Abstract
Background
The occurrence of concomitant multiple cerebral arteriovenous malformations (mAVMs) is often associated with hereditary hemorrhagic telangiectasia (HHT) or craniofacial arteriovenous metameric syndrome (CAMS) and frequently occurs in the pediatric population.
Methods
Between 1995 and 2013, demographic, clinical, and angiographic data of cerebral AVMs have been prospectively collected. We retrospectively analyzed data of patients presenting multiple cerebral AVMs.
Results
Six patients (mean age, 44 years, male-to-female ratio, 5) presented an angiographic diagnosis of cerebral mAVMs. Only one of them was known to have a HHT. Five patients presented two cerebral AVMs and one patient had three. Three AVMs (23.1 %) presented bleeding at admission. Three patients had supratentorial mAVMs only and the three others had supra and infratentorial AVMs. Only one patient suffered from bleeding of more than one of his mAVMs with an interval of 23 years.
Conclusions
For asymptomatic AVMs discovered incidentally without angiographic bleeding risk, we propose a therapeutic abstention. In case of AVM rupture and bleeding, the other “associated” AVMs (discovered through a complete angiographic assessment) should also be treated if they are not located in an eloquent area and if the treatment does not present technical difficulties. AVMs with a history of bleeding or associated to angiographic risks have to be treated more aggressively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The presence of concomitant multiple cerebral arteriovenous malformations (cAVMs) has already been described, in particular in the pediatric population [3]. Rendu–Osler–Weber disease (also known as hereditary hemorrhagic telangiectasia, HHT) and cerebrofacial arteriovenous metameric syndromes (CAMS) are the first etiology of multiple cAVMs [3, 15]. However, in the adult population, the discovery of multiple cAVMs is rarely reported and the pathophysiology remains unknown [2, 10, 14, 15]. We present herein our experience with six patients, each presenting multiple cAVMs and discuss the management of each possible situation based on our experience and on the literature available: ruptured AVM, concomitant unruptured AVM discovered in the context of rupture of another cerebral AVM (“associated” AVM) and purely incidental AVMs.
Case series
We have maintained an ongoing prospective database where demographic, clinical, and angiographic information regarding 794 patients harboring a cerebral arteriovenous malformation. From 1995 to 2013, six patients with multiple brain AVMs have been treated in our institution. Demographic data recorded for each patient included age, sex, and clinical presentation. The diagnosis of brain AVMs was confirmed by digital subtraction angiography (DSA) for all patients. Table 1 summarizes the patient demographic data and the angiographic characteristics of each arteriovenous malformation.
Case 1
A right-handed 37-year-old male without a relevant medical history underwent a cerebral magnetic resonance imaging (MRI) for vertigo, which revealed a left occipital AVM. Left vertebral digital subtraction angiography (DSA) showed a 40-mm compact nidus involving the visual cortex, fed by the calcarine and the parieto-occipital arteries with the presence of a 6-mm flow-related aneurysm at the junction of the second and third segment of the left posterior cerebral artery (PCA). The venous drainage was superficial through the superior sagittal sinus (SSS) via the left occipital vein (Spetzler–Martin grade 3 [11]). Left internal carotid artery DSA also showed another frontal AVM formed by a little cortical nidus of less than 5 mm in the intermediate frontal gyrus fed by the pre-frontal artery with a superficial venous drainage assumed by the precentral vein to the SSS (Spetzler–Martin grade 1 [11]). We decided to coil the inflow aneurysm of the left P2-P3 junction. The procedure was performed without complication. We decided not to treat the two AVMs, given the absence of bleeding episode and the absence of angiographic risk factor for bleeding. The left occipital AVM was localized in an eloquent area, which reinforced our conservative therapeutic decision. Angiographic follow-up performed 26 months after the aneurysm treatment did not show aneurysm recurrence or evolution of the AVMs. The patient presented a modified Rankin score (mRS) 0 at the last visit 5 years after the treatment.
