Mizrahi R, Rusjan P, Vitcu I, Seeman P, Wilson AA, Kapur S (2008) Brain region binding of the D2/3 agonist [11C]-(+)-PHNO and the D2/3 antagonist [11C]raclopride in healthy humans. Hum Brain Mapp 29(4):400–410
Graybiel AM (2005) The basal ganglia: learning new tricks and loving it. Curr Opin Neurobiol 15:638–644
Gregorios-Pippas L, Tobler PN, Schultz W (2009) Short-term temporal discounting of reward value in human ventral striatum. J Neurophysiol 101:1507–1523
Grupe DW, Nitschke JB (2013) Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat Rev Neurosci 14(7):488–501
Haahr ME, Rasmussen PM, Madsen K, Marner L, Ratner C, Gillings N, Baaré WF, Knudsen GM (2012) Obesity is associated with high serotonin 4 receptor availability in the brain reward circuitry. Neuroimage 61(4):884–888
Haahr ME, Fisher PM, Jensen CG, Frokjaer VG, Mahon BM, Madsen K, Baaré WF, Lehel S, Norremolle A, Rabiner EA, Knudsen GM (2014) Central 5-HT4 receptor binding as biomarker of serotonergic tonus in humans: a [11C]SB207145 PET study. Mol Psychiatry 19(4):427–432
Haber SN (2003) The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat 26(4):317–330
Haber SN, Knutson B (2010) The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35(1):4–26
Heal D, Gosden J, Smith S (2011) The 5-HT6 receptor as a target for developing novel antiobesity drugs. Int Rev Neurobiol 96:73–109
Heller AS, Johnstone T, Light SN, Peterson MJ, Kolden GG, Kalin NH, Davidson RJ (2013) Relationships between changes in sustained fronto-striatal connectivity and positive affect in major depression resulting from antidepressant treatment. Am J Psychiatry 170:197–206
Hinton EC, Parkinson JA, Holland AJ, Arana FS, Roberts AC, Owen AM (2004) Neural contributions to the motivational control of appetite in humans. Eur J Neurosci 20(5):1411–1418
Hollerman JR, Tremblay L, Schultz W (1998) Influence of reward expectation on behavior-related neuronal activity in primate striatum. J Neurophysiol 80(2):947–963
Holtkamp K, Konrad K, Kaiser N, Ploenes Y, Heussen N, Grzella I, Herpertz-Dahlmann B (2005) A retrospective study of SSRI treatment in adolescent anorexia nervosa: insufficient evidence for efficacy. J Psychiatr Res 39:303–310
Huot P, Johnston TH, Darr T, Hazrati L-N, Visanji NP, Pires D, Brotchie JM, Fox SH (2010) Increased 5-HT2A receptors in the temporal cortex of parkinsonian patients with visual hallucinations. Mov Disord 25:1399–1408
Huot P, Fox SH, Brotchie JM (2011a) The serotonergic system in Parkinson’s disease. Prog Neurobiol 95:163–212
Huot P, Johnston TH, Lewis KD, Koprich JB, Reyes MG, Fox SH, Piggott MJ, Brotchie JM (2011b) Characterization of 3,4-methylenedioxymethamphetamine (MDMA) enantiomers in vitro and in the MPTP-lesioned primate: R-MDMA reduces severity of dyskinesia, whereas S-MDMA extends duration of ON-time. J Neurosci 3:7190–7198
Imbert C, Bezard E, Guitraud S, Boraud T, Gross CE (2000) Comparison of eight clinical rating scales used for the assessment of MPTP-induced parkinsonism in the Macaque monkey. J Neurosci Methods 96:71–76
Iravani MM, Jackson MJ, Kuoppamäki M, Smith LA, Jenner P (2003) 3,4-methylenedioxymethamphetamine (ecstasy) inhibits dyskinesia expression and normalizes motor activity in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primates. J Neurosci 23:9107–9115
Ismail Z, Herrmann N, Rothenburg LS, Cotter A, Leibovitch FS, Rafi-Tari S, Black SE, Lanctôt KL (2004) A functional neuroimaging study of appetite loss in Alzheimer’s disease. J Neurol Sci 271:97–103
Jean A, Conductier G, Manrique C, Bouras C, Berta P, Hen R, Charnay Y, Bockaert J, Compan V (2007) Anorexia induced by activation of serotonin 5-HT4 receptors is mediated by increases in CART in the nucleus accumbens. Proc Natl Acad Sci USA. 104(41):16335–16340
