Abstract
Cobalt hydroxide nanoparticles (Co(OH)2 NPs) were uniformly deposited on flexible carbon cloth substrate (Co(OH)2@CC) rapidly by a facile one-step electrodeposition, which can act as an enzyme-free glucose and uric acid sensor in an alkaline electrolyte. Compositional and morphological characterization were examined by X-ray diffraction (XRD), scanning electron microscopy (SEM), and energy-dispersive X-ray spectroscopy (EDS), which confirmed the deposited nanospheres were Co(OH)2 nanoparticles (NPs). The electrochemical oxidation of glucose and uric acid at Co(OH)2@CC electrode was investigated by electrochemical impedance spectroscopy (EIS), cyclic voltammetry (CV), differential pulse voltammetry (DPV), and chronoamperometry methods. The results revealed a remarkable electrocatalytic activity toward the single and simultaneous determination of glucose and uric acid at about 0.6 V and 0.3 V (vs. Ag/AgCl), respectively, which is attributed to a noticeable synergy effect between Co(OH)2 NPs and CC with good repeatability, satisfactory reproducibility, considerable long-term stability, superior selectivity, outstanding sensitivity, and wide linear detection range from 1 uM to 2 mM and 25 nM to 1.5 uM for glucose and UA, respectively. The detection limits were 0.36 nM for UA and 0.24 μM for glucose (S/N = 3). Finally, the Co(OH)2@CC electrode was utilized for glucose and uric acid determination in human blood samples and satisfying results were obtained. The relative standard derivations (RSDs) for glucose and UA were in the range 6 to 14% and 0 to 3%, respectively. The recovery ranges for glucose an UA were 97 to 103% and 95 and 101%, respectively. These features make the novel Co(OH)2@CC sensor developed by a low-cost, efficient, and eco-friendly preparation method a potentially practical candidate for application to biosensors.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Glucose and uric acid are two important metabolic intermediates that can be used as biomarkers for various diseases, such as gout and glucose metabolism disorders. The quantitative detection of the two biomolecules has become an object of comprehensive study owing to their great worth in environmental monitoring, biological process commanding, food analysis, and human health monitoring [1]. As far as human health management is concerned, glucose is known as a marker for diverse diseases, such as diabetes [2], and detecting blood glucose levels regularly and closely is becoming vital for diabetics [3]. Like glucose, uric acid is also an essential metabolic end product of purines in human blood. Uric acid is treated as a crucial biomarker for diseases [4, 5], and people who are suffering from gout and uric acid nephropathy, the uric acid is as low as 6 mg L−1 [6]. Therefore, the quantitative monitoring of uric acid is indispensable for the treatment of some diseases.
To date, a great number of glucose and uric acid detection methods have been applied, such as chromatography, fluorescence spectroscopy [5, 7], colorimetry, capillary electrophoresis-amperometry, and electrochemical methods [8]. Among them, electrochemical technology has obtained more attention ascribed to its brilliant performance and real-time in vitro/in vivo detection abilities [9,10,11]. Although diverse biosensors have arisen, detection methods with high sensitivity and low limit are still the objectives chased by researchers. Liu et al. [12] have constructed layered double hydroxide (LDH)/rGO electrochemical biosensor which demonstrated amazing performance such as wide linear range and low detection limit in detection of AA, UA, and DA.
For the past few years, great efforts have been put in exploiting nanostructured materials, such as transition metals (Mn, Cu, Co, Pt, and Ni) in non-enzymatic electrochemical determination of uric acid and glucose [13] because of their great specific surface area, efficient electron transfer, and high electrocatalytic activity. These nanomaterials intrinsically serve as artificial enzymes to catalyze the electro-oxidation of uric acid and glucose on the electrode surface. Cobalt and its derivations such as Co(OH)2 excel in sensing applications, involving good limit of detection, high sensitivity, and catalytic property [14]. Regardless of the growing advancement in Co(OH)2 materials, they still suffer from low conductivity [15] owing to semiconductive nature [16] and highly insulating nature [9] of these transition metal hydroxides which confines the efficiency of the redox reactions. Therefore, combining Co(OH)2 with conductive supporting such as graphene, carbon nanotubes (CNTs), carbon paste electrode (CPE), carbon cloth, and glassy carbon electrode (GCE) can often enhance the properties since the composite electrodes have electron transport ways with low resistance. Javad et al. [17] have constructed polymeric graphitic carbon nitride/nanolayered Co(OH)2/CPE-based glucose sensors using chemical bath deposition with a wide concentration range (25–420 mM). Wang et al. [18] have synthesized Co(OH)F nanoflower on carbon cloth based on microplasma synthesis method for glucose detection. Liu et al. [19] have synthesized Co(OH)2 nanosheet precursor–modified GCE by hydrothermal method which displayed excellent catalytic ability toward glucose oxidation. Iman et al. [20] have fabricated a kind of Co(OH)2 nanorods deposited on a 3D graphene network by chemical bath deposition which provided a detection limit of 16 nM and a high sensitivity for glucose (3.69 mA mM−1 cm−2). Among different conductive substrates, development of flexible electrodes that can tolerate remarkable shape alterations have appealed wide spectrum of research curiosity for wearable electronics, compact sensing platforms, and miniaturized portable devices. Particularly, composite electrodes with multifunctional nanomaterials deposited on flexible carbon substrates have attracted widespread attention in sensing [21]. It is noteworthy that flexible carbon cloth is attracting more and more attention attributed to the advantages of sufficiently large surface area and good conductivity [16], which can reduce the internal resistance, accelerate charge transfer in electrochemical reaction, and increase the quantity of Co(OH)2 active centers and contact area for adsorption and diffusion of glucose and uric acid molecules, thus promoting the oxidation of glucose and uric acid. On the other hand, electrodeposition method without adding any binder at substrates is a promising strategy to construct composite electrodes because of its low cost, simplicity, and high efficiency.
