Abstract
Rationale
Although serotonin (5-HT) dysregulation is implicated in the pathophysiology of major depressive disorder (MDD), the role of specific receptor subtypes remains to be elucidated. Emerging preclinical research suggests an important role for the 5-HT1B receptor in behavioral regulation and depressive phenotypes. In particular, 5-HT1B heteroreceptors located within the striatum have been shown to play an essential role in antidepressant action.
Objectives
The objective of this study was to determine 5-HT1B receptor binding potential (BP ND) in the region of the ventral striatum/ventral pallidum (VS/VP) in individuals with MDD and healthy control participants.
Methods
Ten participants with MDD (30.8 ± 9.5 years, five men/five women) in a current major depressive episode (MDE) and ten healthy control participants (30.7 ± 10.5 years, five men/five women) underwent positron emission tomography (PET) scanning with the selective 5-HT1B receptor radioligand [11C]P943.
Results
Within the VS/VP region of interest, [11C]P943 BP ND was significantly reduced in the MDD group compared with the healthy control group (1.37 ± 0.13 and 1.68 ± 0.16, respectively; 18.7% between-group difference; p < 0.001).
Conclusions
Consistent with preclinical and postmortem data, our findings suggest abnormally reduced function of VS/VP 5-HT1B receptors in humans with MDD. Abnormal 5-HT1B heteroreceptor function may contribute to dysfunctional reward signaling within the striatum, including the nucleus accumbens, via interaction with dopamine, γ-amino-butyric acid, or glutamate systems. Our findings suggest reduced 5-HT1B receptor signaling in the VS/VP in MDD and contribute to the therapeutic rationale for testing 5-HT1B agonists as a novel class of antidepressants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Abundant research implicates altered serotonin (5-HT) function in major depressive disorder (MDD) (Heninger et al. 1984; Jans et al. 2007; Belmaker and Agam 2008; aan het Rot et al. 2009). Replicated findings in support of deficient 5-HT function include reduced 5-HT metabolites in the plasma and cerebrospinal fluid (CSF) of depressed patients, depressiogenic effects of acute tryptophan depletion (ATD) in vulnerable individuals, and the efficacy of 5-HT reuptake inhibitors in the treatment of depressive symptoms (Neumeister et al. 2004, 2006; Jans et al. 2007). However, the precise role of 5-HT or its numerous receptor subtypes in the pathophysiology of depression remains to be characterized.
Positron emission tomography (PET) neuroreceptor studies of the 5-HT1A receptor in humans have demonstrated reduced receptor binding potential (BP ND) (Innis et al. 2007) within a cortico-limbic-striatal circuit during both acute depressive episode and remission (Drevets et al. 1999; Bhagwagar et al. 2004; Drevets et al. 2007; Savitz et al. 2009). Although changes in the 1A receptor appear to be associated with depression, it is clear that alterations in this receptor would account for only a portion of the putative dysregulated 5-HT neurotransmission in the pathophysiology of the disorder.
Growing preclinical evidence suggests an important role of the 5-HT1B receptor in depression (Sari 2004; Svenningsson et al. 2006; Chenu et al. 2008; Ruf and Bhagwagar 2009). The 5-HT1B receptor is an inhibitory G protein-coupled metabotropic receptor found primarily as presynaptic terminal auto- and heteroreceptors on 5-HT and non-5-HT neurons, respectively (Pauwels 1997; Hoyer et al. 2002; Hannon and Hoyer 2008). In the mammalian central nervous system (CNS), 5-HT1B receptors are widely distributed, with particularly high densities occurring in the striatum and pallidum (Pauwels 1997; Hoyer et al. 2002). Given the emerging role of the striatum and globus pallidus, particularly ventral regions encompassing the nucleus accumbens (NAc), in the functional neuroanatomy of depression (Nestler and Carlezon 2006; Krishnan and Nestler 2008; Carlezon and Thomas 2009), the high levels of 5-HT1B receptors in these regions may further suggest an important role for this receptor subtype in the pathophysiology of the disorder.
