Abstract
The psychostimulant methylphenidate (MPH) is the first choice of treatment in attention-deficit hyperactivity disorder and is based mainly on inhibition of dopamine transporter (DAT). Nonetheless, the complete cellular effects of MPH are still unknown. We attempted to determine whether MPH influences neurotransmitter levels, synaptic gene expression, and cell proliferation in a dose-dependent manner in rat pheochromocytoma cells (PC12) lacking DAT. PC12 were treated in a dose-dependent manner with MPH. Gene expression level of synaptotagmin (Syt) 1 and 4, syntaxin 1a (Stx1a), and synaptic vesicle glycoprotein 2C (SV2C) was measured using quantitative real-time RT-PCR. Different Neurotransmitter release was measured using high-performance liquid chromatography (HPLC). Differences in cell proliferation were evaluated via BrdU incorporation. Treatment with low-dose MPH (1–100 nM) altered intra-/extracellular neurotransmitter levels, down-regulated all investigated genes as well as enhanced cell proliferation significantly. These data point to diverse effects of MPH on cell metabolism independent of inhibiting DAT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is the most frequent psychiatric disorder in children and adolescents, with up to 6–12% affected worldwide (Biederman and Faraone 2005). ADHD is characterized by the constitutive development of inappropriate levels of inattention, hyperactivity, and/or impulsiveness. The cause and pathophysiology of ADHD are still unknown, but there is immense evidence that ADHD has multiple aetiologies (e.g. genetic, environmental, neurobiological, and neurochemical) in which catecholamine dysfunction, including afferent dopaminergic and noradrenergic neurotransmission, has an important underlying feature (Wilens 2008). The noradrenergic system modulates higher cortical functions, such as attention, alertness, vigilance, and executive functions, which are impaired in a variety of psychiatric disorders such as ADHD (Hahn, Steele et al. 2009). The norepinephrine transporter (NET), an important component of the noradrenergic system, involves in the reuptake of norepinephrine (NE) into the presynaptic terminals. A recent report implicates the involvement of NET in ADHD (Kim, Hahn et al. 2006), in which they identified a functional polymorphism in the human NET gene that decreases promoter function and was significantly associated with ADHD. Considering the neurobiology of ADHD, it is reasonable to hypothesize that medications that increase dopamine (DA) or NE availability and/or decrease their breakdown should improve ADHD symptoms.
Stimulants are part of the standard treatment for ADHD. Methylphenidate (MPH), a central nervous system stimulant with an approximately 70-year history of use, is the most frequently prescribed drug for the symptomatic treatment of ADHD and has consistently shown efficacy. However, the mechanism by which low doses of MPH reduce the activity and increase the attention is still unknown. Functionally, MPH is a high-affinity inhibitor of dopamine transporter (DAT) and NET, blocking the inward transport of DA and NE (Gatley, Pan et al. 1996; Han and Gu 2006). A recent study showed the effect of MPH on vesicular monoamine transporter 2 (VMAT-2) in rats after in vivo administration. The results of the study indicated that a single dose of MPH redistributes VMAT-2 and associated vesicles within nerve terminals from the synaptosomal membranes to the cytoplasm. In addition, MPH administration leads to increased DA in the vesicle fraction (Volz, Farnsworth et al. 2008). Considering these findings and the knowledge that MPH function is not solely the inhibition of DAT, we decided to investigate the effects of in vitro MPH treatment in neuronal cell line devoid of DAT expression, on neurotransmitter levels, gene expression profile of synaptic proteins, and cell proliferation, in order to elucidate the additional molecular mechanism of MPH. The cell culture model chosen for this investigation was the rat PC12 cell line, derived from rat pheochromocytoma, a tumor arising from the chromaffin cells of the adrenal medulla. PC12 cells are a common model for studying neurobiochemical and neurobiological events (Jaeger 1985). These cells have been widely used as an in vitro experimental model to study the effects of various neuroactive compounds and contain many membrane-bound and cytosolic neuron-associated macromolecules (Shafer and Atchison 1991). Previously it was reported that proliferating PC12 cells highly express NET and in very low levels express DAT, while in differentiated PC12 cells DAT expression was detected (Kadota, Yamaai et al. 1996). We used proliferating PC12 cells (originated form Israel, old generation ATCC) in which we could detect high expression of NET but no DAT expression at all whatsoever (see Suppl. Figure S1 and S2), which makes this model an ideal model to study MPH effects not associated with DAT inhibition.