Herein, we prepared a non-enzymatic electrochemical sensor that can detect glucose and uric acid by uniform electrodeposition low-cost, non-toxic, high electrocatalytic active Co(OH)2 nanoparticles onto carbon cloth rapidly. Compared with other procedures to fabricate electrode in the application of catalytic oxidation, this method is relatively fast, efficient, and eco-friendly. As a result of its rough surface and unique material structure, this composite electrode can provide a broad contact area and abundant active centers for adsorption and diffusion of glucose and uric acid molecules, thereby enabling efficient electrochemical reactions.
Experimental
Materials
Carbon cloth (CC) with a thickness of 0.33 mm was obtained from China Suzhou Sinero Technology Co., Ltd. Trisodium citrate dihydrate (C6H5Na3O7·2H2O, 99.5%), nitric acid (HNO3, 68%), and glucose (C6H12O6⋅H2O) were gotten from Nanjing Chemical Reagent Co., Ltd. Cobalt chloride hexahydrate (CoCl2⋅6H2O, 99%), ammonium sulfate ((NH4)2SO4, 99%), lactic acid (C3H6O3, 85%), sulfuric acid (H2SO4, 98%), and L( +)-ascorbic acid (C6H8O6, 99.7%) were purchased from Sinopharm Chemical Reagent Co., Ltd. Dopamine hydrochloride (C8H11NO2⋅HCl, 98%) and uric acid (C5H4N4O3, 99%) were obtained from Shanghai Aladdin Biochemical Technology Co., Ltd. Sodium hydroxide (NaOH, 96%) was gotten from Xilong Science Co., Ltd. All reagents in the experiment were analytical grade. The real samples (human blood) were received from the local hospital.
Fabrication of Co(OH)2@CC
Hydrophilicity of the carbon cloth surface was increased by acidification. Typically, 75 mL of HNO3 solution and 25 mL of H2SO4 solution (V/V = 3:1) with a mass fraction of 10% were taken into a beaker, and a 2 cm × 2 cm carbon cloth was immersed in the acidic solution for 1 h. Then, the immersed carbon cloth was ultrasonically cleaned with deionized water and absolute ethanol in turn, and dried at room temperature. The dried carbon cloth was cut to make sure that the effective working area of each carbon cloth was 0.5 cm × 0.5 cm.
In a mixed solution composed of 0.1 M CoCl2, 0.45 M (NH4)2SO4, and 0.2 M C6H5Na3O7 solution, the amperometric method was carried out to electrodeposit cobalt hydroxide on the surface of carbon cloth under an applied potential of − 1 V. Stirring was applied while electrodeposition, so that the Co2+ in the electrodeposition solution was evenly distributed and the concentration was consistent everywhere. The above electrodeposition process can be expressed by Eqs. (1)–(3).
After electrodeposition, the carbon cloth was rinsed with deionized water, and then was soaked in deionized water for 5 min to wash away most of the interfering substances.
In order to improve the sensitivity and stability of Co(OH)2@CC electrode, we employed cyclic voltammetry (CV) to electrochemically activate the electrode. The Co(OH)2@CC electrode was cycled 40 times through CV in the voltage range of − 0.2 to 0.8 V in 0.1 M NaOH.
Characteristics and electrochemical measures
A contact-angle analyzer (JC2000D1, Shanghai, China) was applied to define the contact angles (CAs) of carbon cloth and Co(OH)2@CC electrodes. We employed a thermogravimetric analyzer (DTG-60AH, Japan) to research the thermal performance of carbon cloth and Co(OH)2@CC electrodes at a temperature range of 25 to 600 ℃, a heating rate of 10 ℃/min, and a nitrogen flow rate of 10 mL/min. A scanning electron microscopy (SEM, Tokyo, Japan) was carried out to observe the morphologies and microstructures of Co(OH)2@CC electrodes. The energy-dispersive X-ray spectrometer (EDS) was exploited to determine the elements and relative content contained in the modified electrode. A combined multifunctional horizontal X-ray diffractometer (XRD, Japan) was utilized to research the crystal structure and phase composition of the modified electrode in a range of 5° to 70°. We made use of an electrochemical workstation (CHI 760E) to test the electrochemical performance of modified electrodes. We adopted different test methods, involving cyclic voltammetry (CV) (the voltage range was from − 0.2 to 0.8 V and the scan rate was 50 mV/s), amperometric method (i-t) (the applied voltage for glucose detection was 0.6 V), electrochemical impedance spectroscopy (EIS) (the electrolyte was 5 mM [Fe(CN)6]3−/4− containing 0.1 M KCl; applied amplitude was ± 5 mV and the frequency range was from 0.01 to 100,000 Hz) and differential pulse voltammetry (DPV) (the voltage range was from 0.1 to 0.5 V) throughout the experiment. The whole system was a traditional three-electrode system, and it was composed of three electrodes, in which Pt mesh was the counter electrode, the modified electrode was the working electrode, and a saturated Ag/AgCl was the reference electrode. Electrolyte in the experiment was 0.1 M sodium hydroxide solution.