The aim of the current study was to characterize 5-HT1B receptor function in the region of the ventral striatum/ventral pallidum (VS/VP) in MDD using the selective 5-HT1B receptor radioligand [11C]P943 (Nabulsi et al. 2010). Recent evidence suggests that 5-HT1B receptor expression is decreased in an animal model of depression and that p11, an important intracellular protein involved in 5-HT1B signaling, is decreased in postmortem brains of depressed patients (Svenningsson et al. 2006). Therefore, we hypothesized that patients with MDD would exhibit reduced [11C]P943 BP ND in VS/VP, reflecting low levels of 5-HT1B expression in this region.
Methods and materials
Subjects
Ten participants with MDD in a current major depressive episode (MDE) and ten healthy control participants were recruited through public advertisement. Participants were screened and diagnosed using Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria and the Structured Clinical Interview for DSM-IV (SCID) administered by an experienced clinician (First et al. 1995; APA 2000). Depression and anxiety severity were assessed using the Montgomery–Asberg Depression Rating Scale (MADRS) and the Hamilton Rating Scale for Anxiety (HAM-A), respectively (Hamilton 1959; Montgomery and Asberg 1979). Smoking status was assessed with the Fagerstrom Test for Nicotine Dependence (FTND) (Heatherton et al. 1991); family history of depression was assessed using a modified version of the Family Interview for Genetic Studies (FIGS) (Maxwell 1992). All participants were evaluated by physical examination, electrocardiogram, standard laboratory tests, urine analysis, and toxicology. Participants with significant medical or neurological conditions or with history of head injury with loss of consciousness were excluded from the study. All scans with female participants were conducted, while participants were in the follicular phase of their menstrual cycle in order to control for the effects of hormonal variation on PET measures. The protocol was approved by the Yale University School of Medicine Human Investigation Committee, the Human Subjects Subcommittee of the Veterans Affairs Connecticut Healthcare System, the Magnetic Resonance Research Center, and the Yale New Haven Hospital Radiation Safety Committee. Written informed consent was obtained from all participants after full explanation of study procedures.
Scanning and imaging procedures
Subject preparation for the PET scan consisted of indwelling venous catheter placement. A transmission scan using a 137Cs point source was obtained before the emission scan. The PET scans were acquired for 120 min at rest using a single intravenous injection of high-specific activity [11C]P943, a selective 5-HT1B receptor antagonist radiotracer (Nabulsi et al. 2010), on an HRRT PET scanner (207 slices, resolution less than 3 mm full-width at half-maximum in 3D acquisition mode). Dynamic scan data were reconstructed with corrections (attenuation, normalization, scatter, randoms, and deadtime). Motion correction of PET data was performed by coregistering each reconstructed frame to an early summed image (0–10 min postinjection) using a six-parameter mutual information algorithm and FMRIB's Linear Image Registration Tool (FLIRT, FSL 3.2, Analysis Group, FMRIB, Oxford, UK).
Magnetic resonance (MR) images were obtained for each subject on a Siemens 3T Trio system to exclude individuals with anatomical abnormalities and for co-registration. A second summed image (0–10 min postinjection) was created from the motion-corrected PET data and registered to the subject's MR image, which in turn, was registered (12-parameter affine transformation) to an MR template (MNI space). The VS/VP region of interest (ROI) was taken from the template for SPM2 (Anatomical Automatic Labeling) and applied to the PET data to produce time–activity curves for the ROI, in reference to the cerebellum (Tzourio-Mazoyer et al. 2002). Pixel-by-pixel analysis was performed using the multilinear reference tissue model, MRTM2 (Ichise et al. 2003), to produce images of BP ND (Innis et al. 2007). The interpretation of BP ND is f ND * Bavail / Kd where f ND is the tracer-free fraction in a region without specific binding, Bavail is the unoccupied receptor concentration, and Kd is the dissociation equilibrium constant of the tracer. The cerebellum was used as the reference region since it is essentially devoid of 5-HT1B receptors (Varnas et al. 2005). Assuming that there is no change in affinity or non-specific binding between subject groups, changes in BP ND were interpreted as changes in receptor concentration. BP ND values from MRTM2 have provided highly comparable results to those obtained with arterial input functions (Gallezot et al. 2010).
