Abstract
Multiple biological processes throughout development require intracellular vesicular trafficking, where the SNARE (soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein (SNAP) receptors) complex plays a major role. The core proteins forming the SNARE complex are SNAP-25 (synaptosomal-associated protein 25), VAMP (vesicle-associated membrane protein) and Syntaxins, besides its regulatory proteins, such as Synaptotagmin. Genes encoding these proteins (SNAP25, VAMP1, VAMP2, STX1A, SYT1 and SYT2) have been studied in relation to psychiatric disorders susceptibility. Here, we review physiological aspects of SNARE complex and genetic association results reported for attention deficit hyperactivity disorder, both in children and adults, autism spectrum disorders, major depressive disorder, bipolar disorder and schizophrenia. Moreover, we included findings from expression, pharmacogenetics and animal model studies regarding these clinical phenotypes. The overall scenario depicted here suggests that the SNARE complex may exert distinct roles throughout development, with age-specific effects of genetic variants in psychiatric disorders. Such perspective should be considered in future studies regarding SNARE complex genes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
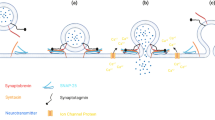
SNARE (soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptors) complex is a large family of proteins that plays a major role in intracellular vesicular trafficking in eukaryotic cells. Such process is essential in different biological events, such as cell division, maintenance of subcellular compartments, protein and hormone secretion and neurotransmitter release (Zylbersztejn and Galli 2011). The SNARE complex is formed by members of the SNAP-25 (Synaptosomal-Associated Protein 25), VAMP (Vesicle-Associated Membrane Protein) and Syntaxins families. These proteins interact creating a four-helix bundle, formed by two helices of SNAP-25, one vesicle-transmembrane VAMP and one presynaptic plasma membrane Syntaxin that approximates the vesicle and plasmatic membranes (Sutton et al. 1998; Brunger 2000) (Fig. 1). Other proteins interact with the SNARE complex and regulate it, such as Munc-18, Complexin, Synaptophysin and the better studied Syt (Synaptotagmin) (Südhof 2013).
SNARE complex assembly at the presynaptic terminal allowing neurotransmitter release. a Components of the core SNARE complex—SNAP-25 (green), Syntaxin 1A (orange) and VAMP (-1 or -2) (purple) -, as well as the regulatory protein Synaptotagmin (I or II) (red), are shown individually. b Assembly of the core SNARE complex, approximating plasma and vesicle membranes; the cytoplasmic SNAP-25 forms a heterodimer with Syntaxin 1A through the binding of SNARE domains (represented by the rectangles). Next, VAMP (-1 or -2) binds to the second SNARE domain of SNAP-25 forming a parallel four-helix bundle. Squares represent plasma (for Syntaxin-1A) and vesicle (for VAMP and Synaptotagmin) transmembrane domains. The SNARE complex is completely assembled. c Upon Calcium (Ca2+) influx, plasma and vesicle membranes are joined and fusion pore is opened through the binding of Ca2+ to the two calcium-binding domains (red balloons) of Synaptotagmin, allowing neurotransmitter release (blue dots). Other regulatory proteins involved in the process are not shown
According to cell tissue and developmental stage, distinct family members of SNARE complex present different expression profiles. SNAP-25 family members are characterized by the presence of two SNARE domains, which are the binding sites between SNAP-25 and VAMP and Syntaxin SNARE domains, in order to form the core SNARE complex. The most studied member of this protein family is SNAP-25, which is expressed in neurons and directly involved in neurotransmitter release. It is anchored to the presynaptic plasma membrane through palmitoylation of cysteine residues. SNAP-25 paralogs, such as SNAP-23, -29, -47, present slightly different properties, and although they may also be expressed in the brain in a distinct distribution (Ravichandran et al. 1996; Steegmaier et al. 1998; Holt et al. 2006; Yamamori et al. 2011), their genetic variants are far less studied. The VAMP family is comprised by 7 proteins that are involved in vesicle fusion (VAMP-1/Synaptobrevin 1, VAMP-2/Synaptobrevin 2, VAMP-3/Cellubrevin, VAMP-4, VAMP-5/Myobrevin, VAMP-7/Ti-VAMP, VAMP-8/Endobrevin) (Larra and Khan 2014). They are characterized by one SNARE domain and one C-terminal transmembrane domain. VAMP-1 and -2 are expressed in the synaptic vesicles of neurons and secretory granules of endocrine and exocrine cells (Trimble 1993). In humans, the Syntaxins family is composed by 16 members (Syntaxin-1A, -1B, -2, -3, -4, -5, -6, -7, -8, -10, -11, -12, -16, -17, -18 and -19). Among them, Syntaxin-1A, -1B and -3 are expressed at presynaptic nerve terminals and involved in synchronous neurotransmitter release, possibly acting on vesicle docking (Ullrich et al. 2015). While Syntaxin-1A and 1B are the major Syntaxin isoforms in brain, Syntaxin 3, for example, is most important for retinal exocytosis (Curtis et al. 2010). They are characterized by a single SNARE domain, a C-terminal transmembrane domain and an N-terminal regulatory domain (Teng et al. 2001). Furthermore, an important family of proteins that interacts with the SNARE complex is the Syt family, which consists of 17 members (SytI to SytXVII), characterized by an N-terminal intravesicular domain, a transmembrane domain and two C-terminal calcium-binding C2-domains (Ullrich et al. 1994; Südhof 2002). It has been shown that SytI, -II and -IX play a critical role in fast Ca2+ triggered exocytosis, which is essential for appropriate neurotransmission (Geppert et al. 1994; Sun et al. 2007; Xu et al. 2007). These three members of Syt family are differentially expressed in the brain, which confer distinct release properties. It has been described that SytII promotes the fastest synapse (Xu et al. 2007), which is consistent with its major localization in neurons mediating auditory circuits (Xiao et al. 2010), while SytIX is the slowest and it is located predominantly in the striatum and limbic system, mediating emotional responses. SytI is slightly slower that SytII and it is equally distributed in all forebrain regions (Xu et al. 2007).
On the following sections we will focus on functional characteristics of specific members of the core SNARE complex (SNAP-25, VAMP-1/-2 and Syntaxin 1A) that mediate neuronal exocytosis (e.g. neurotransmitter release and growth of neuronal membranes), as well as on the most studied regulatory protein (Syt) occurring on neuronal surfaces. We also review the role of their genetic variants on the susceptibility to five major psychiatric disorders: Attention Deficit Hyperactivity Disorder (ADHD), Autism Spectrum Disorders (ASD), Major Depressive Disorder, Bipolar Disorder and Schizophrenia. The role of other regulatory and/or less studied SNARE proteins will not be discussed.
SNARE proteins and its multiple biological functions
SNARE proteins are involved in biological processes throughout several stages of the development, from fertilization to neural development and synaptic plasticity, and even during adulthood and aging (Hepp and Langley 2001). The first stage of the development could be considered the fertilization process itself, where SNARE proteins participate in the main membrane fusion events: acrosomal exocytosis (De Blas et al. 2005) and fusion of male and female gametes (Gadella and Evans 2011). Additionally, following gametes fusion, the SNARE complex acts on cortical reaction (granules exocytosis), an essential process in order to avoid polyspermic fertilization (Gadella and Evans 2011). Moreover, studies focusing on the Syntaxins family have demonstrated that it also mediates essential cell fusion and division events during early embryogenesis in Drosophila (Burgess et al. 1997), in the two-cell stage of sea urchin (Conner and Wessel 1999) and in plants (Touihri et al. 2011). Supporting an essential role of Syntaxins in embryogenesis, knockout mice of Stx1a showed that most of Stx1a (-/-) die in utero having a reduced body size and abnormal development (McRory et al. 2008). Furthermore, a recent study has also suggested that Syntaxin-1A influences the sexual maturity and egg size in Locusta migratoria (Chen et al. 2015).
The nervous system development also requires SNARE proteins. It has been suggested that SNAP-25 may be involved in neurite sprouting (Shirasu et al. 2000; Kimura et al. 2003) and elongation (Osen-Sand et al. 1993), whereas VAMP-2 might promote neurite elongation (Shirasu et al. 2000; Kimura et al. 2003) and Syntaxin-1A could be related to axonal growth (Igarashi et al. 1996) and neurite sprouting as well (Yamaguchi et al. 1996). SNARE-regulatory proteins also seem to participate in the neural development, as demonstrated by the involvement of Syt1 and Syt2 in promoting neurite outgrowth in in vitro studies conducted with rat adrenal pheochromocytoma PC12 cell line, a well-established cellular model used in studies of neuronal function and development (Fukuda et al. 2002). Furthermore, Syt1 might also be involved in axon branching, as reported by a study with cultured chicken forebrain neurons (Greif et al. 2013). Moreover, the expression of SNARE proteins changes throughout life stages suggesting a different role of these proteins during development. For example, experiments with rats demonstrated that Vamp has very low expression levels in the prenatal period, increasing after birth until adulthood; while Syntaxins are highly expressed in the prenatal period and in a short period after birth, decreasing rapidly and then maintaining a constant baseline level (Shimohama et al. 1998). SNAP-25 seems to have an important role in synaptogenesis, since animal studies have shown that Snap-25 is highly expressed in early postnatal maturing rat brains and it shifts its subcellular localization from axons and cell bodies to presynaptic terminals in adults (Oyler et al. 1991).
Moreover, to evaluate the SNAP-25 function, Hess et al. (1992) used mutant mice with a deletion on the distal portion of chromosome 2, which encompasses the Snap25 gene. This animal model is known as Coloboma mouse and was described for the first time in 1964 by Dr. Margaret Dickie in the Jackson Laboratory (Theiler et al. 1978). The homozygous deletion was shown to be lethal, leading to the animal’s death during the embryonary period. Experiments with heterozygous Coloboma mice demonstrated that they had a 50 % decrease on the Snap25 mRNA and protein levels, without affecting its normal tissue distribution profile. These mice also presented small eyes due to a failure in separating cornea and lens epithelium during eye development. Additionally, they displayed head bobbing and locomotor hyperactivity (Hess et al. 1992). It has been strongly suggested that the lack of Snap25 in Coloboma mice is implicated on this hyperactive phenotype since a Snap25 transgene insertion normalizes locomotor activity levels (Hess et al. 1996). Further studies with Coloboma mice reported delayed achievement of neurodevelopmental milestones, such as righting reflex and motor coordination (Heyser et al. 1995) and an abnormal presynaptic catecholamine regulation (Jones et al. 2001).
SNARE proteins are also essential for neuronal maintenance. It has been demonstrated that SNAP-25 and Syntaxin-1 are directly required for neuronal survival as they are responsible for the recycling of proteins on plasma membrane surface (Peng et al. 2013). Further Syntaxin-1 studies with animal model indicated that Stx1b seems to be the most involved in neuronal survival and development (Kofuji et al. 2014; Wu et al. 2015). SNARE proteins seem also to participate in the Brain-Derived Neurotrophic Factor (BDNF) secretion, which is a hormone that regulates neuronal development and plasticity (Shimojo et al. 2015).