Materials and methods
Cell culture
PC12 cells used in this study were generous gift from the laboratory of Dr. Silvia Mandel, Technion Faculty of Medicine, Haifa, Israel (old generation originating from ATCC) and grown in buffered Dulbecco`s modified Eagle medium (DMEM) (Pan Biotech GmbH, Aidenbach, Germany) supplemented with 10% fetal bovine serum, 5% horse serum, and 0.3% gentamycin (50 mg/ml) (Invitrogen, Karlsruhe, Germany) in a humidified incubator (5% CO2) at 37°C.
MPH treatment
For high-performance liquid chromatography (HPLC), quantitative real-time polymerase chain reaction (QRT-PCR), and BrdU incorporation experiments, PC12 cells were treated with varying concentrations of MPH (Sigma–Aldrich, Schnellendorf, Germany) (1, 10, 100 nM, 1, 10, 100 μM), or purified water as a control, by adding it to the culture medium.
For BrdU incorporation analysis, the MPH incubation was stopped after 24 h. For QRT-PCR and HPLC, the reaction was stopped after 48 h. MPH was protected from light for all treatments, and the dilutions were always freshly prepared. The cells were incubated with MPH under dark conditions in order to reduce MPH degradation. Handling MPH in the dark delays the drug’s degradation reaction (Suppl. Figure S3).
Catecholamine determination via HPLC
PC12 cells were plated at a density of 4 × 105 cells/mL into a 24-well plate (Sarstedt, Nuembrecht, germany) and treated with MPH as described above. Reverse-phase HPLC was used to measure the intracellular and extracellular concentrations of DA, its metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA); NE, its metabolite 3-methoxy-4-hydroxy-phenylglycol (MHPG); and serotonin (5-HT) and its metabolite 5-hydroxyindole acetic acid (5-HIAA). Cells were sonicated in ice-cold 90% (v/v) 0.1 mM Na2PO4 (pH 4.0) and 10% (v/v) methanol containing 0.1 mM EDTA (Sigma, Munich, Germany), 0.65 mM octanesulfonic acid, and 0.5 mM triethylamine. Homogenates were centrifuged at 19,000g for 20 min at 4°C and the supernatants were used for the measurements. For the analysis of neurotransmitters and metabolites, the supernatants from cell homogenates or medium from the cell culture suspensions were measured using HPLC as described by Riederer and Reynolds (1981). The concentrations of DA, DOPAC, HVA, NE, MHPG, 5-HT, and 5-HIAA in the cell culture medium were measured before the experiment and subtracted from the concentration measured at the end of the experiment to obtain the amount of neurotransmitter released. Probes were quantified using an external standard that contained all detected neurotransmitters and metabolites at different concentrations (80 ng/mL, 40 ng/mL, and 20 ng/mL).
Immunocytochemistry
PC12 cells were plated at a density of 1.5 × 105 cells/mL in poly-d-lysine/laminin-coated culture slides (BD Biosciences, Heidelberg, Germany) and differentiated with 50 ng/mL human NGF-β (Sigma, Munich, Germany) for 7 days (this procedure was conducted only for the immunocytochemical analysis). After incubation, the cells were washed in phosphate-buffered saline (PBS) and fixed with ice-cold acetone/methanol (1:1) for 10 min. After three PBS washes, the cells were incubated with a blocking solution containing 5% goat serum and 0.3% Triton X-100 in PBS for 2 h at room temperature to block non-specific binding. The cells were then incubated overnight in antiserum diluted in blocking solution 1:50 for synaptotagmin 1 (syt1) (Abcam, Cambridge, MA, USA), synaptotagmin 4 (syt4) (Santa Cruz Biotechnology, Heidelberg, Germany), and syntaxin 1A (stx1a) (Lifespan Biosciences, Seattle, WA) and 1:100 for synaptophysin (syp) (Sigma). Cells were subsequently incubated with cyanine 2- and 3 (Cy3 and Cy2)-labeled secondary antibodies diluted (1:200) in blocking solution for 2 h in the dark at room temperature to visualize the bound primary antibody. After washing, cells were mounted with Vectashield (Vector Laboratories, Burlingame, CA, USA). Immunofluorescence was observed using a Leica TCS SP2 confocal microscope.