Statistical analysis
Independent sample t tests were used to compare continuous clinical and demographic variables and [11C]P943 BP ND values between MDD and control groups. Data were normally distributed as determined by visual inspection and the Kolmogorov–Smirnov D test. Chi-square was used in the case of dichotomous variables. Tests of association between continuous variables were performed using Pearson's product-moment correlations. All tests were performed two-tailed, with results considered significant at p < 0.05. Means and standard deviations are reported unless otherwise noted. All statistical analyses were conducted using SPSS version 16.0 (SPSS Inc, Chicago, IL, USA).
Results
Demographics and clinical characteristics
Participants were matched for age and gender (HC, 30.7 ± 10.5 years, 5M/5F; MDD, 30.8 ± 9.5 years, 5M/5F; see Table 1). Participants with MDD were outpatients with moderate severity depression (MARDS score, 23.9 ± 5.0) and were free of comorbid axis I disorders. All MDD participants had recurrent episodes, onset of illness before 21 years of age, and a family history positive for depression (at least one first degree blood relative afflicted). The mean duration of the current major depressive episode was 9.4 ± 5.3 months (range, 4 to 20 months). Participants with MDD were antidepressant treatment naïve, except one participant who had a previous trial of an SSRI; all participants were free of any psychotropic medication for at least 4 weeks prior to the time of the PET scan.
Neuroreceptor imaging
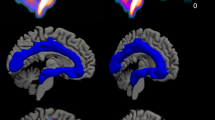
Within our bilateral combined VS/VP ROI, [11C]P943 BP ND was significantly reduced in the MDD group compared to the healthy control group (1.37 ± 0.13 and 1.68 ± 0.16, respectively; 18.7% between-group difference; p < 0.001) (Fig. 1). Both right and left individual ROIs were also significantly different between groups (left hemisphere, 1.35 ± 0.13 and 1.61 ± 0.15, respectively; 16.1% between-group difference; p = 0.001; right hemisphere, 1.38 ± 0.24 and 1.75 ± 0.26, respectively; 21.1% between-group difference; p = 0.004).
Upper panel: plot showing significant differences in ventral striatum/ventral pallidum (VS/VP) region of interest [11C]P943 binding potential (BPND) between patients with major depressive disorder (MDD) and healthy control subjects (HC). Lower panel: average [11C]P943 BPND co-registered positron emission tomography images illustrate reduced VS/VP [11C]P943 BPND in MDD (right) relative to HC (left)
[11C]P943 BP ND did not correlate with any clinical or demographic measures or the PET injection parameters.
Discussion
In this study, we found significantly reduced [11C]P943 BP ND in VS/VP in MDD compared with healthy control participants. To our knowledge, this report is the first direct evidence that abnormal brain 5-HT1B receptor expression is associated with depression in humans.
Our findings are consistent with the recent report of low postmortem levels of the 5-HT1B related protein p11 in brains of depressed patients (Svenningsson et al. 2006). P11 appears to play a critical role in 5-HT1B receptor intracellular trafficking and cell surface expression specifically, and low p11 mRNA transcription directly corresponds to low 5-HT1B receptor function in animals (Svenningsson et al. 2006). Notably, antidepressant medications or electroconvulsive administration enhanced p11 mRNA in mice, and over-expression of p11 conferred antidepressant properties in a mouse model, whereas p11 knockout resulted in a depressive phenotype (Svenningsson et al. 2006). These preclinical findings are in agreement with previous studies demonstrating decreased depressive and anxious behavior in animal models as a result of 5-HT1B agonists (Tatarczynska et al. 2004; Chenu et al. 2008) (however, conflicting results are reported, see (Ruf and Bhagwagar 2009) for review). Previous studies in humans have suggested 5-HT1B receptor hypofunction in depression based upon blunted growth hormone (GH) responses to administration of the 5-HT1D/1B agonists sumatriptan or zolmitriptan (Cleare et al. 1998; Whale et al. 2001).
