Abstract
Rationale
The 5-HT transporter (5-HTT) is implicated in the regulation of appetite. Expression of the 5-HTT varies in the human population, and this variation may determine both individual differences in feeding and abnormal feeding behaviours such as eating disorders.
Objectives
The effects of 5-HTT expression on feeding and satiety were examined in a transgenic mouse model of 5-HTT overexpression.
Materials and methods
We measured free-feeding food intake and observed the behavioural satiety sequence (BSS) after food deprivation in mice at baseline and after administration of the anorectic drug fenfluramine.
Results
5-HTT overexpressing mice were both lighter and shorter than their wildtype littermates. Despite this size difference, food intake by transgenic and wildtype mice did not differ. There was no effect of genotype on the BSS or on food intake during the test at baseline. Increasing doses of fenfluramine reduced food intake in a similar manner in both transgenic and wildtype mice. After 0.3 and 1 mg/kg fenfluramine, the temporal pattern of the BSS was the same for both groups, whereas 3 and 10 mg/kg fenfluramine disrupted the BSS. In transgenic mice, this disruption was evident at the 3 mg/kg dose, while in wildtypes, it emerged only at the 10-mg/kg dose.
Conclusion
These data suggest that overexpression of the 5-HTT does not lead to alterations in feeding or satiety in food-deprived mice but does increase the occurrence of other non-feeding behaviours in response to the 5-HT releasing agent fenfluramine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Evidence suggests that 5-hydroxytryptamine (5-HT, serotonin) pathways are important in both normal and abnormal feeding behaviours (for reviews, see Blundell 1992; Steiger 2004; Halford et al 2007; Kaye et al 2005a). The 5-HT transporter (5-HTT) is an important regulator of brain 5-HT function as it is responsible for the high-affinity reuptake of synaptic 5-HT (Blakely et al. 1994). There is a large degree of variation in the level of 5-HTT expression in the human population, with 2–3-fold differences in 5-HTT binding found in healthy populations (Malison et al. 1998; Mann et al. 2000). A number of commonly occurring polymorphisms in the 5-HTT gene have been identified, which may account for this variation (Heils et al. 1996; Ogilvie et al. 1996; Heinz et al. 2000; Kilic et al. 2003; Ozaki et al. 2003). Variation in food intake and feeding behaviour is well documented and may be, in part, genetic in origin (Rankinen and Bouchard 2006). It is therefore possible that variation in 5-HTT expression may drive both individual differences in feeding behaviour and represent a vulnerability factor for abnormal feeding behaviour.
Evidence from human studies suggests that the 5-HTT is important in regulating feeding behaviours. Administration of selective 5-HT reuptake inhibitors (SSRIs), which target the 5-HTT, reduces food intake and induces weight loss in both obese and lean individuals (Mcguirk and Silverstone 1990; Lawton et al. 1995). Additionally, whilst the primary use of SSRIs is to treat depression and anxiety, these drugs are also used clinically to treat bulimia nervosa (Walsh et al. 2004) and to prevent relapse in anorexia nervosa (Kaye et al 2001), disorders in which disturbances of satiety may be important. It is also of note that reduced 5-HTT binding sites, observed in both platelets and brain, have been associated with eating disorders (Tauscher et al. 2001; Bruce et al. 2006; Ekman et al. 2006). These results might be evidence that disordered eating during illness leads to state-dependent effects; however, other evidence suggest that these differences in 5-HT expression persist after recovery (Kaye et al. 2005b; Steiger et al. 2005). Therefore, alterations of the 5-HT system may be a trait marker for these disorders and could possibly contribute to vulnerability. Finally, gene association studies suggest that polymorphic variants of the 5-HTT gene, which are thought to influence 5-HTT expression, may confer vulnerability to eating disorders and to obesity, although these findings are not confirmed (Di Bella et al. 2000; Fumeron et al. 2001; Matsushita et al. 2004; Monteleone et al. 2006; Sookoian et al 2007).
Studies in rodents examining a well-validated specific sequence of behaviours associated with feeding and satiety [known as the behavioural satiety sequence (BSS); Antin et al. 1975] support findings in humans. Thus, the anorectic agent fenfluramine, which releases 5-HT following entry into the nerve terminal via the 5-HTT, enhances satiety and reduces food intake in the BSS (Vickers et al. 1996, 1999). Moreover, inhibitors of the 5-HTT also reduce food intake and enhance satiety in the BSS (McGuirk et al. 1992; Halford and Blundell 1996). It is also of interest that the 5-HTT knockout mouse develops an obese phenotype at around 3 months (Murphy and Lesch 2008; Holmes et al 2002; Warden et al. 2005).
Given that the 5-HTT is important in regulating appetite and satiety, as well as in the pathophysiology of eating disorders (disorders in which there may be changes in satiety), the current study examined the effects of variation of 5-HTT expression on feeding and satiety using a recently reported genetically engineered 5-HTT overexpressing mouse (Jennings et al. 2006). These mice exhibit a 2–3-fold higher 5-HTT expression, along with reduced brain 5-HT levels, compared to their wildtype littermates (Jennings et al. 2006). Food intake was assessed in free-feeding animals, and the BSS was measured under baseline conditions and in response to fenfluramine following food deprivation.
