Abstract
A new electrocatalytic biosensor (MOF-74(Cu) NS-CC) based on the in situ deposition of MOF-74(Cu) nanosheet on carbon cloth via a bottom-up synthetic approach in a glass tube was developed. The electrocatalytic activity of the deposited MOF-74(Cu) NS was demonstrated in the oxidation of glucose to gluconate under alkaline conditions. The results revealed that the proposed method of in situ formation of MOF-74(Cu) NS onto a carbon cloth surface in a multi-layer solution is capable to generate a stable MOF-74(Cu) NS-CC electrode with excellent sensing performance. When the as-synthesized MOF-74(Cu) NS-CC was applied directly as the working electrode for glucose sensing, it showed much higher conductivity and redox activity than MOF-74(Cu) NS-GCE. With the potential applied at 0.55 V (vs. Ag/AgCl), this new electrocatalytic biosensor exhibits an excellent linear relationship between current density and concentration of glucose. Moreover, a wide linear range of detection (1.0 to 1000 μM) was observed. The limit of detection was found to be 0.41 μM (S/N = 3). The response sensitivity is 3.35 mA mM−1 cm−2 when the concentration of glucose is in the range 1–100 μM and 3.81 mA mM−1 cm−2 for the 100–1000 μM concentration range. This study provides a low-cost, easy to prepare, and reproducible methodology for the synthesis of highly redox-active nanomaterials based on the in situ formation of two-dimensional MOF-74(Cu) NS for the development of new electrocatalytic biosensors.

Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metal-organic frameworks (MOFs) are multi-functional materials that have great plasticity in nano-engineering to create unique functionality and are widely applied in a broad area of material science [1,2,3,4] and sensing [5, 6]. Some recent developments of MOFs have extended their applications in electrocatalysis and electroanalysis because a number of advantages could be achieved by integrating MOFs to develop new materials for these important applications. MOFs are porous crystalline materials and may act as host substrates for the immobilization of catalysts, such as nanoparticles and enzymes [7, 8]. Moreover, MOFs can be used as the sacrificial templates for calcinations into metallic or carbonaceous nanomaterials to produce structurally defined electrocatalysts [9,10,11]. However, most investigations find that MOFs suffer from some critical limitations, such as hindered active sites and poor electron transfer that significantly reduce the redox activity of the catalyst [12]. These adverse factors may restrict the potential application of MOFs in the field of electrocatalysis and electroanalysis, and thus, further advancement is required.
Recently, two-dimensional (2D) MOFs have been demonstrated to generate special structures that are able to offer tailor-made chemical and physical properties. The 2D MOFs also possess many highly accessible active sites on the surface, which is significant for the applications in electrocatalysis [13] and electrosensing [14] as the redox activity of the metallic sites in the 2D-MOF materials can be enhanced [15]. Therefore, many new generations of electrosensor have been fabricated with 2D MOFs such as NCST-1 ([Co(DCTP)(L)(H2O)2]n) [14]. Most nanomaterial-based electrochemical sensors are currently fabricated through the modification of nanomaterials and followed immobilizing them with Nafion onto the surface of the working electrode [16, 17]. This process for assembling of sensing systems is time-consuming and also causes significant drawbacks. In particular, for the immobilization of nanomaterials that have low conductivity, Nafion usually causes higher resistance of the composites than that of the pure nanomaterial. The activity of the nanomaterial is therefore significantly reduced in the applications of electrocatalysis and electrosensing. The recent developments have shown that direct growth (in situ deposition) of nanomaterials onto the surface of some conductive substrates, such as carbon cloth (CC), metal foam (nickel foam and copper foam), and indium tin oxide glass (ITO), as the working electrode is able to overcome the problem. This strategy for nanomaterials deposition is a recognized method to enhance the catalyst–substrate interaction for efficient electron transport during the electrocatalytic reactions [18]. Recently, many studies have been reported on the growth of 3D-MOF onto a surface of conductive substrates for electrocatalytic applications. For example, MOF-74(Co,Fe)/Co nanosheet was in situ deposited to the surface of carbon cloth to form Co,Fe-MOF-74/Co/CC. The material was applied as hybrid electrode for water splitting under alkaline conditions [19]. In addition, the 2D-MOF with a conductive substrate was fabricated, such as the core-shell Co3O4/Ni-MOFs NS that was deposited to the surface of carbon cloth by a facile two-step hydrothermal strategy to form Co3O4/Ni-MOFs-CC. The material was demonstrated as a supercapacitor under alkaline conditions and also utilized as a catalyst in the near-infrared photocatalytic hydrogen evolution [20]. However, no example of electrocatalytic biosensing system developed with in situ deposition of 2D MOFs onto the surface of conductive substrate has been reported thus far.