RNA extraction and QRT-PCR
PC12 cells were plated at a density of 4 × 105 cells/mL into a 24-well plate (Sarstedt, Nuembrecht, germany) and treated with MPH as described above. Total RNA was extracted from PC12 cells using the RNeasy Plus MiniKit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Total RNA (500 ng) was reverse transcribed using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Munich, Germany). QRT-PCR was performed using an iCycler iQTM Real Time PCR Detection System (Bio-Rad) and the SYBR-Green detection method. The QRT-PCR reaction was optimized according to the manufacturer’s instructions. QuantiTech Primer assays for syt1 (catalog no. QT01573019), syt4 (QT00195664), stx1a (QT01083229), NET (QT00184604), β-actin (Actb; QT00193473), aminolevulinic acid synthase 1 (Alas1; QT00436310), and ribosomal protein L13A (Rpl13a; QT00425873) were purchased from Qiagen. Actb, Alas1, and Rpl13a were used for normalization according to GeNorm (Vandesompele et al. 2002).
The amplified transcripts were quantified using the comparative threshold cycle (Ct) analyzed using the BioRad iCycler iQ program.
BrdU incorporation
PC12 cells were plated at a density of 1 × 105 cells/mL in poly-d-lysine/laminin-coated culture slides (BD Biosciences) and incubated at 37°C overnight in a humidified atmosphere of 5% CO2. The next day, settled cells were treated with different doses of MPH and incubated for 24 h under the same conditions.
To identify the proliferating cells and cells in S-phase of the cell cycle under proliferating conditions, cells were exposed to 10 μM BrdU (Roche) for 4 h at 37°C for incorporation into newly synthesized DNA. Cells were then fixed with 4% paraformaldehyde for 20 min. Three PBS washes were performed prior to DNA denaturation with 2 M HCl for 45 min at room temperature. Neutralization was carried out by incubating cells in 0.15 M sodium borate buffer for 10 min at room temperature. The cells were then washed three times with PBS. The cells were incubated with blocking solution for 2.5 h at room temperature to block non-specific binding. The cells were then incubated with primary mouse anti-BrdU (1:100) overnight at 4°C. Antibody detection was performed as described in the “Immunocytochemistry” part. Sixteen independent experiments were conducted, and BrdU incorporation was done in duplicate with four evaluated pictures for each dose (n = 16). The BrdU-positive cells were calculated as percentage to all stained cells.
Statistical analysis
Statistical analyses of the HPLC, QRT-PCR, and proliferation data were performed using StatView for Windows (SAS Institute Inc., version 5). Statistical tests included analysis of variance (ANOVA) with post hoc Scheffe Test. Significance was set as P < 0.05. For a better comparison of the data, we normalized the results and converted the data into percentages. The control group was set as 100%.
Results
Using Western blot and real-time PCR for the detection of DAT and NET in PC12, we could show that the PC12 obtained from Israel presented no DAT expression but a high NET expression (Suppl. Figure S1 and S2).
Neurotransmitter alteration in MPH-treated PC12 cells
Alteration of NE and MHPG levels
The intracellular NE concentration was 3–19 ng/mL. Higher NE concentrations were measured in the cell medium (extracellular) (50–120 ng/mL). The extracellular MHPG concentration was below the level of detection (<2.2 ng/mL).