Jean A, Laurent L, Bockaert J, Charnay Y, Dusticier N, Nieoullon A, Barrot M, Neve R, Compan V (2012) The nucleus accumbens 5-HTR4-CART pathway ties anorexia to hyperactivity. Transl Psychiatry 2(12):e203
Jellinger KA (1999) Post mortem studies in Parkinson’s disease—Is it possible to detect brain areas or specific symptoms? J Neural Transm 56:1–29
Jensen J, McIntosh AR, Crawley AP, Mikulis DJ, Remington G, Kapur S (2003) Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron 40:1251–1257
Jezzini A, Caruana F, Stoianov I, Gallese V, Rizzolatti G (2012) Functional organization of the insula and inner perisylvian regions. Proc Natl Acad Sci USA. 109(25):10077–10082
Kalin NH, Shelton SE, Fox AS, Oakes TR, Davidson RJ (2005) Brain regions associated with the expression and contextual regulation of anxiety in primates. Biol Psychiatry 58(10):796–804
Kaye WH, Nagata T, Weltzin TE, Hsu LG, Sokol MS, McConaha C, Plotnicov KH, Weise J, Deep D (2001) Double-blind placebo-controlled administration of fluoxetine in restricting-and restricting-purging-type anorexia nervosa. Biol Psychiatry 49:644–652
Kaye WH, Fudge JL, Paulus M (2009) New insights into symptoms and neurocircuit function of anorexia nervosa. Nat Rev Neurosci 10(8):573–584
Kish SJ, Shannak K, Hornykiewicz O (1988) Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications. N Engl J Med 318(14):876–880
Knutson B, Fong GW, Adams CM, Varner JL, Hommer D (2001) Dissociation of reward anticipation and outcome with event-related fMRI. NeuroReport 12(17):3683–3687
Lavoie B, Parent A (1990) Immunohistochemical study of the serotoninergic innervation of the basal ganglia in the squirrel monkey. J Comp Neurol 99(1):1–16
Lee JY, Lee EK, Park SS, Lim JY, Kim HJ, Kim JS, Jeon BS (2009) Association of DRD3 and GRIN2B with impulse control and related behaviors in Parkinson’s disease. Mov Disord 24(12):1803–1810
Lehéricy S, Bardinet E, Tremblay L, Van de Moortele PF, Pochon JB, Dormont D, Kim DS, Yelnik J, Ugurbil K (2006) Motor control in basal ganglia circuits using fMRI and brain atlas approaches. Cereb Cortex 16(2):149–161
Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, Samara M, Barbui C, Engel RR, Geddes JR, Kissling W, Stapf MP, Lässig B, Salanti G, Davis JM (2013) Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 382(9896):951–962
Loane C, Wu K, Bain P, Brooks DJ, Piccini P, Politis M (2013) Serotonergic loss in motor circuitries correlates with severity of action-postural tremor in PD. Neurology 80:1850–1855
Maillet A, Krack P, Lhommée E, Météreau E, Klinger H, Favre E, Le Bars D, Schmitt E, Bichon A, Pelissier P, Fraix V, Castrioto A, Sgambato-Faure V, Broussolle E, Tremblay L, Thobois S (2016) The prominent role of serotonergic degeneration in apathy, anxiety and depression in de novo Parkinson’s disease. Brain 139(Pt 9):2486–2502
Mallet L, Polosan M, Jaafari N, Baup N, Welter ML, Fontaine D, du Montcel ST, Yelnik J, Chéreau I, Arbus C, Raoul S, Aouizerate B, Damier P, Chabardès S, Czernecki V, Ardouin C, Krebs MO, Bardinet E, Chaynes P, Burbaud P, Cornu P, Derost P, Bougerol T, Bataille B, Mattei V, Dormont D, Devaux B, Vérin M, Houeto JL, Pollak P, Benabid AL, Agid Y, Krack P, Millet B, Pelissolo A, STOC Study Group (2008) Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med 359(20):2121–2134
Marinus J, Visser M, Verwey NA, Verhey FR, Middelkoop HA, Stiggelbout AM, van Hilten JJ (2003) Assessment of cognition in Parkinson’s disease. Neurology 61(9):1222–1228