Materials and methods
Animals
Male CBAxC57BL6J 5-HTT overexpressing transgenic (generated as previously described Jennings et al. 2006) and wildtype littermates [2–5 months, n = 8 per group, bred from wildtype (female), transgenic (male) pairs] were housed in wildtype/transgenic pairs under a 12-h light/dark cycle (lights on 0700) in a temperature-controlled environment (21 ± 1°C) with ad libitum access to standard mouse food and water, except where stated under procedures. Home cages were 16 × 47 × 12 cm. The experimenter was blind to genotype for the duration of the study period. Experiments were carried out in accordance with the Animals Scientific Procedures Act (1986) and Home Office regulations.
Animal size
Weights of the mice were taken at the start of each experiment; this amounted to approximately one measurement per week over the course of 13 weeks. Body lengths (nose to anus) of mice were measured on day 1 of the study, at which point mice had reached adult size. Body mass index was calculated over the course of the study as follows: weight/length2.
Habituation and baseline BSS data
Mice were habituated in cohorts of four (two transgenic and two wildtype) at a time over a period of 12 days during the second half of the light phase of the light/dark cycle. During habituation, each mouse was individually placed into a clear plastic box (17.5 cm × 28 cm × 25 cm) and presented with a clear plastic Petri dish lid containing 10 g of a palatable wet mash (one part expanded diet pellets to one part tap water) over a period of 40 min. At the end of this period, any spilt food was collected from the boxes, and uneaten food was weighed to the nearest 0.1 g. Test boxes were wiped with a damp paper towel and dried between testing of different cohorts.
BSS measurements followed a similar protocol to that of Vickers et al. (1999), beginning immediately after wet mash was placed in the clear boxes. Mice were observed individually once every 30 s over the 40 min and categorised into one of four mutually exclusive behaviours: immobility (absence of movement with or without eyes closed), feeding (the acquisition and eating of the food substrate, including holding, sniffing or transporting of food), grooming (cleaning of face and body, including scratching), exploration [movement around box, sniffing (excluding sniffing of food) and rearing (lifting of fore legs from the floor of the box)].
Initial data from the habituation period indicated that consumption of the wet mash remained low (<1 g per test period), and as a result, the BSS did not emerge. Cumulative manipulations of experimental parameters were applied in order to increase wet mash consumption (low lighting, dark phase testing and 1-, 11- and 24-h food deprivation), revealing that 24-h food deprivation and testing during the dark phase under low-light conditions provided the optimum conditions for emergence of the BSS in the present study. For collection of baseline and all subsequent BSS data, food was removed from the home cage at 1900 h on the day prior to testing, and mice were tested during the first 2 h of the dark phase. Following testing, mice were returned to free feeding until the evening prior to their next test session. This method of food deprivation resulted in stable, reproducible increases in food intake across the course of the study without significant effects on body weight (data not shown).
Fenfluramine study
Each animal received all treatments (fenfluramine 0.3, 1 and 3 mg/kg and vehicle). Fenfluramine was dissolved in 0.9% saline, and drug administrations were in the volume of 10 ml/kg, which were administered i.p. in a latin square randomised order 30 min prior to testing as described above. A minimum of 72 h separated successive drug administrations. In a further separate study, animals received fenfluramine (10 mg/kg) or vehicle according to the same design.
Food and water consumption
At the end of the BSS studies, mice were returned to free feeding, individually housed and habituated to the new cages over 48 h. Testing began 1 h before the beginning of the dark phase of the light/dark cycle. Mice were supplied with 5 g of standard mouse food, and remaining food was weighed in the morning. Water bottles were also weighed at the beginning of testing and the following morning. This procedure was repeated on three separate nights. Data from two of this cohort were missing for one of the overnight measurements. In a separate cohort of mice, food intake was examined in both light and dark phases on three separate nights according to the same protocol.
Body temperature
Core body temperature of a separate cohort of male 5-HTT overexpressing and wildtype mice (n = 12 per group) was measured by inserting a probe (V004 mouse probe) coupled to a thermometer (Digitron 2006 T thermocouple thermometer) 1–1.5 cm into the rectum whilst gently restraining the animal. Animals were moved into the testing room (ambient temperature 25 ± 1°C) at least 2 h prior to testing.
Statistical analysis
Data were analysed using ANOVA. The data for the BSS were collated into 8 × 5-min time bins and were analysed separately for each behaviour. Where homogeneity of variance could not be assumed, the Huynh–Feldt correction was used. Interpretation of significant effects was assisted by the use of simple main effects. Data analysis was performed using SPSS version 14.