The intracellular NE concentration was significantly reduced by MPH treatment compared to control at doses of 10 nM–100 μM (−30% for 10 nM; −80% for 100 nM, 1, and 10 μM; and −88% for 100 μM; P < 0.001; Fig. 1a). On the other hand, intracellular MHPG levels increased at low doses of MPH (1–100 nM; Fig. 1a). The turnover rate of NE seems to increase as a consequence of MPH treatment (Suppl. Table S1). The extracellular concentration of NE significantly increased at MPH doses of 1–100 nM (+70–80% compared to control, P < 0.01). Since the extracellular MHPG levels were below the limits of HPLC sensitivity, no alterations could be observed in the extracellular samples.
Dose-dependent alteration of neurotransmitter levels in MPH-treated PC12 cells represented as a percent of the control (MPH = 0 nM). a Norepinephrine (NE) and its metabolite 3-methoxy-4-hydroxyphenylglycol (MHPG), b dopamine (DA) and its metabolites dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA), c serotonin (5-HT) and its metabolite 5-hydroxyindole acetic acid (5-HIAA) were measured in pellets (P = intracellular) and the medium (M = extracellular). Independent experiments were conducted eight times, and HPLC measurements were done in duplicate (n = 8). **P < 0.0001 vs. MPH = 0 nM; *P < 0.05 vs. MPH = 0 nM using one-way ANOVA with post hoc Scheffe test. Error bars represent standard error in percent
Alterations of DA, DOPAC, and HVA levels
The intracellular DA concentration was 30–120 ng/mL, whereas the extracellular concentration was 9–160 ng/mL. Intracellular DOPAC concentrations were 3–5 ng/mL, and the extracellular concentration was 26–120 ng/mL. The intracellular HVA concentration was below the level of detection, whereas the extracellular concentration was 400–1,300 ng/mL.
The intracellular DA concentration increased significantly with MPH doses of 1–100 nM (1 nM +35%, 10 nM +37%, 100 nM +58% compared to control; P < 0.02; Fig. 1b) and decreased after treatment with 10 μM or 100 μM MPH, but the decrease was only significant for 100 μM (−52% compared to control, P < 0.0001; Fig. 1B). No significant effect on intracellular DOPAC and HVA levels was observed after MPH treatment (Fig. 1b). Similarly, the turnover of DA into DOPAC was not significantly altered as a consequence of MPH treatment (Suppl. Table S1).
The extracellular concentration of DA was significantly decreased after treatment with 1–100 nM MPH (−90 to −95% compared to control, P < 0.0001), whereas no alteration in the extracellular DA concentration was observed at high doses (Fig. 1b). A significant decrease in the extracellular DOPAC concentration (−70 to −75% compared to control, P < 0.0005) was observed only at high MPH doses (1–100 μM). After treatment with low doses of MPH, the extracellular HVA concentration significantly increased (1 nM +180%, P < 0.0001; 10 nM +187%, P < 0.0001; 100 nM +220%, P < 0.0001), whereas treatment with high doses of MPH led to no relevant effect on the extracellular HVA concentration (Fig. 1b). An increased turnover of DA to HVA was observed at the lower doses of MPH (1–100 nM), whereas decreased turnover to DOPAC was observed at higher doses (Suppl. Table S1).
Alteration of 5-HT and 5-HIAA levels
The intracellular 5-HT concentration was 3–5 ng/mL, but intracellular 5-HIAA was below the level of detection. The intracellular concentration of 5-HT and 5-HIAA was not significantly altered as a consequence of treatment with MPH at any dose (Fig. 1c).
The extracellular 5-HT concentration was 40–115 ng/mL, and the extracellular 5-HIAA concentration was 14–107 ng/mL. The 5-HT concentration was significantly decreased after treatment with 1–100 μM MPH (1 μM, −65%; 10 μM, −60%; 100 μM, −40% compared to control, P < 0.009), and the 5-HIAA concentration was significantly increased after treatment with lower doses of MPH (1 nM +430%; 10 nM +440%; 100 nM +380% compared to control, P < 0.0001). Extracellular 5-HT turnover to 5-HIAA was increased, especially at lower MPH doses (1–100 nM; Suppl. Table S1).