Matsumura M, Tremblay L, Richard H, Filion M (1995) Activity of pallidal neurons in the monkey during dyskinesia induced by injection of bicuculline in the external pallidum. Neuroscience 65(1):59–70
Mazzucchi S, Frosini D, Ripoli A, Nicoletti V, Linsalata G, Bonuccelli U, Ceravolo R (2015) Serotonergic antidepressant drugs and l-DOPA-induced dyskinesias in Parkinson’s disease. Acta Neurol Scand 131(3):191–195
McCabe C, Mishor Z, Cowen PJ, Harmer CJ (2010) Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biol Psychiatry 67:439–445
Meneses A (2014) Memory formation and memory alterations: 5-HT6 and 5-HT7 receptors, novel alternative. Rev Neurosci 25(3):325–356
Middleton FA, Strick PL (1996) The temporal lobe is a target of output from the basal ganglia. Proc Natl Acad Sci USA 93:8683–8687
Middleton FA, Strick PL (2000) Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain Cogn 42(2):183–200
Moore TJ, Glenmullen J, Mattison DR (2014) Reports of pathological gambling, hypersexuality, and compulsive shopping associated with dopamine receptor agonist drugs. JAMA Intern Med 174(12):1930–1933
Morissette M, Goulet M, Grondin R, Blanchet P, Bédard PJ, Di Paolo T, Lévesque D (1998) Associative and limbic regions of monkey striatum express high levels of dopamine D3 receptors: effects of MPTP and dopamine agonist replacement therapies. Eur J Neurosci 10(8):2565–2573
Mounayar S, Boulet S, Tandé D, Jan C, Pessiglione M, Hirsch EC, Féger J, Savasta M, François C, Tremblay L (2007) A new model to study compensatory mechanisms in MPTP-treated monkeys exhibiting recovery. Brain 130(Pt 11):2898–2914
Murrough JW, Henry S, Hu J, Gallezot JD, Planeta-Wilson B, Neumaier JF, Neumeister A (2011) Reduced ventral striatal/ventral pallidal serotonin1B receptor binding potential in major depressive disorder. Psychopharmacology 213(2–3):547–553
Napadow V, Sheehan JD, Kim J, Lacount LT, Park K, Kaptchuk TJ, Rosen BR, Kuo B (2013) The brain circuitry underlying the temporal evolution of nausea in humans. Cereb Cortex 23(4):806–813
Neumane S, Mounayar S, Jan C, Epinat J, Ballanger B, Costes N, Féger J, Thobois S, François C, Sgambato-Faure V, Tremblay L (2012) Effects of dopamine and serotonin antagonist injections into the striatopallidal complex of asymptomatic MPTP-treated monkeys. Neurobiol Dis 48(1):27–39
Nichols DE, Nichols CD (2008) Serotonin receptors. Chem Rev 108(5):1614–1641
O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ (2002) Neural responses during anticipation of a primary taste reward. Neuron 33(5):815–826
O’Sullivan SS, Wu K, Politis M, Lawrence AD, Evans AH, Bose SK, Djamshidian A, Lees AJ, Piccini P (2011) Cue-induced striatal dopamine release in Parkinson’s disease-associated impulsive-compulsive behaviours. Brain 134:969–978
Obeso JA, Olanow CW, Nutt JG (2000) Levodopa motor complications in Parkinson’s disease. Trends Neurosci 23:S2–S7
Parent A, Mackey A, De Bellefeuille L (1983) The subcortical afferents to caudate nucleus and putamen in primate: a fluorescence retrograde double labeling study. Neuroscience 10(4):1137–1150
Paulus W, Jellinger K (1991) The neuropathologic basis of different clinical subgroups of Parkinson’s disease. J Neuropathol Exp Neurol 50(6):743–755
Pavese N, Simpson BS, Metta V, Ramlackhansingh A, Chaudhuri KR, Brooks DJ (2012) [18F]FDOPA uptake in the raphe nuclei complex reflects serotonin transporter availability. A combined [18F]FDOPA and [11C]DASB PET study in Parkinson’s disease. Neuroimage 59(2):1080–1084
Payer DE, Guttman M, Kish SJ, Tong J, Strafella A, Zack M, Adams JR, Rusjan P, Houle S, Furukawa Y, Wilson AA, Boileau I (2015) [11C]-(+)-PHNO PET imaging of dopamine D(2/3) receptors in Parkinson’s disease with impulse control disorders. Mov Disord 30(2):160–166