Results and discussion
Characterization of composite electrode
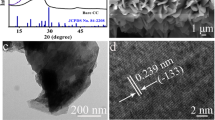
Figure 1a exhibits schematic measures applied for electrodeposition of fabricated Co(OH)2@CC electrode. The morphological, compositional, and structural analysis of the composite electrode were investigated by SEM, EDS, XRD, and thermogravimetric analysis. As displayed in Fig. 1b, carbon cloths with three-dimensional microstructural morphologies can be noticed, and the diameter of carbon fiber was approximately 5–10 µm. Under higher magnification in Fig. 1c, the surface of the CC was coated with abundant dense-packed spherical electro-deposition particles, and the tiny particles with an average diameter of about 300 nm from Fig. 1d. Owing to its rough surface and particular structure, this composite can supply numerous active sites and large specific surface area for the adsorption and spread of uric acid and glucose molecules, thus implementing efficient electrochemical reactions. The elemental composition of samples was surveyed by energy-dispersive X-ray spectroscopy (EDS). The results shown in Fig. 1e definitely demonstrated that C, O, and Co were three main elements.
a Schematic diagram of the synthetic process of the Co(OH)2@CC electrode. b, c, d SEM micrographs of Co(OH)2@CC at low and high magnification. e EDS analysis of fabricated Co(OH)2@CC. f Typical XRD diffraction graph of CC and constructed Co(OH)2@CC. g TGA diagram of CC and Co(OH)2@CC samples obtained in N2 between room temperature and 600 °C at a heating rate of 10 °C min.−1
In order to further investigate the structure of composites, the XRD patterns are displayed in Fig. 1f. Three diffraction peaks (labelled with ★) appeared at the 2θ value of 26°, 43°, and 54° were indicative of the (002), (100), and (004) planes of the hexagonal structure of the carbon fiber (JCPDS file no. 041–1487), respectively [22]. The characteristic peak emerged at 2θ = 26.3° was ascribed to the amorphous construction of carbon frameworks. In the curve of composite electrode, six characteristic peaks emerged at about 10°, 18°, 20°, 31°, 33°, and 36° (labelled with ▲) that was a representative XRD pattern of Co(OH)2 indexed to (003), (111), (006), (220), (100), and (311) planes (JCPDS file no. 42–1467), which was in compliance with previous reported results [17]. This result indicated that Co(OH)2 NPs have been successfully deposited on carbon cloth.
Furthermore, the thermostability of carbon cloth and Co(OH)2@CC electrode was forecasted utilizing thermogravimetric analysis under a N2 flow between room temperature and 600 °C; the result is shown in Fig. 1g. Compared to the CC, the decomposition of Co(OH)2 exhibited three main weight loss processes; the first stage was the desorption of physically adsorbed substances such as water and the volatile ingredients. Afterwards from 120 °C, which was assigned to the thermal decomposition of Co(OH)2, and the final result was Co3O4 [23]. The weight of sample left at 600 °C was above 92 wt%, demonstrating the outstanding thermostability of Co(OH)2@CC electrode.
Electrochemical measurement
The amperometry and cyclic voltammetry were carried out to investigate the electrocatalytic behaviors of Co(OH)2@CC toward glucose oxidation. The results are displayed in Fig. 2. An electrochemical activation process by repetitive CV measurements has been confirmed to be indispensable for the increment of the sensitivity and stability of the Co(OH)2@CC electrode toward glucose detection [24]. Therefore, our Co(OH)2@CC electrode was CV-activated treatment for 40 consecutive cycles in 0.1 M NaOH first, as shown in Fig. 2a. It was featured by the first abnormal sweep followed by a steady state where the current of anodic peak decreased to about 30% of the initial value assigned to the instability of the electrocatalytic layer in the course of repeated cyclic voltammetry scanning. With the increment of the cycle numbers, the stability of Co(OH)2@CC electrode also enhanced.
a The CV treatment of Co(OH)2@CC electrode for 40 cycles in 0.1 M NaOH media with a scan rate of 50 mV s−1. b Typical electrochemical impedance spectroscopy of CC and Co(OH)2@CC electrode before and after CV activation treatment in the presence of 5 mM of [Fe(CN)6]3−/4− containing 0.1 M KCl within a frequency range of 0.01 Hz to 100,000 Hz with applied amplitude of ± 5 mV. c Cyclic voltammograms of CC and Co(OH)2@CC electrodes with the presence and absence of 1 mM glucose. d The amperometric response of the as-fabricated Co(OH)2@CC electrode before and after CV activation treatment for successive injection of 10 uL of 1 mM glucose at + 0.6 V. e Cyclic voltammograms (CVs) of Co(OH)2@CC samples obtained by electrodeposition for different times with 1 mM glucose. f Real-time amperometric responses of Co(OH)2@CC sensor with continuous addition of 10 uL of 1 mM glucose solution under different applied potentials (0.3–0.65 V). g CVs investigated for determination different concentrations of glucose using Co(OH)2@CC electrodes as working electrode. h The CV curves of Co(OH)2@CC electrode at various scan rates from 10 to 100 mV/s containing 1 mM glucose. i Linearity curve based on anodic peak current vs. square root of the scan rate (v.1/2)
Electrochemical impedance spectroscopy (EIS) was utilized as a well-known mean to investigate the transform of electrode surface state, effective fabrication process, and charge transfer properties of sensing electrodes with an electron transfer probe [Fe(CN)6]3−/4−. The EIS spectra was composed of an inclined linear portion at the low-frequency region and a semicircle at high frequencies, in accordance with diffusion-limited process and the electron transfer-restricted process, respectively. The diameter of the semicircle corresponds to the charge transfer resistance (Rct), which represents the electron transfer kinetics of the redox needle at the electrode surface. Figure 2b depicts the obtained Nyquist plots of CC and Co(OH)2@CC electrode before and after cyclic voltammetry treatment. The semicircle of the bare carbon cloth electrode was approximately small, exhibiting good charge transfer rate with a low Rct value of 200 Ω owing to its high conductivity. However, post-electrodeposition of Co(OH)2, the semicircle somewhat became larger and the Rct was obtained as 300 Ω, while the semicircle further increased after it was electrochemically activated and the Rct was obtained as 325 Ω, which may be attributed to the mutual exclusion between negative charge of Co(OH)2-modified material and [Fe(CN)6]3−/4− needle that reduced electron transfer rate.