Localization of syt1, syt4, and stx1a in PC12 cells
The synaptic proteins syt1, syt4, and stx1a were detected in untreated PC12 cells via immunocytochemistry. Figure 2 shows the distribution and localization of the proteins with the neuronal vesicle protein syp (Fig. 2a, d, g) within the cells. Syt1 staining was located mainly in the soma (Fig. 2b). Similarly, syt4 expression was found in the cell body and punctuated in neurite-like processes (Fig. 2e). A high density of accumulated protein was perinuclear, possibly in the Golgi apparatus (Fig. 2f, arrow). The distribution pattern of syp and syt4 mostly correlated with one another. Stx1a was spread in most areas of the PC12 cells (Fig. 2h). Stx1a showed a similar distribution pattern as syp with a high level of coincidence (Fig. 2i). The cell’s nucleus was unstained in all cases.
Colocalization of synaptic proteins with synaptophysin (syp) on poly-d-lysine/laminin in PC12 cells. The immunolocalization of syp (a, d, g) revealed by a Cy2-coupled secondary antibody (green) was compared with syt1 (b), syt4 (e), and stx1a (h) immunostaining revealed by a Cy3-coupled secondary antibody (red) and superimposed (yellow; c, f, i). The arrow indicates strong perinuclear syt4 staining. Scale bar 100 μm
Gene expression profiles for synaptic proteins in MPH-treated PC12 cells
The influence of dose-dependent treatment with MPH on the expression of syt1, syt4, stx1a, and NET in the rat PC12 cell line was tested using QRT-PCR (Fig. 3). The expression of mRNA for all investigated genes was significantly reduced after treatment with 1 nM MPH. Syt1 was down-regulated 43% (P < 0.0001) compared to the control group. Additionally, the expression of syt4 (−20%, P = 0.04), stx1a (−15%, P = 0.045), and NET (−27%, P = 0.0014) was down-regulated compared to the control. Higher doses of MPH did not show significant changes after ANOVA one-way post hoc Scheffe, but a significant down-regulation of NET after treatment with 10 (−20%), 100 nM (−26%), and with 10 μM (−15%) was observed using a less conservative test (the student’s t-test).
Methylphenidate (MPH) dose-dependent gene expression profiles in PC12 cells represented as the percent of control cells (MPH = 0 nM). Gene expression was normalized to three housekeeping genes using the GeNorm method. Independent experiments were conducted twelve times, and QRT-PCR measurements were done in triplicate (n = 12). Syntaxin 1a stx1a; synaptotagmin 1 syt1; synaptotagmin 4 syt4; norepinephrine transporter NET. *P < 0.05 vs. MPH = 0 nM using one-way ANOVA with post hoc Scheffe test. Error bars represent standard error in percent
Low doses of MPH enhance PC12 cell proliferation
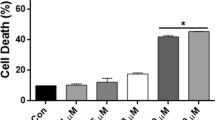
Because cell proliferation is associated with de novo DNA synthesis, we stained MPH-treated and untreated cells with BrdU to visualize its incorporation into DNA. A significantly larger population of PC12 cells displayed active DNA synthesis when treated with lower doses of MPH (1, 10, 100 nM) compared to control (+10–20% enhanced proliferation versus control, P < 0.05; Fig. 4). Higher doses of MPH (1, 10, 100 μM) had no effect on cell proliferation.