Pessiglione M, Guehl D, Agid Y, Hirsch EC, Féger J, Tremblay L (2003) Impairment of context-adapted movement selection in a primate model of presymptomatic Parkinson’s disease. Brain 126:1392–1408
Pessiglione M, Guehl D, Jan C, François C, Hirsch EC, Féger J, Tremblay L (2004) Disruption of self-organized actions in monkeys with progressive MPTP-induced parkinsonism: II. Effects of reward preference. Eur J Neurosci 19:437–446
Pifl C, Schingnitz G, Hornykiewicz O (1991) Effect of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine on the regional distribution of brain monoamines in the rhesus monkey. Neuroscience 44(3):591–605
Pine A, Shiner T, Seymour B, Dolan RJ (2010) Dopamine, time, and impulsivity in humans. J Neurosci 30:8888–8896
Politis M (2014) Neuroimaging in Parkinson disease: from research setting to clinical practice. Nat Rev Neurol 10:708–722
Politis M, Niccolini F (2015) Serotonin in Parkinson’s disease. Behav Brain Res 277:136–145
Politis M, Loane C, Wu K, O’Sullivan SS, Woodhead Z, Kiferle L, Lawrence AD, Lees AJ, Piccini P (2013) Neural response to visual sexual cues in dopamine treatment-linked hypersexuality in Parkinson’s disease. Brain 136(2):400–411
Politis M, Wu K, Loane C, Brooks DJ, Kiferle L, Turkheimer FE et al (2014) Serotonergic mechanisms responsible for levodopa-induced dyskinesias in Parkinson’s disease patients. J Clin Invest 124:1340–1349
Ponseti J, Bosinski HA, Wolff S, Peller M, Jansen O, Mehdorn HM, Büchel C, Siebner HR (2006) A functional endophenotype for sexual orientation in humans. Neuroimage 33(3):825–833
Porras G, Li Q, Bezard E (2012) Modeling Parkinson’s disease in primates: the MPTP model. Cold Spring Harb Perspect Med 2:a009308
Pringle A, Jennings KA, Line S, Bannerman DM, Higgs S, Sharp T (2008) Mice overexpressing the 5- hydroxytryptamine transporter show no alterations in feeding behaviour and increased non-feeding responses to fenfluramine. Psychopharmacology 200(2):291–300
Radua J, van den Heuvel OA, Surguladze S, Mataix-Cols D (2010) Meta-analytical comparison of voxel-based morphometry studies in obsessive-compulsive disorder vs other anxiety disorders. Arch Gen Psychiatry 67(7):701–711
Remy P, Doder M, Lees A, Turjanski N, Brooks D (2005) Depression in Parkinson’s disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain 128(Pt 6):1314–1322
Ricaurte GA, Yuan J, McCann UD (2000) (+/-)3,4-Methylenedioxymethamphetamine (’Ecstasy’)-induced serotonin neurotoxicity: studies in animals. Neuropsychobiology 42:5–10
Romo RE, Scarnati E, Schultz W (1992) Role of primate basal ganglia and frontal cortex in the internal generation of movements. II. Movement-related activity in the anterior striatum. Exp Brain Res 91(3):385–395
Rylander D, Parent M, O’Sullivan SS, Dovero S, Lees AJ, Bézard E, Cenci MA (2010) Maladaptive plasticity of serotonin axon terminals in levodopa-induced dyskinesia. Ann Neurol 68:619–628
Saga Y, Tremblay L (2016) Ventral Striatopallidal pathways involved in appetitive and aversive motivational processes. In: Dreher JC, Tremblay L (eds) Decision neuroscience. An integrative perspective, pp 47–58
Saga Y, Richard A, Sgambato-Faure V, Hoshi E, Tobler PN, Tremblay L. (2016) Ventral pallidum encodes contextual information and controls aversive behaviors. Cereb Cortex [pii: bhw107]
Scatton B, Javoy-Agid F, Rouquier L, Dubois B, Agid Y (1983) Reduction of cortical dopamine, noradrenaline, serotonin and their metabolites in Parkinson’s disease. Brain Res 275(2):321–328
Schatzberg AF, Nemeroff CB (2009) The American Psychiatric Publishing Textbook of Psychopharmacology, 4th edn. American Psychiatric Publishing Inc, Arlington