Case 2
A 49-year-old man known for a chronic hepatitis and heroine addiction presented a spontaneous comatose state (World Federation Neurosurgical Societies WFNS score 5). Admission computerized tomography (CT) revealed a subarachnoid hemorrhage secondary to the rupture of an aneurysm located at junction between the first and the second segment of the right PCA. Left vertebral DSA showed the aneurysm to be flow-related, associated with a right atrial and peduncular AVM fed by the right postero-lateral choroidal artery and by PCA perforators. This AVM had a 23-mm compact nidus involving the mesencephalon with a venous drainage through the right basal vein (Spetzler–Martin 3). Left carotid DSA also revealed the presence of a left central sulcal AVM with a nidus measuring less than 5 mm fed by the left central artery and drained by the central vein into the SSS (Spetzler–Martin 2). According to the patient’s clinical status, we decided to embolize the ruptured aneurysm by a balloon-assisted coiling in the first session after the positioning of an external ventricular drainage. Despite the treatment of an acute hydrocephalus and a standard neurointensive therapy, the patient did not recover, and died 11 days after his admission. No treatment had been performed for his cerebral AVMs.
Case 3
A right-handed 24-year-old male already operated 23 years ago for a left ruptured frontal cortical AVM was admitted for a new intraparenchymal hemorrhage. At the same time, a left thalamic AVM was also diagnosed but conservative management was decided. After microsurgical resection, the left frontal AVM was considered completely cured and the patient had fully recovered from a right hemiparesis. Admission CT revealed a left thalamic hematoma unrelated to the porencephalic left frontal cavity in keeping with the previous AVM resection. Left carotid DSA showed a left capsulo-thalamic AVM fed by thalamic perforators of the posterior communicating artery. The nidus was compact and measured 33 mm. Its venous drainage involved the galenic system via the left thalamostriate vein (Spetzler–Martin 2). It was decided to treat the capsulo-thalamic AVM by radiosurgery. A complete cure was confirmed by a DSA performed 25 months after the treatment. The patient kept a discrete right hemiparesis (mRS 2). The latest DSA performed 45 months after the treatment confirmed the complete obliteration of the AVM.
Case 4
A 20-year-old man who suffered from a unilateral headache underwent a cerebral MRI, which revealed two cAVMs. This patient was already known to have HHT disease with a pulmonary AVM. Right carotid DSA showed a 12-mm right cortical nidus of the inferior parietal gyrus fed by branches of the inferior parietal artery and drained by two cortical veins (Spetzler–Martin grade 1). The 3D reconstruction of the right internal carotid DSA revealed the presence of two nidal aneurysms. Left vertebral angiogram also highlighted a fistulous communication between a basilar artery perforator and a tributary vein of the left basal vein located in the tegmentum (Spetzler–Martin grade 3). Regarding the angiographic risk factors of the supratentorial AVM, we decided to treat it endovascularly. This treatment was conducted with one embolization session using 0.7 ml of NBCA (concentration 50 %) (Glubran, GEM, Viareggio, Italy) without complication. The treatment risks for the mesencephalic AVM were considered too high to plan a curative treatment for this asymptomatic lesion and observational follow-up was decided. The latest DSA was performed 35 months after the treatment and highlighted no reperfusion of the supratentorial AVM and no modification of the mesencephalic AVM.
Case 5
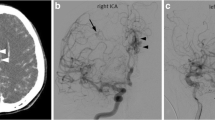
A right-handed 63-year-old woman was admitted for a thunderclap headache associated with an expressive aphasia. Admission CT showed a left frontal hemorrhage with intraventricular bleeding secondary to the rupture of an AVM. Left carotid angiogram (Fig. 1a, b) confirmed the presence of a 25-mm compact nidus in the first frontal gyrus fed by branches of the calloso-marginal artery with a unique cortical venous drainage that converged to the SSS (Spetzler–Martin grade 1). Right vertebral DSA (Fig. 1c, d) revealed two other posterior fossa AVMs. The first one was located in the inferior vermis (13 mm). It was fed by branches of the left postero-inferior and anterior-inferior cerebellar arteries and drained by the occipital sinus via a medial cerebellar hemispheric vein (Spetzler–Martin grade 1). The other AVM was a fistulous type located in the left cerebral peduncle fed by perforators of the basilar artery and the left superior cerebellar artery and drained into a tributary of the left lateral mesencephalic vein (Spetzler–Martin grade 3). We decided to treat endovascularly the ruptured left frontal AVM. Its complete obliteration was achieved after two embolization sessions using Onyx (Covidien, Irvine, CA, USA) by an arterial approach. No complication was noted during this treatment and no recurrence of the arteriovenous shunt was viewed at the control DSA performed 12 months after the last session. The two other AVMs were located in highly eloquent areas, were asymptomatic and presented no angiographic risk factor of bleeding. Consequently, we decided to perform a simple follow-up by angio-MR. The latest follow-up performed 4 years after the discovery of these AVMs showed a stability of the lesions. The patient was neurologically symptom-free (mRS = 0).
