Abstract
Background
Multiple AVMs are exceptionally rare lesions and only a few larger series have been published, including other vascular pathologies, such as arterio-venous fistulae (AVF) or patients with hereditary syndromes. Our study presents clinical, angiographic, and therapeutic characteristics of patients harboring sporadic multiple AVMs.
Methods
Basic demographic data, vascular architecture, clinical presentation, treatment strategies, and treatment outcome were analyzed retrospectively from patients with cerebral AVMs treated in our department between 1990 and 2015.
Results
Six out of 539 patients (1.1 %) harbored 15 multiple and distinct cerebral lesions. Nidus size was predominantly small, consequently determining a Spetzler–Martin grade °I–°II (three-tier grading system). In three patients, AVMs shared a proximal feeding artery supply, whereas each AVM displayed its own venous drainage. Five of six patients (83 %) presented with hemorrhage. Four patients received therapy of the AVMs with complete elimination in 3/4 patients (75 %) and 8/9 treated AVMs (89 %). All patients with treatment of the AVM showed good-to-excellent recovery (n = 4, mRS ≤ 2).
Conclusions
Multiple cerebral AVMs are complex vascular lesions. The multiplicity of hemodynamic and malformation-related variables influence treatment strategy and sequence. Thus, awareness of these parameters (of various malformations before and during treatment) is important. The high number of hemorrhagic events in the present series might justify a more aggressive treatment of multiple AVMs than previously thought.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebral arterio-venous malformations (AVMs) are vascular lesions, typically appearing solitarily. Multiplicity of AVMs is rarely encountered, accounting for only 0.3–3.2 % of all brain AVMs [19]. Multiple and discrete AVMs are more frequently seen in association with syndromes like hereditary hemorrhagic telangiectasia (HHT) or Wyborn–Mason syndrome (WMS) [10, 14, 17]. In contrast, only small numbers of small series and case reports exist for sporadic cases [4, 9, 15, 30]. Despite their genetic background, the treatment of multiple AVMs is most challenging. The effect of multiplicity not only complicates estimating the future risk for hemorrhage [10] but it also compounds the decision for treatment indication and treatment strategies in particular for unruptured lesions.
Our series presents clinical and angiographic characteristics, treatment strategies, and treatment outcome of six patients harboring sporadic multiple AVMs and thus a reputable number of cases, given the exceptional rarity of this entity.
Materials and methods
Six out of 539 patients with cerebral AVMs, admitted to our institution between 1990 and 2015, harbored multiple and distinct cerebral lesions (1.1 %). There were three males and three females, with a mean age of 41.2 ± 22.4 years at first diagnosis of the AVMs, ranging from 7 to 71 years. Only one patient was younger than 18 years (case 3, 7 years). No patient had a medical history of epistaxis or family history for HHT and no patient presented with telangiectasia at typical locations on clinical examination. Therefore, no individual met the clinical diagnostic criteria of HHT, known as the “Curaçao Criteria” [6, 22].
Basic demographic data, vascular architecture, clinical presentation, and treatment strategies were compiled from our departmental database and evaluated retrospectively. Depending on clinical presentation and condition, patients were examined with computed tomography (CT) and/or magnetic resonance imaging (MRI), followed by cerebral digital subtraction angiography (DSA) on admission and prior to treatment. Patients were evaluated in an interdisciplinary approach by experienced neurosurgeons, neuroradiologists, and radiosurgeons. Treatment decisions were based on clinical presentation, the angio-architecture of the AVMs, the source of hemorrhage in case of rupture, the presumed risk for hemorrhage from unruptured lesions and the patients’ informed consent. In case of hemorrhage from a ruptured AVM, treatment was primarily directed towards the symptomatic lesion.