As shown in Fig. 2c, the electrochemical properties of pure CC and Co(OH)2@CC electrodes were surveyed by CV scanning in the range of − 0.2 V to 0.8 V. From the results, Co(OH)2@CC exhibited two definite anodic peaks in 1 mM glucose at around 0.3 V (vs. Ag/AgCl) and 0.6 V (vs. Ag/AgCl), while there was not any apparent redox peak at bare carbon cloth, both with and without glucose, which revealed that CC had no electrocatalytic activity for oxidation of glucose. During electrodeposition of Co(OH)2, electrodeposition solution consists of cobalt chloride, sodium citrate, and ammonium sulfate. Among them, CoCl2 served as a main salt providing cobalt source. (NH4)2SO4 supplies an alkaline environment, and the presence of SO42− groups may facilitate the adsorption process of Co complexes on CC electrodes, while NH4+ ions adsorbed on the electrode surface can prevent the diffusion of Co(OH)2 into growing clusters [25]. As a good complexing agent, Na3C6H5O7 can increase the polarization in the process of electrodeposition through the coordination of citrate, thereby reducing the internal stress of the coating. Moreover, sodium citrate is beneficial to the deposition of Co(OH)2 with fine grains.
Subsequently, the effects of CV activation treatment, different electrodeposition time, and applied voltage on electrocatalytic behaviors of Co(OH)2@CC were discussed aiming at optimizing synthetic and experimental conditions. Figure 2d describes the obtained amperometric response of the as-fabricated Co(OH)2@CC electrode before and after CV activation treatment under an applied bias voltage of + 0.6 V (vs. Ag/AgCl). Both of them displayed a stair-step response and Co(OH)2@CC-CV electrode showed a much higher response current with successive injection of 10 uL of 1 mM glucose into stirred electrolyte. The sensitivities of the Co(OH)2@CC-CV and Co(OH)2@CC electrodes were 1779.92 μA mM−1 cm−2 and 927.08 μA mM−1 cm−2, respectively, which could be calculated utilizing Eq. (4), confirming the activation of electrodes was beneficial for the improvement of sensitivity. The reason could be attributed to the conversion of Co(OH)2 to CoOOH/CoO2 during CV activation treatment, promoting electrocatalytic performance.
where k is the slope of calibration curve at the detection ranges of lower glucose concentration [26] and S is the geometric area of carbon cloth.
As obviously seen in Fig. 2e, two oxidation peaks appeared in CVs of all samples with different electrodeposition times exposed to 1 mM glucose. The peak current response of Co(OH)2(25)@CC was the least obvious in all electrodes, implying the worst electrocatalytic property for glucose, which can be assigned to the least active centers in the sample. With the extension of electrodeposition time, the active sites became abundant and the anodic peak current increased step-by-step. However, the catalytic property for glucose was not improving any longer when the deposition time increased up to 150 s, implying the limited active centers on the electrode surface. Moreover, the thickness of electrode samples increased gradually, bringing about the increment in the internal resistance of the electrode, which can result in the decline of the electrochemical property of Co(OH)2@CC electrode in turn.
Different amperometric response curves were obtained with working potential varying from 0.30 to 0.65 V, as illustrated in Fig. 2f. The response current increased in steps under different applied bias potentials upon the successive addition of glucose. Although the acquired results indicated that the higher operating potential brought about stronger response current, the sensitivity of sensors did not always improve with the increment of the applied voltage. The calculated sensitivities in the potential range of 0.3 V to 0.65 V were 7.56, 114.34, 600.6, 1648.28, and 1528.8 μA uM−1 cm−2, respectively (calculated by Eq. (4)). Hence, 0.6 V was selected as the optimum working potential for glucose determination.
Furthermore, the dependent response of the modified electrode on various glucose concentrations is depicted in Fig. 2g. As glucose concentration increases, the oxidation peak current at a voltage of around 0.6 V (vs. Ag/AgCl) that was originating from Co4+/ Co3+ redox couple increased, indicating additional current ascribed to the oxidation of glucose. Moreover, the peak potential value shifted toward a more negative direction, which attributed to the enhancement of the electrochemical oxidation reaction rate, while the reduction peak also transferred towards the consistent value. After the oxidation of glucose by Co4+, it was reduced to Co3+ and then Co3+ was reduced to Co2+, which formed two cathodic peaks at 0.2 V (vs. Ag/AgCl) and 0.5 V (vs. Ag/AgCl), respectively. In the present research, the proposed electrocatalytic mechanism was based on the following [17]:
CVs of Co(OH)2@CC electrode in 0.1 M NaOH solution containing 1 mM glucose with the scan rate increasing from 10 to 100 mV/s are depicted in Fig. 2h. As the scan rate rose, the strength of the oxidation and reduction peak ascribed to the redox couples of Co(II)/Co(III) and Co(III)/Co(IV) increased and the peak potential shifted toward a higher value. Figure 2i displays the relationship between the current response and root square of the scan rate. It showed that the oxidation peak current at 0.6 V (vs. Ag/AgCl) was proportional to the root square of the scan rate, in accordance with an equation I (uA) = 54.6 v1/2 + 26.4 (correlation coefficient of 0.997). The results revealed that the electrochemical oxidation of glucose on surface of Co(OH)2@CC electrode was a diffusion-controlled process [27].