Our previous studies demonstrated that copper-based MOFs can be developed as the sensitive and selective biosensors for rapid detection of biologically important analysts [21]. Monitoring glucose levels in blood are a very essential part of managing diabetes because glucose is an important biological analyst for clinical diagnosis [22, 23]. The development of catalysts with high activity and selectivity to promote the catalytic oxidation of glucose is important in the field of biofuel cells [24] and nonenzymatic sensors [25, 26]. Up to now, various precious transition metals and their complexes have been shown very good electrocatalytic activity towards nonenzyme glucose oxidation [27, 28]. However, to improve their electrical conductivity, redox activity, and sensitivity, most of these electrocatalytic glucose-sensing systems are generally developed based on nanomaterial-carbon nanotubes or grapheme composites. To advance further these sensing systems for practical and wider applications, a facile protocol for surface deposition or immobilization of electrocatalysts to achieve excellent conductivity and redox activity is crucial [29,30,31].
In the present study, a new copper-based MOF nanosheet (MOF-74(Cu) NS) was synthesized under room temperature conditions via a bottom-up synthesis strategy. The in situ fabricated MOF-74(Cu) NS-CC electrode applying Cu(II) ions as the metal node, which is also the active site for electrocatalysis, was used directly (no further calcination is required) as the nonenzymatic electrosensor for direct determination of glucose contents in human blood or serum samples. The synthesized materials of MOF-74(Cu) NS and MOF-74(Cu) NS-CC were fully characterized by TEM, SEM, AFM, XPS, XRD, FTIR, and BET. The electrocatalytic activity, selectivity, sensitivity, and stability of the new MOF-74(Cu) NS-CC electrosensor towards glucose in alkaline media were also investigated.
Experimental section
Reagents and instruments
Sodium hydroxide (0.1 M in water), copper nitrate trihydrate (Cu(NO3)2·3H2O), 2,5-dihydroxyterephthalic acid (DHTP), N,N-dimethylformamide (DMF), acetonitrile, ethanol, glucose (Glu), ascorbic acid (AA), fructose (Fru), lactose (Lac) and uric acid (UA), glyphosate (Gly), hydroquinone (HQ), catechol (CTH), calcium chloride dihydrate (CaCl2·2H2O), and magnesium chloride hexahydrate (MgCl2·6H2O) were purchased from Aladdin (Shanghai, China). Carbon cloth was purchased from Wuhan Gaoss Union technology Co., Ltd. Human serum was obtained from Xiamen Hospital of Traditional Chinese Medicine. All chemicals and solvents used were analytical grade. The de-ionized water (18.2 MΩ cm) used was from a Milli-Q water purification system.
Synthesis of MOF-74(Cu) NS-CC
Firstly, a piece of carbon cloth (Ø = 1.2 cm) was pre-cleaned successively by sonication in a diluted hydrochloric acid solution (3 M), acetone, and ethanol; the pre-cleaned carbon cloth was dried at 333 K under vacuum condition. The PTFE seal tape was torn into thin lines and passing through the middle of the carbon cloth; paper clip was used as a pendant to suspend the carbon cloth in the linker layer. The solutions for in situ growth of MOF-74(Cu) NS onto carbon cloth surface were prepared as described in the “Supplementary information” section for the synthesis of MOF-74(Cu) NS.
Fabrication of MOF-74(Cu) NS-CC as a working electrode
MOF-74(Cu) NS-CC (1.0 × 0.5 cm) was directly applied as a working electrode for electrochemical tests, in which a Pt wire was used as the counter electrode and a Ag/AgCl electrode was used as a reference electrode. A MOF-74(Cu) NS-GCE was prepared with MOF-74(Cu) NS and using Nafion (0.5 wt%) as the binder to deposit onto the surface of a glassy carbon electrode (GCE). In order to compare the glucose determination performance between MOF-74(Cu) NS-CC and MOF-74(Cu) NS-GCE, MOF-74(Cu) NS-GCE was also fabricated by following the previous reported procedures [32].
Electrochemical testing
The amperometric i-t curve was carried out for the determination of glucose in 10 mL NaOH aqueous solution (0.1 M). To this NaOH aqueous solution, the glucose solution (10 μL) at different concentration was added. The current signal at 0.55 V (vs Ag/AgCl) of the oxidation of glucose for each addition was recorded by an electrochemical work station (CHI660).