The SNARE complex might also have a function on behavioral response. For example, Stx1a is involved in abnormal behavioral response in mice possibly due to dysregulation in the hypothalamic-pituitary adrenal axis response (Fujiwara et al. 2011), reinforcing the role of Stx1a in the hormone regulation during life events. This system plays a central role in modulating response to stimuli and has been implicated with abnormal development and psychiatric disorders (Caspi et al. 2003; Jiang et al. 2009; Bortoluzzi et al. 2015; Roberts et al. 2015). Additionally, a rare human variant in SYT1 gene (I368T) has been recently described in a case-report as implicated with severe behavioral impairments and cognitive deficits (Baker et al. 2015).
Concerning the role of SNARE proteins during aging, studies have demonstrated that SNAP-25 is necessary for cognitive functioning (Gosso et al. 2006) and long-term memory consolidation (Hou et al. 2004; 2006). A non-pathological age-dependent cognitive decline is observed in humans, yet little is known about the neurobiological processes underlying such decline. Observing hippocampal protein expression levels from rats at different ages, Vanguilder et al. (2010) demonstrated a significant decrease in a large subset of proteins involved with neurotransmitter release between adulthood and advanced-age stage, including Snap-25, Vamp-2, Syntaxin-1, Syt1 and Synaptophysin. The hippocampus is responsible for learning and memory consolidation, therefore both functions could be impaired by the reduced expression of proteins involved in neurotransmitter release. In fact, there is evidence that reduced Synaptophysin expression in hippocampus is related to impaired spatial learning (Smith et al. 2000) and memory decline (Bennett et al. 2006) with aging.
Post-translational modifications of SNARE proteins
Palmitoylation is an important post-translational modification in SNARE proteins that is essential to regulation and their correct attachment in the membrane (el-Husseini Ael-D and Bredt 2002; Greaves and Chamberlain 2007). Most of SNARE proteins are palmitoylated in adult brain (Veit et al. 1996, 2000; Lane and Liu 1997; Vogel and Roche 1999; Kang et al. 2004). For example, SNAP-25 has a rich-cysteine domain wherein one or more cysteines are palmitoylated and this is essential for the plasma membrane targeting (Veit et al. 1996; Gonelle-Gispert et al. 2000). Studies with embryonic rat brains showed that in this early stage Syt1 and Vamp-2 lack this post-translational modification. This different palmitoylation profile in embryonic cells indicates that it could be developmentally regulated, influencing SNARE function across life stages (Veit et al. 2000). For example, it has been suggested that SytI palmitoylation might affect its interaction with other proteins and specific microdomains of the presynaptic membrane (Prescott et al. 2009). Moreover, dysregulated palmitoylation has been implicated in a number of psychiatric phenotypes, such as Schizophrenia and Intellectual Disability (Young et al. 2012).
Protein phosphorylation is another important post-translational mechanism for regulating synaptic activity. It has been shown that Cyclic AMP-dependent Protein Kinase A and Casein Kinase II can phosphorylate SNAP-25 and Syntaxin-1A, respectively (Risinger and Bennett 1999). Although phosphorylation itself has minimal effects on the in vitro assembly of the SNARE complex, it was demonstrated that it enhances the interaction between Syntaxin-1A and SytI (Risinger and Bennett 1999). SNAP-25 is also phosphorylated by Protein Kinase C (Nagy et al. 2002) and such mechanism might affect the modulation of neuronal voltage-gated calcium channels (Pozzi et al. 2008) and seems to improve fast exocytosis triggering by recruiting secretory vesicles to the plasma membrane (Nagy et al. 2002). Furthermore, Snap-25 phosphorylation is dynamically regulated by stress in stress-related regions in mouse brain, such as cerebral cortex, hippocampus and amygdala (Yamamori et al. 2014). Mice with sleep deprivation, for example, which are animal models of mania, exhibited increased levels of phosphorylated Snap-25 in the hippocampus and prefrontal cortex (Abrial et al. 2015). Another study with animal models demonstrated that when Snap-25 could not be correctly phosphorylated due to a homozygous mutation, mice showed strong anxiety-related behavior, convulsive seizures and lower serotonin and dopamine levels in the amygdala (Kataoka et al. 2011). Additionally, another post-translational modification that has recently been described is the SUMOylation in Syntaxin-1A. It is essential to appropriate temporal and spatial regulation of neurotransmitter release and synaptic function, where the SUMOylation leads to a decreased affinity of Syntaxin-1A to other SNARE proteins and influence the balance of synaptic vesicle endo/exocytosis, increasing the endocytosis (vesicle recycle) (Craig et al. 2015).
Distinct expression profiles among isoforms and family members
Distinct isoforms of SNARE proteins have been shown to display temporal differences in expression profiles. SNAP-25 has two main isoforms, SNAP-25a and SNAP-25b, which are generated by alternative splicing of the mutually exclusive duplicated exon 5 (5a and 5b) and differ by only 9 of the 39 amino acids from this exon (Bark 1993). Such duplication of exon 5 occurred more than 400 million years ago, during early bony fish development, in which sensory and motor systems were well developed (Johansson et al. 2008). Different from what was initially thought, the functional difference between SNAP-25a and SNAP-25b seems to be due to two nonconservative substitutions at the first SNAP-25 SNARE domain, and not due to the different localization of one palmitoylated cysteine (Nagy et al. 2005).
The relative expression levels of the two isoforms in rat brain drastically changes between embryogenesis and postnatal period; Snap-25a is the main isoform expressed in mouse brain in embrionary stage but 2 weeks after birth, Snap-25b becomes the major splice variant and later in adulthood this isoform represents more than 90 % of total Snap-25 expressed in the brain (Bark et al. 1995; Boschert et al. 1996). This switch between major isoforms throughout development suggests that SNAP-25a may participate mainly in axonal growth, while SNAP-25b could be primarily associated with fast neurotransmitter release. Snap-25b seems to be better than Snap-25a in priming synaptic vesicles, a key step for efficient exocytosis (Sørensen et al. 2003), thus supporting a more prominent role of Snap-25b in neurotransmission.
There are also spatial differences between the isoforms expression profiles during adulthood. Although most brain regions express mainly Snap-25b, in the pituitary gland the Snap-25a remains as the major isoform (Bark et al. 1995; Prescott and Chamberlain 2011). In humans, a post-mortem study showed similar pattern of results, in which SNAP-25b was the most expressed isoform in almost all adult brain areas (Prescott and Chamberlain 2011). Experiments with mutant mice demonstrated that animals without Snap-25b showed developmental anomalies, spontaneous seizures, and impaired short-term synaptic plasticity (Johansson et al. 2008). Moreover, adult mutants had morphological changes in the hippocampus with a severe impairment of spatial learning, supporting the importance of this duplication to enhanced functional plasticity in higher eukaryotes (Johansson et al. 2008).
What leads to this expression switch between the SNAP-25 isoforms remains unclear. It has been suggested that electrical activity could be responsible for such isoform expression profile changes, based on evidences that chronic depolarization induces the Snap-25b expression on PC12 cells, which would be expressing Snap-25a under normal culture conditions (Hepp et al. 2001). Other factors might also influence this process since in adult brain regions involved with more plasticity (olfactory bulb, hippocampus, pineal gland, substantia nigra/pars compacta), Snap-25a and Snap-25b are almost equally expressed in rat brain (Boschert et al. 1996). In this sense, it has been suggested that growth factors, such as Nerve Growth Factor (NGF) or Glial Derived Neurotrophic Factor (GDNF), or other molecules may also regulate the relative levels of SNAP-25 isoforms (Hepp et al. 2001).
Different expression patterns between proteins of the same family have also been shown. Vamp-1 and -2, for example, are differentially expressed in the mature rat brain; while Vamp-2 is widely expressed and more abundant in most brain areas, Vamp-1 predominates in a few particular brain areas (Raptis et al. 2005). This could indicate that distinct family members could exert different functions, which is supported by the fact that Vamp-2 crucial activity in neuroexocytosis at early stages of brain development cannot be replaced by Vamp-1 (Schoch et al. 2001). Additionally, a wider range of Synaptotagmin members is expressed in the first postnatal period (days 3–6) than in postnatal days 12–15 in neurons of rat models (Xiao et al. 2010). This different expression profile of Synaptotagmin proteins during development (higher vs. lower diversity) could indicate distinct roles of these proteins on neural development (Xiao et al. 2010).
SNARE proteins in glial cells
In addition to their role as supportive cells, glial cells can modulate neuronal activity levels, release chemical transmitters (gliotransmitters) and may also contribute to the maintenance of extracellular ion levels (Fellin 2009; Rossi and Volterra 2009). Gliotransmitters are able to activate neuronal receptors and consequently modify neuronal excitability and synaptic transmission (Rossi and Volterra 2009). Several SNARE proteins are expressed in astrocytes (Parpura et al. 1995; Zhang et al. 2004a), the most abundant glial cells in the central nervous system, and are essential to exocytosis of glutamate, d-Serine, BDNF and ATP; all of them stored in VAMP-2-containing vesicles (Araque et al. 2000; Zhang et al. 2004b; Mothet et al. 2005; Parpura et al. 2010; Parpura and Zorec 2010).
Apart from astrocytes, microglia are also important constituents of glia that play a role in neuron surveillance and maintenance in the brain (Hanisch and Kettenmann 2007; Graeber 2010). Moreover, there is evidence that they might be involved in synapse maturation and/or elimination (synapse pruning) (Graeber 2010; Paolicelli et al. 2011). SNARE proteins SNAP-25, SNAP-23, SytI and VAMP-2 are expressed in microglial cells (Hepp et al. 1999; Paolicelli et al. 2011). Myelin is formed by another type of glial cells in the central nervous system, the oligodendrocytes, and it is important to protect and increase the electrical impulses speed along the neuronal fiber (Sherman and Brophy 2005). Evidence of SNARE proteins expression in these glial cells suggests its involvement in the trafficking machinery that is essential in myelinating cells to allow temporal and spatial control by environmental cues (Feldmann et al. 2009; Baron and Hoekstra 2010).
SNARE complex and psychiatric disorders
The core SNARE complex proteins involved in neurotransmission are SNAP-25, VAMP (-1 or -2) and Syntaxin-1A. Cytoplasmic SNAP-25 forms a heterodimer with Syntaxin-1A, a plasma transmembrane protein, at the presynaptic terminal. Next, the vesicle transmembrane protein VAMP binds to this heterodimer forming the SNARE complex, joining plasmatic and vesicle membranes. An important regulatory protein to such membrane fusion event is Syt (I or II) that, upon calcium inflowing, interacts with the SNARE complex, inducing fusion and allowing neurotransmitter release (Südhof 2013) (Fig. 1).