Images of case 5. Left carotid angiograms on anterior-posterior (a) and lateral (b) projections showing a left frontal compact nidus (25 mm) fed by branches of the calloso-marginal artery. Left vertebral angiograms on anterior-posterior (c) and lateral (d) projections showing a left cerebellar nidus and also an arteriovenous shunt located in the pons on the right side
Case 6
A 63-year-old male with a history of human immunodeficiency virus (HIV) infection treated by triple therapy underwent a cerebral MRI for non-specific vertigo revealing a left AVM located in the central region (Fig. 2e). Left carotid angiogram (Fig. 2a, b, d) confirmed the presence of this compact nidus (20 mm) fed by homolateral branches of the calloso-marginal artery and drained by the SSS via the central and pre-central veins (Spetzler–Martin grade 1). Left vertebral injection DSA (Fig. 2c) also showed a little AVM located in the right cerebellar hemisphere between a branch of the superior cerebellar artery and a superior cerebellar hemispheric vein (Spetzler–Martin grade 1). Retrospectively, the fistulous AVM located in the right cerebellar hemisphere was already visible in the initial MRI performed for the vertigo exploration, thanks to the arterial spin labeling (ASL) sequence (Fig. 2f). These two AVMs were discovered incidentally and were asymptomatic therefore we decided to perform a simple follow-up with MRI. One year after the diagnosis, the patient had not presented signs of bleeding and his neurological examination was normal.
Images of the case 6. Right carotid angiograms on lateral (a), anterior-posterior (d) projections revealing the central AVM fed by a calloso-marginal branch. The 3D reconstruction of the DSA highlights the arterial supply (colored in orange) and the two cortical draining veins (in blue). Left vertebral angiogram on lateral projection (c) showing an arteriovenous shunt between branches of the right superior cerebellar artery and a superficial cerebellar vein. MRI with the sequences TOF (e) and ASL (f) permit to localize and also to reveal little arteriovenous shunt (hypersignal in f)
Discussion
Brain AVM is often diagnosed alone or in association with another neurovascular disease (cerebral aneurysm, dural arteriovenous fistulas) but the presence of multiple cerebral AVMs is very rare. Most of the publications on this topic are centered on the pediatric population [3, 4, 15]. For infants and young children, the presence of concomitant cerebral AVMs is encountered in HHT and CAMS [3]. On the contrary, adult cases with multiple AVMs are only described in isolated cases and have an idiopathic origin [2, 15].
In our series, only one patient was known for an HHT, and the five others did not present vascular risk factors. The overage incidence in our AVM database of patients harboring multiple AVMs in the cerebral AVM Rothschild Foundation Hospital database was 0.9 % (six patients/695). This is lower than described in the literature (3.2–4 %) [14]. We could explain this discrepancy by the absence of the pediatric population in our AVM database and, consequently, the low number of syndromic cases. Furthermore, we have noted that an associated pathology was present in four of the six patients.
Five of our patients were male but the analysis of other case reports of the literature did not reveal any sex predominance. We do not have any explanation for the male predominance in our series and we think that this is only due to hazard. The circumstances of diagnosis, the age of patients, and the anatomical distribution of the lesions are similar to those of patients with single arteriovenous malformation [5, 14].