Schoenbaum G, Setlow B, Ramus SJ (2003) A systems approach to orbitofrontal cortex function: recordings in rat orbitofrontal cortex reveal interactions with different learning systems. Behav Brain Res 146(1–2):19–29
Schultz W, Romo R (1992) Role of primate basal ganglia and frontal cortex in the internal generation of movements. I. Preparatory activity in the anterior striatum. Exp Brain Res 91(3):363–384
Schultz W, Apicella P, Scarnati E, Ljungberg T (1992) Neuronal activity in monkey ventral striatum related to the expectation of reward. J Neurosci 12(12):4595–4610
Schultz W, Dayan P, Montague PR (1997) A neural substrate of prediction and reward. Science 275:1593–1599
Schultz W, Tremblay L, Hollerman JR (1998) Reward prediction in primate basal ganglia and frontal cortex. Neuropharmacology 37:421–429
Seeman P (2015) Parkinson’s disease treatment may cause impulse-control disorder via dopamine D3 receptors. Synapse 69(4):183–189
Sgambato-Faure V, Worbe Y, Epinat J, Féger J, Tremblay L (2016) Cortico-basal ganglia circuits involved in different motivation disorders in non-human primates. Brain Struct Funct. 221(1):345–364
St Onge JR, Floresco SB (2009) Dopaminergic modulation of risk-based decision making. Neuropsychopharmacology 34:681–697
Stark P, Hardison CD (1985) A review of multicenter controlled studies of fluoxetine vs. imipramine and placebo in outpatients with major depressive disorder. J Clin Psychiatry 46:53–58
Stefano GB, Ptáček R, Kuželová H, Mantione KJ, Raboch J, Papezova H, Kream RM (2013) Convergent dysregulation of frontal cortical cognitive and reward systems in eating disorders. Med Sci Monit 19:353–358
Szabo Z, McCann UD, Wilson AA, Scheffel U, Owonikoko T, Mathews WB, Ravert HT, Hilton J, Dannals RF, Ricaurte GA (2002) Comparison of (+)-(11)C-McN5652 and (11)C-DASB as serotonin transporter radioligands under various experimental conditions. J Nucl Med 43:678–692
Tanaka SC, Schweighofer N, Asahi S, Shishida K, Okamoto Y, Yamawaki S, Doya K (2007) Serotonin differentially regulates short- and long-term prediction of rewards in the ventral and dorsal striatum. PLoS One 2(12):e1333
Tavares AA, Caillé F, Barret O, Papin C, Lee H, Morley TJ, Fowles K, Holden D, Seibyl JP, Alagille D, Tamagnan GD (2014) In vivo evaluation of 18F-MNI698: an 18F-labeled radiotracer for imaging of serotonin 4 receptors in brain. J Nucl Med 55:858–864
Thobois S, Ardouin C, Lhommée E, Klinger H, Lagrange C, Xie J, Fraix V, Coelho Braga MC, Hassani R, Kistner A, Juphard A, Seigneuret E, Chabardes S, Mertens P, Polo G, Reilhac A, Costes N, LeBars D, Savasta M, Tremblay L, Quesada JL, Bosson JL, Benabid AL, Broussolle E, Pollak P, Krack P (2010) Non-motor dopamine withdrawal syndrome after surgery for Parkinson’s disease: predictors and underlying mesolimbic denervation. Brain 133(Pt 4):1111–1127
Thobois S, Lhommée E, Klinger H, Ardouin C, Schmitt E, Bichon A, Kistner A, Castrioto A, Xie J, Fraix V, Pelissier P, Chabardes S, Mertens P, Quesada JL, Bosson JL, Pollak P, Broussolle E, Krack P (2013) Parkinsonian apathy responds to dopaminergic stimulation of D2/D3 receptors with piribedil. Brain 136(5):1568–1577
Tobler PN, Fiorillo CD, Schultz W (2005) Adaptive coding of reward value by dopamine neurons. Science 307:1642–1645
Tom SM, Fox CR, Trepel C, Poldrack RA (2007) The neural basis of loss aversion in decision-making under risk. Science 315(5811):515–518
Tomasi D, Volkow ND (2013) Striatocortical pathway dysfunction in addiction and obesity: differences and similarities. Crit Rev Biochem Mol Biol 48(1):1–19