In order to define the linear range, sensitivity and limit of detection (LOD) of our suggested sensor towards glucose, the amperometric response of Co(OH)2@CC is illustrated in Fig. 3. As displayed in Fig. 3a, the proposed sensor displayed a rapid and stepwise increase in current response with the addition of glucose, demonstrating the constructed sensor had a property of quick-response for glucose determination owing to the fast charge transfer rate and large specific surface area of Co(OH)2@CC. Figure 3b displays the corresponding regression curve of Co(OH)2@CC with two linear detection ranges from 1 uM to 0.4 mM and 0.4 mM to 2 mM, from which linear equations can be expressed as I (uA) = 34.3 + 450.2 CGLU (mM) and I (uA) = 146.8 + 181.2 CGLU (mM) in accordance with a correlation coefficient of 0.985 and 0.988, respectively. On one hand, the phenomenon of oxidation kinetics of catalytic processes is controlled by glucose absorption at extreme low concentration and by activation of glucose at high concentration, which is a rate-determining step, whereas in the middle region, the oxidation current is controlled by combined effect of absorption and activation of glucose on electrode surface [28]. On the other hand, the proposed electrocatalytic mechanism of Co(OH)2@CC electrode is based on reaction between Co(OH)2 and CoO2. With the concentration of glucose increased, more and more active sites were occupied, and then the rate of increase was slower than that in the first stage, so the electrode sensitivity became lower. At a signal-to-noise ratio of 3 (S/N = 3), the limit of detection (LOD) and sensitivity were calculated on the basis of Eqs. (4) and (8). A low LOD of 0.24 uM was obtained; the calculated sensitivities of Co(OH)2@CC were 1800.95 and 724.87 μA mM−1 cm−2, respectively. In addition, the sensor also achieved a fast response time of 0.1 s, as shown in Inset (ii). The acquired results suggested that Co(OH)2-modified CC exhibited outstanding electrocatalytic activity for glucose detection.
where SD is the standard deviation of blank signals (nB = 11) [29].
a The typical amperometric I-t curve of Co(OH)2@CC recorded in 0.1 M NaOH upon continuous injection of glucose with stable stirring at 0.6 V. Inset (i): amplification of amperometric response in the range of low concentration. Inset (ii): exhibiting the response time of Co(OH)2@CC. b The generated regression curve for current response vs. glucose concentration with two linear ranges. Inset (iii): showing magnified analysis. c The anti-interference test of Co(OH)2@CC electrode with successive addition of 1 mM glucose and 0.1 mM interferents such as DA, AA, and LA at + 0.6 V under constant stirring. d The CV curves of Co(OH)2@CC electrode with different bending angles (0°, 45°, 90°, and return to 0°) in the presence of 1 mM glucose. Inset (IV): the photographs of Co(OH)2@CC electrode under different bending angles clamped by electrode clamp
The performance of our fabricated Co(OH)2@CC composite electrode was also evaluated by some other significant parameters such as selectivity and anti-bending capability. The normal concentration of glucose in human blood is 3–8 mM, which is at least 10 times higher than those of potential interferents [2]. Our anti-interference test was conducted by adding 1 mM glucose and 0.1 mM other possible interfering substances such as ascorbic acid (AA), dopamine (DA), and lactic acid (LA) in NaOH electrolyte. Figure 3c exhibits that DA, AA, and LA would produce slight current response in comparison with glucose addition, indicating the superior anti-interference capability of Co(OH)2@CC composite electrode for glucose determination.
Furthermore, considering the deformation of sensing electrode attributed to the adverse external forces, the effect of electrode bending on the electrochemical property of Co(OH)2@CC sensor was assessed. Figure 3d displays the CV curves of composite electrode bending from 0 to 90°, and then back to 0°, and a negligible change could be observed, proving that Co(OH)2@CC electrode can detect target glucose molecules even if deformed.
The electrochemical behavior of CC and Co(OH)2@CC toward UA determination were further assessed through differential pulse voltammetry (DPV) method. As shown in Fig. 4a, DPV curve exhibited a well-defined oxidative peak at around 0.2 V (vs. Ag/AgCl) for Co(OH)2@CC, which corresponded to the conversion of Co2+ to Co3+, while CC showed almost none response current. Then, DPVs with various UA concentrations in a range of 0–1.5 uM using Co(OH)2@CC electrode were recorded in Fig. 4b. As observed, the oxidation peak current densities increased with UA concentration increasing, demonstrating well electro-chemical UA sensing performance of Co(OH)2@CC. The peak potential value transferred towards a more negative direction assigned to the enhancement of the electrochemical oxidation reaction rate [30]. The proposed mechanism for the direct oxidation of UA on Co(OH)2@CC electrode was expressed by Eqs. (9) and (10) [31]:
a DPV curves of CC and Co(OH)2@CC in 0.1 M NaOH. b DPV profiles of the composite electrode Co(OH)2@CC in 0.1 M NaOH containing 0.5, 1, or 1.5 μM UA. c DPV plots recorded of Co(OH)2@CC upon various different concentrations of UA (25 nM–5 µM). d The related calibration line of peak current data as a function of the corresponding concentration of UA derived from (c)
According to previously reported work [14], differential pulse voltammetry (DPV) can detect a very low concentration of UA accurately in the NaOH electrolyte owing to its better resolution and higher current sensitivity. To investigate the activity for determination of UA at Co(OH)2@CC, the DPV curves were conducted with continuous addition of uric acid from 25 nM to 5 μM, as shown in Fig. 4c. The oxidation peak current at potential of 0.2 V (vs. Ag/AgCl) enhanced with the continuous addition of UA, and calibration curve was plotted in Fig. 4d which demonstrated two ranges of 25 to 250 nM and 250 nM to 1.5 uM given by the linear regression equations of I (uA) = 94.1 CUA (μM) + 321.8 (R2 = 0.996) and I (uA) = 5.58 CUA (μM) + 343.6 (R2 = 0.990), respectively. The sensitivity of composite electrode calculated by Eq. (4) were 376.44 μA μM−1 cm−2 and 22.31 μA μM−1 cm−2, respectively. The LOD was computed to be 0.36 nM with signal-to-noise of 3 according to Eq. (8).
Table 1 demonstrates a comparison of the detection for glucose and uric acid in the present research and some other reported materials previously, indicating the linear detection ranges and the limits of detection of the proposed electrode are comparable with the most reported ones.
For ascertaining the anti-interference ability of the modified electrode, several potential interferents such as DA, AA, and LA that coexist with UA were assessed for their electrochemical behavior by DPV technique. The concentrations of UA and interfering substances are all 1 uM. The test results are depicted in Fig. 5, showing negligible changes in peak current in the presence of abovementioned interferents, exhibiting that Co(OH)2@CC electrode had prominent anti-interference ability for UA determination.