Results
Body weight, size and temperature
Transgenic mice were 14 ± 4% lighter and 11 ± 3% shorter than their wildtype littermates [main effects of genotype for weight: F(1, 14) = 5.05, p < 0.05 and length: F(1, 14) = 9.33, p < 0.05; Table 1], and this effect was maintained throughout the 13-week study [Fig. 1; effect of time F(5.2, 52.8) = 13.9, p < 0.05 and genotype F(1, 10) = 8.6, p < 0.05, no time × genotype interaction]. However, body mass index did not differ significantly between 5-HTT overexpressing and wildtype mice [main effect of genotype: F(1, 14) = 2.15, p = 0.17; Table 1]. There was no difference in body temperature between transgenic and wildtype mice [main effect of genotype: F(1, 22) = 2.52, p = 0.13; Table 1].
Food intake measurements taken over the course of 13 weeks plotted against animal’s age. Measurements began at 13 weeks old (young adulthood) and were taken at least once per week (although up to four times per week, in which case these values were averaged to produce a weight for that week) up to 26 weeks of age. Note that this difference between genotypes is stable over the course of the 2.5 months period (p < 0.05). Values represent mean±SEM of six animals per genotype
Baseline BSS
In experiments with 24-h food deprivation, food intake over the observation period of the BSS was not significantly different between 5-HTT overexpressing and wildtype mice under baseline conditions [main effect of genotype: F(1, 14) = 0.32, p = 0.58; mean + SEM TG 1.94 ± 0.21 g, WT 2.14 ± 0.17 g]. Similarly, there was no difference between transgenic and wildtype mice when food intake over the observation period was calculated as a function of body weight [main effect of genotype: F(1, 14) = 0.15, p = 0.71].
In initial experiments involving no or minimal food deprivation, there was also no significant difference in food intake (Fig. 2) or the pattern of feeding behaviour in the BSS between genotypes (data not shown).
Food intake during habituation/BSS testing over the course of cumulative manipulations. Each value represents mean±SEM from eight animals per genotype. Measurements were taken on at least two different days per animal per condition. Note that the lack of effect of genotype on food intake before any manipulations are applied and that this is not altered by the manipulations (p > 0.05)
The expected pattern of the BSS was clearly evident after 24-h food deprivation (Fig. 3). Thus, feeding behaviour significantly decreased with time whilst immobility increased [feeding—main effect of time: F(7, 98) = 20.64, p < 0.01; immobility—main effect of time: F(3.12, 43.60) = 4.57, p < 0.01]. Transgenic mice showed no difference from wildtype mice in the incidence of either feeding or immobility, and the temporal pattern of these behaviours was similar for both genotypes [feeding—main effect of genotype: F(1, 14) = 3.22, p = 0.09; immobility—main effect of genotype: F(1, 14) = 0.07, p = 0.80; feeding—time × genotype interaction: F(7, 98) = 1.35, p = 0.24; immobility—time × genotype interaction: F(3.16, 43.60) = 1.10, p = 0.36]. Overall, 5-HTT overexpressing mice exhibited less exploratory behaviour than wildtype mice; however, the temporal pattern of exploratory behaviour was similar in both genotypes [main effect of genotype: F(1, 14) = 14.52, p < 0.01, time × genotype interaction: F(7, 98) = 1.14, p = 0.35].
The BSS measured after 24 h of food deprivation. Wildtype (left panel; n = 8) and transgenic animals (right panel; n = 8). Each mouse was observed every 30 s for 40 min, and behaviour was categorised as feeding  , exploration
, exploration  , grooming
, grooming  or immobility
or immobility  . The graphs represent the mean of measurements taken on two separate occasions
. The graphs represent the mean of measurements taken on two separate occasions
Effect of d-fenfluramine challenge on the BSS
Dose response study
The amount of food eaten decreased with increasing doses of fenfluramine after food deprivation [main effect of dose F(3, 42) = 3.01, p < 0.05; Fig. 4]. This effect was maintained as a trend when food eaten was expressed as a function of body weight [main effect of dose F(3, 42) = 2.60, p = 0.07]. Compared to wildtypes, transgenic mice showed no difference in the amount of food consumed at any dose of fenfluramine [main effect of genotype: F(1, 14) = 0.53, p = 0.48; dose × genotype interaction: F(3, 42) = 0.61, p = 0.61]. Similarly, when food intake was examined in relation to body weight, no difference was seen between genotypes [main effect of genotype: F(1, 14) < 0.001, p = 1.00].
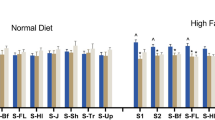
The effect of fenfluramine on wet mash consumption in wildtype (white bars; n = 8) and transgenic mice (shaded bars; n = 8). The amount of food consumed was significantly reduced at the highest (3 and 10 mg/kg) doses of fenfluramine. The data reported in this figure represent two separate studies. In study 1, doses of up to 3 mg/kg were compared to vehicle in a latin square design; in study 2, a dose of 10 mg/kg was compared to vehicle. Mean±SEM values are shown; *p < 0.05 vs vehicle, #p < 0.05 vs 0.3 mg/kg fenfluramine, Fisher’s LSD post hoc testing
After fenfluramine administration, the pattern of behaviours expected in the BSS remained evident (Fig. 5). Despite reducing food intake, fenfluramine did not have a significant effect on the incidence of feeding [main effect of dose: F(2.35, 32.93) = 2.18, p = 0.12]. Similarly, the offset of feeding was not brought forward in response to increasing doses of the drug (dose × time interaction: F(13.45, 188.33) = 1.20, p = 0.28]. Importantly, however, neither the incidence of feeding nor the temporal pattern of feeding behaviour differed between 5-HTT overexpressing and wildtype mice after fenfluramine [main effect of genotype: F(1, 14) = 0.82, p = 0.38; dose × genotype interaction: F(2.35, 32.93) = 0.61, p = 0.58; dose × time × genotype interaction: F(13.45, 188.33) = 0.98, p = 0.47].