Results and discussion
Characterization of MOF-74(Cu) NS and MOF-74(Cu) NS-CC
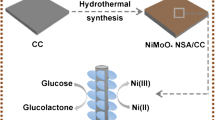
The synthesis of MOF-74(Cu) NS-CC was conducted in a glass tube, as shown in Fig. S1, using multi-layer of solutions for the in situ formation of MOF-74(Cu) NS onto the carbon cloth surface. The as-synthesized materials were characterized with SEM and TEM. Figure 1 a and b shows the SEM images of MOF-74(Cu) NS-CC. The images indicate that MOF-74 (Cu) NS was grown evenly on the surface. Figure 1c shows clearly that the morphology of MOF-74 (Cu) NS is a honeycomb-like nanosheet array. As indicated from the AFM image of MOF-74 (Cu) NS shown in Fig. S2, the thickness of the nanosheet is about 4.5 nm. Moreover, Fig. 1d reveals that the MOF-74(Cu) NS was an ultra-thin nanosheet. From Fig. 1 e–h, the EDS mapping images of MOF-74 (Cu) NS-CC reveal an extremely homogeneous distribution of Cu, O, and C in MOF-74 (Cu) NS-CC. The characterization of MOF-74(Cu) NS was investigated in detail with XPS, XRD, FT-IR, and BET (Fig. S3–S4). These characterization results support evidently the in situ formation of MOF-74(Cu) NS on carbon cloth.
The working mechanism of the proposed electrocatalytic biosensor based on MOF-74(Cu) NS-CC
A facile protocol to assemble the new MOF-74(Cu) NS-based electrocatalytic biosensor for the detection of glucose was shown in Scheme 1. The sensing system was developed with respect to the electrocatalytic activity of MOF-74(Cu) NS to catalyze the oxidation of glucose to gluconate under alkaline conditions. In this sensing system, glucose was oxidized by the Cu3+ species, which were produced in situ via the electrochemical oxidation of Cu2+ species under alkaline conditions [33]. The redox process could be detected by electrochemical workstation, in which the oxidation peak for glucose was observed at 0.55 V (vs Ag/AgCl). The higher the concentration of glucose present in the solution, the higher the oxidation current can be observed. When the electrocatalyst (MOF-74(Cu) NS) was in situ deposited onto carbon cloth, the electrode (MOF-74(Cu) NS-CC) is able to provide a much better conductivity and electrocatalytic performance than MOF-74(Cu) NS-GCE. In addition, MOF-74(Cu) NS-CC is able to make the sensing process more convenient than that with the modified-GCE electrode because our new sensing system does not require additional electrode modification process such as calcinations.
Electrochemical characterization of MOF-74(Cu) NS-CC for glucose detection
Electrochemical impedance spectroscopy (EIS) is widely used to study the characteristics of electrodes. As shown in Fig. 2a, the bare carbon cloth (CC) exhibited very low resistance while MOF-74(Cu) NS-CC has higher resistance compared to the bare CC. The result indicates that MOF-74(Cu) NS was successfully grown onto the CC surface. The MOF-74(Cu) NS was also deposited onto GCE with Nafion for comparison. The MOF-74(Cu) NS-GCE exhibited the highest resistance because the existence of Nafion in the composite further reduced the conductivity of MOF-74(Cu) NS. The high resistance observed for MOF-74(Cu) NS-GCE also supports that MOF-74(Cu) NS was successfully immobilized onto the GCE surface [34].