The repeatability, reproducibility, and storage stability of Co(OH)2@CC electrode for glucose and uric acid detection were also estimated in 0.1 M NaOH. Specifically, repeatability was conducted by the same electrode in five repetitive measurements, and the reproducibility was performed in five independent measurements using five different electrodes [38]. As shown in Fig. 6a, d, the calculated relative standard deviation (RSD) values of repeatability test for 1 mM glucose and 1 uM uric acid were 1.7% and 1.1%, respectively, while RSDs of reproducibility test were calculated to be 3.0% and 0.65%, respectively in Fig. 6b, e. The long-term stability was assessed by testing the sensing electrode with addition of 1 mM of glucose and 1 uM of uric acid for every 2 days over 15 days, as shown in Fig. 6c, f. Co(OH)2@CC sensors were stored in a vacuum environment, and the RSDs towards glucose and uric acid were computed to be 4.3% and 3.1%, respectively. The oxidation peak current responses retained 112.93% and 107.41% of the initial responses after 15 days for glucose and uric acid, respectively. Therefore, the above analyses confirmed that the fabricated sensor had satisfactory storage stability, all-right repeatability, and reliable reproducibility.
In the end, the applicability of Co(OH)2@CC was assessed by detecting the concentration of glucose and uric acid in four different drug-free human blood samples collected from Nanjing Integrated Traditional Chinese and Western Medicine Hospital. In the process of detecting glucose, the blood samples were diluted 26 times with 0.1 M NaOH by the addition of 0.8 mL blood sample into 20 mL 0.1 M NaOH. The amperometric tests were conducted with or without the blood samples, respectively. The obtained results (computed by the above linear equation) are summarized in Table 2. Each sample was measured three times to compute their relative standard deviation (RSD). DPV technique was applied to detect UA concentration, and the results are shown in Table 2. Based on Eq. (11), the relative standard derivations (RSDs) for glucose ranged from 6 to 14% and the recovery was found to range from 97 to 103%, while the RSDs for UA ranged from 0 to 3% and recoveries was between 95 and 101%.
where Ca is the concentration of glucose or UA measured by the Co(OH)2@CC biosensor and Cb represents the glucose or UA concentration obtained from the hospital [39]. The obtained results using Co(OH)2@CC biosensor consisted with those received from the hospital basically, demonstrating that the fabricated biosensor had good practical application attributed to its electrocatalytic performance. Conventional blood analysis is the recommended biological medium to monitor different biomarkers, but it involves inconvenient and painful processes to draw the sample which deter patients from frequent testing. Therefore, the non-invasive monitoring of both key biomarkers (GLU and UA) is of great significance, such as the emerging wearable sensing technologies, have the potential to extend our perceptions about those molecular signs easily, rapidly, and non-invasively.
Conclusions
This work put forward a straightforward method to synthesize the sphere-like Co(OH)2 nanoparticles deposited on the carbon cloth (Co(OH)2@CC) using electrodeposition method. Structural and morphological studies proved the successful synthesis of Co(OH)2 NPs with an average diameter of 300 nm. The experiments have confirmed that Co(OH)2@CC composite electrode was capable of detecting UA and glucose effectively as a non-enzymatic sensor. These superior detection parameters are mainly ascribed to the certain synergistic effect of the carbon cloth substrate and Co(OH)2 NPs, in which the carbon cloth with three-dimensional structure possesses large surface area and high electrical conductivity, while Co(OH)2 NPs provide abundant active centers and good electrocatalytic activity for the catalytic oxidation reaction of glucose and UA on the surface of electrode. Analysis of real human blood samples indicated that the composite electrode had decent recovery rate. Thus, the synthesized novel Co(OH)2@CC may be a kind of promising electrode material for practical detection of UA and glucose in real human samples.
References
Zhao MG, Shang JH, Qu HY, Gao RJ, Li H, Chen SG (2020) Fabrication of the Ni/ZnO/BiOI foam for the improved electrochemical biosensing performance to glucose. Anal Chim Acta 1095:93–98. https://doi.org/10.1016/j.aca.2019.10.033
Liu SL, Zeng W, Li YQ (2020) Synthesis of ZnCo2O4 microrods grown on nickel foam for non-enzymatic glucose sensing. Mater Lett 259:126820. https://doi.org/10.1016/j.matlet.2019.126820
Hazhir T, Abbas B, Joseph W (2020) Electrochemical glucose sensors in diabetes management: an updated review (2010–2020). Chem Soc Rev 49(21):7671–7709. https://doi.org/10.1039/D0CS00304B
Vasiliou F, Plessas AK, Economou A, Thomaidis N, Papaefstathiou GS, Kokkinos C (2022) Graphite paste sensor modified with a Cu(II)-complex for the enzyme-free simultaneous voltammetric determination of glucose and uric acid in sweat. J Electroanal Chem 917:116393. https://doi.org/10.1016/j.jelechem.2022.116393