Results
Clinical presentation
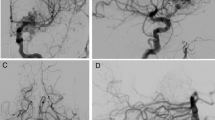
Five of six patients (83 %) presented with hemorrhage from a ruptured AVM. Among these, two patients were admitted in a poor clinical state. One patient (case 5) with bilateral AVMs, partially embolized twice in a different institution 3 and 13 years ago, suffered from fatal recurrent hemorrhage of the right deep-seated AVM. After admission in our hospital with fixed and dilated pupils, he received urgent decompressive craniectomy without targeting the AVMs. The second patient (case 2) harbored four AVMs (Fig. 1) and was admitted in comatose state with a right intracerebral hematoma (ICH). She developed multiple cerebral territorial infarctions and conservative management was adopted, following the patient’s provision. Patients’ demographic characteristics and clinical details are summarized in Table 1.
Radiological findings in a patient harboring four distinct AVMs (case 2). a CT angiography of a patient with a right intracerebral hemorrhage showing an adjacent deep frontal AVM (arrow) and an additional vascular anomaly in the midline (arrowheads). b Right carotid angiogram confirming a small deep right frontal AVM (arrow) and a second callosal AVM, fed by branches of the right ACA. c Angiogram of the left ICA depicting two left hemispheric AVMs temporal (arrowheads) and deep frontal (arrow)
Radiological findings
The six patients included in our study suffered from 15 AVMs in total. Four patients carried two, one carried three, and one carried four AVMs, respectively. Four patients harbored bilateral AVMs, with one patient presenting a mirror-like location in both temporal lobes (case 4). In this patient, the asymptomatic right temporal lesion also showed signs of previous hemorrhage (Fig. 2). There was no AVM located in the posterior fossa. AVM size was predominantly small according to the Spetzler–Martin grading system (SMG) [23], consequently determining a SMG °I–°II (three-tier grading system) [24]. In three patients, AVMs where fed through a common proximal arterial supply (cases 2, 3, 6). However, a “sharing” of distal feeding arteries was not observed. No associated feeding artery aneurysms could be found and each AVM displayed its distinct venous drainage. A detailed overview of radiological findings is presented in Table 1.
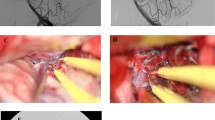
Radiological findings of a patient suffering from two mirror-like AVMs in both temporal lobes. a MRI shows an acute hemorrhage on the left site and signs of previous hemorrhage on the right site (white arrow). b Angiogram of both ICAs depicting small superficial AVMs in both temporal lobes (arrows). c DSA after neuroradiological intervention showing a complete occlusion of the left symptomatic lesion. d Late angiogram of the left ICA 2 weeks later revealing an early draining vein of the previously embolized AVM (arrow). e DSA after surgery confirming complete resection of both lesions
Treatment and outcome
Except for those two patients in a poor initial clinical state, all other patients (n = 4) received treatment of all AVMs. A multimodal approach was chosen in 3/4 patients for 3/9 treated lesions and one patient was scheduled for radiosurgery of two AVMs. All patients received treatment of their second/third lesion within 1 month after eliminating the symptomatic/ruptured AVM. Complete elimination could be achieved in 3/4 patients and 8/9 treated AVMs). All patients with treatment of the AVM in our institution showed good-to-excellent recovery (n = 4, mRS ≤ 2) except for those two patients mentioned above. An overview of treatment modalities and clinical outcome is shown in Table 2.
Discussion
Patients and clinical presentation
Multiple and distinct AVMs are exceptionally rare lesions. Only a few larger series have been published, including other vascular pathologies, such as arteriovenous fistulae (AVF) and patients with hereditary syndromes [7, 29]. To the best of our knowledge, our series of six consecutive patients represents one of the largest series of sporadic multiple AVMs and is of clinical relevance in several aspects.