Anatomically, we chose to focus on the VS/VP due to the high levels of 5-HT1B receptor expression in this region (Sari 2004) and the prominent role of this region in neurocircuitry models of depression (Nestler and Carlezon 2006; Krishnan and Nestler 2008; Carlezon and Thomas 2009). The NAc (anatomically overlapping with the ventral striatum), a key substrate for reward and motivated behavior, receives 5-HTergic innervation from the dorsal raphe and DAergic innervation from the ventral tegmental area (VTA), as well as glutamateric input from prefrontal cortex (PFC) and hippocampus (Nestler and Carlezon 2006). Recently, attenuated activation in this region has been observed in response to positive stimuli in patients with MDD using functional magnetic resonance imaging (Epstein et al. 2006; Pizzagalli et al. 2009). Serotonin1B terminal autoreceptors in this region would be expected to reduce activity of 5-HT neurons, while terminal heteroreceptors would be expected to reduce activity in non-5-HT neurons, for example DAergic, glutamatergic, or GABAergic (Hoyer et al. 2002). However, 5-HT1B agonists were shown to facilitate, rather than inhibit, DA release in the NAc of rats (Yan and Yan 2001). Further, the antidepressant effects of the 5-HT1B agonist anpirtoline in the forced swim test were found to specifically depend on the 5-HT1B heteroreceptor, rather than the autoreceptor, suggesting that 5-HT1B modulation of DAergic function may mediate the antidepressant effect (Chenu et al. 2008). Our finding of low VS/VP 5-HT1B receptor density is consistent with hypothesized dysfunction of DA transmission, in addition to 5-HT transmission, in MDD (Meyer et al. 2006; Nestler and Carlezon 2006; Hasler et al. 2008).
The finding of low [11C]P943 BP ND in MDD extends the preclinical findings of low p11 and 5-HT1B receptor function in animal models (Svenningsson et al. 2006) and adds to the therapeutic rationale for testing 5-HT1B agonists as a novel class of antidepressants. Although preclinical data is mixed regarding the antidepressant effects of 5-HT1B agonists (Ruf and Bhagwagar 2009), receptor localization needs to be considered to determine the impact this receptor subtype has on depression and antidepressant mechanisms. It is quite likely that 5-HT1B receptors are involved in distinct if not opposing processes in depression, potentially accounting for conflicting preclinical results. For example, Chenu et al. demonstrated that 5-HT1B heteroreceptors on non-serotoninergic neurons were specifically required for the antidepressant effects of an SRI in the forced swim test (Chenu et al. 2008). Further, the authors reported a specific antidepressant effect of a 5-HT1B agonist when infused into the caudate and putamen, however, not when infused into the hippocampus, substantia nigra, or frontal cortex (Chenu et al. 2008). Our finding of regional VS/VP reduction of 5-HT1B receptors may support the hypothesis that 5-HT1B agonists would possess antidepressant properties specifically via their interaction with 5-HT1B heteroreceptors located on DAergic neurons in limbic regions of the basal ganglia.
This study has several limitations. The relatively small sample size of the groups limits the inference that can be drawn regarding depression pathophysiology in the broader population. Our MDD sample was very well characterized and relatively homogenous, early-onset, recurrent with positive family history of depression, and treatment naïve with one exception. This homogeneity may have enhanced our ability to detect a biological signal. However, there is a need to expand the sample to include a broader range of depressive illness phenotypes. Further, we are unable to determine if our finding represents a trait or state feature for MDD. Likewise, we are unable to establish if low 5-HT1B receptor binding represents a preexisting vulnerability factor, or rather develops as a consequence of the illness. Lastly, tobacco use was comorbid in our MDD sample (three MDD participants were current smokers vs none in the HC group) and may potentially impact 5-HT1B signaling by virtue of its influence on striatal DA function (Brody et al. 2009). Our sample size did not allow us to address the influence of smoking status on our findings, and future studies will be needed to clarify the potential role of smoking on 5-HT1B function sufficiently.