Methylphenidate (MPH) dose-dependent effect on PC12 cell proliferation represented as the percent of control cells (MPH = 0 nM). Independent experiments were conducted sixteen times, and BrdU incorporation was done in duplicate with four evaluated pictures for each dose (n = 16). The BrdU-positive cells were percental calculated to all stained cells **P < 0.01, *P < 0.05 vs. MPH = 0 nM using one-way ANOVA with post hoc Scheffe test. Error bars represent standard error in percent
Discussion
In this work, we presented the effects of low-dose MPH treatments after a short time at the level of extracellular and intracellular neurotransmitters and their metabolites, as well as its dose-dependent transcriptional effects on the expression of synaptic protein genes, excluding its inhibitory effects on the DAT (Suppl. Figure S1 and S2). A recent study used low doses of MPH (lowest dose ~130 nM) to treat human neuroblastoma cell line to investigate the effect on cell survival (Schmidt et al. 2009). However, to the best of our knowledge, the present study is the first to deal with the impact of even lower doses of MPH. We showed that very low doses of MPH (1–100 nM) affect intra and extracellular neurotransmitter levels. The NE concentration was significantly higher (70–80%) in the medium of treated PC12 cells compared to untreated cells, whereas the intracellular levels of NE were significantly lower after treatment with nanomolar concentrations of MPH. This outcome is generally consistent with the literature describing the interactions of this psychostimulant with biogenic amine transporter systems (Berridge, Devilbiss et al. 2006). MPH has been shown to inhibit NE uptake (Ferris, Tang et al. 1972; Wall, Gu et al. 1995) and bind to DAT and NET with high affinity, which leads to a higher extracellular monoaminergic level (Gatley, Pan et al. 1996; Volkow et al. 2002). Here, we report a novel finding; the intracellular DA concentration was significantly higher, whereas lower extracellular DA levels were detected after treatment with low doses of MPH. These results confirm with our additional finding that syt1 (one of our investigated synaptic protein) was also down-regulated after nanomolar treatment with MPH. Roden et al. (Roden, Papke et al. 2007) could show that after a stable RNA interference of syt1 in PC12 cells, the DA release was 50% lower. They also found that NE release was lower, but because MPH is a potential NET blocker it seems that this effect is abrogated in our experiment with the PC12 lacking DAT (Suppl. Figure S1 and S2). MPH activation of the synthesizing enzyme, tyrosine hydroxyls, might also be one of the reasons to the increased DA levels, but this must be investigated for confirmation. Another potential reason for the higher intracellular DA level could be our findings regarding cell proliferation, although this must be interpreted cautiously since we would have expected than that all neurotransmitters would have increased at this dose treatment. Cell proliferation was enhanced significantly by 1–100 nM of MPH, whereas higher doses (1–100 μM) caused no alteration. In contrast to (Lagace, Yee et al. 2006), who demonstrated an inhibition of neurogenesis in the juvenile rat brain after MPH administration, we could show higher proliferation rate in PC12 cells after low doses of MPH. Our present study as well as the other cell culture studies (Ludolph, Schaz et al. 2006) revealed mainly increased cell survival and proliferation after MPH treatment. However, one has to be cautious about constructing a link between in vitro cell culture results and in vivo mechanisms. In addition, we demonstrated that the change in extracellular DA metabolites DOPAC and HVA correlated with the breakdown of DA. After nanomolar MPH treatment, the extracellular DOPAC and HVA levels were higher compared to the intracellular level. The degradation of DA seems to be enhanced after MPH treatment; which might be through the activation of monoamine oxidase-B or catechol-O-methyltransferase activities via MPH. But such hypothesis should be further investigated.
The result obtained for the 5-HT metabolite 5-HIAA is an additional evidence for this potential hypothesis. In this case, we obtained also extracellularly the most significant results at nanomolar doses for 5-HIAA (5-HT metabolite). Interestingly, as soon as we raised the MPH doses to the micromolar range, the extracellular DA level was higher than the intracellular level, which is in accordance with previous reports showing that MPH increases the extracellular level of DA (Kuczenski and Segal 1997; Easton, Steward et al. 2007; Volz, Farnsworth et al. 2008).