Whereas the occurrence of multiple cerebral AVMs in our series is within range of the others (0.7–3 %), the number of hemorrhagic events (83 %) is remarkably different, compared to previous publications and compared to hemorrhage rates of solitary supratentorial AVMs [16, 18]. In a literature review, Okada et al. summarized 22 cases of multiple AVMs where ten patients (45 %) suffered from rupture of an AVM [15]. Iizuka et al. found a hemorrhage rate of nearly 40 %, emphasizing that multiple AVMs do not carry an increased risk for hemorrhage, compared to single AVMs [7]. Since a simple “summation hypothesis” seems to be uncertain for AVM-associated pathologies such as associated feeding artery aneurysms, it remains questionable whether it can be adopted for multiple AVMs [2]. We cannot provide a simple conclusion for the high rate of hemorrhages in our series. One reason might be a different biology of these pathologies compared to single lesions. This hypothesis finds support in some reports of de novo development during follow-up and a differential clinical behavior after radiosurgery with lower occlusion rates [11, 15, 20, 25, 26]. On the other side, Kim et al. recently described a potentially lower risk for hemorrhage in patients with HHT and cerebral AVM compared to patients with sporadic AVMs [8]. Since we did not include HHT patients in our series (according to the clinical data), our high number of hemorrhages might have been induced through this “selection bias”.
Radiological findings
Interestingly, all detected AVMs in our series were ≤3 cm in nidus size. This finding is in accordance with previous publications, reporting of predominantly small AVMs [30]. In their review, Okada and coworkers found small AVMs in 6/7 patients, presenting with intracerebral hemorrhage [15]. Thus, a small nidus size in this particular patient group stresses out the need for careful angiographic evaluation of each patient’s neurovascular conditions in order to prevent a potential overlooking of multiple AVMs [3, 5, 13].
The historical phenomenon of micro-AVMs [7, 29] could not be found in our series. All patients harbored true and distinct AVMs with typical nidal structures, except for one patient (case 6) with an additional fistula-like lesion. Whether micro-AVMs represent true malformations rather than reactive micro-arteriovenous shunts caused by hemodynamic alterations remains controversial [29]. Again, these lesions might have biased the number of cases in previous reports, as well as the difficulty to distinguish multi-compartmental single AVMs from true multiple AVMs [7, 30].
Treatment considerations and strategy
Some authors assume the same treatment principles for multiple and single AVMs, postulating that size and structures involved are more important factors in the prognosis than multiplicity of the lesions [13, 27]. We only partially agree with these considerations. First, the indication for treatment in our series was based on the high number of hemorrhagic events and led to consequently approach the symptomatic lesion in three patients. Second, the remaining AVMs in our cohort displayed changed or altered flow patterns in three of four treated patients (Table 1, Figs. 3, 4, and 5). Resecting the hemorrhagic AVM in a given patient does not necessarily imply to eliminate the remaining lesion. Nevertheless, potential hemodynamic interactions remarkably influence treatment strategies as well as the treatment sequence [21]. A number of case reports note an elevated risk for hemorrhage from the second AVM after eliminating the first. The authors postulate an altered or increased flow within the remaining AVM, thus recommending prompt elimination [4, 13, 15]. In contrast, hemodynamic changes might also lead to disappearance of the second AVM. A consecutive thrombosis could be a result of normalized flow rate or decrease of blood flow after resection [28, 31]. Undoubtedly, it is both crucial to (1) understand the potential hemodynamic interactions between distinct AVMs and (2) to obtain a detailed picture of AVM angio-architecture [13, 27]. Based on these findings, we treated the remaining AVMs more aggressively than unruptured single AVMs and as implicated by the ARUBA trial [12]. Robert et al. [18] recently emphasized the same approach. Although this strategy so far has not been proven to be superior, we deem this approach reasonable when risks of treatment are carefully balanced.
