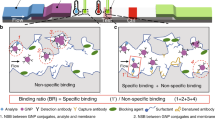
Abstract
Human immunodeficiency virus (HIV) infection inflicts significant economic and social burdens on many countries worldwide. Given the substantial morbidity and mortality from HIV infection, there is an urgent need for accurate and early detection of the virus. In this study, immunofluorescence and visual techniques are described that detect the HIV-1 p24 antigen, which relied on selective recognition of Ag+/Ag nanoparticles (Ag NPs) and Cu2+/Cu+ using cadmium telluride quantum dots (CdTe QDs). After the sandwich immunoreactions were accomplished, the alkaline phosphatase (ALP) hydrolyzed L-ascorbic acid 2-phosphate (AAP) to form ascorbic acid (AA) that further reduces Ag+ and Cu2+ to Ag NPs and Cu+, respectively. This method was highly sensitive and selective and could detect as low as 1 pg/mL of p24 antigen by naked eyes and had a good linearity in the concentration range 1–100 pg/mL. When using Ag+ and Cu2+ as media, the limit of detection (LOD) of the new method was 0.3 pg/mL and 0.2 pg/mL, respectively. Compared with clinical electrochemiluminescence immunoassay (ECLIA) results and clinical data, this method demonstrated good consistency for the quantification of HIV-1 p24 antigen in 34 clinical serum samples. In addition, this method could accurately distinguish HIV from other viruses and infections such as hepatitis B virus, systemic lupus erythematosus, hepatitis C virus, Epstein-Barr virus, cytomegalovirus, lipemia, and hemolysis. Therefore, our dual-mode analysis method may provide additional solutions to identify clinical HIV infection.
Graphical abstract
An immunofluorescence and visualization dual-mode strategy for the detection of p24 antigen was constructed based on immune recognition reaction and a phenomenon that cadmium telluride quantum dots (CdTe QDs) can selectively recognize Ag+/Ag nanoparticles (Ag NPs) and Cu2+/Cu+.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
HIV infection/acquired immune deficiency syndrome is one of the major diseases causing significant morbidity and mortality worldwide. Effective management of HIV infection relies on early and accurate detection of the disease [1, 2]. The current HIV diagnosis techniques mainly detect the virus antigen and nucleic acid. Electrochemiluminescence immunoassay (ECLIA) is a highly sensitive and specific technique for quantitative detection of the HIV-1 p24 antigen and corresponding antibodies in human serum or plasma [3]. Despite its sensitivity of pg/mL, the technique is relatively expensive. The urgent need for rapid HIV diagnosis has accelerated the development of several HIV detection techniques, which include visual, colorimetric, fluorescence, photoelectrochemical, ECL, and electrochemical [4,5,6,7,8,9,10,11]. These methods offer unique and varied advantages in HIV diagnosis such as point-of-care testing (POCT) among others.
For POCT analysis, the choice of the signal readouts is extremely important. Various luminescent nanomaterials including QDs [12, 13], copper nanoclusters [14], copper nano-assemblies [15], silver nanoclusters [16], and gold nanoclusters [17, 18] are being applied in disease diagnosis and biological imaging among other functions. Among the luminescent nanomaterials, QDs offer significant advantages such as simple and rapid synthesis and low cost, require non-complicated or inexpensive devices, and have a long shelf life. Certain QDs (CdTe/CdSe QDs, etc.) have unique properties such cation exchange with metal ions (Ag+/Hg2+/Cu2+). Cation exchange reaction (CER) readily and rapidly occurs at room temperature (RT), and the color of the solution before and after the reaction can be distinguished with the naked eyes [19,20,21]. QDs can identify metal ions, accurately distinguish metal ions of different valences, and identify complexes of metal ions such as Cu2+/Cu+, Ag+/Ag NPs, Ag+/C–Ag+-C, and Hg2+/T-Hg2+-T [19, 22,23,24,25]. The selective CER property of QDs has been applied in analysis of AA and ALP, as well as in the diagnosis of urinary stone diseases by measuring the level of oxalate in urine in our preliminary work.