We used much lower doses of MPH to demonstrate the potency of MPH at lower doses than what was expected until now. Using a cell line model, we must take into account that doses used in vitro should be conferred to an in vivo model; therefore, an adaptation of the drug doses from the micromolar range to the nanomolar range has to be assumed. We showed that low doses of MPH not only alter neurotransmitter release, but also influence synaptic gene expression. We investigated the expression of NET and synaptic protein genes, including syt1, syt4, and stx1a, which is a part of the soluble N-ethylmaleimide-sensitive factor attachment receptor (SNARE) core complex. SNARE proteins assemble into a very stable α-helical complex between two membranes. The formation of the SNARE complex brings two membranes closer and thus leads to membrane fusion. A previous study (Sung and Blakely 2007) demonstrated a direct interaction between NET and stx1a, a protein involved in vesicle trafficking and docking. Importantly, the NET/stx1a interaction appears to be not irrevocable, but rather can be modulated rapidly by extracellular stimuli such as a psychostimulant. Stx1a regulates NET through two mechanisms, one involving the delivery of NET to the plasma membrane and a second involving the inactivation of its catalytic function. How these mechanisms are linked to neuronal activation or second messenger responses remains to be elucidated. Our results show that stx1a mRNA decreases after all MPH doses, but a significant effect was observed only with 1 nM MPH. Interestingly, all investigated synaptic proteins and NET are down-regulated at this low concentration. The significant down-regulation of synaptic proteins by 1 nM MPH shows a strong link to the extracellular DA level. We measured a much lower DA level in the medium after treatment with 1 nM MPH compared to untreated cells (Fig. 1b). Synaptic protein expression can be regulated by the dopamine receptor pathway. DA can activate extracellular signal-regulated kinase (ERK) signaling by stimulating both the D1- and the D2-family receptors. Zachor and colleagues demonstrated for the first time that wild-type PC12 cells express D1 and D2 receptor proteins (Zachor, Moore et al. 2000). Activated ERK induces cAMP response element-binding protein (CREB) phosphorylation and c-fos expression, which further regulate target gene expression, including synaptic proteins. Changes in the expression of these genes may contribute to different aspects of long-term neuro-adaptation (Zhang and Xu 2006). The consequence of low levels of DA is a decreased expression of synaptic proteins. Comparing the gene expression profiles of syt1 and NET after treatment with MPH, they have almost the same progression, which might indicate a common mechanism of action. This theory must be further investigated.
Summarizing our results, we showed that much lower MPH doses than expected are biologically active and lead to an alteration of neurotransmitter release, neurotransmitter metabolism, cell proliferation, and the expression of certain genes. Additional investigations into intermediate nanomolar doses of MPH are necessary for further confirmation.
References
Berridge CW, Devilbiss DM et al (2006) Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry 60(10):1111–1120
Biederman J, Faraone SV (2005) Attention-deficit hyperactivity disorder. Lancet 366(9481):237–248
Easton N, Steward C et al (2007) Effects of amphetamine isomers, methylphenidate and atomoxetine on synaptosomal and synaptic vesicle accumulation and release of dopamine and noradrenaline in vitro in the rat brain. Neuropharmacology 52(2):405–414
Ferris RM, Tang FL et al (1972) A comparison of the capacities of isomers of amphetamine, deoxypipradrol and methylphenidate to inhibit the uptake of tritiated catecholamines into rat cerebral cortex slices, synaptosomal preparations of rat cerebral cortex, hypothalamus and striatum and into adrenergic nerves of rabbit aorta. J Pharmacol Exp Ther 181(3):407–416
Gatley SJ, Pan D et al (1996) Affinities of methylphenidate derivatives for dopamine, norepinephrine and serotonin transporters. Life Sci 58(12):231–239