Hemodynamic changes during AVM treatment (case 3). a Cranial CT scan of a patient presenting with a right cerebral and intraventricular hemorrhage. b Corresponding angiogram depicting two AVMs (arrows) in the right temporal and right parietal lobe, supplied by feeders of the right PCA. c Angiogram after initially complete endovascular embolization of the ruptured temporal AVM. The nidus of the remaining parietal AVM shows an increase in flow patterns (arrow). d Control after 1 month shows recanalization of the initially obliterated temporal AVM (arrow) and the patient was scheduled for a near-term two-step microsurgical resection of both AVMs
Hemodynamic changes 3 years after radiosurgery (case 1). a Cranial MRI of a patient with two distinct unruptured AVMs (right dorsal basal ganglia, white arrowheads; left parietal, white arrow). b Right ICA angiogram depicting the AVM of the right hemisphere, mainly supplied by feeders of the right PcomA. c Left ICA angiogram shows the small left parietal AVM, exclusively supplied by one MCA branch (arrow). The dorsal nidal compartments of the right AVM are faintly perfused (arrows). d MRI follow-up 3 years after radiosurgery (Cyberknife, 16 Gy) shows complete disappearance of the left AVM (arrow). e The right AVM nidus still remains patent, verified by DSA. f Whereas the left ICA angiogram demonstrates complete obliteration of the left AVM (arrowheads on the discontinued former MCA feeder), nidal perfusion of the right AVM from the left site appears increased (arrow)
Treatment sequence in a patient with three AVMs. a CT scan shows an intracerebral hemorrhage from a left occipital paramedian AVM. b Complimentary MRI detects two additional vascular pathologies in the left dorsal temporal lobe. c Verification of two typical distinct AVMs (arrows) and one AVM with fistulous morphology (arrowhead). d DSA after endovascular embolization of the symptomatic lesion. Flow within the fistula like AVM appears increased (arrowhead). e After partial embolization of the second left temporal dorsal AVM, delicate nidus-like vessels within the remaining fistula-like AVM are visible. f Intraoperative DSA during the same setting revealing complete elimination of all three AVMs
The conducted treatment modalities for multiple AVMs can be widely adopted from the treatment of single AVMs, as described in detail by Bradac and coworkers [1]. In this context, the problem of small numbers (of a rare disease), collected over a long period needs to be stressed as well as the heterogeneity of the subgroup. Our database contains patients treated from 1990 to 2015. Not only treatment modalities changed over this stretch of time but also new (endovascular) techniques were implemented. As a consequence, a consistent algorithm for the applied modalities is difficult in our series.
Finally, a correctly and well-informed patient has the final decision in the therapeutic decision-making process [1]. Consultation comprises the center’s own experiences as well as a comprehensive picture with all facets of the disease (including hereditary syndromes), based on the body of literature. Whether a remaining and unruptured AVM in case of AVM multiplicity can be considered an “incidental” lesion, is uncertain. Therefore, the conclusions of ARUBA might be of little help when counseling an individual patient suffering from multiple AVMs [12].
Conclusions
Multiple cerebral AVMs are complex vascular lesions. The multiplicity of hemodynamic and malformation-related variables influence the treatment strategy and sequence. Thus, awareness of these parameters (of various malformations before and during treatment) is important. The high number of hemorrhagic events in the present series might justify a more aggressive treatment of multiple AVMs than previously thought.
References
Bradac O, Charvat F, Benes V (2013) Treatment for brain arteriovenous malformation in the 1998–2011 period and review of the literature. Acta Neurochir 155(2):199–209
da Costa L, Wallace MC, Ter Brugge KG, O’Kelly C, Willinsky RA, Tymianski M (2009) The natural history and predictive features of hemorrhage from brain arteriovenous malformations. Stroke J Cereb Circ 40(1):100–105