Due to its central role on neurotransmitter release, genes related to the SNARE complex and its regulatory proteins have been investigated in psychiatric disorders. Here, we review the overall findings regarding SNAP25, VAMP1, VAMP2, STX1A, SYT1 and SYT2 genes in relation to Attention Deficit Hyperactivity Disorder (ADHD), Autism Spectrum Disorders (ASD), Major Depressive Disorder, Bipolar Disorder and Schizophrenia susceptibilities, as well as animal model and expression studies. Given the aforementioned central role of SNARE complex on neurodevelopment, results for children and adult samples are presented separately whenever possible (summarized in Table 1) and distinct age-related effects are highlighted and discussed throughout the manuscript. Although genome-wide association studies have not yet implicated the reviewed genes on these disorders, several SNARE polymorphisms have been associated with such phenotypes in candidate gene studies, some of which being supported by meta-analytic studies (Supplementary Table S1).
Synaptosomal-associated protein 25 (SNAP25)
As mentioned above, the first evidence of a possible effect of SNAP-25 on abnormal neuropsychiatric development came from the Coloboma mouse, which was then considered an animal model for ADHD due to their hyperactive behavior. Based on this preliminary observation, the role of SNAP25 gene variants (Chr.20p12.2) has been extensively investigated in relation to different psychiatric disorders (Table 1 and Supplementary Table S1). Studies on childhood ADHD have found multiple polymorphisms associated with the disorder (Feng et al. 2005; Kim et al. 2007; Guan et al. 2009; Zhang et al. 2011; Sarkar et al. 2012; Hawi et al. 2013) most of which, however, were not consistently replicated by independent studies (Sánchez-Mora et al. 2013; Gao et al. 2015).
Evidence implicating a functional role of some SNAP25 variants may give additional support to these association findings. This is the case of rs362990 and rs6108461 SNPs, associated with childhood ADHD and for which a genotype-dependent SNAP25 mRNA expression profile was observed (Hawi et al. 2013). The study reported a dose-dependent effect, where the increased presence of ADHD implicated alleles (A-rs362990 and A-rs6108461) were associated with decreased SNAP25 transcript levels in the brain (Hawi et al. 2013). For other SNPs, apart from their association with childhood ADHD, a broader effect on psychiatric phenotypes has been suggested. For example, it was demonstrated that rs3787283 was nominally associated with ADHD in children and with comorbid Major Depressive Disorder in the same sample, thus affecting the clinical heterogeneity of childhood ADHD (Kim et al. 2007). Furthermore, a subsequent study found that the same SNP was also associated with Major Depressive Disorder itself in adults (Wang et al. 2015).
Moreover, there are two meta-analyses indicating a significant association between the rs3746544 SNP and childhood ADHD (Forero et al. 2009; Gizer et al. 2009), which was further replicated by a subsequent study (Sarkar et al. 2012). Although others have failed in retrieving such result (Hawi et al. 2013; Gálvez et al. 2014; Gao et al. 2015), among the multiple SNAP25 associations reported, this is indubitably the most robust finding on childhood ADHD susceptibility and, as depicted by meta-analytic studies, the T-allele can be considered as a risk-allele for ADHD in children (Supplementary Table S1).
Contrasting with this finding and the overall multiple-SNP implications scenario of SNAP25 on childhood ADHD and despite having several SNPs tested, there is an almost absolute lack of significant findings regarding ADHD in adults (Sánchez-Mora et al. 2013; Olgiati et al. 2014). Such distinct effects of SNAP25 variation on children and adults might be related to its developmentally dependent pattern of expression as discussed on the Distinct expression profiles among isoforms and family members topic. In fact, the only association of a SNAP25 SNP with ADHD in adults was found precisely with rs3746544 (Herken et al. 2014); however, the observed effects of these SNP alleles for adults were in the opposite direction of what was observed for children samples (G for adults, while T for children as risk-allele). Such effect of the G-allele on adulthood ADHD is consistent with findings reported for other psychiatric disorders in adults, where it was also implicated on the susceptibility to Schizophrenia in two meta-analyses (Dai et al. 2014; Wang et al. 2015) and to Major Depressive Disorder (Wang et al. 2015).
Other SNPs spanning the SNAP25 gene have also been associated with Schizophrenia (Carroll et al. 2009; Lochman et al. 2013), as well as with a broader construct of Schizophrenia-related phenotypes (Fanous et al. 2010). Furthermore, evidence of SNAP-25 expression studies in post-mortem brain support its relationship with Schizophrenia. SNAP-25 expression was demonstrated to be increased in a segment of the frontal cortex (Broadmann’s Area 9) (Thompson et al. 1998—not replicated in Scarr et al. 2006), cingulate cortex (Gabriel et al. 1997) and orbitofrontal cortex (Ramos-Miguel et al. 2014). On the other hand, expression was decreased in cerebellum (Mukaetova-Ladinska et al. 2002), olfactory bulb (Egbujo et al. 2015), hippocampus (Fatemi et al. 2001), and segments of the temporal cortex (Broadmann’s Area 20) and anterior prefrontal cortex (Broadmann’s Area 10) (Thompson et al. 1998—not replicated by Gray et al. 2010) of Schizophrenia patients compared to controls. Moreover, Duric et al. (2013) showed that subjects with Major Depressive Disorder had reduced expression levels of SNAP-25 in the hippocampus. However, when antidepressant usage and cause of death were considered, the results did not remain significant, suggesting that the expression pattern could be related to these factors. The same study demonstrated that rats with chronic unpredictable stress (a rat model for depression) presented a trend for decreased levels of Snap-25 in hippocampus.
Furthermore, no association was found with any SNAP25 SNP tested with ASD (Guerini et al. 2011; Braida et al. 2015), although associations with hyperactivity (Guerini et al. 2011) and with cognitive function (Braida et al. 2015) within ASD samples have been reported; and only one study assessed the role of SNAP25 variants on Bipolar Disorder (Etain et al. 2010). This later study found an association of rs6039769 with early-onset Bipolar Disorder; this same SNP was tested for childhood and adulthood ADHD and Schizophrenia with no significant results (Renner et al. 2008; Carroll et al. 2009; Sánchez-Mora et al. 2013) (Supplementary Table S1).
Apart from psychiatric disorder diagnoses, neurocognitive measures were also evaluated regarding SNAP25 genotypes. The rs363039 SNP was associated with working memory in childhood ADHD samples (Gao et al. 2015), and also in samples of healthy children and adults (Söderqvist et al. 2010). This SNP, along with other SNAP25 polymorphisms, was also associated with intelligence quotient (IQ) (Gosso et al. 2006, 2008) but with none of the psychiatric disorders reviewed here (Supplementary Table S1). Thus, it could be suggested that this SNP might influence neurocognitive measures across psychiatric and cognitive phenotypes.
It is noteworthy that most SNPs associated with a wide array of psychiatric disorders are located on the same genetic region, the 3′ end of the gene, that was shown to be implicated in miRNA binding and expression control (Németh et al. 2013). It has been demonstrated that the T–T haplotype for rs3746544-rs1051312 forms a perfect binding site for miR-641, which increases mRNA degradation regulating SNAP-25 expression (Németh et al. 2013). Such T–T haplotype was previously associated with ADHD in children (Mill et al. 2004). Taking into account the known SNAP-25 role on axonal growth and synaptic plasticity, it seems plausible that lower SNAP-25 expression caused by the T–T haplotype might be related to ADHD susceptibility during childhood, a developmental stage where such neuronal processes are remarkably important. Interestingly, the same effect was not observed in adults; in fact, as noted above, the opposite rs3746544 allele (G) seems to confer risk to psychiatric disorders during adulthood, as it has been implicated in ADHD (Herken et al. 2014), Major Depressive Disorder (Wang et al. 2015) and Schizophrenia (Wang et al. 2015) in adults.
Altogether, SNAP25 findings show an interesting pleiotropic scenario pinpointing variants associated to the susceptibility to several psychiatric disorders at distinct developmental stages and suggest that fine control of SNAP-25 expression plays a central role on normal development.
Vesicle-associated membrane protein (VAMP1 and VAMP2)
VAMP-1 is involved in neurotransmitter exocytosis at the presynaptic terminal (Bourassa et al. 2012). The association of VAMP1 gene (Chr.12p13.31) polymorphisms has been tested in regards to ADHD susceptibility, both in children and adults (Sánchez-Mora et al. 2013); however, none of the tested SNPs showed significant effects (Table 1). Expression studies in post-mortem brains, on the other hand, have indicated that VAMP1 mRNA levels are increased on the temporal lobe of Schizophrenia patients when compared to controls (Sokolov et al. 2000), and mRNA levels of a VAMP1 orthologue in rats (Vamp1) were decreased in hippocampus of animal models of depression (Müller et al. 2011).
VAMP2 gene is located at Chr.17p13.1 region and is highly similar to VAMP1 (McNew et al. 2000). The relationship between VAMP2 and psychiatric disorders and related animal model phenotypes has been the focus of several studies. Regarding ADHD in children, several VAMP2 SNPs were evaluated but none of them revealed any association (Brookes et al. 2005, 2006; Sánchez-Mora et al. 2013; Table 1). There was, however, a nominal association between one of these polymorphisms (rs1150) with visual working memory in a sample of children with ADHD, in which homozygotes for the minor allele (A) had better performance scores (Gao et al. 2015). On the other hand, only two variants have been investigated in respect to ADHD in adult samples; while Sánchez-Mora et al. (2013) did not observe association with the single VAMP2 SNP addressed (rs8067606), Kenar et al. (2014) described a significant association with the 26 bp insertion/deletion (26pb Ins/Del) polymorphism of VAMP2. The 26pb Ins/Del is located at 2 kb from 3′ region of VAMP2, in an intergenic region (Falbo et al. 2002), and the Ins-allele was significantly more frequent in adults with ADHD (Kenar et al. 2014) (Table 1 and Supplementary Table S1). Studies regarding other psychiatric disorders did not retrieve significant associations, such as for Schizophrenia (Kawashima et al. 2008) or Bipolar Disorder (Jamra et al. 2008). However, similarly to what was observed for VAMP1, increased VAMP2 mRNA levels on the temporal lobe of Schizophrenia patients were found when compared to controls (Sokolov et al. 2000).
Mice models of depression induced by different types of stress (endogenous stress or unpredictable chronic stress) showed reduced Vamp2 mRNA levels in hippocampus (Malki et al. 2014) and Vamp2 mRNA and protein levels in adrenal medulla (Santana et al. 2015). In rats, however, chronic restraint stress induced an increase of Vamp2 mRNA levels in prefrontal cortex and in hippocampus, as well as increased protein levels in prefrontal cortex (Müller et al. 2011). In hippocampus, while Gao et al. (2006) also reported an increase in Vamp-2 protein levels, Müller et al. (2011) observed decreased Vamp-2. Such controversial findings between Vamp2 mRNA (increased) and Vamp-2 protein levels (decreased) in rat hippocampus reported by Müller et al. (2011) might be due to possible post-transcriptional regulation of Vamp-2, as suggested by the authors. However, more studies are necessary to elucidate the opposite findings for Vamp-2 protein levels between Gao et al. (2006) and Müller et al. (2011) studies. Elfving et al. (2008) assessing a model of electroconvulsive therapy, a known treatment for depression, in rats observed an upregulation of Vamp2 in hippocampus after seizures. Additionally, human studies with post-mortem brain tissue of patients with Major Depressive Disorder revealed that VAMP2 mRNA levels were decreased in the prefrontal cortex compared to controls (Malki et al. 2014).