We could note in our series the usual association of a supratentorial AVM with an infratentorial one. Among the 32 cases described in the literature [1–4, 7, 10, 13–16], the same distribution has been seen in ten patients and particularly in the adult population [2, 14, 15]. It could be anecdotic, but this could be the sign of a general vascular pathology instead of a local or regional vascular fragility because it affects vascular territories of completely different embryological origins.
In our series, only one patient presented two AVMs with nidi of equal size. In all the other patients, the nidus of one of the two AVMs measured less than 5 mm. Moreover, in two cases, there was a fistulous AVM between a parenchymal artery and a cerebral vein. Hasegawa et al. [2] underlined this characteristic in a description of a patient with multiple cerebral AVMs and also a spinal AVM. Willinsky et al. [15] evoked the possibility that the hemodynamic changes provoked by the biggest nidus could lead to the opening of little arteriovenous shunts. Other authors [3, 4, 10, 15] have not discussed this element. The diagnosis of AVMs with a little nidus could be difficult, even when achieving a systematic DSA, especially when the interventionist attention is focused on another area of the brain, for example in a context of intra-parenchymal hematoma and the search for an underlying AVM. As a consequence, we could imagine that multiple cerebral AVMs were under-diagnosed. Arterial spin labeling (ASL) magnetic resonance imaging could be helpful [13] in diagnosing these arteriovenous shunts without true nidus.
The management of patients harboring multiple AVMs could be problematic. The poor literature concerning these patients did not give information regarding the risk of bleeding of each AVM. Does a patient with two cerebral AVMs simply have twice the bleeding risk of a patient with only one AVM? Should we consider a vascular failure for this patient that increased the risk of bleeding? The response is difficult and we think that we have to consider that patients with multiple cAVMs have the same risk of bleeding as patients with a unique AVM. Consequently, we recommend treating unruptured cerebral AVMs only if angiographic risk factors are present (associated aneurysm, deep location, only deep venous drainage, and single draining vein). For patients with multiple AVMs, the decision should be taken case-by-case regarding the location of the AVMs and the possible risk of treatment complications.
In our series, we report the case of a patient who has presented one bleeding of each of the two AVMs he had (case 3). We have found two other similar cases in the literature [14, 15]. The first patient, reported by Utsuki et al. [14], had presented two hemorrhages at an interval of 10 days. Our patient presented the second hemorrhage 23 years after the first one. Even if it is only three isolated cases, it represents an important rate of patients with multiple AVMs. Consequently, the majority of authors [4, 9, 10, 12, 16, 17] interested in multiple cAVMs advocated treating unruptured AVMs associated with a ruptured AVM in the same patient. We shared the opinion that an associated AVM has to be treated, but the risks of the treatment have to be balanced. As we have managed in cases 1, 4, and 5, the therapeutic abstention is reasonable in case of eloquent area nidus or technical difficulties of the treatment. We proposed treating these associated AVMs more aggressively than idiopathic unruptured AVMs [6] but to keep in mind that the risks of treatment have to be well evaluated.
In general, every ruptured AVM has to be treated with the most appropriate modality of treatment in each case [6]. In case of high flow aneurysmal ruptures, we suggest treating only the aneurysm and to treat the AVM later if it is indicated (case 1). There is no consensus regarding the treatment indication of the unruptured AVM in these conditions [1, 6].
Patients with multiple cerebral AVMs have nidi that are separated by normal brain tissue. In some cases, it could be difficult to distinguish a single AVM with multiple nidal compartments as we could see in particular for AVMs involving the corpus callosum [8]. The bleeding of an AVM could also fragment the nidus and it could be misdiagnosed as multiple AVMs.
Conclusions
The pathophysiology and natural history of multiple cerebral AVMs remain unclear because the number of cases reported in the literature is low. Our series brings new epidemiologic information for idiopathic multiple AVMs in the adult population. A case-by-case approach is recommended, but we could suggest an aggressive treatment for ruptured AVM and associated AVM if it is located in a non-eloquent area. For incidentally diagnosed multiple AVMs, the abstention seems to be the better option, although the risk of bleeding is unknown.