We developed a highly sensitive visual and fluorescent immunoassay for HIV-1 p24 antigen by combining multiple QD-based selective recognition reactions. The application of this method for the clinical diagnosis of HIV was also assessed with Ag+ and Cu2+ as signal media, respectively [19, 23]. In this work, ALP hydrolyzes its substrate, AAP to produce reductant AA that further reduced Ag+ and Cu2+ to Ag NPs and Cu+. Ag+/Ag NPs and Cu2+/Cu+ could then be identified using QDs, respectively (Scheme 1). Preliminary findings demonstrated the high sensitivity of the technique, which was comparable to that of the ECL method. As low as 1 pg/mL of p24 antigen could be recognized by naked eyes. The p24 antigen contents of 13 clinical HIV-positive samples which had been detected by our method were consistent with that of clinical ECLIA and RT-PCR findings. The specificity of the technique using 14 samples from the patients with other diseases (hepatitis B virus, Epstein-Barr virus, cytomegalovirus, hepatitis C virus, systemic lupus erythematosus, lipemia, and hemolysis) and 7 healthy individuals was also satisfactory. Therefore, the novel fluorescence method is an alternative technique for accurate HIV infection diagnosis.
Experimental section
Reagents
All reagents used in this study were of analytical grade level and were used without further purification. The 96-well plates were purchased from Boruijie Biotechnology Company (Chengdu, China). Purity CdCl2, KBH4, Na3C6H5O7·2H2O, NaNO3, Mg(NO3)2, HCl, and NaOH were purchased from Kelong Chemical Reagents (Chengdu, China). 3-(N-Morpholino) propanesulfonic acid (MOPS) was purchased from Solarbio Technology Co., Ltd (Beijing, China). Anti-HIV-1 p24 antibody (Ab1), anti-HIV-1 p24 antibody (Biotin-Ab2), and HIV-1 p24 protein were purchased from Abcam Company (Shanghai, China). Streptavidin–alkaline phosphatase (SA-ALP), bovine serum albumin (BSA), human serum albumin (HSA), thrombin, transferrin, and papain were purchased from Sigma Company (Shanghai, China). Tween-20 was obtained from Sangon Biotechnology Co., Ltd (Shanghai, China). Na2TeO3, 3-mercaptopropionic acid (MPA), L-ascorbic acid-2-phosphate (AAP), AgNO3, and CuSO4 were purchased from Aladdin Reagent Co., Ltd (Shanghai, China). The buffers include blocking buffer, 1% (w/v, g mL−1) BSA in 10 mM pH 7.4 MOPS buffer; assay buffer, 10 mM MOPS buffer (100 mM NaNO3, 2.5 mM Mg (NO3)2) at pH 7.4; and washing buffer, 10 mM MOPS buffer (100 mM NaNO3, 2.5 mM Mg (NO3)2) at pH 7.4 supplemented with 0.05% (v/v) Tween-20. Purity deionized water (18.2 MΩ-cm) was obtained from the Milli-Q water system (Chengdu Ultrapure Technology Co., Ltd., Chengdu, China). All stock solutions and regents were stored in a refrigerator at 4 °C before use.
Synthesis of CdTe QDs
The CdTe QDs were synthesized as previously described [25,26,27]. Briefly, 52 μL of MPA was added into freshly prepared 50 mL solution containing 0.5 mmol CdCl2 and 0.2 g trisodium citrate before adjusting the pH to 10.5 using NaOH. Na2TeO3 (0.1 mmol) and KBH4 (50 mg) were added into the above mixture before refluxing the final solution for 1 h to obtain the CdTe QDs. The CdTe QDs were precipitated using n-propanol and centrifuged at 11,000 rpm to obtain pure CdTe QDs. The red CdTe QDs were re-dispersed in high purity water before use.