Syntaxin-1A (STX1A)
STX1A (Chr.7q11.23) polymorphisms have been investigated in relation to ADHD susceptibility in both children and adult samples. It is noteworthy that, although there are more studies with children, most associations were found with adult samples of ADHD. Of the five childhood ADHD studies, only one of them found a significant result (Table 1). The single associated STX1A SNP (rs875342) was found by Gao et al. (2015) comparing Chinese children with and without ADHD.
Regarding adults, the three studies that investigated the relationship between STX1A and ADHD have found significant associations (Sánchez-Mora et al. 2013; Kenar et al. 2014; Olgiati et al. 2014); nevertheless the implicated SNPs were not always the same across studies. Sánchez-Mora et al. (2013) have evaluated several SNPs, both in children and adult samples, and found four STX1A SNPs associated with ADHD during adulthood (Supplementary Table S1). The association of rs2228607 to ADHD observed in adults (Sánchez-Mora et al. 2013) was also found in an independent sample of adults, but in the opposite direction (Olgiati et al. 2014). These results are especially interesting since this polymorphism has been shown to be functionally relevant, where the G-allele is able to affect the mRNA splicing process, favoring the inclusion of intron 3 and resulting in decreased mRNA stability by enhancing nonsense-mediated mRNA decay (von Känel et al. 2013). A third study has implicated a different STX1A SNP (rs35459363) on adult susceptibility to ADHD (Kenar et al. 2014). A possible implication of this SNP in psychiatric disorders during adulthood has also been suggested by a Schizophrenia susceptibility study in a sample from Canada and Portugal (Wong et al. 2004); however, this was not replicated in a Japanese sample (Kawashima et al. 2008) (Supplementary Table S1). Childhood ADHD studies have not directly investigated the effect of this SNP; nevertheless, no association was observed when its effect was assessed in ASD, which is another childhood onset psychiatric disorder (Nakamura et al. 2008).
High functioning autism was nominally associated to the functional STX1A rs2228607 SNP and rs4717806 in children in a Caucasian sample (Nakamura et al. 2008) and to rs6951030 in a Japanese sample (Nakamura et al. 2011). Asperger’s Syndrome was associated to rs4717806 and rs941298 in adults (Durdiaková et al. 2014) (Table 1 and Supplementary Table S1). Moreover, the rs1569061 T-allele was over-transmitted to ASD probands, but other SNPs were not associated (Malenfant et al. 2012). Furthermore, copy number variations encompassing Chr.7q11.23 region have been associated with intellectual and developmental disabilities (Kaminsky et al. 2011), Schizophrenia (Mulle et al. 2014) and ASD (Malenfant et al. 2012; Tordjman et al. 2013; Roberts et al. 2014).
Animals submitted to unpredictable stress to induce depressive-like behavior presented lower Stx1A mRNA levels in the adrenal medulla when compared to controls (Santana et al. 2015). Concerning other expression studies, a lower STX1A mRNA expression was found in post-mortem brain tissues of adults with ASD, specifically in the anterior cingulate gyrus (Nakamura et al. 2011). Nevertheless, Schizophrenia patients present higher Syntaxin-1A levels in cingulate cortex (Gabriel et al. 1997; Honer et al. 1997) and in dorsolateral prefrontal cortex (Gil-Pisa et al. 2012—not found by Gray et al. 2010). Additionally, changes in the protein levels were not found in parietal cortex (Gabriel et al. 1997; Gray et al. 2010), frontal cortex (Gabriel et al. 1997) and prefrontal cortex (Gray et al. 2010). Moreover, in Schizophrenia patients a negative correlation between age and STX1A mRNA levels was demonstrated in the temporal cortex (Sokolov et al. 2000). Regarding Bipolar Disorder, an expression study did not find significant differences in the Syntaxin-1A levels between patients and controls in prefrontal cortex, dorsolateral prefrontal cortex and parietal cortex (Gray et al. 2010).
Synaptotagmin (SYT1 and SYT2)
The SytI protein, encoded by the SYT1 gene (Chr.12q21.2), acts as a Ca2+ sensor in vesicular trafficking and neurotransmitter release (Fernández-Chacón et al. 2001). Although earlier childhood ADHD studies did not find any association (Brookes et al. 2005, 2006), subsequent studies found several SYT1 SNPs nominally associated with the disorder (Guan et al. 2009; Sánchez-Mora et al. 2013) as well as with age of ADHD onset (Lasky-Su et al. 2008) (Supplementary Table S1). For adulthood ADHD, only one study has been conducted so far. Sánchez-Mora et al. (2013) found a nominal association between SYT1 rs2251214 and ADHD in adults, but not in children. None of the other SNPs tested was associated with ADHD in adults (Sánchez-Mora et al. 2013). In addition, copy number variations were evaluated for ASD where several chromosomal regions were implicated, including a segment encompassing the SYT1 gene (Szatmari et al. 2007).
Analyses of SYT1 mRNA levels in post-mortem brains of elderly Schizophrenia patients and controls showed that there was an increased expression in the left superior temporal gyrus of the patients (Sokolov et al. 2000). Moreover, rats with depressive-like symptoms present an increase in Syt1 mRNA levels in the hypothalamus (Ge et al. 2013). Despite that, rats that were submitted to models of electroconvulsive therapy did not show changes regarding Syt1 mRNA expression levels in frontal cortex and hippocampus (Elfving et al. 2008). There was, however, a significant decrease in the paralogue Syt3 mRNA levels in rat hippocampus after both single and repeated treatment exposure (Elfving et al. 2008).
SytII seems to play a role similar to SytI on the SNARE complex assembly (Nagy et al. 2006). SYT2 SNPs (rs6427957-G and rs907697-T) were significantly associated with ADHD in children, but not in adults (Sánchez-Mora et al. 2013). The conflicting results between childhood and adulthood ADHD found for SYT2 may indicate that some SNPs in genes related to neurotransmitter release might be age-specific factors, interfering on the age of onset or diagnosis persistence. More studies regarding this gene in other psychiatric disorders are needed to further elucidate its role on neurodevelopment.
SNARE genes and pharmacogenetics
The interindividual variability in treatment response to psychiatric drugs may be better understood through pharmacogenetic studies. Since the majority of the medications used to treat psychiatric disorders target components of the neurotransmitter systems, genes related to this pathway have earned attention and raised prominent results in psychiatric pharmacogenetics (Kitzmiller et al. 2011). In this context, genes encoding components of the SNARE complex and its regulatory proteins are candidates with potential effects on treatment response to psychiatric medications, since they play a key role in neurotransmitter release (Südhof 2013). Unfortunately, scarce pharmacogenetic studies focusing on the components of this complex have been conducted so far.
Evidence from animal model studies evaluating SNARE complex mRNA or protein have supported the importance of this system in response to a variety of psychiatric drugs. Hess et al. (1996) evaluated the effects of the widely used psychostimulants in ADHD treatment, methylphenidate (MPH) and amphetamine, on locomotor activity using the Coloboma mice mentioned above compared to controls. Administration of amphetamine reduced the hyperactivity symptoms of Coloboma mice, but increased the locomotor activity of controls. On the other hand, MPH increased locomotor activity in both Coloboma and control mice, suggesting a more important role of Snap25 on ADHD treatment with amphetamine than with MPH. Additionally, PC12 cells treated with MPH presented reduced Syt1, Syt4 and Stx1a mRNA levels (Bartl et al. 2010). Thus, one could speculate that the SNARE complex may be involved in the response to commonly used ADHD medications.
There is also evidence of changes on SNARE proteins expression levels in different regions of the rat brain under antipsychotic treatment. More specifically, it has been shown that the use of typical antipsychotic drugs (chlorpromazine, haloperidol or trifluoperazine) alters Snap-25 and Synaptophysin protein levels in a drug- and region-specific manner in the rat hippocampus (Barr et al. 2006). Moreover, Barakauskas et al. (2010) observed that treatment with haloperidol led to increased Snap-25, Syntaxin and Vamp protein levels in striatal regions of rats, whereas clozapine only increased levels of Vamp in the same region (Barakauskas et al. 2010). In the same sense, clozapine seems to downregulate Stx1a expression in frontal cortex whereas it upregulates expression in the parietal cortex (Sommer et al. 2010). Additionally, antidepressants have also been associated with changes in SNARE proteins expression levels. For example, Yamada et al. (2002) showed that chronic antidepressant treatment with imipramine and sertraline increased Vamp-2 protein levels in the frontal cortex of rat, but reported no significant differences on Snap-25 and Stx1 protein levels. Taken together, these results from animal models suggest that components of SNARE complex may exert important functions on the treatment response to different psychiatric drugs and that genes encoding these components are considered good candidates for pharmacogenetic studies.
In clinical psychiatric studies, the most promising results regarding pharmacogenetics of SNARE complex genes are related to SNAP25. The rs3746544 polymorphism, in addition to their robust associations with childhood ADHD susceptibility (Forero et al. 2009; Gizer et al. 2009) was also investigated in studies evaluating the response to MPH in patients with ADHD. In preschoolers aged 3–5 years, the TT genotype of rs3746544 was associated with better response to IR-MPH (immediate release MPH) when compared to G carriers. Additionally, this study also showed association between the G-allele and risk to irritability as treatment side effect (McGough et al. 2006). However, these results were not replicated in a subsequent study in patients with ADHD aged 6–17 years (McGough et al. 2009). More recently, this same SNP was associated with OROS-MPH (osmotic controlled release oral delivery system MPH) treatment response in a sample of children and adolescents aged 6–18 years, in which the TT and TG genotypes were associated with better therapeutic response (Song et al. 2014). These findings are in agreement with the results reported in preschoolers with IR-MPH (McGough et al. 2006). However, the single study that evaluated the role of this SNAP25 SNP on MPH response in adults with ADHD did not retrieve any significant association (Contini et al. 2012). The rs3746544 polymorphism was also associated with efficacy of treatment and weight gain after 14 weeks of treatment in Schizophrenia patients with history of poor response to antipsychotics (Müller et al. 2005). Patients under treatment with clozapine, olanzapine, risperidone or haloperidol that carried the TT genotype showed a better clinical response, but also increased weight gain when compared to TG and GG genotypes. Such findings are in line with the results supported by two meta-analyses reporting the G-allele as risk to Schizophrenia susceptibility as described earlier (Dai et al. 2014; Wang et al. 2015). However, this SNP was not associated with treatment response in another study that evaluated patients with Schizophrenia being treated with quetiapine, risperidone, aripiprazole and olanzapine over a period of 8–12 weeks (Spellman et al. 2008).