Future neuroreceptor studies will be needed to address several important research questions, including the specificity of 5-HT1B receptor dysfunction to MDD. Serotonin dysfunction appears to be an important biological factor in several psychiatric disorders, including anxiety and substance disorders, in addition to MDD (Jans et al. 2007). Our group recently published an initial finding of elevated [11C]P943 BP ND in VS/VP in alcohol dependence (Hu et al. 2010). Although the pathological implications of these changes in MDD and alcohol dependence remain unclear, the opposite directionality of the signal in the two disorders may suggest the potential for diagnostic specificity.
In conclusion, we have demonstrated a significant reduction in VS/VP [11C]P943 BP ND in MDD, suggestive of reduced 5-HT1B receptor expression in the disorder. Despite its complex pharmacology, the 5-HT1B receptor may represent an important new target in depression research and therapeutics.
References
aan het Rot M, Mathew SJ, Charney DS (2009) Neurobiological mechanisms in major depressive disorder. CMAJ 180:305–313. doi:10.1503/cmaj.080697
American Psychiatric Association. Task Force on DSM-IV (2000) Diagnostic and statistical manual of mental disorders: DSM-IV-TR. American Psychiatric Association, Washington
Belmaker RH, Agam G (2008) Major depressive disorder. N Engl J Med 358:55–68. doi:10.1056/NEJMra073096
Bhagwagar Z, Rabiner EA, Sargent PA, Grasby PM, Cowen PJ (2004) Persistent reduction in brain serotonin1A receptor binding in recovered depressed men measured by positron emission tomography with [11C]WAY-100635. Mol Psychiatry 9:386–392. doi:10.1038/sj.mp.4001401
Brody AL, Olmstead RE, Abrams AL, Costello MR, Khan A, Kozman D, Saxena S, Farahi J, London ED, Mandelkern MA (2009) Effect of a history of major depressive disorder on smoking-induced dopamine release. Biol Psychiatry 66:898–901. doi:10.1016/j.biopsych.2009.06.011
Carlezon WA Jr, Thomas MJ (2009) Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology 56(Suppl 1):122–132. doi:10.1016/j.neuropharm.2008.06.075
Chenu F, David DJ, Leroux-Nicollet I, Le Maitre E, Gardier AM, Bourin M (2008) Serotonin1B heteroreceptor activation induces an antidepressant-like effect in mice with an alteration of the serotonergic system. J Psychiatry Neurosci 33:541–550
Cleare AJ, Murray RM, Sherwood RA, O’Keane V (1998) Abnormal 5-HT1D receptor function in major depression: a neuropharmacological challenge study using sumatriptan. Psychol Med 28:295–300
Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, Huang Y, Gautier C, Mathis C (1999) PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry 46:1375–1387
Drevets WC, Thase ME, Moses-Kolko EL, Price J, Frank E, Kupfer DJ, Mathis C (2007) Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nucl Med Biol 34:865–877. doi:10.1016/j.nucmedbio.2007.06.008
Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J, Hochberg H, Murrough J, Strohmayer E, Stern E, Silbersweig DA (2006) Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiatry 163:1784–1790. doi:10.1176/appi.ajp.163.10.1784
First MB, Spitzer RL, Gibbon M, Williams JBW (1995) Structured Clinical Interview for DSM-IV Axis Disorders (SCID). New York State Psychiatric Institute, Biometrics Research, New York
Gallezot JD, Nabulsi N, Neumeister A, Planeta-Wilson B, Williams WA, Singhal T, Kim S, Maguire RP, McCarthy T, Frost JJ, Huang Y, Ding YS, Carson RE (2010) Kinetic modeling of the serotonin 5-HT(1B) receptor radioligand [(11)C]P943 in humans. J Cereb Blood Flow Metab 30:196–210. doi:10.1038/jcbfm.2009.195