Immunoassay
The process was performed using a 96-well plate. Briefly, 10 μL of 20 μg mL−1 Ab1 and 30 μL assay buffer were added in each well and incubated overnight at 4 °C. The plate was washed three times using 200 μL of washing buffer to remove unbound antibodies. Uncoated active sites were saturated using 100 μL of blocking buffer at 37 °C for 1 h.
Forty microliters of HIV-1 p24 protein or serum samples with 60 μL assay buffer were added in the wells and incubated for 1 h at 37 °C. The wells were rinsed three times before adding 40 μL biotin-Ab2 (5 μg mL−1) and 60 μL assay buffer. The wells were rinsed three times after incubation for 1 h at 37 °C. To form the Ab1-p24-Ab2-SA-ALP sandwich complexes, 40 μL SA-ALP (5 μg mL−1) and 60 μL assay buffer were added in the plate wells and incubated at RT for 15 min.
Fifty microliters of AAP (1 mM) and 100 μL assay buffer were added to the wells and incubated at 37 °C for 20 min before transferring the solution into EP tubes. CER was performed using either AgNO3 or CuSO4.
CER was performed as follows: For AgNO3, 50 μL AgNO3 (5 μM) was added into the tubes and incubated for 20 min at RT. After adding 0.5 μL CdTe QDs, a further 10 min incubation at RT was performed. For CuSO4, 5 μL CuSO4 (50 μM) was added into the tubes and incubated at RT for 2.5 min. Then, 0.3 μL CdTe QDs was added and incubated 5 min at RT. Fluorescence was detected with 365 nm as excitation wavelength.
Results and discussion
Principles and feasibility of HIV-1 p24 antigen analysis
The dual-mode fluorescence and visual of HIV-1 p24 antigen analysis strategy are mainly based on immune reaction to form Ab1-p24-Ab2-SA-ALP sandwich complexes. The p24 antigen content positively correlated with ALP content. ALP catalyzes the production of reducing AA from AAP. AA reduces Ag+ to Ag NPs or Cu2+ to Cu+. QDs selectively react with Ag+ and Ag NPs (Scheme 1A, the quenching power of Ag+ on QDs is stronger than that of Ag NPs, mode 1) and Cu2+ and Cu+ (Scheme 1B, the quenching power of Cu+ on QDs is stronger than that of Cu2+, mode 2). P24 antigen is then detected based on the fluorescence signals (Scheme 1C). Herein, Ag+ was used in the turn-on mode, whereas Cu2+ was used in the turn-off mode in the p24 antigen analysis strategy.
In our previous work, we found that QDs can selectively detect Ag+ and Ag NPs and Cu2+ and Cu+ [19, 23]. In this study, transmission electron microscopy (TEM) analysis of QDs under different conditions further validated these findings (Fig. 1). When Ag+ was added to QDs (Fig. 1A), spherical QDs reunited to Ag2Te significantly (Fig. 1B). Energy dispersive spectroscopy (EDS) (Fig. 1C and D) revealed that Ag could be found after CER. This implies that ion exchange occurred between Ag+ and Cd2+, resulting in the formation of Ag2Te. Both Cu2+ and Cu+ can trigger the CER resulting agglomeration of QDs to form CuTe. The agglomeration power of Cu+ on QDs was superior to that of Cu2+ (Fig. 1E vs. 1F) but comparable to that of Ag+. Fluorescence experiments further verified the above phenomenon in the absence of immune reaction (Fig. 1G). Ag+, Cu2+, and Cu+ significantly reduced the fluorescence signal of QDs. AA inhibits the quenching of QDs by Ag+ (Fig. 1G-b vs. 1G-c). The fluorescence quenching ability of Cu+ to QDs is superior to that of Cu2+ (Fig. 1G-j vs. 1G-k, l, and m). Furthermore, AA produced by hydrolyzation of AAP by ALP inhibited the quenching of QDs by Ag+ with similar efficiency as directly added AA (Fig. 1G-c vs. 1G-d).