Another well-studied SNAP25 SNP, rs1051312, was also investigated in most studies mentioned above. This SNP was associated with IR-MPH treatment response and side effects in preschoolers with ADHD (3–5 years old), in which the T-allele was associated with worse treatment response and the C-allele predicted motor tics as a side effect (McGough et al. 2006). However, no association of this SNP with treatment response and side effects was found in a sample of children and adolescents (McGough et al. 2009). Regarding antipsychotics, this polymorphism was not significantly associated with clinical response or weight gain in patients with Schizophrenia (Müller et al. 2005; Spellman et al. 2008). Apart from SNAP25, other SNARE components are still poorly investigated in clinical pharmacogenetic studies. In one of the few studies, VAMP2 gene polymorphisms were investigated on clinical response to the antidepressant fluvoxamine in a Japanese sample of adults with Major Depressive Disorder, but no significant association was found (Saito et al. 2007).
Given the relative scarcity of clinical pharmacogenetic studies, expression experiments may raise interesting possibilities for further investigations. An interesting example comes from the post-mortem analysis of protein expression levels in the prefrontal cortex of subjects with and without Schizophrenia, treated or not with antipsychotics. It was demonstrated that Syntaxin-1A protein levels were increased in Schizophrenia, but showed a slightly reduction with antipsychotic treatment (Gil-Pisa et al. 2012). SNAP-25, VAMP, Syt, Munc18-1a and Synaptophysin expression was not altered in the brain of Schizophrenia patients without treatment, while in patients treated with antipsychotics drugs only VAMP had reduced expression levels (Gil-Pisa et al. 2012).
Despite more replication studies being warranted, the evidence up to now involving animal model, expression and clinical studies suggests that SNARE complex genes are important candidates to be investigated on response to different psychiatric drugs, including antipsychotics, psychostimulants and antidepressants. Provided that the SNARE proteins have different expression patterns throughout life, it is worth mentioning the importance of considering age groups in such pharmacogenetics studies, at least regarding MPH response. Thus, elucidating genetic variants that influence the effectiveness of treatment or the incidence of adverse effects will allow to perform early pharmacological intervention and improve the patient’s prognosis.
Conclusion
In this review we addressed the state-of-the-art of genetic association findings and biological evidence linking SNARE complex to developmental psychiatry. We revisited studies exploring the relationship between SNARE genes (SNAP25, VAMP1, VAMP2, STX1A, SYT1 and SYT2) and several psychiatric disorders and their treatments. Although still inconclusive, genetic association findings on psychiatric disorders susceptibility depict a promising overall scenario, with variants exerting distinct effects in children and in adults. Additionally, despite SNARE proteins usually being known by their role in neurotransmitter release, they participate in a wide variety of processes throughout life stages and are essential to neuropsychiatric development.
References
Abrial E, Betourne A, Etievant A et al (2015) Protein kinase C inhibition rescues manic-like behaviors and hippocampal cell proliferation deficits in the sleep deprivation model of mania. Int J Neuropsychoph 18:1–11. doi:10.1093/ijnp/pyu031
Araque A, Li N, Doyle RT, Haydon PG (2000) SNARE protein-dependent glutamate release from astrocytes. J Neurosci 20:666–673
Baker K, Gordon S, Grozeva D et al (2015) Identification of a human synaptotagmin-1 mutation that perturbs synaptic vesicle cycling. J Clin Invest 125:1670–1678. doi:10.1172/JCI79765
Barakauskas VE, Beasley CL, Barr AM et al (2010) A novel mechanism and treatment target for presynaptic abnormalities in specific striatal regions in schizophrenia. Neuropsychopharmacol 35(5):1226–1238
Bark IC (1993) Structure of the chicken gene for SNAP-25 reveals duplicated exons encoding distinct isoforms of the protein. J Mol Biol 233:67–76
Bark IC, Hahn KM, Ryabinin AE, Wilson MC (1995) Differential expression of SNAP-25 protein isoforms during divergent vesicle fusion events of neural development. Proc Natl Acad Sci USA 92:1510–1514. doi:10.1073/pnas.92.5.1510
Baron W, Hoekstra D (2010) On the biogenesis of myelin membranes: sorting, trafficking and cell polarity. FEBS Lett 584:1760–1770. doi:10.1016/j.febslet.2009.10.085
Barr AM, Young CE, Phillips AG, Honer WG (2006) Selective effects of typical antipsychotic drugs on SNAP-25 and synaptophysin in the hippocampal trisynaptic pathway. Int J Neuropsychopharmacol 9:457–463. doi:10.1017/S1461145705006000
Bartl J, Link P, Schlosser C et al (2010) Effects of methylphenidate: the cellular point of view. Atten Deficit Hyperact Disord 2:225–232. doi:10.1007/s12402-010-0039-6
Bennett JC, McRae PA, Levy LJ, Frick KM (2006) Long-term continuous, but not daily, environmental enrichment reduces spatial memory decline in aged male mice. Neurobiol Learn Mem 85:139–152. doi:10.1016/j.nlm.2005.09.003
Bortoluzzi A, Blaya C, Rosa ED et al (2015) What can HPA axis-linked genes tell us about anxiety disorders in adolescents? Trends Psychiatry Psychother 37(4):232–237. doi:10.1590/2237-6089-2015-0035
Boschert U, O’Shaughnessy C, Dickinson R et al (1996) Developmental and plasticity-related differential expression of two SNAP-25 isoforms in the rat brain. J Comp Neurol 367:177–193. doi:10.1002/(SICI)1096-9861(19960401)367:2<177:AID-CNE2>3.0.CO;2-2
Bourassa CV, Meijer IA, Merner ND et al (2012) VAMP1 mutation causes dominant hereditary spastic ataxia in newfoundland families. Am J Hum Genet 91:548–552. doi:10.1016/j.ajhg.2012.07.018
Braida D, Guerini FR, Ponzoni L et al (2015) Association between SNAP-25 gene polymorphisms and cognition in autism: functional consequences and potential therapeutic strategies. Transl Psychiatry 5(1):e500. doi:10.1038/tp.2014.136
Brookes KJ, Knight J, Xu X, Asherson P (2005) DNA pooling analysis of ADHD and genes regulating vesicle release of neurotransmitters. Am J Med Genet B 139B:33–37. doi:10.1002/ajmg.b.30216
Brookes K, Xu X, Chen W et al (2006) The analysis of 51 genes in DSM-IV combined type attention deficit hyperactivity disorder: association signals in DRD4, DAT1 and 16 other genes. Mol Psychiatry 11:934–953. doi:10.1038/sj.mp.4001869
Brunger AT (2000) Structural insights into the molecular mechanism of Ca(2 +) dependent exocytosis. Curr Opin Neurobiol 10(3):293–302. doi:10.1016/S0959-4388(00)00098-2
Burgess RW, Deitcher DL, Schwarz TL (1997) The synaptic protein syntaxin1 is required for cellularization of Drosophila embryos. J Cell Biol 138:861–875. doi:10.1083/jcb.138.4.861
Carroll LS, Kendall K, O’Donovan MC et al (2009) Evidence that putative ADHD low risk alleles at SNAP25 may increase the risk of schizophrenia. Am J Med Genet B 150:893–899. doi:10.1002/ajmg.b.30915
Caspi A, Sugden K, Moffitt TE et al (2003) Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301(5631):386–389
Chen Q, He J, Ma C et al (2015) Syntaxin 1A modulates the sexual maturity rate and progeny egg size related to phase changes in locusts. Insect Biochem Mol Biol 56:1–8. doi:10.1016/j.ibmb.2014.11.001
Conner SD, Wessel GM (1999) Syntaxin is required for cell division. Mol Biol Cell 10:2735–2743. doi:10.1091/mbc.10.8.2735
Contini V, Victor MM, Bertuzzi GP et al (2012) No significant association between genetic variants in 7 candidate genes and response to methylphenidate treatment in adult patients with ADHD. J Clin Psychopharmacol 32:820–823. doi:10.1097/JCP.0b013e318270e727
Craig TJ, Anderson D, Evans AJ et al (2015) SUMOylation of Syntaxin1A regulates presynaptic endocytosis. Sci Rep. 4(5):17669. doi:10.1038/srep17669
Curtis L, Datta P, Liu X et al (2010) Syntaxin 3B is essential for the exocytosis of synaptic vesicles in ribbon synapses of the retina. Neuroscience 166(3):832–841. doi:10.1016/i.neuroscience.2009.12.075
Dai D, Wang Y, Yuan J et al (2014) Meta-analyses of 10 polymorphisms associated with the risk of schizophrenia. Biomedical Reports. doi:10.3892/br.2014.308
De Blas GA, Roggero CM, Tomes CN, Mayorga LS (2005) Dynamics of SNARE assembly and disassembly during sperm acrosomal exocytosis. PLoS Biol. doi:10.1371/journal.pbio.0030323
Durdiaková J, Warrier V, Banerjee-Basu S et al (2014) STX1A and Asperger syndrome: a replication study. Mol Autism 5:14. doi:10.1186/2040-2392-5-14
Duric V, Banasr M, Stockmeier CA et al (2013) Altered expression of synapse and glutamate related genes in post-mortem hippocampus of depressed subjects. Int J Neuropsychopharmacol 16:69–82
Egbujo CN, Sinclair D, Borgmann-Winter KE et al (2015) Molecular evidence for decreased synaptic efficacy in the postmortem olfactory bulb of individuals with schizophrenia. Schizophr Res. doi:10.1016/j.schres.2015.07.026
Elfving B, Bonefeld BE, Rosenberg R, Wegener G (2008) Differential expression of synaptic vesicle proteins after repeated electroconvulsive seizures in rat frontal cortex and hippocampus. Synapse 62:662–670. doi:10.1002/syn.20538
el-Husseini Ael-D, Bredt DS (2002) Protein palmitoylation: a regulator of neuronal development and function. Nat Rev Neurosci 3(10):791–780
Etain B, Dumaine A, Mathieu F et al (2010) A SNAP25 promoter variant is associated with early-onset bipolar disorder and a high expression level in brain. Mol Psychiatry 15:748–755. doi:10.1038/mp.2008.148