Hamilton M (1959) The assessment of anxiety states by rating. Br J Med Psychol 32:50–55
Hannon J, Hoyer D (2008) Molecular biology of 5-HT receptors. Behav Brain Res 195:198–213. doi:10.1016/j.bbr.2008.03.020
Hasler G, Fromm S, Carlson PJ, Luckenbaugh DA, Waldeck T, Geraci M, Roiser JP, Neumeister A, Meyers N, Charney DS, Drevets WC (2008) Neural response to catecholamine depletion in unmedicated subjects with major depressive disorder in remission and healthy subjects. Arch Gen Psychiatry 65:521–531. doi:10.1001/archpsyc.65.5.521
Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO (1991) The Fagerstrom Test for nicotine dependence: a revision of the Fagerstrom Tolerance questionnaire. Br J Addict 86:1119–1127
Heninger GR, Charney DS, Sternberg DE (1984) Serotonergic function in depression. Prolactin response to intravenous tryptophan in depressed patients and healthy subjects. Arch Gen Psychiatry 41:398–402
Hoyer D, Hannon JP, Martin GR (2002) Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav 71:533–554
Hu J, Henry S, Gallezot JD, Ropchan J, Neumaier JF, Potenza MN, Sinha R, Krystal JH, Huang Y, Ding YS, Carson RE, Neumeister A (2010) Serotonin 1B receptor imaging in alcohol dependence. Biol Psychiatry. doi:10.1016/j.biopsych.2009.12.028
Ichise M, Liow JS, Lu JQ, Takano A, Model K, Toyama H, Suhara T, Suzuki K, Innis RB, Carson RE (2003) Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab 23:1096–1112. doi:10.1097/01.WCB.0000085441.37552.CA
Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE (2007) Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 27:1533–1539. doi:10.1038/sj.jcbfm.9600493
Jans LA, Riedel WJ, Markus CR, Blokland A (2007) Serotonergic vulnerability and depression: assumptions, experimental evidence and implications. Mol Psychiatry 12:522–543. doi:10.1038/sj.mp.4001920
Krishnan V, Nestler EJ (2008) The molecular neurobiology of depression. Nature 455:894–902. doi:10.1038/nature07455
Maxwell ME (1992) Family Interview for Genetic Studies (FIGS): Manual For FIGS. Clinical Neurogenetics Branch, Intramural Research Program, National Institute of Mental Health, Bethesda, MD
Meyer JH, McNeely HE, Sagrati S, Boovariwala A, Martin K, Verhoeff NP, Wilson AA, Houle S (2006) Elevated putamen D(2) receptor binding potential in major depression with motor retardation: an [11C]raclopride positron emission tomography study. Am J Psychiatry 163:1594–1602. doi:10.1176/appi.ajp.163.9.1594
Montgomery SA, Asberg M (1979) A new depression scale designed to be sensitive to change. Br J Psychiatry 134:382–389
Nabulsi N, Huang Y, Weinzimmer D, Ropchan J, Frost JJ, McCarthy T, Carson RE, Ding YS (2010) High-resolution imaging of brain 5-HT 1B receptors in the rhesus monkey using [11C]P943. Nucl Med Biol 37:205–214. doi:10.1016/j.nucmedbio.2009.10.007
Nestler EJ, Carlezon WA Jr (2006) The mesolimbic dopamine reward circuit in depression. Biol Psychiatry 59:1151–1159. doi:10.1016/j.biopsych.2005.09.018
Neumeister A, Nugent AC, Waldeck T, Geraci M, Schwarz M, Bonne O, Bain EE, Luckenbaugh DA, Herscovitch P, Charney DS, Drevets WC (2004) Neural and behavioral responses to tryptophan depletion in unmedicated patients with remitted major depressive disorder and controls. Arch Gen Psychiatry 61:765–773. doi:10.1001/archpsyc.61.8.765