Regarding the feasibility of p24 immunoassay using Ag+ and Cu2+, the concentration of p24 antigen (0, 10 pg/mL, 100 pg/mL, and 1 ng/mL) was directly proportional to the intensity of the fluorescence signals. Therefore, Ag+ can potentially be used for p24 antigen analysis (Fig. 1G-e, f, g, and h). Different from Ag+, when using Cu2+, the fluorescence signal of the solution decreased (Fig. 1G-n, o, p) with an increase in p24 antigen concentration of (1–100 pg/mL). This resulted from the generation of Cu+. Therefore, Cu2+ is also efficient for p24 antigen analysis, and its sensitivity is similar to that of Ag+.
Analytical performance of HIV-1 p24 antigen
Under the optimized conditions (Figs. S1 and S2), analytical performance of p24 antigen using Ag+ as a medium was investigated. Different p24 concentrations generated varied color intensities under UV light irradiation, visible to the naked eye. For instance, the emission generated by 1 pg/mL p24 antigen was significantly stronger than 0.1 pg/mL p24 (Fig. 2A). Between 1 and 100 pg/mL, the red color of the solutions deepened with increase in p24 concentration. We found a perfect linear relationship between the intensity of the fluorescence signals and the logarithm of p24 concentration (1–100 pg/mL) (Fig. 2B and C). The linear equation of the relationship can be expressed as Y = 163LogC + 135 (R2 = 0.996). The LOD of this method was 0.3 pg/mL (3 S/N, n = 11). The LOD was calculated without p24 antigen given that the fluorescence signal was three standard deviations higher than the background signals [28, 29] and comparable to most existing analytical methods (Table 1). Although the sensitivity of this strategy is not optimal, the advantages such as simple to use at room temperature, without instruments, and uses low-cost reagents with visual analysis make it stand out. The reproducibility of this method was assessed using 10 pg/mL of HIV-1 p24 antigen. The relative standard deviation (RSD) of five consecutive detections was 3.6%. The specificity of the method was assessed using proteins potentially present in clinical samples for HIV-1 diagnosis. P24 antigen (10 and 100 pg/mL) induced significantly stronger fluorescence, relative to other interference proteins used at higher concentrations (1 ng/mL, Fig. 2D and E).
Analytical performance and selectivity of p24 antigen via multiple selective CER. Mode 1 (Ag+-mediated recognition): A Color change caused by adding increasing concentration of p24 antigen, B, C fluorescence intensity change caused the addition of different concentration of p24 antigen, and D, E fluorescence intensity change caused by adding different types and concentrations of interfering proteins. Mode 2 (Cu2+-mediated recognition): F Color change caused by adding increasing concentration of p24 antigen and G, H Fluorescence intensity change caused by the addition of different concentration of p24 antigen. The error bars originated from at least three repeated tests
When using Cu2+ for p24 antigen detection, in contrast with Ag+, the color of the solution weakened gradually with increase in p24 concentration 1–100 pg/mL (Fig. 2F). The sensitivity using Cu2+ was similar to that of Ag+, with the lower limit of visual reading of 1 pg/mL. The fluorescence intensity of the solutions and the logarithm of p24 concentration (1–100 pg/mL) also displayed a good linear relationship (Fig. 2G and H). The LOD when using Cu2+ was 0.2 pg/mL, similar to that of Ag+. This method was reproducible, and the RSD of five consecutive detections of 10 pg/mL HIV-1 p24 antigen was 4.2%.
In summary, the p24 antigen analysis system based on immune recognition and visual/fluorescent changes of QDs caused by Ag+ or Cu2+ is highly sensitive and selective. In contrast, the Ag+-assisted mode is more stable, but the medium should avoid halogen elements. The Cu2+-assisted mode analysis is fast and requires high levels of operators. The method we presented is a proof-of-concept study. With the automatic reaction and instrumentations applied in commercial or clinical applications, future low-cost, high sensitivity, and integrated detection machines could be applied in this study.