Falbo V, Floridia G, Gaudi S et al (2002) A new polymorphism in the flanking region of human VAMP2 and hPer1 genes. Mol Cell Probes 16:391–392. doi:10.1006/mcpr.2002.0430
Fanous AH, Zhao Z, Van Den Oord EJCG et al (2010) Association study of SNAP25 and schizophrenia in Irish family and case-control samples. Am J Med Genet B 153:663–674
Fatemi SH, Earle JA, Stary JM et al (2001) Altered levels of the synaptosomal associated protein SNAP-25 in hippocampus of subjects with mood disorders and schizophrenia. NeuroReport 12:3257–3262
Feldmann A, Winterstein C, White R et al (2009) Comprehensive analysis of expression, subcellular localization, and cognate pairing of SNARE proteins in oligodendrocytes. J Neurosci Res 87:1760–1772. doi:10.1002/jnr.22020
Fellin T (2009) Communication between neurons and astrocytes: relevance to the modulation of synaptic and network activity. J Neurochem 108:533–544. doi:10.1111/j.1471-4159.2008.05830.x
Feng Y, Crosbie J, Wigg K et al (2005) The SNAP25 gene as a susceptibility gene contributing to attention-deficit hyperactivity disorder. Mol Psychiatry 10(11):998–1005
Fernández-Chacón R, Königstorfer A, Gerber SH et al (2001) Synaptotagmin I functions as a calcium regulator of release probability. Nature 410:41–49. doi:10.1038/35065004
Forero DA, Arboleda GH, Vasquez R, Arboleda H (2009) Candidate genes involved in neural plasticity and the risk for attention-deficit hyperactivity disorder: a meta-analysis of 8 common variants. J Psychiatry Neurosci 34:361–366
Fujiwara T, Kofuji T, Akagawa K (2011) Dysfunction of the hypothalamic-pituitary-adrenal axis in STX1A knockout mice. J Neuroendocrinol 23:1222–1230. doi:10.1111/j.1365-2826.2011.02214.x
Fukuda M, Ogata Y, Saegusa C et al (2002) Alternative splicing isoforms of synaptotagmin VII in the mouse, rat and human. Biochem J 365:173–180. doi:10.1042/BJ20011877
Gabriel SM, Haroutunian V, Powchih P et al (1997) Increased concentrations of presynaptic proteins in the cingulate cortex of subjects with Schizophrenia. Arch Gen Psychiatry 54:559–566. doi:10.1001/archpsyc.1997.01830180077010
Gadella BM, Evans JP (2011) Membrane fusions during mammalian fertilization. In: Dittmar T, Zänker KS (eds) Chapter 5—Cell fusion in health and disease, Springer, Netherlands 713: 65–80. doi:10.1007/978-94-007-0763-4_5
Gálvez JM, Forero DA, Fonseca DJ et al (2014) Evidence of association between SNAP25 gene and attention deficit hyperactivity disorder in a Latin American sample. Atten Deficit Hyperact Disord 6:19–23. doi:10.1007/s12402-013-0123-9
Gao Y, Bezchlibnyk YB, Sun X et al (2006) Effects of restraint stress on the expression of proteins involved in synaptic vesicle exocytosis in the hippocampus. Neuroscience 141:1139–1148. doi:10.1016/j.neuroscience.2006.04.066
Gao Q, Liu L, Chen Y et al (2015) Synaptosome-related (SNARE) genes and their interactions contribute to the susceptibility and working memory of attention-deficit/hyperactivity disorder in males. Prog Neuro Psychopha Biol Psychiatry 57:132–139. doi:10.1016/j.pnpbp.2014.11.001
Ge JF, Qi CC, Zhou JN (2013) Imbalance of leptin pathway and hypothalamus synaptic plasticity markers are associated with stress-induced depression in rats. Behav Brain Res 249:38–43. doi:10.1016/j.bbr.2013.04.020
Geppert M, Goda Y, Hammer RE et al (1994) Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell 79:717–727. doi:10.1016/0092-8674(94)90556-8
Gil-Pisa I, Munarriz-Cuezva E, Ramos-Miguel A et al (2012) Regulation of munc18-1 and syntaxin-1A interactive partners in schizophrenia prefrontal cortex: down-regulation of munc18-1a isoform and 75 kDa SNARE complex after antipsychotic treatment. Int J Neuropsychopharmacol 15:573–588. doi:10.1017/S1461145711000861
Gizer IR, Ficks C, Waldman ID (2009) Candidate gene studies of ADHD: a meta-analytic review. Hum Genet 126:51–90. doi:10.1007/s00439-009-0694-x
Gonelle-Gispert C, Molinete M, Halban PA, Sadoul K (2000) Membrane localization and biological activity of SNAP-25 cysteine mutants in insulin-secreting cells. J Cell Sci 113:3197–3205
Gosso MF, de Geus EJC, van Belzen MJ et al (2006) The SNAP-25 gene is associated with cognitive ability: evidence from a family-based study in two independent Dutch cohorts. Mol Psychiatry 11:878–886. doi:10.1038/sj.mp.4001868
Gosso MF, De Geus EJC, Polderman TJC et al (2008) Common variants underlying cognitive ability: further evidence for association between the SNAP-25 gene and cognition using a family-based study in two independent Dutch cohorts. Genes Brain Behav 7:355–364. doi:10.1111/j.1601-183X.2007.00359.x
Graeber MB (2010) Changing face of microglia. Science 330:783–788. doi:10.1126/science.1190929
Gray LJ, Dean B, Kronsbein HC et al (2010) Region and diagnosis-specific changes in synaptic proteins in schizophrenia and bipolar I disorder. Psychiatry Res 178:374–380. doi:10.1016/j.psychres.2008.07.012
Greaves J, Chamberlain LH (2007) Palmitoylation-dependent protein sorting. J Cell Biol 176:249–254. doi:10.1083/jcb.200610151
Greif KF, Asabere N, Lutz GJ et al (2013) Synaptotagmin-1 promotes the formation of axonal filopodia and branches along the developing axons of forebrain neurons. Dev Neurobiol 73:27–44. doi:10.1002/dneu.22033
Guan L, Wang B, Chen Y et al (2009) A high-density single-nucleotide polymorphism screen of 23 candidate genes in attention deficit hyperactivity disorder: suggesting multiple susceptibility genes among Chinese Han population. Mol Psychiatry 14:546–554. doi:10.1038/sj.mp.4002139
Guerini FR, Bolognesi E, Chiappedi M et al (2011) SNAP-25 single nucleotide polymorphisms are associated with hyperactivity in autism spectrum disorders. Pharmacol Res 64:283–288. doi:10.1016/j.phrs.2011.03.015
Hanisch U-K, Kettenmann H (2007) Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10:1387–1394. doi:10.1038/nn1997
Hawi Z, Matthews N, Wagner J et al (2013) DNA variation in the SNAP25 gene confers risk to ADHD and is associated with reduced expression in prefrontal cortex. PLoS One 8:1–8. doi:10.1371/journal.pone.0060274
Hepp R, Langley K (2001) SNAREs during development. Cell Tissue Res 305:247–253. doi:10.1007/s004410100359
Hepp R, Perraut M, Chasserot-Golaz S et al (1999) Cultured glial cells express the SNAP-25 analogue SNAP-23. Glia 27:181–187. doi:10.1002/(SICI)1098-1136(199908)27:2<181:AID-GLIA8>3.0.CO;2-9
Hepp R, Dupont JL, Aunis D et al (2001) NGF enhances depolarization effects on SNAP-25 expression: induction of SNAP-25b isoform. NeuroReport 12:673–677. doi:10.1097/00001756-200103260-00011
Herken H, Erdal M, Kenar A (2014) Association of SNAP-25 gene Ddel and Mnll polymorphisms with adult attention deficit hyperactivity disorder. Psychiatry Investig 11:476–480. doi:10.4306/pi.2014.11.4.476
Hess EJ, Jinnah HA, Kozak CA, Wilson MC (1992) Spontaneous locomotor hyperactivity in a mouse mutant with a deletion including the Snap gene on chromosome 2. J Neurosci 12:2865–2874
Hess EJ, Collins KA, Wilson MC (1996) Mouse model of hyperkinesis implicates SNAP-25 in behavioral regulation. J Neurosci 16(9):3104–3111
Heyser CJ, Wilson MC, Gold LH (1995) Coloboma hyperactive mutant exhibits delayed neurobehavioral developmental milestones. Dev Brain Res 89:264–269. doi:10.1016/0165-3806(95)00130-6
Holt M, Varoqueaux F, Wiederhold K et al (2006) Identification of SNAP-47, a novel Qbc-SNARE with ubiquitous expression. J Biol Chem 281:17076–17083
Honer WG, Falkai P, Young C et al (1997) Cingulate cortex synaptic terminal proteins and neural cell adhesion molecule in schizophrenia. Neuroscience 78:99–110. doi:10.1016/S0306-4522(96)00489-7
Hou Q, Gao X, Zhang X et al (2004) SNAP-25 in hippocampal CA1 region is involved in memory consolidation. Eur J Neurosci 20:1593–1603. doi:10.1111/j.1460-9568.2004.03600.x
Hou QL, Gao X, Lu Q et al (2006) SNAP-25 in hippocampal CA3 region is required for long-term memory formation. Biochem Biophys Res Commun 347:955–962. doi:10.1016/j.bbrc.2006.06.184
Igarashi M, Terakawa S, Ide C, Iomiya Y (1996) 1224 A t-Snare is involved in axonal growth: botulinum neurotoxin C1 induces growth cone collapse. Neurosci Res 25:S134. doi:10.1016/0168-0102(96)88931-0
Jamra RA, Gobina CM, Becker T et al (2008) Association study between genetic variants at the VAMP2 and VAMP3 loci and bipolar affective disorder. Psychiat Genet 18:199–203. doi:10.1097/YPG.0b013e3283050a83
Jiang X, Wang J, Luo T et al (2009) Impaired hypothalamic-pituitary-adrenal axis and its feedback regulation in serotonin transporter knockout mice. Psychoneuroendocrino 34(3):317–331
Johansson JU, Ericsson J, Janson J et al (2008) An ancient duplication of exon 5 in the Snap25 gene is required for complex neuronal development/function. PLoS Genet 4(11):e1000278. doi:10.1371/journal.pgen.1000278
Jones MD, Williams ME, Hess EJ (2001) Abnormal presynaptic catecholamine regulation in a hyperactive SNAP-25-deficient mouse mutant. Pharmacol Biochem Behav 68:669–676. doi:10.1016/S0091-3057(01)00481-6
Kaminsky EB, Kaul V, Paschall J et al (2011) An evidence-based approach to establish the functional and clinical significance of copy number variants in intellectual and developmental disabilities. Genet Med 13:777–784. doi:10.1097/GIM.0b013e31822c79f9
Kang R, Swayze R, Lise MF et al (2004) Presynaptic trafficking of synaptotagmin I is regulated by protein palmitoylation. J Biol Chem 279:50524–50536. doi:10.1074/jbc.M404981200
Kataoka M, Yamamori S, Suzuki E et al (2011) A single amino acid mutation in SNAP-25 induces anxiety-related behavior in mouse. PLoS One. doi:10.1371/journal.pone.0025158
Kawashima K, Kishi T, Ikeda M et al (2008) No association between tagging SNPs of SNARE complex genes (STX1A, VAMP2 and SNAP25) and schizophrenia in a Japanese population. Am J Med Genet Part B Neuropsychiatr Genet 147:1327–1331. doi:10.1002/ajmg.b.30781