Dammann P, Breyer T, Wrede KH, Stein KP, Wanke I, Grams AE, Gizewski ER, Schlamann M, Forsting M, Sandalcioglu IE, Sure U (2014) Treatment of complex neurovascular lesions: an interdisciplinary angio suite approach. Ther Adv Neurol Disord 7(1):60–70
de Sousa AA, Dantas F (1992) Bilateral arteriovenous malformations: case report. Neurosurgery 30(6):940–943
Ericson K, Soderman M, Karlsson B, Guo WY, Lindquist C (1994) Multiple intracranial arteriovenous malformations: a case report. Neuroradiology 36(2):157–159
Faughnan ME, Palda VA, Garcia-Tsao G, Geisthoff UW, McDonald J, Proctor DD, Spears J, Brown DH, Buscarini E, Chesnutt MS, Cottin V, Ganguly A, Gossage JR, Guttmacher AE, Hyland RH, Kennedy SJ, Korzenik J, Mager JJ, Ozanne AP, Piccirillo JF, Picus D, Plauchu H, Porteous ME, Pyeritz RE, Ross DA, Sabba C, Swanson K, Terry P, Wallace MC, Westermann CJ, White RI, Young LH, Zarrabeitia R (2011) International guidelines for the diagnosis and management of hereditary haemorrhagic telangiectasia. J Med Genet 48(2):73–87
Iizuka Y, Rodesch G, Garcia-Monaco R, Alvarez H, Burrows P, Hui F, Lasjaunias P (1992) Multiple cerebral arteriovenous shunts in children: report of 13 cases. Child’s Nerv Syst: Off J Int Soc Pediatr Neurosurg 8(8):437–444
Kim H, Nelson J, Krings T, terBrugge KG, McCulloch CE, Lawton MT, Young WL, Faughnan ME (2015) Hemorrhage rates from brain arteriovenous malformation in patients with hereditary hemorrhagic telangiectasia. Stroke J Cereb Circ 46(5):1362–1364
Kohmura E, Taki T, Tanioka T (1990) Multiple intracerebral arteriovenous malformations in deep structure—case report. Neurol Med Chir 30(8):624–627
Kuo YH, Santoreneos S, Roos D, Brophy BP (2007) Treatment of multiple arteriovenous malformations in pediatric patients with hereditary hemorrhagic telangiectasia and spontaneous hemorrhage. Report of two cases. J Neurosurg 107(6 Suppl):489–494
Miyasaka Y, Nakahara K, Takagi H, Hagiwara H (2003) Development of multiple cerebral arteriovenous malformations documented in an adult by serial angiography. Case report. J Neurosurg 98(1):190–193
Mohr JP, Parides MK, Stapf C, Moquete E, Moy CS, Overbey JR, Al-Shahi Salman R, Vicaut E, Young WL, Houdart E, Cordonnier C, Stefani MA, Hartmann A, von Kummer R, Biondi A, Berkefeld J, Klijn CJ, Harkness K, Libman R, Barreau X, Moskowitz AJ (2014) Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet 383(9917):614–621
Nakayama Y, Tanaka A, Yoshinaga S, Tomonaga M, Maehara F, Ohkawa M (1989) Multiple intracerebral arteriovenous malformations: report of two cases. Neurosurgery 25(2):281–286
Nishida T, Faughnan ME, Krings T, Chakinala M, Gossage JR, Young WL, Kim H, Pourmohamad T, Henderson KJ, Schrum SD, James M, Quinnine N, Bharatha A, Terbrugge KG, White RI Jr (2012) Brain arteriovenous malformations associated with hereditary hemorrhagic telangiectasia: gene-phenotype correlations. Am J Med Genet A 158A(11):2829–2834
Okada Y, Shima T, Nishida M, Yamane K (1992) Bilateral symmetrical cerebral arteriovenous malformations in the basal ganglia--case report. Neurol Med Chir 32(2):88–92
Perret G, Nishioka H (1966) Report on the cooperative study of intracranial aneurysms and subarachnoid hemorrhage. Section VI. Arteriovenous malformations. An analysis of 545 cases of cranio-cerebral arteriovenous malformations and fistulae reported to the cooperative study. J Neurosurg 25(4):467–490
Putman CM, Chaloupka JC, Fulbright RK, Awad IA, White RI Jr, Fayad PB (1996) Exceptional multiplicity of cerebral arteriovenous malformations associated with hereditary hemorrhagic telangiectasia (Osler–Weber–Rendu syndrome). AJNR Am J Neuroradiol 17(9):1733–1742
Robert T, Blanc R, Botta D, Ciccio G, Smajda S, Redjem H, Fahed R, Piotin M (2016) Management of multiple cerebral arteriovenous malformations in a non-pediatric population. Acta Neurochir 158(6):1019–1025
Salcman M, Scholtz H, Numaguchi Y (1992) Multiple intracerebral arteriovenous malformations: report of three cases and review of the literature. Surg Neurol 38(2):121–128