Tremblay L, Worbe Y, Hollerman R (2009) The ventral striatum: a heterogeneous structure involved in reward processing, motivation and decision-making. In: Dreher JC, Tremblay L (eds) Handbook of Reward and Decision Making. Academic Press, Oxford, pp 51–77
Tremblay L, Worbe Y, Thobois S, Sgambato-Faure V, Féger J (2015) Selective dysfunction of basal ganglia subterritories: from movement to behavioral disorders. Mov Disord 30(9):1155–1170
Varnäs K, Nyberg S, Karlsson P, Pierson ME, Kågedal M, Cselényi Z, McCarthy D, Xiao A, Zhang M, Halldin C, Farde L (2011) Dose-dependent binding of AZD3783 to brain 5-HT1B receptors in non-human primates and human subjects: a positron emission tomography study with [11C]AZ10419369. Psychopharmacology 213(2–3):533–545
Verbaan D, Marinus J, Visser M, van Rooden SM, Stiggelbout AM, Middelkoop HA, van Hilten JJ (2007) Cognitive impairment in Parkinson’s disease. J Neurol Neurosurg Psychiatry 78(11):1182–1187
Voon V, Fernagut PO, Wickens J, Baunez C, Rodriguez M, Pavon N, Juncos JL, Obeso JA, Bezard E (2009) Chronic dopaminergic stimulation in Parkinson’s disease: from dyskinesias to impulse control disorders. Lancet Neurol 8(12):1140–1149
Voon V, Napier C, Frank M, Sgambato-Faure V, Grace AA, Rodriguez-Oroz M, Obeso J, Bezard E, Fernagut P-O (2016) Impulse control disorders and dyskinesias in Parkinson’s disease: an update. Lancet Neurol (in press)
Walsh BT, Fairburn CG, Mickley D, Sysko R, Parides MK (2004) Treatment of bulimia nervosa in a primary care setting. Am J Psychiatry 161(3):556–561
Weintraub D, David AS, Evans AH, Grant JE, Stacy M (2015) Clinical spectrum of impulse control disorders in Parkinson’s disease. Mov Disord 30(2):121–127
Whone AL, Moore RY, Piccini PP, Brooks DJ (2003) Plasticity of the nigropallidal pathway in Parkinson’s disease. Ann Neurol 53(2):206–213
Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G (2003) Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron 40(3):655–664
Woolley ML, Marsden CA, Fone KC (2004) 5-ht6 receptors. Curr Drug Targets CNS Neurol Disord 3(1):59–79
Worbe Y, Baup N, Grabli D, Chaigneau M, Mounayar S, McCairn K, Féger J, Tremblay L (2009) Behavioral and movement disorders induced by local inhibitory dysfunction in primate striatum. Cereb Cortex 19:1844–1856
Worbe Y, Sgambato-Faure V, Epinat J, Chaigneau M, Tandé D, François C, Féger J, Tremblay L (2013) Towards a primate model of Gilles de la Tourette syndrome: anatomo-behavioural correlation of disorders induced by striatal dysfunction. Cortex 49:1126–1140
Yanagimoto K, Maeda H (2003) The nucleus accumbens unit activities related to the emotional significance of complex environmental stimuli in freely moving cats. Neurosci Res 46(2):183–189
Zahodne LB, Fernandez HH (2008) Pathophysiology and treatment of psychosis in Parkinson’s disease: a review. Drugs Aging 25(8):665–682
Acknowledgements
The authors thank Maude Beaudoin-Gobert, Audrey Maillet and Elise Metereau for technical support. Dr V. Sgambato-Faure is supported by INSERM (Institut National de la Santé et de la Recherche Médicale) and Dr L Tremblay by CNRS (Centre National de Recherche Scientifique).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This work was supported by Grants from Agence Nationale de la Recherche (Grant Number ANR-09-MNPS-018), Fondation de France (Grant Numbers 201234497 and 00016818) and Labex Cortex (ANR-11-LABX-0042).
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Sgambato-Faure, V., Tremblay, L. Dopamine and serotonin modulation of motor and non-motor functions of the non-human primate striato-pallidal circuits in normal and pathological states. J Neural Transm 125, 485–500 (2018). https://doi.org/10.1007/s00702-017-1693-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-017-1693-z