a Nyquist diagrams of 0.1 mM NaOH at GCE, MOF-74(Cu) NS-GCE, carbon cloth, and MOF-74(Cu) NS-CC; b Feasibility analysis of the electrocatalytic sensor to detect Glu with MOF-74(Cu) NS-CC; c CV spectra of MOF-74 (Cu) NS-CC in different pH; d CV curves of MOF-74(Cu) NS-CC in 25 mM PBS (pH 7.0); e CV spectra of MOF-74 (Cu) NS-CC with 1 mM glucose in different scan rate; f The plot of the relationship between square root of scan rates and the current density
The feasibility of direct use MOF-74(Cu) NS-CC as an electrosensor for glucose determination was investigated in a 0.1 M NaOH aqueous solution with or without glucose. As shown in Fig. 2b, upon the addition of glucose, a clear oxidation current with the peak potential at 0.55 V (vs Ag/AgCl) was observed, which indicated that MOF-74(Cu) NS is active to catalyze the oxidation of glucose (Glu). The electrochemical response of MOF-74(Cu) NS-CC towards Glu (1 mM) in the range of pH 9–14 was also investigated. As shown in Fig. 2c, there was no obvious glucose oxidation peak found under pH 9–12 because no Cu3+ species was produced to oxidize glucose under these pH conditions. As increasing the pH to 13 or 14, the Cu2+ ions of MOF-74(Cu) NS-CC were oxidized to Cu3+ upon the current was applied. The results support that the electrochemically generated Cu3+ species are able to oxidize glucose to gluconate efficiently at pH 13–14 and a current peak at 0.55 V (vs Ag/AgCl) was observed. It was found that the electrochemical response of MOF-74(Cu) NS-CC towards 1 mM Glu at pH 13 was the best condition. The corresponding catalytic mechanism is illustrated by the following equations:
In order to further investigate the mechanism of the electrosensor, the cyclic voltammogram (CV) curves of MOF-74(Cu) NS-CC in 25 mM PBS (pH 7.0) were obtained. As shown in Fig. 2d, when it was scan from 0 to 0.7 V (vs Ag/AgCl), the Cu(II) in MOF-74(Cu) NS was oxidized to Cu(III) and an oxidation peak was recorded at 0.55 V (vs Ag/AgCl). The Cu(III) species is the active catalyst to oxidize glucose to gluconate in alkaline solution and the Cu(III) species was reduced to Cu(II) during the reaction. The corresponding reduction signal was recorded at 0.4 V when it was scanned from 0.7 to 0 V (vs Ag/AgCl). Moreover, the electron transfer number was calculated according to the following equation [35]:
where Ep is the peak potential, Ep/2 is the half peak potential, F is the Faraday constant (96,485 C mol−1), R is a universal gas constant (8.314 J K−1 mol−1), and T is the Kelvin temperature (298 K). As shown in Fig. 2d, the values of |Ep-Ep/2| for the oxidation peak and reduction peak were determined to be 55.8 mV and 56 mV, respectively. Thus according to Eq. (4), the values of n for both oxidation and reduction were calculated to be 1.01 and 1.008, respectively. The results indicate that it is a single-electron transfer system when applying MOF-74(Cu) NS-CC as the working electrode.
The cyclic voltammograms of using MOF-74(Cu) NS-CC as the working electrode in a NaOH aqueous solution (0.1 M) containing glucose (1 mM) were obtained at different scan rates. As shown in Fig. 2 e and f, the current density increases with the increasing of scan rates from 10 to 100 mV s−1. A linear relationship between the peak current densities and the square root of scan rates was established (Fig. 2f). The results may imply that the glucose oxidation occurred on MOF-74(Cu) NS-CC electrode is probably a diffusion controlled process [36].
Calibration curve for the determination of glucose
Figure 3a shows the current density responses of MOF-74 (Cu) NS-CC after the reaction with glucose at different concentrations. The current density increased gradually with respect to the increasing concentration of glucose in the range of 1.0 to 1000 μM. Moreover, Fig. 3b shows a good linear relationship between the current density and glucose concentrations. The linear equations were established as follows:
a Amperometric sensing and the corresponding calibration curve of Glu by successive addition of Glu at MOF-74 (Cu) NS-CC at 0.55 V (vs Ag/AgCl) in 0.1 M NaOH solution; b The corresponding calibration curve of Glu by successive addition of Glu at MOF-74 (Cu) NS-CC, the insert plot was the fitting curve in the range of 1–80 μM; c Amperometric sensing and the corresponding calibration curve of Glu by successive addition of Glu at MOF-74 (Cu) NS-GCE at 0.55 V (vs Ag/AgCl) in 0.1 M NaOH solution; d The corresponding calibration curve of Glu by successive addition of Glu at MOF-74 (Cu) NS-GCE
The limit of detection (LOD) was 0.41 μM (S/N = 3) and the response sensitivity calculated was 3.35 mA mM−1 cm−2 when the glucose concentration was in the range of 1–100 μM and 3.81 mA mM−1 cm−2 for the 100–1000 μM.
We further investigated the performance of MOF-74(Cu) NS-GCE in the determination for glucose for comparison. As show in Fig. 3c, the current density increased gradually with respect to the concentration of glucose increasing from 5.0 to 100 μM. The linear equation obtained was ΔJ = 2.25[C(Glu)](μM) − 1.7 with a R2 = 0.997. The LOD was 1.9 μM (S/N = 3). The response sensitivity was calculated to be 2.25 mA mM−1 cm−2, which is significantly lower than that of MOF-74 (Cu) NS-CC. The results reveal that MOF-74(Cu) NS-CC exhibits better performance in the determination of glucose, which may be attributed to its high conductivity. The performance of this electrosensor was also compared with other nanomaterial-based sensors reported recently. As listed in Table 1, we found that MOF-74 (Cu) NS-CC exhibits higher sensitivity, lower detection limit, and wider linear range than most reported sensing systems in the detection of glucose. For the palladium-based nanomaterials such as Pd/PEDOT [37], PdFe alloy [38] and Pd–Ni alloy [40] do not exhibit better performance than the present study. Moreover, palladium as a noble metal presents some drawbacks such as high cost and low abundance. MOF-74(Cu) NS is the copper-based materials, which is the abundant metal and thus it has more advantage than the palladium-based materials. Another electrocatalytic glucose sensor based on CuO-NA-GCE, which is constructed by calcination of Cu-MOF [43], gave a slightly better LOD (0.15 μM) than MOF-74 (Cu) NS-CC (0.41 μM). However, MOF-74 (Cu) NS-CC biosensor can be in situ fabricated by one-step synthesis and also can be utilized directly as the working electrode without modification process required.