References
Ericson K, Soderman M, Karlsson B, Guo WY, Lindquist C (1994) Multiple intracranial arteriovenous malformations: a case report. Neuroradiology 36:157–159
Hasegawa S, Hamada JI, Morioka M, Kai Y, Takaki S, Ushio Y (1999) Multiple cerebral arteriovenous malformations (AVMs) associated with spinal AVM. Acta Neurochir (Wien) 141:315–319
Iizuka Y, Rodesch G, Garcia-Monaco R, Alvarez H, Burrows P, Hui F, Lasjaunias P (1992) Multiple cerebral arteriovenous shunts in children: report of 13 cases. Childs Nerv Syst 8:437–444
Kuo YH, Santoreneos S, Roos D, Brophy BP (2007) Treatment of multiple arteriovenous malformations in pediatric patients with hereditary hemorrhagic telangiectasia and spontaneous hemorrhage. Report of two cases. J Neurosurg 107:489–494
Mansmann U, Meisel J, Brock M, Rodesch G, Alvarez H, Lasjaunias P (2000) Factors associated with intracranial hemorrhage in cases of cerebral arteriovenous malformation. Neurosurgery 46:272–279, discussion 279–281
Mohr JP, Parides MK, Stapf C, Moquete E, Moy CS, Overbey JR, Al-Shahi Salman R, Vicaut E, Young WL, Houdart E, Cordonnier C, Stefani MA, Hartmann A, von Kummer R, Biondi A, Berkefeld J, Klijn CJ, Harkness K, Libman R, Barreau X, Moskowitz AJ, International Ai (2014) Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet 383:614–621
Rigamonti D, Spetzler RF (1988) The association of venous and cavernous malformations. Report of four cases and discussion of the pathophysiological, diagnostic, and therapeutic implications. Acta Neurochir (Wien) 92:100–105
Robert T, Blanc R, Ciccio G, Gilboa B, Fahed R, Redjem H, Pistocchi S, Bartolini B, Piotin M (2015) Angiographic factors influencing the success of endovascular treatment of arteriovenous malformations involving the corpus callosum. J Neurointerv Surg 7(10):715–720
Romero FJ, Ibarra B, Rovira M (1988) Double intracranial arteriovenous malformation in the same patient. Neuroradiology 30:87
Salcman M, Scholtz H, Numaguchi Y (1992) Multiple intracerebral arteriovenous malformations: report of three cases and review of the literature. Surg Neurol 38:121–128
Spetzler RF, Martin NA (1986) A proposed grading system for arteriovenous malformations. J Neurosurg 65:476–483
Stone JL, Crowell RM, Lisner BM, Naseem M, Oldershaw JB (1983) Bilateral parietal arteriovenous malformations: report of a case. Neurosurgery 13:587–592
Sunwoo L, Sohn CH, Lee JY, Yi KS, Yun TJ, Choi SH, Cho YD, Kim JH, Park SW, Han MH, Paek SH, Kim YH, Kim JW, Chung HT, Kim DG (2015) Evaluation of the degree of arteriovenous shunting in intracranial arteriovenous malformations using pseudo-continuous arterial spin labeling magnetic resonance imaging. Neuroradiology 57(8):775–782
Utsuki S, Kurata A, Miyasaka Y, Takano M, Ootaka H, Fujii K (2002) Multiple arteriovenous malformations with hemorrhage. Acta Neurochir (Wien) 144:97–101
Willinsky RA, Lasjaunias P, Terbrugge K, Burrows P (1990) Multiple cerebral arteriovenous malformations (AVMs). Review of our experience from 203 patients with cerebral vascular lesions. Neuroradiology 32:207–210
Yoshimoto T, Kashiwaba T, Houkin K, Abe H (1996) Spontaneous disappearance of arteriovenous malformation during staged treatment of multiple cerebral arteriovenous malformations—case report. Neurol Med Chir (Tokyo) 36:812–814
Zellem RT, Buchheit WA (1985) Multiple intracranial arteriovenous malformations: case report. Neurosurgery 17:88–93
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Funding
No funding was received for this research.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Robert, T., Blanc, R., Botta, D. et al. Management of multiple cerebral arteriovenous malformations in a non-pediatric population. Acta Neurochir 158, 1019–1025 (2016). https://doi.org/10.1007/s00701-016-2785-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-016-2785-5