Neumeister A, Hu XZ, Luckenbaugh DA, Schwarz M, Nugent AC, Bonne O, Herscovitch P, Goldman D, Drevets WC, Charney DS (2006) Differential effects of 5-HTTLPR genotypes on the behavioral and neural responses to tryptophan depletion in patients with major depression and controls. Arch Gen Psychiatry 63:978–986. doi:10.1001/archpsyc.63.9.978
Pauwels PJ (1997) 5-HT 1B/D receptor antagonists. Gen Pharmacol 29:293–303
Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Iosifescu DV, Rauch SL, Fava M (2009) Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry 166:702–710. doi:10.1176/appi.ajp.2008.08081201
Ruf BM, Bhagwagar Z (2009) The 5-HT(1B) receptor: a novel target for the pathophysiology of depression. Curr Drug Targets 10:1118–1138
Sari Y (2004) Serotonin1B receptors: from protein to physiological function and behavior. Neurosci Biobehav Rev 28:565–582. doi:10.1016/j.neubiorev.2004.08.008
Savitz J, Lucki I, Drevets WC (2009) 5-HT(1A) receptor function in major depressive disorder. Prog Neurobiol 88:17–31. doi:10.1016/j.pneurobio.2009.01.009
Svenningsson P, Chergui K, Rachleff I, Flajolet M, Zhang X, El Yacoubi M, Vaugeois JM, Nomikos GG, Greengard P (2006) Alterations in 5-HT1B receptor function by p11 in depression-like states. Science 311:77–80. doi:10.1126/science.1117571
Tatarczynska E, Klodzinska A, Stachowicz K, Chojnacka-Wojcik E (2004) Effects of a selective 5-HT1B receptor agonist and antagonists in animal models of anxiety and depression. Behav Pharmacol 15:523–534
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289. doi:10.1006/nimg.2001.0978
Varnas K, Hurd YL, Hall H (2005) Regional expression of 5-HT1B receptor mRNA in the human brain. Synapse 56:21–28. doi:10.1002/syn.20128
Whale R, Clifford EM, Bhagwagar Z, Cowen PJ (2001) Decreased sensitivity of 5-HT(1D) receptors in melancholic depression. Br J Psychiatry 178:454–457
Yan QS, Yan SE (2001) Activation of 5-HT(1B/1D) receptors in the mesolimbic dopamine system increases dopamine release from the nucleus accumbens: a microdialysis study. Eur J Pharmacol 418:55–64
Acknowledgments
This study was supported by the National Institute of Mental Health grant R21 MH081103 (ARRA) and grant R21 MH085627, and the VA National Center for Posttraumatic Stress Disorder at the West Haven VA Connecticut Clinical Neurosciences Division.
The authors acknowledge the excellent work of the staff of the Yale PET Center and the nursing support from Sue Kasserman, R.N. for help in recruitment and patient care and Brenda Breault, R.N., B.S.N. for her contributions with patient care during the PET scans. In addition, we acknowledge the Yale-Pfizer Bioimaging Alliance for support in the development of [11C]P943.
Conflict of interest
Dr. Neumaier reports lecture fees from Eli Lilly and Wyeth, and Dr. Neumeister grant support from Pfizer Inc., Eli Lilly, UCB Pharma Inc., and Ortho-McNeil Janssen Scientific Affairs, LLC.; Dr. Neumeister has grant support from Pfizer Inc., Eli Lilly, UCB Pharma Inc., and Ortho-McNeil Janssen Scientific Affairs, LLC. No other potential conflict of interest relevant to this article was reported. The contents of the manuscript are solely the responsibility of the authors and do not necessarily represent the official views of any of the funding agencies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Murrough, J.W., Henry, S., Hu, J. et al. Reduced ventral striatal/ventral pallidal serotonin1B receptor binding potential in major depressive disorder. Psychopharmacology 213, 547–553 (2011). https://doi.org/10.1007/s00213-010-1881-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-010-1881-0