The study of selectivity, reproducibility, and stability of the sensor
To avoid the interference responses for nonenzymatic sensors is one of the great challenges in analysis of glucose in human serum samples. The primary interferents such as lactose (Lac), fructose (Fru), glyphosate (Gly), hydroquinone (HQ), and catechol (CTH) exhibit a comparable electroactive activity to glucose. In addition, ascorbic acid (AA), calcium ion (Ca2+), magnesium ions (Mg2+), and uric acid (UA) are also present in the blood serum samples and all these interferents may affect the performance of sensors [36]. Therefore, to evaluate the anti-interference performance of our new biosensor towards these potential interfering species is essential. Figure 4a exhibits the amperometric responses of the MOF-74(Cu) NS-CC at 0.55 V in 0.1 M NaOH aqueous solution after the addition of 50.0 μM Glu, 5.0 mM AA, 10 mg mL−1 Lac, 10 mM Ca2+, 10 mM Mg2+, 5.0 mM Fru, 5.0 mM UA, 0.5 mM Gly, 0.5 mM HQ, and 0.5 mM CTH successively. The results clearly showed that MOF-74(Cu) NS-CC gives response selectively for Glu only, while the response for the interferents examined was weak and negligible. The previous work reported that Gly, HQ, and CTH could be oxidized with electrocatalysts to produce current response under either weak acid or weak base conditions [44, 45]. However, the sensing condition of MOF-74(Cu) NS-CC was conducted under highly alkaline conditions (pH 13–14); therefore, this electrocatalytic biosensor showed no response to Gly, HQ, and CTH. Moreover, the current density can increased again with second addition of glucose (50 μM) as shown in Fig. 4 a and b. The results may indicate that MOF-74(Cu) NS-CC biosensor has very high selectivity towards glucose.
The manufacturing reproducibility was assessed by comparing the current generated from proposed electrocatalytic biosensors fabricated independently in a 0.1 M NaOH aqueous solution with 50 μM glucose for six times. The relative standard deviation (RSD) obtained is 5.6%, indicating that our synthetic protocol has very good reproducibility. In addition, to demonstrate the stability and long-term stability of the biosensor, MOF-74(Cu) NS-CC electrode was freshly fabricated and was tested in a 0.1 M NaOH aqueous solution with 50 μM glucose and then was stored at ambient conditions for 30 days. After that, the biosensor was re-assessed under the same conditions and only 6% of the current density was decreased compared to its original as shown in Fig. S5. The results show that the electrocatalytic biosensor has high stability.
Determination of glucose in serum samples
To verify the feasibility of using MOF-74(Cu) NS-CC for routine and practical analysis, the sensor was utilized to detect glucose in human serum. The test procedures and conditions were briefly described as follows. Three human serum samples (0.1 mL each), which were obtained from Xiamen Hospital of Traditional Chinese Medicine, were diluted with 9.9 mL of 0.1 M NaOH aqueous solution independently. The concentrations of glucose determined in these samples were 4.95 mM, 5.39 mM, and 8.07 mM, respectively, which were very comparable with the sample concentrations given by the hospital (Table 2). In addition, the recoveries were examined using the method of standard addition. Three serum samples given by the hospital were spiked with standard solution of glucose (1.0 mM, 2.0 mM, and 4.0 mM, respectively). The spiked concentration was chosen based on the normal concentration of glucose in blood, which is in the range of 80–120 mg dL−1 (4.4–6.6 mM) [46]. The recoveries observed with MOF-74(Cu) NS-CC biosensor were in the range of 96–104% (Table 2). These results indicate that the present protocol is feasible and reliable to fabricate biosensors for the determination of glucose from human serum samples.