The BSS at four different levels of fenfluramine dose and vehicle. Wildtype (left panel; n = 8) and transgenic mice (right panel; n = 8). Each mouse was observed every 30 s for 40 min, and behaviour was categorised as feeding  , exploration
, exploration  , grooming
, grooming  or immobility
or immobility  . The data reported in this figure represent two separate studies. In study 1, doses of up to 3 mg/kg were compared to vehicle in a latin square design; in study 2, a dose of 10 mg/kg was compared to vehicle. As there were no significant differences in the vehicle data for both of these studies, only the vehicle data for study 1 have been reported
. The data reported in this figure represent two separate studies. In study 1, doses of up to 3 mg/kg were compared to vehicle in a latin square design; in study 2, a dose of 10 mg/kg was compared to vehicle. As there were no significant differences in the vehicle data for both of these studies, only the vehicle data for study 1 have been reported
In both transgenic and wildtype mice, the incidence of immobility increased with increasing doses of fenfluramine [main effect of dose: F(3, 42) = 10.48, p < 0.01], and the temporal pattern of immobility changed [dose × time interaction: F(7.13, 99.88) = 2.33, p < 0.05; Fig. 5]. Interestingly, the temporal pattern of immobility differed between genotypes, resulting in an earlier peak in the incidence of immobility in 5-HTT overexpressing mice [genotype × time interaction: F(5.22, 73.09) = 2.71, p < 0.05; Fig. 5]. Moreover, 3 mg/kg (but not 0.3 or 1 mg/kg) fenfluramine induced a significantly greater incidence of immobility in transgenic mice compared to wildtype mice [simple main effect of genotype at 3 mg/kg: F(1, 14) = 7.20, p < 0.05; mean immobility at 3 mg/kg—TG: 3.34 ± 0.43; WT: 1.33 ± 0.26]. This analysis also revealed that the effect of dose was significant in transgenic but not wildtype mice [simple main effect of dose for TG: F(3, 12) = 18.60, p < 0.01); simple main effect of dose for WT: F(3, 12) = 2.41, p = 0.12]. As at baseline, there was decreased exploratory behaviour in 5-HTT overexpressing compared to wildtype mice [main effect of genotype: F(1, 14) = 11.80, p < 0.01].
Single dose study
The findings of the dose response study were further clarified by a single dose study at the higher dose of 10 mg/kg. Compared to vehicle, 10 mg/kg fenfluramine induced a significant reduction in food consumption in food-deprived mice during assessment of the BSS [main effect of dose: F(1, 14) = 53.71, p < 0.01]. However, there was no difference in food intake between genotypes [main effect of genotype: F(1, 14) = 0.01, p = 0.93], nor did the dose-dependent reduction in food intake differ between genotypes [dose × genotype interaction: F(1, 14) = 0.89, p = 0.36; Fig. 4]. These results remained unchanged when food intake was calculated as a function of body weight [main effect of dose: F(1, 14) = 40.93, p < 0.01; main effect of genotype: F(1, 14) = 0.67, p = 0.43; dose × genotype interaction: F(1, 14) = 0.11, p = 0.75].
Fenfluramine (10 mg/kg) significantly reduced the incidence of feeding behaviour compared to vehicle [main effect of dose: F(1, 14) = 24.66, p < 0.01), as well as the temporal pattern of feeding behaviour [dose × time interaction: F(7, 98) = 2.48, p < 0.05]. The dose × genotype interaction was driven by a disruption of the BSS, such that the incidence of feeding was reduced throughout the observation period (Fig. 5). This disruption was apparent in both 5-HTT overexpressing and wildtype mice, with no differences between genotypes observed either in the incidence or the temporal offset of feeding after fenfluramine [10 mg/kg; main effect of genotype: F(1, 14) = 2.02, p = 0.18; dose × genotype interaction: F(1, 14) = 0.24, p = 0.63; dose × time × genotype interaction: F(7, 98) = 0.51, p = 0.82].
As at 3 mg/kg, 10 mg/kg fenfluramine significantly increased immobility [main effect of dose: F(1, 14) = 22.62, p < 0.01]. Furthermore, this effect was again enhanced in transgenic relative to wildtype mice [dose × genotype interaction: F(1, 14) = 7.49, p < 0.05; mean immobility at 10 mg/kg TG: 4.58 ± 0.50; WT: 1.82 ± 0.30]. However, no difference in the temporal onset of immobility was observed between wildtype and transgenic mice [dose × time interaction: F(3.38, 47.28) = 0.87, p = 0.48; dose × time × genotype interaction: F(3.38, 47.28) = 0.46, p = 0.73]. As before, transgenic mice exhibited significantly less exploratory behaviour than wildtype mice [main effect of genotype: F(1, 14) = 24.99, p < 0.01].