Sandalcioglu IE, Asgari S, Wende D, van de Nes JA, Dumitru CA, Zhu Y, Gizewski ER, Stolke D, Sure U (2010) Proliferation activity is significantly elevated in partially embolized cerebral arteriovenous malformations. Cerebrovasc Dis 30(4):396–401
Sandalcioglu IE, Wanke I, Zappala V, Forsting M, Sure U (2011) The management of arteriovenous malformations. J Neurosurg Sci 55(1):57–69
Shovlin CL, Guttmacher AE, Buscarini E, Faughnan ME, Hyland RH, Westermann CJ, Kjeldsen AD, Plauchu H (2000) Diagnostic criteria for hereditary hemorrhagic telangiectasia (Osler–Weber–Rendu syndrome). Am J Med Genet 91(1):66–67
Spetzler RF, Martin NA (1986) A proposed grading system for arteriovenous malformations. J Neurosurg 65(4):476–483
Spetzler RF, Ponce FA (2011) A 3-tier classification of cerebral arteriovenous malformations. Clinical article. J Neurosurg 114(3):842–849
Sure U, Butz N, Schlegel J, Siegel AM, Wakat JP, Mennel HD, Bien S, Bertalanffy H (2001) Endothelial proliferation, neoangiogenesis, and potential de novo generation of cerebrovascular malformations. J Neurosurg 94(6):972–977
Sure U, Butz N, Siegel AM, Mennel HD, Bien S, Bertalanffy H (2001) Treatment-induced neoangiogenesis in cerebral arteriovenous malformations. Clin Neurol Neurosurg 103(1):29–32
Tada T, Sugita K, Kobayashi S, Watanabe N (1986) Supra- and infratentorial arteriovenous malformations with an aneurysmal dilatation: a case report. Neurosurgery 19(5):831–834
Utsuki S, Kurata A, Miyasaka Y, Takano M, Ootaka H, Fujii K (2002) Multiple arteriovenous malformations with hemorrhage. Acta Neurochir 144(1):97–101
Willinsky RA, Lasjaunias P, Terbrugge K, Burrows P (1990) Multiple cerebral arteriovenous malformations (AVMs). Review of our experience from 203 patients with cerebral vascular lesions. Neuroradiology 32(3):207–210
Yamashita K, Suzuki Y, Yoshizumi H, Takahashi J, Nogawa T (1993) Multiple cerebral arteriovenous malformations—case report. Neurol Med Chir 33(1):24–27
Yoshimoto T, Kashiwaba T, Houkin K, Abe H (1996) Spontaneous disappearance of arteriovenous malformation during staged treatment of multiple cerebral arteriovenous malformations—case report. Neurol Med Chir 36(11):812–814
Acknowledgments
The authors thank Mike Sucker and Tobias Schoemberg for assistance in preparing the figures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
No IRB approval is needed for retrospective cohorts in academic centers in Germany, so no informed consent was required from any of the patients.
Additional information
Comments
The authors present an interesting series of six patients with multiple AVMs who were managed with variety of treatment strategies including conservative management. While two patients had a very poor outcome, four survived with good/reasonable function. The authors encourage the treatment of patients with multiple AVM based on their apparent higher risk of bleed (which is based on small series of multiple AVMs). They also suggest caution in terms of hemodynamic change in multiple AVMs treatment when one is treated and the other is left alone (whether this increases the risk of bleed or decreases is unclear). Theoretically, one could imagine that multiple AVMs in a given patient will put that patient at higher risk of hemorrhagic event than a patient with a single AVM. On the other hand, treatment of all AVMs has undeniably an accumulated risk of complications, especially if multi-modality treatments are undertaken. I personally lean towards the treatment of multiple AVMs (and more so if one has bled) if the neurological morbidity is considered low or acceptable especially in younger patients. Aruba trial is easily criticized for its non-applicability to surgical treatment of AVMs, and certainly cannot be brought as a validation of observation vs. treatment in multiple AVM scenarios.
Amir Dehdashti
NY, USA
Rights and permissions
About this article
Cite this article
Stein, KP., Wanke, I., Oezkan, N. et al. Multiple cerebral arterio-venous malformations: impact of multiplicity and hemodynamics on treatment strategies. Acta Neurochir 158, 2399–2407 (2016). https://doi.org/10.1007/s00701-016-2989-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-016-2989-8