Application of this strategy in clinical samples
The core of an analysis method is its practical application. We compared the utility of this method with that of ECLIA methods using 34 clinical samples, including 13 HIV-positive samples (different ages, gender, and different periods), 14 samples from patients with other diseases, and 7 healthy individuals. The accuracy of our method (mode 1, Fig. 3A) was comparable to that of the ECLIA method (Roche, COI, Fig. 3B), where the linear correlation coefficient was as high as 0.996 (Fig. 3C). The performance of our test was also consistent with that of PCR findings (Fiebig stage and RNA results of PCR analysis, Table S1) [34]. Analysis of samples from patients with hepatitis B virus, hepatitis C virus, Epstein-Barr virus, cytomegalovirus, systemic lupus erythematosus, lipemia, and hemolysis demonstrated that the method was highly specific (Fig. 3D). The specificity of the method was further validated using 7 samples from healthy individuals (Table S1, Fig. 3D). Overall, the system is highly sensitive and specific for p24 antigen detection in clinical serum samples. It is therefore a feasible alternative for HIV infection diagnosis.
Analysis results of clinical serum samples. The concentration of p24 antigen in HIV-positive patients’ serums detected by our method (A) and clinical immune-ECL strategy (B). C Consistency of clinical ECL and our method on HIV-positive samples. D Anti-interference ability of the method was evaluated by using serum samples from patients with different diseases and healthy persons. The error bars resulted from at least three repeated measurements
Conclusions
We successfully developed a highly sensitive and specific immunofluorescence and visual method for HIV diagnosis by detecting the p24 antigen. The method relied on selective detection of Ag+/Ag NPs and Cu2+/Cu+ using QDs. The method is relatively economic, with a LOD of pg/mL level, visible to the naked eyes. The performance of this method for HIV infection diagnosis from clinical samples was comparable to the traditional ECLIA and PCR techniques. Given the superior luminescence, the stability of QDs, and the portability of the fluorometer, this new method offers a strong alternative for HIV diagnosis. Given the analytical sensitivity of this strategy for p24 antigen, if combining with nucleic acid signal amplification strategies, it will be promising to achieve ultra-early HIV infection screening [35,36,37,38,39]. In addition, the color recognition POCT mode has certain limitations for color blind or color weak people, which can be improved by changing the reading mode, such as distance or height.
References
Cornett JK, Kirn TJ (2013) Laboratory diagnosis of HIV in adults: a review of current methods. Clin Infect Dis 57:712–718
Tominski D, Katchanov J, Driesch D, Daley MB, Liedtke A, Schneider A, Slevogt H, Arasteh K, Stocker H (2017) The late-presenting HIV-infected patient 30 years after the introduction of HIV testing: spectrum of opportunistic diseases and missed opportunities for early diagnosis. HIV Med 18:125–132
Zhang SY, Garcia-D’Angeli A, Brennan JP, Huo Q (2014) Predicting detection limits of enzyme-linked immunosorbent assay (ELISA) and bioanalytical techniques in general. Analyst 139:439–445
Cao HY, Liu Y, Sun HY, Li ZL, Gao Y, Deng XL, Shao YM, Cong YL, Jiang XY (2020) Increasing the assembly efficacy of peptidic beta-sheets for a highly-sensitive HIV detection. Anal Chem 92:11089–11094
De La Rica R, Stevens MM (2012) Plasmonic ELISA for the ultrasensitive detection of disease biomarkers with the naked eye. Nat Nanotechnol 7:821–824
Du MY, Li NX, Mao GB, Liu YC, Wang XX, Tian SB, Hu QX, Ji XH, Liu YL, He ZK (2019) Self-assembled fluorescent Ce(III) coordination polymer as ratiometric probe for HIV antigen detection. Anal Chim Acta 1084:116–122
Fang YS, Huang XJ, Wang LS, Wang JF (2015) An enhanced sensitive electrochemical immunosensor based on efficient encapsulation of enzyme in silica matrix for the detection of human immunodeficiency virus p24. Biosens Bioelectron 64:324–332
Kurdekar AD, Chunduri LA, Manohar CS, Haleyurgirisetty MK, Hewlett IK, Venkataramaniah K (2018) Streptavidin-conjugated gold nanoclusters as ultrasensitive fluorescent sensors for early diagnosis of HIV infection. Sci Adv 4:eaar6280.