Food and water consumption
Food and water intake in free-feeding mice did not differ between genotypes over the 3 days of testing [main effects of genotype for food: F(1, 12) = 0.20, p = 0.66 and water: F(1, 12) = 0.67, p = 0.43]. Calculating overnight food and water consumption as a function of body weight revealed a marginal trend towards transgenics consuming more than wildtype mice [main effects of genotype for food: F(1, 12) = 3.28, p = 0.10 and water: F(1, 12) = 4.28, p = 0.06; Table 1]. In a separate cohort of mice, food consumption was measured separately during light (day) and dark (night) phases. Food consumption during the day was markedly lower than during the night (Table 2). As in previous experiments, food consumption did not differ significantly between genotypes in the night period, nor did it differ during the day (Table 2). Additionally, total food intake over the whole period did not differ between genotypes.
Discussion
The current study investigated the hypothesis that altered 5-HTT expression might affect feeding behaviour and satiety in a genetically engineered mouse model of 5-HTT overexpression (Jennings et al. 2006). 5-HTT overexpressing mice are smaller and lighter than their wildtype littermates but showed no difference in food intake (in the presence and absence of food deprivation) or in feeding during the BSS (in food-deprived mice both at baseline and after fenfluramine administration). This suggests that body size differences between transgenic and wildtype mice are unlikely to be due to altered satiety. The transgenic mice did, however, demonstrate an enhanced response to fenfluramine challenge in the form of increased immobility in the BSS compared to wildtype mice. The lack of effect of 5-HTT overexpression on feeding behaviour and satiety, as measured in the present study, is interesting in the context of existing data about the importance of the 5-HTT in regulating feeding behaviours and its possible role in eating disorders.
The finding that 5-HTT overexpressing mice are both smaller and lighter than their wildtype littermates might suggest that transgenic mice would have decreased energy needs. However, short-term intake of a wet mash diet when food-deprived did not differ between transgenic and wildtype mice (BSS studies) and in overnight measurements in non-food-deprived animals, there were no differences in either food or water consumption. Moreover, when overnight food and water intake were calculated as a function of body weight, there was no evidence that the transgenic mice consumed less than wildtype mice.
We also found no evidence for altered satiety, as measured by the BSS, in transgenic mice. This finding was confirmed in separate studies in both drug naïve and saline-treated food-deprived mice. Under these conditions, the development of the BSS did not differ between transgenic and wildtype mice. The pattern of feeding and non-feeding behaviours seen in the BSS is affected by manipulations that influence satiety. Thus, prefeeding, which presumably enhances satiety, temporally advances the sequence (Blundell et al. 1985; Ishii et al. 2003), while food deprivation delays the sequence (Ishii et al. 2003). This BSS pattern was clearly evident in the present study, but neither the temporal onset nor offset of these behaviours differed between transgenic and wildtype mice. Increasing doses of fenfluramine decreased food intake in a similar manner in both transgenic and wildtype mice. Furthermore, across all doses of fenfluramine, there were no differences between transgenic and wildtype mice in terms of either the amount of time spent in feeding behaviour or the temporal offset of feeding.
It is worth noting that, in addition to the above experiments, initial experiments involving no or minimal food deprivation also showed no difference in the amount of food intake between genotypes (Fig. 2). This finding suggests that the lack of difference in feeding behaviour between wildtype and transgenic mice reported in the present study is not a result of food deprivation and other manipulations (necessary for the emergence of the BSS) masking genotype differences.
Changes in feeding behaviour might have been expected as 5-HTT overexpressing mice exhibit reductions in regional brain tissue and extracellular 5-HT along with a 2–3-fold increase in 5-HTT expression (Jennings et al. 2006). Moreover, 5-HT in general (Blundell 1992) and the 5-HTT in particular (Mcguirk and Silverstone 1990; McGuirk et al. 1992; Lawton et al. 1995; Halford and Blundell 1996) are strongly implicated in the regulation of appetite and satiety (see “Introduction”). It is possible that the transgenic mice undergo neuroadaptive changes, which compensate for elevated 5-HTT expression and low 5-HT levels, and thus no effect on satiety is seen. Indeed, transgenic mice have been shown to exhibit changes in the sensitivity of postsynaptic 5-HT2A receptors that may compensate for low presynaptic 5-HT function (Jennings et al. 2008).
Gene association studies in humans report that polymorphic variants of the 5-HTT gene confer susceptibility to eating disorders such as bulimia nervosa and anorexia nervosa and also to obesity (Di Bella et al. 2000; Fumeron et al. 2001; Matsushita et al. 2004; Monteleone et al. 2006; Sookoian et al. 2007). In as much as satiety mechanisms contribute to eating disorders, the finding that the 5-HTT overexpressing mice did not exhibit altered satiety might suggest that the latter associations are not due to changes in 5-HTT expression. On the other hand, various studies report reduced 5-HTT expression both in the brain and platelets of both currently ill and recovered eating disorder patients (Tauscher et al. 2001; Kaye et al. 2005b; Steiger et al. 2005; Bruce et al. 2006; Ekman et al. 2006). The data reported here suggest that if altered 5-HTT expression does represent a trait marker for eating disorders, it is unlikely to confer vulnerability via differences in satiety.