Conclusion
In conclusion, a new MOF-74(Cu) NS-CC–based electrocatalytic biosensor with excellent sensing performance was developed through one-step in situ formation of MOF-74(Cu) NS onto the surface of carbon cloth in a multi-layer solution. Compared with the use of a glassy carbon electrode modified with MOF-74(Cu) NS as a working electrode for the determination of glucose, MOF-74(Cu) NS-CC exhibited much higher conductivity and sensitivity. More importantly, the method of in situ deposition MOF-74(Cu) NS onto a conductive substrate with no calcination process required is more convenient than that of using glassy carbon electrode modification. The MOF-74(Cu) NS-CC biosensor was also demonstrated in the determination of glucose directly from real human serum samples. The results showed that the biosensor is accurate and reliable. The present study also demonstrates the potential application of two-dimensional metal-organic frameworks to fabricate electrochemical sensors in situ for analytical applications.
References
Pang Y, Zang X, Li H, Liu J, Chang Q, Zhang S, Wang C, Wang Z (2020) Solid-phase microextraction of organophosphorous pesticides from food samples with a nitrogen-doped porous carbon derived from g-C3N4 templated MOF as the fiber coating. J Hazard Mater 384:121430
Li JT, Huang WZ, Wang MM, Xi SB, Meng JS, Zhao KN, Jin J, Xu WW, Wang ZY, Liu X, Chen Q, Xu LH, Liao XB, Jiang YL, Owusu KA, Jiang BL, Chen CX, Fan DNA, Zhou L, Mai LQ (2019) Low-crystalline bimetallic metal-organic framework electrocatalysts with rich active sites for oxygen evolution. Acs Energy Letters 4:285–292
Fan WD, Wang X, Liu XP, Xu B, Zhang XR, Wang WJ, Wang XK, Wang YT, Dai FN, Yuan DQ, Sun DF (2019) Regulating C2H2 and CO2 storage and separation through pore environment modification in a microporous Ni-MOF. ACS Sustain Chem Eng 7:2134–2140
Cabrera-Garcia A, Checa-Chavarria E, Rivero-Buceta E, Moreno V, Fernandez E, Botella P (2019) Amino modified metal-organic frameworks as pH-responsive nanoplatforms for safe delivery of camptothecin. J Colloid Interface Sci 541:163–174
Lai H, Li G, Xu F, Zhang Z (2020) Metal–organic frameworks: opportunities and challenges for surface-enhanced Raman scattering–a review. J Mater Chem C 8:2952–2963
Hu S, Ouyang W, Guo L, Lin Z, Jiang X, Qiu B, Chen G (2017) Facile synthesis of Fe3O4/g-C3N4/HKUST-1 composites as a novel biosensor platform for ochratoxin A. Biosens Bioelectron 92:718–723
Song JY, He WT, Shen H, Zhou ZX, Li MQ, Su P, Yang Y (2019) Construction of multiple enzyme metal-organic frameworks biocatalyst via DNA scaffold: a promising strategy for enzyme encapsulation. Chem Eng J 363:174–182
Gkaniatsou E, Sicard C, Ricoux R, Benahmed L, Bourdreux F, Zhang Q, Serre C, Mahy JP, Steunou N (2018) Enzyme encapsulation in mesoporous metal-organic frameworks for selective biodegradation of harmful dye molecules. Angewandte Chemie-International Edition 57:16141–16146
Yi XR, He XB, Yin FX, Chen BH, Li GR, Yin HQ (2019) Co-CoO-Co3O4/N-doped carbon derived from metal-organic framework: the addition of carbon black for boosting oxygen electrocatalysis and Zn-air battery. Electrochim Acta 295:966–977
Mukherjee S, Cullen DA, Karakalos S, Liu KX, Zhang H, Zhao S, Xu H, More KL, Wang GF, Wu G (2018) Metal-organic framework- derived nitrogen-doped highly disordered carbon for electrochemical ammonia synthesis using N-2 and H2O in alkaline electrolytes. Nano Energy 48:217–226
Hu SS, Yan JJ, Huang XM, Guo LH, Lin ZY, Luo F, Qiu B, Wong KY, Chen GN (2018) A sensing platform for hypoxanthine detection based on amino-functionalized metal organic framework nanosheet with peroxidase mimic and fluorescence properties. Sensors and Actuators B-Chemical 267:312–319
Salunkhe RR, Kaneti YV, Kim J, Kim JH, Yamauchi Y (2016) Nanoarchitectures for metal-organic framework-derived nanoporous carbons toward supercapacitor applications. Acc Chem Res 49:2796–2806