Kenar ANI, Ay ÖI, Herken H, Erdal ME (2014) Association of VAMP-2 and syntaxin 1A genes with adult attention deficit hyperactivity disorder. Psychiatry Investig 11:76–83. doi:10.4306/pi.2014.11.1.76
Kim JW, Biederman J, Arbeitman L et al (2007) Investigation of variation in SNAP-25 and ADHD and relationship to co-morbid major depressive disorder. Am J Med Genet Part B Neuropsychiatr Genet 144:781–790. doi:10.1002/ajmg.b.30522
Kimura K, Mizoguchi A, Ide C (2003) Regulation of growth cone extension by SNARE proteins. J Histochem Cytochem 51:429–433. doi:10.1177/002215540305100404
Kitzmiller JP, Groen DK, Phelps MA, Sadee W (2011) Pharmacogenomic testing: relevance in medical practice: why drugs in some patients but not in others. Cleve Clin J Med 78:243–257. doi:10.1016/j.biotechadv.2011.08.021.Secreted
Kofuji T, Fujiwara T, Sanada M et al (2014) HPC-1/syntaxin 1A and syntaxin 1B play distinct roles in neuronal survival. J Neurochem 130:514–525. doi:10.1111/jnc.12722
Lane SR, Liu Y (1997) Characterization of the palmitoylation domain of SNAP-25. J Neurochem 69:1864–1869. doi:10.1046/j.1471-4159.1997.69051864.x
Larra SA, Khan SAF (2014) Phylogenetic history analysis of Vamp gene family. J Public Heal Biol Sci 3(1):17–23
Lasky-Su J, Anney RJL, Neale BM et al (2008) Genome-wide association scan of the time to onset of attention deficit hyperactivity disorder. Am J Med Genet Part B Neuropsychiatr Genet 147:1355–1358. doi:10.1002/ajmg.b.30869
Lochman J, Balcar VJ, Šťastný F, Šerý O (2013) Preliminary evidence for association between schizophrenia and polymorphisms in the regulatory Regions of the ADRA2A, DRD3 and SNAP-25 Genes. Psychiatry Res 205:7–12. doi:10.1016/j.psychres.2012.08.003
Malenfant P, Liu X, Hudson ML et al (2012) Association of GTF2i in the Williams-Beuren syndrome critical region with autism spectrum disorders. J Autism Dev Disord 42:1459–1469. doi:10.1007/s10803-011-1389-4
Malki K, Keers R, Tosto MG et al (2014) The endogenous and reactive depression subtypes revisited: integrative animal and human studies implicate multiple distinct molecular mechanisms underlying major depressive disorder. BMC Med 12:73. doi:10.1186/1741-7015-12-73
McGough J, McCracken J, Swanson J et al (2006) Pharmacogenetics of methylphenidate response in preschoolers with ADHD. J Am Acad Child Adolesc Psychiatry 45:1314–1322. doi:10.1097/01.chi.0000235083.40285.08
McGough J, McCracken JT, Loo SK et al (2009) An evidence-based approach to establish the functional and clinical significance of copy number variants in intellectual and developmental disabilities. J Am Acad Child Adolesc Psychiatry 48:1155–1164. doi:10.1097/CHI.0b013e3181bc72e3.A
McNew JA, Parlati F, Fukuda R et al (2000) Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature 407:153–159. doi:10.1038/35025000
McRory JE, Rehak R, Simms B et al (2008) Syntaxin 1A is required for normal in utero development. Biochem Biophys Res Commun 375:372–377. doi:10.1016/j.bbrc.2008.08.031
Mill J, Curran S, Kent L et al (2002) Association study of a SNAP-25 microsatellite and attention deficit hyperactivity disorder. Am J Med Genet Part B Neuropsychiatr Genet 114(3):269–271. doi:10.1002/ajmg.10253
Mill J, Richards S, Knight J et al (2004) Haplotype analysis of SNAP-25 suggests a role in the aetiology of ADHD. Mol Psychiatry 9:801–810. doi:10.1038/sj.mp.4001482
Mill J, Xu X, Ronald A et al (2005) Quantitative trait locus analysis of candidate gene alleles associated with attention deficit hyperactivity disorder (ADHD) in five genes: DRD4, DAT1, DRD5, SNAP-25, and 5HT1B. Am J Med Genet Part B Neuropsychiatr Genet 133(1):68–73. doi:10.1002/ajmg.b.30107
Mothet J-P, Pollegioni L, Ouanounou G et al (2005) Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter d-serine. P Natl Acad Sci USA 102:5606–5611. doi:10.1073/pnas.0408483102
Mukaetova-Ladinska EB, Hurt J, Honer WG et al (2002) Loss of synaptic but not cytoskeletal proteins in the cerebellum of chronic schizophrenics. Neurosci Lett 317:161–165. doi:10.1016/S0304-3940(01)02458-2
Mulle JG, Pulver AE, McGrath JA et al (2014) Reciprocal duplication of the williams-beuren syndrome deletion on chromosome 7q11.23 is associated with schizophrenia. Biol Psychiatry 75:371–377. doi:10.1016/j.biopsych.2013.05.040
Müller DJ, Klempan TA, De Luca V et al (2005) The SNAP-25 gene may be associated with clinical response and weight gain in antipsychotic treatment of schizophrenia. Neurosci Lett 379:81–89. doi:10.1016/j.neulet.2004.12.037
Müller HK, Wegener G, Popoli M, Elfving B (2011) Differential expression of synaptic proteins after chronic restraint stress in rat prefrontal cortex and hippocampus. Brain Res 1385:26–37. doi:10.1016/j.brainres.2011.02.048
Nagy G, Matti U, Nehring RB et al (2002) Protein kinase C-dependent phosphorylation of synaptosome-associated protein of 25 kDa at Ser187 potentiates vesicle recruitment. J Neurosci 22:9278–9286
Nagy G, Milosevic I, Fasshauer D et al (2005) Alternative splicing of SNAP-25 regulates secretion through nonconservative substitutions in the SNARE domain. Mol Biol Cell 16:5675–5685. doi:10.1091/mbc.E05-07-0595
Nagy G, Kim JH, Pang ZP et al (2006) Different effects on fast exocytosis induced by synaptotagmin 1 and 2 isoforms and abundance but not by phosphorylation. J Neurosci 26:632–643. doi:10.1523/JNEUROSCI.2589-05.2006
Nakamura K, Anitha A, Yamada K et al (2008) Genetic and expression analyses reveal elevated expression of syntaxin 1A (STX1A) in high functioning autism. Int J Neuropsychopharmacol 11:1073–1084. doi:10.1017/S1461145708009036
Nakamura K, Iwata Y, Anitha A et al (2011) Replication study of Japanese cohorts supports the role of STX1A in autism susceptibility. Prog Neuro-Psychopharmacology Biol Psychiatry 35:454–458. doi:10.1016/j.pnpbp.2010.11.033
Németh N, Kovács-Nagy R, Székely A et al (2013) Association of impulsivity and polymorphic MicroRNA-641 target sites in the SNAP-25 gene. PLoS One 8:8–13. doi:10.1371/journal.pone.0084207
Olgiati P, Mandelli L, Alberti S et al (2014) Role of synaptosome-related (SNARE) genes in adults with attention deficit hyperactivity disorder. Psychiatry Res 215:799–800. doi:10.1016/j.psychres.2013.06.025
Osen-Sand A, Catsicas M, Staple JK et al (1993) Inhibition of axonal growth by SNAP-25 antisense oligonucleotides in vitro and in vivo. Lett Nat 364:445–448. doi:10.1038/364445a0
Oyler GA, Polli JW, Wilson MC, Billingsley ML (1991) Developmental expression of the 25-kDa synaptosomal-associated protein (SNAP-25) in rat brain. Proc Natl Acad Sci USA 88:5247–5251. doi:10.1073/pnas.88.12.5247
Paolicelli RC, Bolasco G, Pagani F et al (2011) Synaptic pruning by microglia is necessary for normal brain development. Science 333:1456–1458. doi:10.1126/science.1202529
Parpura V, Zorec R (2010) Gliotransmission: exocytotic release from astrocytes. Brain Res Rev 63:83–92. doi:10.1016/j.brainresrev.2009.11.008
Parpura V, Fang Y, Basarsky T et al (1995) Expression of synaptobrevin II, cellubrevin and syntaxin but not SNAP-25 in cultured astrocytes. FEBS Lett 377:489–492. doi:10.1016/0014-5793(95)01401-2
Parpura V, Baker BJ, Jeras M, Zorec R (2010) Regulated exocytosis in astrocytic signal integration. Neurochem Int 57:451–459. doi:10.1016/j.neuint.2010.02.007
Peng L, Liu H, Ruan H et al (2013) Cytotoxicity of botulinum neurotoxins reveals a direct role of syntaxin 1 and SNAP-25 in neuron survival. Nat Commun 4:1472. doi:10.1038/ncomms2462
Pozzi D, Condliffe S, Bozzi Y et al (2008) Activity-dependent phosphorylation of Ser187 is required for SNAP-25-negative modulation of neuronal voltage-gated calcium channels. Proc Natl Acad Sci USA 105:323–328. doi:10.1073/pnas.0706211105
Prescott GR, Chamberlain LH (2011) Regional and developmental brain expression patterns of SNAP25 splice variants. BMC Neurosci 12:35. doi:10.1186/1471-2202-12-35
Prescott GR, Gorleku OA, Greaves J, Chamberlain LH (2009) Palmitoylation of the synaptic vesicle fusion machinery. J Neurochem 110:1135–1149. doi:10.1111/j.1471-4159.2009.06205.x
Ramos-Miguel A, Beasley CL, Dwork AJ et al (2014) Increased SNARE protein–protein interactions in orbitofrontal and anterior cingulate cortices in schizophrenia. Biol Psychiatry. doi:10.1016/j.biopsych.2014.12.012
Raptis A, Torrejón-Escribano B, Gómez De Aranda I, Blasi J (2005) Distribution of synaptobrevin/VAMP 1 and 2 in rat brain. J Chem Neuroanat 30:201–211. doi:10.1016/j.jchemneu.2005.08.002
Ravichandran V, Chawla A, Roche PA (1996) Identification of a novel syntaxin- and synaptobrevin/VAMP-binding protein, SNAP-23, expressed in non-neuronal tissues. J Biol Chem 271:13300–13303
Renner TJ, Walitza S, Dempfle A et al (2008) Allelic variants of SNAP25 in a family-based sample of ADHD. J Neural Transm 115:317–321. doi:10.1007/s00702-007-0840-3
Risinger C, Bennett MK (1999) Differential phosphorylation of syntaxin and synaptosome-associated protein of 25 kDa (SNAP-25) isoforms. J Neurochem 72:614–624. doi:10.1046/j.1471-4159.1999.0720614.x
Roberts JL, Hovanes K, Dasouki M et al (2014) Chromosomal microarray analysis of consecutive individuals with autism spectrum disorders or learning disability presenting for genetic services. Gene 535:70–78. doi:10.1016/j.gene.2013.10.020
Roberts S, Keers R, Lester KJ et al (2015) HPA axis related genes and response to psychological therapies: genetics and epigenetics. Depress Anxiety 32(12):861–870
Rossi D, Volterra A (2009) Astrocytic dysfunction: insights on the role in neurodegeneration. Brain Res Bull 80:224–232. doi:10.1016/j.brainresbull.2009.07.012