Interestingly, whilst transgenic mice did not differ from wildtype mice in their feeding response to fenfluramine, the transgenic mice did demonstrate a greater sensitivity to the effect of the drug on other parameters. Specifically, transgenic mice demonstrated a higher level of immobility in response to fenfluramine than wildtype mice. Changes in immobility, however, were not accompanied by corresponding decreases in feeding, and the temporal pattern of the BSS was disrupted, suggesting non-specific effects of fenfluramine as opposed to altered satiety. An enhanced non-feeding response to fenfluramine in the transgenic mice is in keeping with the fact that the 5-HT releasing effect of fenfluramine is dependent on the drug having access to the nerve terminal via the 5-HTT (Fuller et al 1988). Moreover, in separate studies, fenfluramine evoked a greater expression in the immediate early gene c-Fos (a marker of neural activity) in 5-HTT overexpressing compared to wildtype mice (Jennings et al. unpublished observation). Although fenfluramine is no longer used clinically as an anorectic agent, 5-HTT inhibitors are in frequent use for the treatment of eating disorders, in addition to a variety of other conditions. The current data predict that individuals with higher 5-HTT expression levels would be more sensitive to such drugs.
In the current study, the effect of fenfluramine on feeding behaviour (as measured by the BSS) did not reach statistical significance at lower doses (0.3–3 mg/kg), which is somewhat unexpected given the significant reduction in food intake and the effective doses of fenfluramine demonstrated in previous studies (Vickers et al. 1996; Vickers et al. 1999). However, other studies have also failed to find a significant hypophagic effect of this dose, even under non-food-deprived conditions, and have only reported non-specific effects of a 10 mg/kg dose (e.g. Lee et al. 2004). The discrepancy between the effects of fenfluramine on food intake and on BSS measurements may be explained by the use of a time sampling method of observation in the present study. Time sampling methods are efficient because several animals can be observed at the same time. However, compared with continuous observational analysis, time sampling may slightly overestimate the incidence of event like behaviours (e.g. acquiring and eating food) compared to state-like behaviours (e.g. resting) due to the visual prominence of active event behaviours in the observed period. However, as the same observational methods were applied across both groups, this does not change the finding that transgenic mice exhibit no difference in food intake or feeding behaviour in the BSS compared to wildtype mice. Furthermore, the pattern of behavioural satiety was preserved in both genotypes after 3 mg/kg fenfluramine.
The findings from overnight food intake measurements and BSS studies suggest that the small size of the transgenic mice is not explained by differences in feeding. Moreover, it is unlikely that the small size can be explained in terms of altered levels of locomotor activity. In the BSS studies, wildtype mice displayed more active behaviour (exploration) than transgenic mice, and previously published, as well as unpublished, data from our lab indicate no differences in open field or homecage activity between wildtype and transgenic mice (Jennings et al 2006 and data not shown, respectively). Interestingly, in contrast to the 5-HTT overexpressing mice, 5-HTT knockout mice develop an obese phenotype but also show normal daily food consumption (Murphy and Lesch 2008). Although assessment of locomotor activity in the home cage suggests that these mice may be hypoactive (Holmes et al. 2002; Kalueff et al. 2007), laboratory assessments that include habituation suggest the mice have normal locomotor activity (Bengel et al. 1998; Sora et al 2001). The obesity in the 5-HTT knockout mice is associated with increased plasma leptin and insulin implicating altered metabolism in these mice (Murphy and Lesch 2008). Whilst the finding that the 5-HTT overexpressing mice had a normal core body temperature (compared to wildtype mice) might be indicative of unchanged metabolism, a more detailed analysis of these animals is warranted. Additionally, as the present study examined food intake and satiety in adult animals only, it cannot be ruled out that 5-HTT overexpression causes transient changes in food intake and or satiety during development, which in turn lead to persistent changes in body weight in adult life.
In conclusion, mice that overexpress the 5-HTT are lighter and shorter than their wildtype littermates. However, they do not show changes in free-feeding food intake or in food intake or satiety in the BSS after food deprivation, compared to wildtype mice. Thus, these results do not support the hypothesis that variation in 5-HTT overexpression modulates satiety in adulthood. On the other hand, in keeping with increased 5-HTT overexpression, the transgenic mice demonstrated an increase in fenfluramine-induced immobility.