Hahn MK, Steele A et al (2009) Novel and functional norepinephrine transporter protein variants identified in attention-deficit hyperactivity disorder. Neuropharmacology 57(7–8):694–701
Han DD, Gu HH (2006) Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol 6:6
Jaeger CB (1985) Immunocytochemical study of PC12 cells grafted to the brain of immature rats. Exp Brain Res 59(3):615–624
Kadota T, Yamaai T et al (1996) Expression of dopamine transporter at the tips of growing neurites of PC12 cells. J Histochem Cytochem 44(9):989–996
Kim CH, Hahn MK et al (2006) A polymorphism in the norepinephrine transporter gene alters promoter activity and is associated with attention-deficit hyperactivity disorder. Proc Natl Acad Sci USA 103(50):19164–19169
Kuczenski R, Segal DS (1997) Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: comparison with amphetamine. J Neurochem 68(5):2032–2037
Lagace DC, Yee JK et al (2006) Juvenile administration of methylphenidate attenuates adult hippocampal neurogenesis. Biol Psychiatry 60(10):1121–1130
Ludolph AG, Schaz U et al (2006) Methylphenidate exerts no neurotoxic, but neuroprotective effects in vitro. J Neural Transm 113(12):1927–1934
Riederer P, Reynolds GP (1981) Determination of a wide range of urinary amine metabolites using a simple high-performance liquid chromatographic technique. J Chromatogr 225(1):179–184
Roden WH, Papke JB et al (2007) Stable RNA interference of synaptotagmin I in PC12 cells results in differential regulation of transmitter release. Am J Physiol Cell Physiol 293(6):C1742–C1752
Schmidt A, Krieg J et al. (2009) Impact of drugs approved for treating ADHD on the cell survival and energy metabolism: an in vitro study in human neuronal and immune cells. J Psychopharmacol. doi:10.1177/0269881109105563
Shafer TJ, Atchison WD (1991) Transmitter, ion channel and receptor properties of pheochromocytoma (PC12) cells: a model for neurotoxicological studies. Neurotoxicology 12(3):473–492
Sung U, Blakely RD (2007) Calcium-dependent interactions of the human norepinephrine transporter with syntaxin 1A. Mol Cell Neurosci 34(2):251–260
Vandesompele J, De Preter K et al. (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3(7):RESEARCH0034
Volkow ND, Fowler JS et al. (2002) Mechanism of action of methylphenidate: insights from PET imaging studies. J Atten Disord 6(Suppl 1):S31–S43
Volz TJ, Farnsworth SJ et al (2008) Methylphenidate-induced alterations in synaptic vesicle trafficking and activity. Ann N Y Acad Sci 1139:285–290
Wall SC, Gu H et al (1995) Biogenic amine flux mediated by cloned transporters stably expressed in cultured cell lines: amphetamine specificity for inhibition and efflux. Mol Pharmacol 47(3):544–550
Wilens TE (2008) Effects of methylphenidate on the catecholaminergic system in attention-deficit/hyperactivity disorder. J Clin Psychopharmacol 28(3 Suppl 2):S46–S53
Zachor DA, Moore JF et al (2000) Cocaine inhibits NGF-induced PC12 cells differentiation through D(1)-type dopamine receptors. Brain Res 869(1–2):85–97
Zhang J, Xu M (2006) Opposite regulation of cocaine-induced intracellular signaling and gene expression by dopamine D1 and D3 receptors. Ann N Y Acad Sci 1074:1–12
Acknowledgments
The authors express their appreciation for the funding that was provided by the “Deutsche Forschungsgemeinschaft (DFG)” in the “Klinische Forschergruppe Aufmerksamkeitsdefizit-/Hyperaktivitätssyndrom (ADHS)” KFO-125 http://www.uni-wuerzburg.de/nervenklinik/psychobiologie/kfg/index.html. We would like to thank Dr. Silvia Mandel for the generous gift of the PC12 cells. We would also like to add a special thanks to Mrs. Gabriela Ortega, Ms Miryame Hofmann and Mr. Rainer Burger for their excellent technical work and help. Funding for this study was provided by “Deutsche Forschungsgemeinschaft (DFG)” Grant KFO-125; the DFG had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Conflict of interest
All other authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bartl, J., Link, P., Schlosser, C. et al. Effects of methylphenidate: the cellular point of view. ADHD Atten Def Hyp Disord 2, 225–232 (2010). https://doi.org/10.1007/s12402-010-0039-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12402-010-0039-6