Zhou ZP, Shu T, Sun YF, Si HX, Peng PW, Su L, Zhang XJ (2021) Luminescent wearable biosensors based on gold nanocluster networks for “turn-on” detection of uric acid, glucose and alcohol in sweat. Biosens Bioelectron 192:113530. https://doi.org/10.1016/j.bios.2021.113530
Ahmad R, Tripathy N, Ahn MS, Hahn YB (2017) Solution process synthesis of high aspect ratio ZnO nanorods on electrode surface for sensitive electrochemical detection of uric acid. Sci Rep 7:46475. https://doi.org/10.1038/srep46475
Huang S, Yang EL, Yao JD, Chu X, Liu Y, Zhang Y, Xiao Q (2019) Nitrogen, cobalt Co-doped fluorescent magnetic carbon dots as ratiometric fluorescent probes for cholesterol and uric acid in human blood serum. ACS Omega 4(5):9333–9342. https://doi.org/10.1021/acsomega.9b00874
Balasubramanian P, Annalakshmi M, Chen SM, Chen TW (2019) Ultrasensitive non-enzymatic electrochemical sensing of glucose in noninvasive samples using interconnected nanosheets-like NiMnO3 as a promising electrocatalyst. Sens Actuators B Chem 299:126974. https://doi.org/10.1016/j.snb.2019.126974
Asif M, Aziz A, Ashraf G, Wang ZY, Wang JL, Azeem M, Chen XD, Xiao F, Liu HF (2018) Facet-inspired core-shell gold nanoislands on metal oxide octadecahedral heterostructures: high sensing performance toward sulfide in biotic fluids. ACS Appl Mater Interfaces 10(43):36675–36685. https://doi.org/10.1021/acsami.8b12186
Asif M, Wang HT, Dong S, Aziz A, Zhang GA, Xiao F, Liu HF (2017) Metal oxide intercalated layered double hydroxide nanosphere: with enhanced electrocatalyic activity towards H2O2 for biological applications. Sens Actuators B Chem 239:243–252. https://doi.org/10.1016/j.snb.2016.08.010
Aziz A, Asif M, Azeem M, Ashraf G, Wang ZY, Xiao F, Liu HF (2019) Self-stacking of exfoliated charged nanosheets of LDHs and graphene as biosensor with real-time tracking of dopamine from live cells. Anal Chim Acta 1047:197–207. https://doi.org/10.1016/j.aca.2018.10.008
Asif M, Aziz A, Wang HT, Wang ZY, Wang W, Ajmal M, Xiao F, Chen XD, Liu HF (2019) Superlattice stacking by hybridizing layered double hydroxide nanosheets with layers of reduced graphene oxide for electrochemical simultaneous determination of dopamine, uric acid and ascorbic acid. Mikrochim Acta 186(2):61. https://doi.org/10.1007/s00604-018-3158-y
Wu MY, Zhu JW, Ren YF, Yang N, Hong Y, Wang WJ, Huang W, Si WL, Dong XC (2019) NH2-GQDs-doped nickel-cobalt oxide deposited on carbon cloth for nonenzymatic detection of glucose. Adv Mater Interfaces 7(1):1901578. https://doi.org/10.1002/admi.201901578
Gupta J, Arya S, Verma S, Singh A, Sharma A, Singh B, Prerna Sharma R (2019) Performance of template-assisted electrodeposited Copper/Cobalt bilayered nanowires as an efficient glucose and uric acid senor. Mater Chem Phys 238:121969. https://doi.org/10.1016/j.matchemphys.2019.121969
Asif M, Aziz A, Wang ZY, Ashraf G, Wang JL, Luo HB, Chen XD, Xiao F, Liu HF (2019) Hierarchical CNTs@CuMn layered double hydroxide nanohybrid with enhanced electrochemical performance in H2S detection from live cells. Anal Chem 91(6):3912–3920. https://doi.org/10.1021/acs.analchem.8b04685
Aziz A, Asif M, Ashraf G, Iftikhar T, Hu JL, Xiao F, Wang SQ (2022) Boosting electrocatalytic activity of carbon fiber@fusiform-like copper-nickel LDHs: sensing of nitrate as biomarker for NOB detection. J Hazard Mater 422:126907. https://doi.org/10.1016/j.jhazmat.2021.126907
Javad T, Sayedeh Fatemeh NA, Mojtaba S (2018) A new bifunctional nanostructure based on two-dimensional nanolayered of Co(OH)2 exfoliated graphitic carbon nitride as a high performance enzyme-less glucose sensor: impedimetric and amperometric detection. Anal Chim Acta 1034:63–73. https://doi.org/10.1016/j.aca.2018.06.052
Wang Q, Chen YL, Zhu RX, Luo MF, Zou ZR, Yu HM, Jiang X, Xiong XL (2020) One-step synthesis of Co(OH)F nanoflower based on micro-plasma: as an effective non-enzymatic glucose sensor. Sens Actuators B Chem 304:127282. https://doi.org/10.1016/j.snb.2019.127282
Liu TT, Li M, Dong P, Zhang YJ, Guo LP (2018) Design and facile synthesis of mesoporous cobalt nitride nanosheets modified by pyrolytic carbon for the nonenzymatic glucose detection. Sens Actuators B Chem 255:1983–1994. https://doi.org/10.1016/j.snb.2017.08.218
Iman S, Umakant P, Atiye P, Nanasaheb MS, Seongil I, Seong CJ (2016) Enhanced non-enzymatic amperometric sensing of glucose using Co(OH)2 nanorods deposited on a three dimensional graphene network as an electrode material. Microchim Acta 183(8):2473–2479. https://doi.org/10.1007/s00604-016-1890-8
Asif M, Aziz A, Ashraf G, Iftikhar T, Sun YM, Xiao F, Liu HF (2022) Unveiling microbiologically influenced corrosion engineering to transfigure damages into benefits: a textile sensor for H2O2 detection in clinical cancer tissues. Chem Eng J 427:131398. https://doi.org/10.1016/j.cej.2021.131398
Litkohi HR, Bahari A, Ojani R (2017) Synthesis of Pt-Ni-Fe/CNT/CP nanocomposite as an electrocatalytic electrode for PEM fuel cell cathode. J Nanoparticle Res 19(8):278. https://doi.org/10.1007/s11051-017-3969-5