References
Antin J, Gibbs J, Holt J, Young RC, Smith GP (1975) Cholecystokinin elicits the complete behavioral sequence of satiety in rats. J Comp Physiol Psychol 89:784–790
Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mössner R, Westphal H, Lesch KP (1998) Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol 53:649–655
Blakely R, De Felice L, Hartzell H (1994) Molecular physiology of norepinephrine and serotonin transporters. J Exp Biol 196:263–281
Blundell JE (1992) Serotonin and the biology of feeding. Am J Clin Nutr 55:155S–159S
Blundell JE, Rogers PJ, Hill AJ (1985) Behavioural structure and mechanisms of anorexia: calibration of natural and abnormal inhibition of eating. Brain Res Bull 15:371–376
Bruce KR, Steiger H, Ng Ying Kin NM, Israel M (2006) Reduced platelet [3H]paroxetine binding in anorexia nervosa: relationship to eating symptoms and personality pathology. Psychiatry Res 142:225–232
Di Bella DD, Catalano M, Cavallini MC, Riboldi C, Bellodi L (2000) Serotonin transporter linked polymorphic region in anorexia nervosa and bulimia nervosa. Mol Psychiatry 5:233–234
Ekman A, Sundblad-Elverfors C, Landén M, Eriksson T, Eriksson E (2006) Low density and high affinity of platelet [3H]paroxetine binding in women with bulimia nervosa. Psychiatry Res 142:219–223
Fuller RW, Snoddy HD, Robertson DW (1988) Mechanisms of effects of d-fenfluramine on brain serotonin metabolism in rats: uptake inhibition versus release. Pharmacol Biochem Behav 30:715–721
Fumeron F, Betoulle D, Aubert R, Herbeth B, Siest G, Rigaud D (2001) Association of a functional 5-HT transporter gene polymorphism with anorexia nervosa and food intake. Mol Psychiatry 6:9–10
Halford JC, Blundell JE (1996) Metergoline antagonizes fluoxetine-induced suppression of food intake but not changes in the behavioural satiety sequence. Pharmacol Biochem Behav 54:745–751
Halford JC, Harrold JA, Boyland EJ, Lawton CL, Blundell JE (2007) Serotonergic drugs: effects on appetite expression and use for the treatment of obesity. Drugs 67:27–55
Heils A, Teufel A, Petri S, Stöber G, Riederer P, Bengel D, Lesch KP (1996) Allelic variation of human serotonin transporter gene expression. J Neurochem 66:2621–2624
Heinz A, Jones D, Mazzanti C, Goldman D, Ragan P, Hommer D, Linnoila M, Weinberger D (2000) A relationship between serotonin transporter genotype and in vivo protein expression and alcohol neurotoxicity. Biol Psychiatry 47:643–649
Holmes A, Murphy DL, Crawley JN (2002) Reduced aggression in mice lacking the serotonin transporter. Psychopharmacology 161:160–167
Ishii Y, Blundell JE, Halford JC, Rodgers RJ (2003) Effects of systematic variation in presatiation and fasting on the behavioural satiety sequence in male rats. Physiol Behav 79:227–238
Jennings KA, Loder M, Sheward WJ, Pei Q, Deacon R, Benson M, Olverman H, Hastie N, Harmar AJ, Shen S, Sharp T (2006) Increased expression of the 5-HT transporter confers a low-anxiety phenotype linked to decreased 5-HT transmission. J Neurosci 26:8955–8964
Jennings KA, Sheward WJ, Harmar AJ, Sharp T (2008) Evidence that genetic variation in 5-HT transporter expression is linked to changes in 5-HT2A receptor function. Neuropharmacology 54:776–783
Kalueff AV, Ren-Patterson RF, Murphy DL (2007) The developing use of heterozygous mutant mouse models in brain monoamine transporter research. Trends in Pharmacol Sci 28:122–127
Kaye W, Nagata T, Weltzin T, Hsu L, Sokol M, McConaha C, Plotnicov K, Weise J, Deep D (2001) Double-blind placebo-controlled administration of fluoxetine in restricting- and restricting-purging-type anorexia nervosa. Biol Psychiatry 49:644–652
Kaye WH, Frank GK, Bailer UF, Henry SE (2005a) Neurobiology of anorexia nervosa: clinical implications of alterations of the function of serotonin and other neuronal systems. Int J Eat Disord 37:S15–S19
Kaye WH, Frank GK, Bailer UF, Henry SE, Meltzer CC, Price JC, Mathis CA, Wagner A (2005b) Serotonin alterations in anorexia and bulimia nervosa: new insights from imaging studies. Physiol Behav 85:73–81
Kilic F, Murphy DL, Rudnick G (2003) A human serotonin transporter mutation causes constitutive activation of transport activity. Mol Pharmacol 64:440–446
Lawton CL, Wales JK, Hill AJ, Blundell JE (1995) Serotoninergic manipulation, meal-induced satiety and eating pattern: effect of fluoxetine in obese female subjects. Obes Res 3:345–356
Lee MD, Somerville EM, Kennett GA, Dourish CT, Clifton PG (2004) Reduced hypophagic effects of d-fenfluramine and the 5-HT2C receptor antagonist mCPP in 5-HT1B receptor knockout mice. Psychopharmacology 176:39–49