Zhong H, Ly KH, Wang M, Krupskaya Y, Han X, Zhang J, Zhang J, Kataev V, Büchner B, Weidinger IM (2019) A phthalocyanine-based layered two-dimensional conjugated metal–organic framework as a highly efficient electrocatalyst for the oxygen reduction reaction. Angew Chem 131:10787–10792
Xiao Q-Q, Liu D, Wei Y-L, Cui G-H (2019) A new multifunctional two-dimensional cobalt (II) metal–organic framework for electrochemical detection of hydrogen peroxide, luminescent sensing of metal ions, and photocatalysis. Polyhedron 158:342–351
Zhao M, Wang Y, Ma Q, Huang Y, Zhang X, Ping J, Zhang Z, Lu Q, Yu Y, Xu H (2015) Ultrathin 2D metal–organic framework nanosheets. Adv Mater 27:7372–7378
Wang Q, Yang Y, Gao F, Ni J, Zhang Y, Lin Z (2016) Graphene oxide directed one-step synthesis of flowerlike graphene@ HKUST-1 for enzyme-free detection of hydrogen peroxide in biological samples. ACS Appl Mater Interfaces 8:32477–32487
Yin D, Liu J, Bo X, Li M, Guo L (2017) Porphyrinic metal-organic framework/macroporous carbon composites for electrocatalytic applications. Electrochim Acta 247:41–49
Chaudhari NK, Jin H, Kim B, Lee K (2017) Nanostructured materials on 3D nickel foam as electrocatalysts for water splitting. Nanoscale 9:12231–12247
Zha Q, Li M, Liu Z, Ni Y (2020) Hierarchical Co, Fe-MOF-74/Co/carbon cloth hybrid electrode: simple construction and enhanced catalytic performance in full water splitting. ACS Sustain Chem Eng 8:12025–12035
Zhang L, Zhang Y, Huang S, Yuan Y, Li H, Jin Z, Wu J, Liao Q, Hu L, Lu J (2018) Co3O4/Ni-based MOFs on carbon cloth for flexible alkaline battery-supercapacitor hybrid devices and near-infrared photocatalytic hydrogen evolution. Electrochim Acta 281:189–197
Hu S, Zhu L, Lam CW, Guo L, Lin Z, Qiu B, Wong KY, Chen G, Liu Z (2019) Fluorometric determination of the activity of inorganic pyrophosphatase and its inhibitors by exploiting the peroxidase mimicking properties of a two-dimensional metal organic framework. Microchim Acta 186:190
Chang J, Li H, Hou T, Duan W, Li F (2018) Paper-based fluorescent sensor via aggregation induced emission fluorogen for facile and sensitive visual detection of hydrogen peroxide and glucose. Biosens Bioelectron 104:152–157
Gu C, Gai P, Hou T, Li H, Xue C, Li F (2017) Enzymatic fuel cell-based self-powered homogeneous immunosensing platform via target-induced glucose release: an appealing alternative strategy for turn-on melamine assay. ACS Appl Mater Interfaces 9:35721–35728
Neto SA, Almeida TS, Palma LM, Minteer SD, Andrade ARD (2014) Hybrid nanocatalysts containing enzymes and metallic nanoparticles for ethanol/O 2 biofuel cell. J Power Sources 259:25–32
Mahmoud MA, O’Neil D, El-Sayed MA Hollowand solid metallic nanoparticles in sensingand in nanocatalysis. Chemistry of Materials 26:44–58
Zhao L, Thomas JP, Heinig NF, Abd-Ellah M, Wang X, Leung KT (2014) Au–Pt alloy nanocatalysts for electro-oxidation of methanol and their application for fast-response non-enzymatic alcohol sensing. J Mater Chem C 2:2707–2714
Wu XF, Bao CC, Niu QQ, Lu WB (2019) A novel method to construct a 3D FeWO4 microsphere-array electrode as a non-enzymatic glucose sensor. Nanotechnology 30:165501
Lu WB, Wu XF (2018) Ni-MOF nanosheet arrays: efficient non-noble-metal electrocatalysts for non-enzymatic monosaccharide sensing. New J Chem 42:3180–3183
Shahhoseini L, Mohammadi R, Ghanbari B, Shahrokhian S (2019) Ni(II) 1D-coordination polymer/C-60-modified glassy carbon electrode as a highly sensitive non-enzymatic glucose electrochemical sensor. Appl Surf Sci 478:361–372
Darabdhara G, Bordoloi J, Manna P, Das MR (2019) Biocompatible bimetallic Au-Ni doped graphitic carbon nitride sheets: a novel peroxidase-mimicking artificial enzyme for rapid and highly sensitive colorimetric detection of glucose. Sensors and Actuators B-Chemical 285:277–290
Dong SY, Zhang DD, Cui H, Huang TL (2019) ZnO/porous carbon composite from a mixed-ligand MOF for ultrasensitive electrochemical immunosensing of C-reactive protein. Sensors and Actuators B-Chemical 284:354–361