Zhang LJ, Wang N, Cao PF, Lin M, Xu L, Ma HY (2020) Electrochemical non-enzymatic glucose sensor using ionic liquid incorporated cobalt-based metal-organic framework. Microchem J 159:105343. https://doi.org/10.1016/j.microc.2020.105343
Pak M, Moshaii A, Siampour H, Abbasian S, Nikkhah M (2020) Cobalt-copper bimetallic nanostructures prepared by glancing angle deposition for non-enzymatic voltammetric determination of glucose. Mikrochim Acta 187(5):276. https://doi.org/10.1007/s00604-020-04246-2
Rios-Reyes CH, Granados-Neri M, Mendoza-Huizar LH (2009) Kinetic study of the cobalt electrodeposition onto glassy carbon electrode from ammonium sulfate solutions. Quim Nova 32(9):2382–2386. https://doi.org/10.1590/s0100-40422009000900028
Hu FX, Hu T, Chen SL, Wang DP, Rao QH, Liu YH, Dai FY, Guo CX, Yang HB, Li CM (2020) Single-atom cobalt-based electrochemical biomimetic uric acid sensor with wide linear range and ultralow detection limit. Nanomicro Lett 13(1):7. https://doi.org/10.1007/s40820-020-00536-9
Choi T, Kim SH, Lee CW, Kim H, Choi SK, Kim SH, Kim E, Park J, Kim H (2015) Synthesis of carbon nanotube-nickel nanocomposites using atomic layer deposition for high-performance non-enzymatic glucose sensing. Biosens Bioelectron 63:325–330. https://doi.org/10.1016/j.bios.2014.07.059
Asif M, Liu HW, Aziz A, Wang HT, Wang ZY, Ajmal M, Xiao F, Liu HF (2017) Core-shell iron oxide-layered double hydroxide: high electrochemical sensing performance of H2O2 biomarker in live cancer cells with plasma therapeutics. Biosens Bioelectron 97:352–359. https://doi.org/10.1016/j.bios.2017.05.057
Duan XX, Liu KL, Xu Y, Yuan MT, Gao T, Wang J (2019) Nonenzymatic electrochemical glucose biosensor constructed by NiCo2O4@Ppy nanowires on nickel foam substrate. Sens Actuators B Chem 292:121–128. https://doi.org/10.1016/j.snb.2019.04.107
Hoan NTV, Minh NN, Trang NTH, Thuy LT, Van Hoang C, Mau TX, Vu HXA, Thu PTK, Phong NH, Khieu DQ (2020) Simultaneous voltammetric determination of uric acid, xanthine, and hypoxanthine using CoFe2O4/reduced graphene oxide-modified electrode. J Nanomater 2020:1–15. https://doi.org/10.1155/2020/9797509
Abu Zahed M, Barman SC, Toyabur RM, Sharifuzzaman M, Xuan X, Nah J, Park JY (2019) Ex situ hybridized hexagonal cobalt oxide nanosheets and RGO@MWCNT based nanocomposite for ultra-selective electrochemical detection of ascorbic acid, dopamine, and uric acid. J Electrochem Soc 166(6):304–311. https://doi.org/10.1149/2.0131906jes
Arsalan M, Awais A, Qiao XJ, Sheng QL, Zheng JB (2020) Preparation and comparison of colloid based Ni50Co50(OH)2/BOX electrocatalyst for catalysis and high performance nonenzymatic glucose sensor. Microchem J 159:105486. https://doi.org/10.1016/j.microc.2020.105486
Amin KM, Muench F, Kunz U, Ensinger W (2021) 3D NiCo-Layered double Hydroxide@Ni nanotube networks as integrated free-standing electrodes for nonenzymatic glucose sensing. J Colloid Interface Sci 591:384–395. https://doi.org/10.1016/j.jcis.2021.02.023
Setoudeh N, Jahani S, Kazemipour M, Foroughi MM, Nadiki HH (2020) Zeolitic imidazolate frameworks and cobalt-tannic acid nanocomposite modified carbon paste electrode for simultaneous determination of dopamine, uric acid, acetaminophen and tryptophan: investigation of kinetic parameters of surface electrode and its analytical performance. J Electroanal Chem 863:114045. https://doi.org/10.1016/j.jelechem.2020.114045
Manjula N, Vinothkumar V, Chen SM, Sangili A (2020) Simultaneous and sensitive detection of dopamine and uric acid based on cobalt oxide-decorated graphene oxide composite. J Mater Sci Mater Electron 31(15):12595–12607. https://doi.org/10.1007/s10854-020-03810-z
Mounesh MP, Kumara NYP, Jilani BS, Mruthyunjayachari CD, Reddy KRV (2019) Synthesis and characterization of tetra-ganciclovir cobalt (II) phthalocyanine for electroanalytical applications of AA/DA/UA. Heliyon 5(7):e01946. https://doi.org/10.1016/j.heliyon.2019.e01946
Xu YX, Gao TT, Liang YM, Xiao D (2020) Intercalation lithium cobalt oxide for the facile fabrication of a sensitive dopamine sensor. ChemElectroChem 7(5):1193–1200. https://doi.org/10.1002/celc.202000099
Liu LL, Liu L, Wang YL, Ye BC (2019) A novel electrochemical sensor based on bimetallic metal-organic framework-derived porous carbon for detection of uric acid. Talanta 199:478–484. https://doi.org/10.1016/j.talanta.2019.03.008
Wang KD, Wu C, Wang F, Liao MH, Jiang GQ (2020) Bimetallic nanoparticles decorated hollow nanoporous carbon framework as nanozyme biosensor for highly sensitive electrochemical sensing of uric acid. Biosens Bioelectron 150:111869. https://doi.org/10.1016/j.bios.2019.111869
Acknowledgements
We thank very much advanced analysis and testing center of Nanjing Forestry University for SEM measurements.
Funding
This work was financially supported by the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (21KJA220004).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, F., Shi, F., Chen, C. et al. Electrochemical fabrication of Co(OH)2 nanoparticles decorated carbon cloth for non-enzymatic glucose and uric acid detection. Microchim Acta 189, 385 (2022). https://doi.org/10.1007/s00604-022-05437-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05437-9