Malison RT, Price LH, Berman R, van Dyck CH, Pelton GH, Carpenter L, Sanacora G, Owens MJ, Nemeroff CB, Rajeevan N, Baldwin RM, Seibyl JP, Innis RB, Charney DS (1998) Reduced brain serotonin transporter availability in major depression as measured by [123I]-2 beta-carbomethoxy-3 beta-(4-iodophenyl)tropane and single photon emission computed tomography. Biol Psychiatry 44:1090–1098
Mann JJ, Huang YY, Underwood MD, Kassir SA, Oppenheim S, Kelly TM, Dwork AJ, Arango V (2000) A serotonin transporter gene promoter polymorphism (5-HTTLPR) and prefrontal cortical binding in major depression and suicide. Arch Gen Psychiatry 57:729–738
Matsushita S, Suzuki K, Murayama M, Nishiguchi N, Hishimoto A, Takeda A, Shirakawa O, Higuchi S (2004) Serotonin transporter regulatory region polymorphism is associated with anorexia nervosa. Am J Med Genet B Neuropsychiatr Genet 128:114–117
McGuirk J, Silverstone T (1990) The effect of the 5-HT re-uptake inhibitor fluoxetine on food intake and body weight in healthy male subjects. Int J Obes 14:361–372
McGuirk J, Muscat R, Willner P (1992) Effects of the 5-HT uptake inhibitors, femoxetine and paroxetine, and a 5-HT1A/B agonist, eltoprazine, on the behavioural satiety sequence. Pharmacol Biochem Behav 41:801–805
Monteleone P, Tortorella A, Castaldo E, Maj M (2006) Association of a functional serotonin transporter gene polymorphism with binge eating disorder. Am J Med Genet B Neuropsychiatr Genet 141:7–9
Murphy DL, Lesch K (2008) Targeting the murine serotonin transporter: insights into human neurobiology. Nat Rev Neurosci 9:85–96
Ogilvie AD, Battersby S, Bubb VJ, Fink G, Harmar AJ, Goodwim GM, Smith CA (1996) Polymorphism in serotonin transporter gene associated with susceptibility to major depression. Lancet 347:731–733
Ozaki N, Goldman D, Kaye WH, Plotnicov K, Greenberg BD, Lappalainen J, Rudnick G, Murphy DL (2003) Serotonin transporter missense mutation associated with a complex neuropsychiatric phenotype. Mol Psychiatry 8:933–936
Rankinen T, Bouchard C (2006) Genetics of food intake and eating behavior phenotypes in humans. Annu Rev Nutr 26:413–434
Sookoian S, Gemma C, Garcia SI, Gianotti TF, Dieuzeide G, Roussos A, Tonietti M, Trifone L, Kanevsky D, Gonzalez CD, Pirola CJ (2007) The short allele of the serotonin transporter is a risk factor for obesity in adolescents. Obesity 15:271–276
Sora I, Hall FS, Andrews AM, Itokawa M, Li XF, Wei HB, Wichems C, Lesch KP, Murphy DL, Uhl GR (2001) Molecular mechanisms of cocaine reward: combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc Natl Acad Sci U S A 98:5300–5305
Steiger H (2004) Eating disorders and the serotonin connection: state, trait and developmental effects. J Psychiatry Neurosci 29:20–29
Steiger H, Richardson J, Israel M, Ng Ying Kin NM, Bruce K, Mansour S, Marie Parent A (2005) Reduced density of platelet-binding sites for [3H]paroxetine in remitted bulimic women. Neuropsychopharmacology 30:1028–1032
Tauscher J, Pirker W, Willeit M, de Zwaan M, Bailer U, Neumeister A, Asenbaum S, Lennkh C, Praschak-Rieder N, Brücke T, Kasper S (2001) [123I] beta-CIT and single photon emission computed tomography reveal reduced brain serotonin transporter availability in bulimia nervosa. Biol Psychiatry 49:326–332
Vickers SP, Clifton PG, Dourish CT (1996) Behavioural evidence that d-fenfluramine-induced anorexia in the rat is not mediated by the 5-HT1A receptor subtype. Psychopharmacology (Berl) 125:168–175
Vickers SP, Clifton PG, Dourish CT, Tecott LH (1999) Reduced satiating effect of d-fenfluramine in serotonin 5-HT(2C) receptor mutant mice. Psychopharmacology (Berl) 143:309–314
Walsh BT, Fairburn CG, Mickley D, Sysko R, Parides MK (2004) Treatment of bulimia nervosa in a primary care setting. Am J Psychiatry 161:556–561
Warden SJ, Robling AG, Sanders MS, Bliziotes MM, Turner CH (2005) Inhibition of the serotonin (5-hydroxytryptamine) transporter reduces bone accrual during growth. Endocrinology 146:685–693
Acknowledgements
Supported by an MRC studentship (AP) and EC FP6 Integrated Network (NEWMOOD LSHM-CT-2003-503474).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pringle, A., Jennings, K.A., Line, S. et al. Mice overexpressing the 5-hydroxytryptamine transporter show no alterations in feeding behaviour and increased non-feeding responses to fenfluramine. Psychopharmacology 200, 291–300 (2008). https://doi.org/10.1007/s00213-008-1206-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-008-1206-8