Li Y, Dai H, Zhang Q, Zhang S, Chen S, Hong Z, Lin Y (2016) In situ generation of electron acceptor to amplify the photoelectrochemical signal from poly (dopamine)-sensitized TiO 2 signal crystal for immunoassay. J Mater Chem B 4:2591–2597
Zhang Y, Su L, Manuzzi D, de los Monteros HVE, Jia W, Huo D, Hou C, Lei Y (2012) Ultrasensitive and selective non-enzymatic glucose detection using copper nanowires. Biosens Bioelectron 31:426–432
Kang X, Mai Z, Zou X, Cai P, Mo J (2007) A sensitive nonenzymatic glucose sensor in alkaline media with a copper nanocluster/multiwall carbon nanotube-modified glassy carbon electrode. Anal Biochem 363:143–150
Nenkova R, Wu J, Zhang Y, Godjevargova T (2013) Influence of different nanozeolite particles on the sensitivity of a glucose biosensor. Anal Biochem 439:65–72
Chen T, Liu D, Lu W, Wang K, Du G, Asiri AM, Sun X (2016) Three-dimensional Ni2P nanoarray: an efficient catalyst electrode for sensitive and selective nonenzymatic glucose sensing with high specificity. Anal Chem 88:7885–7889
Hosseini H, Rezaei SJT, Rahmani P, Sharifi R, Nabid MR, Bagheri A (2014) Nonenzymatic glucose and hydrogen peroxide sensors based on catalytic properties of palladium nanoparticles/poly(3,4-ethylenedioxythiophene) nanofibers. Sensors & Actuators B Chemical 195:85–91
Wang J, Wang Z, Zhao D, Xu C (2014) Facile fabrication of nanoporous PdFe alloy for nonenzymatic electrochemical sensing of hydrogen peroxide and glucose. Anal Chim Acta 832:34–43
Liu M, Ru L, Wei C (2013) Graphene wrapped Cu 2 O nanocubes: non-enzymatic electrochemical sensors for the detection of glucose and hydrogen peroxide with enhanced stability. Biosens Bioelectron 45:206–212
Zhao D, Xu C (2015) A nanoporous palladium-nickel alloy with high sensing performance towards hydrogen peroxide and glucose. Journal of Colloid & Interface Science 447:50–57
Li Y, Zhong Y, Zhang Y, Weng W, Li S (2015) Carbon quantum dots/octahedral Cu2O nanocomposites for non-enzymatic glucose and hydrogen peroxide amperometric sensor. Sensors Actuators B Chem 206:735–743
Gao W, Tjiu WW, Wei J, Liu T (2014) Highly sensitive nonenzymatic glucose and H2O2 sensor based on Ni (OH) 2/electroreduced graphene oxide− multiwalled carbon nanotube film modified glass carbon electrode. Talanta 120:484–490
Luo Y, Wang Q, Li J, Xu F, Sun L, Bu Y, Zou Y, Kraatz H-B, Rosei F (2020) Tunable hierarchical surfaces of CuO derived from metal–organic frameworks for non-enzymatic glucose sensing. Inorganic Chemistry Frontiers 7:1512–1525
Gu C, Wang Q, Zhang L, Yang P, Xie Y, Fei J (2020) Ultrasensitive non-enzymatic pesticide electrochemical sensor based on HKUST-1-derived copper oxide@ mesoporous carbon composite. Sensors Actuators B Chem 305:127478
Alshahrani LA, Miao L, Zhang Y, Cheng S, Sathishkumar P, Saravanakumar B, Nan J, Gu FL (2019) 3D-flower-like copper sulfide nanoflake-decorated carbon nanofragments-modified glassy carbon electrodes for simultaneous electrocatalytic sensing of co-existing hydroquinone and catechol. Sensors 19:2289
Wang J (2008) Electrochemical glucose biosensors. Chem Rev 108:814–825
Funding
This study is funded by the STS Key Project of Fujian Province (2017T3007), Nature Sciences Funding of Fujian Province (2018J01682) and the Project of Quangang District Science and Technology Bureau (2019G01), and Natural Science Foundation of Guangdong Province (2019A1515011799).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing of interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 11929 kb)
Rights and permissions
About this article
Cite this article
Hu, S., Lin, Y., Teng, J. et al. In situ deposition of MOF-74(Cu) nanosheet arrays onto carbon cloth to fabricate a sensitive and selective electrocatalytic biosensor and its application for the determination of glucose in human serum. Microchim Acta 187, 670 (2020). https://doi.org/10.1007/s00604-020-04634-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04634-8