Saito S, Takahashi N, Ishihara R et al (2007) Association study between vesicle-associated membrane protein 2 gene polymorphisms and fluvoxamine response in Japanese major depressive patients. Neuropsychobiology 54:226–230. doi:10.1159/000100777
Sánchez-Mora C, Cormand B, Ramos-Quiroga JA et al (2013) Evaluation of common variants in 16 genes involved in the regulation of neurotransmitter release in ADHD. Eur Neuropsychopharmacol 23:426–435. doi:10.1016/j.euroneuro.2012.07.014
Santana MM, Rosmaninho-Salgado J, Cortez V et al (2015) Impaired adrenal medullary function in a mouse model of depression induced by unpredictable chronic stress. Eur Neuropsychopharm 25(10):1753–1766. doi:10.1016/j.euroneuro.2015.06.013
Sarkar K, Bhaduri N, Ghosh P et al (2012) Role of SNAP25 explored in eastern indian attention deficit hyperactivity disorder probands. Neurochem Res 37:349–357. doi:10.1007/s11064-011-0618-8
Scarr E, Gray L, Keriakous D et al (2006) Increased levels of SNAP-25 and synaptophysin in the dorsolateral prefrontal cortex in bipolar I disorder. Bipolar Disord 8:133–143. doi:10.1111/j.1399-5618.2006.00300.x
Schoch S, Deák F, Königstorfer A et al (2001) SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science 294:1117–1122. doi:10.1126/science.1064335
Sherman DL, Brophy PJ (2005) Mechanisms of axon ensheathment and myelin growth. Nat Rev Neurosci 6:683–690. doi:10.1038/nrn1743
Shimohama S, Fujimoto S, Sumida Y et al (1998) Differential expression of rat brain synaptic proteins in development and aging. Biochem Biophys Res Commun 251:394–398. doi:10.1006/bbrc.1998.9480
Shimojo M, Courchet J, Pieraut S et al (2015) SNAREs controlling vesicular release of BDNF and development of callosal axons. Cell Rep 11:1054–1066. doi:10.1016/j.celrep.2015.04.032
Shirasu M, Kimura K, Kataoka M et al (2000) VAMP-2 promotes neurite elongation and SNAP-25A increases neurite sprouting in PC12 cells. Neurosci Res 37:265–275. doi:10.1016/S0168-0102(00)00125-5
Smith TD, Adams MM, Gallagher M et al (2000) Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J Neurosci 20:6587–6593
Söderqvist S, McNab F, Peyrard-Janvid M et al (2010) The SNAP25 gene is linked to working memory capacity and maturation of the posterior cingulate cortex during childhood. Biol Psychiatry 68:1120–1125. doi:10.1016/j.biopsych.2010.07.036
Sokolov BP, Tcherepanov AA, Haroutunian V, Davis KL (2000) Levels of mRNAs encoding synaptic veiscle and synaptic plasma membrane proteins in the temporal cortex of elderly schizophrenia patients. Biol Psychiatry 48:184–196
Sommer JU, Schmitt A, Heck M et al (2010) Differential expression of presynaptic genes in a rat model of postnatal hypoxia: relevance to schizophrenia. Eur Arch Psychiatry Clin Neurosci 260:S81–S89. doi:10.1007/s00406-010-0159-1
Song J, Kim SW, Hong HJ et al (2014) Association of SNAP-25, SLC6A2, and LPHN3 with OROS methylphenidate treatment response in attention-deficit/hyperactivity disorder. Clin Neuropharmacol 37:136–141. doi:10.1097/WNF.0000000000000045
Sørensen JB, Nagy G, Varoqueaux F et al (2003) Differential control of the releasable vesicle pools by SNAP-25 splice variants and SNAP-23. Cell 114:75–86. doi:10.1016/S0092-8674(03)00477-X
Spellman I, Müller N, Musil R et al (2008) Associations of SNAP-25 polymorphisms with cognitive dysfunctions in Caucasian patients with schizophrenia during a brief trail of treatment with atypical antipsychotics. Eur Arch Psy Clin N 258(6):335–344. doi:10.1007/s00406-007-0800-9
Steegmaier M, Yang B, Yoo JS et al (1998) Three novel proteins of the syntaxin/SNAP-25 Family. J Biol Chem 273:34171–34179
Südhof TC (2002) Synaptotagmins: why so many? J Biol Chem 277:7629–7632. doi:10.1074/jbc.R100052200
Südhof TC (2013) Neurotransmitter release: the last millisecond in the life of a synaptic vesicle. Neuron 80:675–690. doi:10.1016/j.neuron.2013.10.022
Sun J, Pang ZP, Qin D et al (2007) A two CA2+ sensor modelo for neurotransmitter release in a central synapse. Nature 450(7120):676–682
Sutton RB, Fasshauer D, Jahn R, Brunger AT (1998) Crystal structure of a SNARE complex involved in synaptic ˚ resolution exocytosis at 2.4A. Nature 395:347–353. doi:10.1038/26412
Szatmari P, Paterson AD, Zwaigenbaum L et al (2007) Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet 39:319–328. doi:10.1038/ng1985
Tachikawa H, Harada S, Kawanishi Y et al (2001) Polymorphism of the 5-upstream region of the human SNAP-25 gene: an association analysis with schizophrenia. Neuropsychobiology 43(3):131–133
Teng FY, Wang Y, Tang BL (2001) The syntaxins. Genome Biol. doi:10.1186/gb-2001-2-11-reviews3012
Theiler K, Varnum DS, Stevens LC (1978) Development of Dickie’s small eye, a mutantion in the house mouse. Anat Embryol 155(1):81
Thompson PM, Sower AC, Perrone-Bizzozero NI (1998) Altered levels of the synaptosomal associated protein SNAP-25 in schizophrenia. Biol Psychiatry 43:239–243. doi:10.1016/S0006-3223(97)00204-7
Tordjman S, Anderson GM, Cohen D et al (2013) Presence of autism, hyperserotonemia, and severe expressive language impairment in Williams-Beuren syndrome. Molecular Autism. doi:10.1186/2040-2392-4-29
Touihri S, Knöll C, Stierhof YD et al (2011) Functional anatomy of the Arabidopsis cytokinesis-specific syntaxin KNOLLE. Plant J 68:755–764. doi:10.1111/j.1365-313X.2011.04736.x
Trimble WS (1993) Analysis of the structure and expression of the VAMP family of synaptic vesicle proteins. J Physiol Paris 87:107–115. doi:10.1016/0928-4257(93)90004-D
Ullrich B, Li C, Zhang JZ et al (1994) Functional properties of multiple synaptotagmins in brain. Neuron 13:1281–1291. doi:10.1016/0896-6273(94)90415-4
Ullrich A, Böhme MA, Schöneberg J et al (2015) Dynamical organization of syntaxin-1A at the presynaptic active zone. PLoS Comput Biol 11(9):e1004407. doi:10.1371/journal.pcbi.1004407
Vanguilder HD, Yan H, Farley JA et al (2010) Aging alters the expression of neurotransmission-regulating proteins in the hippocampal synaptoproteome. J Neurochem 113:1577–1588. doi:10.1111/j.1471-4159.2010.06719.x
Veit M, Söllner TH, Rothman JE (1996) Multiple palmitoylation of synaptotagmin and the t-SNARE SNAP-25. FEBS Lett 385:119–123. doi:10.1016/0014-5793(96)00362-6
Veit M, Becher A, Ahnert-Hilger G (2000) Synaptobrevin 2 is palmitoylated in synaptic vesicles prepared from adult, but not from embryonic brain. Mol Cell Neurosci 15:408–416. doi:10.1006/mcne.1999.0830
Vogel K, Roche PA (1999) SNAP-23 and SNAP-25 are palmitoylated in vivo. Biochem Biophys Res Commun 258:407–410. doi:10.1006/bbrc.1999.0652
Von Känel T, Stanke F, Weber M et al (2013) Clinical and molecular characterization of the CF disease modifier syntaxin 1A. Eur J Hum Genet 21:1462–1466. doi:10.1016/S1569-1993(10)60004-5
Wang Q, Wang Y, Ji W et al (2015) SNAP25 is associated with schizophrenia and major depressive disorder in the han chinese population. J Clin Psychiatry. doi:10.4088/JCP.13m08962
Wong AHC, Trakalo J, Likhodi O et al (2004) Association between schizophrenia and the syntaxin 1A gene. Biol Psychiatry 56:24–29. doi:10.1016/j.biopsych.2004.03.008
Wu YJ, Tejero R, Arancillo M et al (2015) Syntaxin 1B is important for mouse postnatal survival and proper synaptic function at the mouse neuromuscular junctions. J Neurophysiol 114(4):2404–2417. doi:10.1152/jn.00577.2015
Xiao L, Han Y, Runne H et al (2010) Developmental expression of synaptotagmin isoforms in single calyx of Held-generating neurons. Mol Cell Neurosci 44:374–385. doi:10.1016/j.mcn.2010.05.002
Xu J, Mashimo T, Südhof TC (2007) Synaptotagmin-1, -2 and -9: Ca2+ sensors for fast release that specify distinct presynaptic properties in subsets of neurons. Neuron 54:567–581. doi:10.1016/j.neuron.2007.05.004
Yamada M, Takahashi K, Tsunoda M et al (2002) Differential expression of VAMP2/synaptobrevin-2 after antidepressant and electroconvulsive treatment in rat frontal cortex. Pharmacogenomics J 2:377–382. doi:10.1038/sj.tpj.6500135
Yamaguchi K, Nakayama T, Fujiwara T, Akagawa K (1996) Enhancement of neurite-sprouting by suppression of HPC-1/syntaxin 1A activity in cultured vertebrate nerve cells. Brain Res 740:185–192. doi:10.1016/S0006-8993(96)00861-X
Yamamori S, Itakura M, Sugaya D et al (2011) Differential expression of SNAP-25 family proteins in the mouse brain. J Comp Neurol 519:916–932
Yamamori S, Sugaya D, Iida Y et al (2014) Stress-induced phosphorylation of SNAP-25. Neurosci Lett 561:182–187. doi:10.1016/j.neulet.2013.12.044
Young FB, Butland SL, Sanders SS et al (2012) Putting proteins in their place: palmitoylation in Huntington disease and other neuropsychiatric diseases. Prog Neurobiol 97:220–238. doi:10.1016/j.pneurobio.2011.11.002
Zhang Q, Fukuda M, Van Bockstaele E et al (2004a) Synaptotagmin IV regulates glial glutamate release. Proc Natl Acad Sci USA 101:9441–9446. doi:10.1073/pnas.0401960101
Zhang Q, Pangršič T, Kreft M et al (2004b) Fusion-related release of glutamate from astrocytes. J Biol Chem 279:12724–12733. doi:10.1074/jbc.M312845200
Zhang H, Zhu S, Zhu Y et al (2011) An association study between SNAP-25 gene and attention-deficit hyperactivity disorder. Eur J Paediatr Neurol 15:48–52. doi:10.1016/j.ejpn.2010.06.001
Zylbersztejn K, Galli T (2011) Vesicular traffic in cell navigation. FEBS J 278:4497–4505. doi:10.1111/j.1742-4658.2011.08168
Acknowledgments
This work was financially supported by the Brazilian governmental funding agencies CAPES and CNPq.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cupertino, R.B., Kappel, D.B., Bandeira, C.E. et al. SNARE complex in developmental psychiatry: neurotransmitter exocytosis and beyond. J Neural Transm 123, 867–883 (2016). https://doi.org/10.1007/s00702-016-1514-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-016-1514-9