Liu D, Zhang YQ, Zhu MY, Yu ZZ, Ma XM, Song YL, Zhou SF, Yang CY (2020) A microfluidic-integrated multicolor immunosensor for visual detection of HIV-1 p24 antigen with the naked eyes. Anal Chem 92:11826–11833
Zhou LM, Huang JS, Yu B, Liu Y, You TY (2015) A novel electrochemiluminescence immunosensor for the analysis of HIV-1 p24 antigen based on P-RGO@Au@Ru-SiO2 composite. ACS Appl Mater Interfaces 7:24438–24445
Zhuang JY, Han B, Liu WC, Zhou JF, Liu KW, Yang DP, Tang DP (2018) Liposome-amplified photoelectrochemical immunoassay for highly sensitive monitoring of disease biomarkers based on a split-type strategy. Biosens Bioelectron 99:230–236
Liu SY, Yan AM, Guo WYZ, Fang YY, Dong QJ, Li RR, Ni SN, Sun Y, Yang WC, Yang GF (2020) Human neutrophil elastase activated fluorescent probe for pulmonary diseases based on fluorescence resonance energy transfer using CdSe/ZnS quantum dots. ACS Nano 14:4244–4254
Peckys DB, Quint C, de Jonge N (2020) Determining the efficiency of single molecule quantum dot labeling of HER2 in breast cancer cells. Nano Lett 20:7948–7955
Ouyang XY, Wang MF, Guo LJ, Cui CJ, Liu T, Ren YA, Zhao Y, Ge ZL, Guo XN, Xie G, Li J, Fan CH, Wang LH (2020) DNA nanoribbon-templated self-assembly of ultrasmall fluorescent copper nanoclusters with enhanced luminescence. Angew Chem Int Ed 59:11836–11844
Zhou TY, Zhu JY, Gong LS, Nong LT, Liu JB (2019) Amphiphilic block copolymer-guided in situ fabrication of stable and highly controlled luminescent copper nanoassemblies. J Am Chem Soc 141:2852–2856
Wang S, Lu S, Zhao J, Yang X (2019) A ratiometric fluorescent DNA radar based on contrary response of DNA/silver nanoclusters and G-quadruplex/crystal violet. ACS Appl Mater Interfaces 11:25066–25073
Goswami N, Lin FX, Liu YB, Leong DT, Xie JP (2016) Highly luminescent thiolated gold nanoclusters impregnated in nanogel. Chem Mater 28:4009–4016
Zhao Y, Zhuang SL, Liao LW, Wang CM, Xia N, Gan ZB, Gu WM, Li J, Deng HT, Wu ZK (2020) A dual purpose strategy to endow gold nanoclusters with both catalysis activity and water solubility. J Am Chem Soc 142:973–977
Chen PP, Bai YJ, Tang Y, Yan SX, Wang XY, Wei WR, Wang J, Zhang M, Ying BW, Geng J (2020) Rapid and highly sensitive visual detection of oxalate for metabolic assessment of urolithiasis via selective recognition reaction of CdTe quantum dots. J Mater Chem B 8:7677–7684
Li JS, Zhang TR, Ge JP, Yin YD, Zhong WW (2009) Fluorescence signal amplification by cation exchange in ionic nanocrystals. Angew Chem Int Ed 48:1588–1591
Son DH, Hughes SM, Yin YD, Alivisatos AP (2004) Cation exchange reactions-in ionic nanocrystals. Science 306:1009–1012
Chen PP, Huang K, Dai R, Sawyer E, Sun K, Ying BW, Wei XW, Geng J (2019) Sensitive CVG-AFS/ICP-MS label-free nucleic acid and protein assays based on a selective cation exchange reaction and simple filtration separation. Analyst 144:2797–2802
Chen PP, Yan SX, Sawyer E, Ying BW, Wei XW, Wu ZZ, Geng J (2019) Rapid and simple detection of ascorbic acid and alkaline phosphatase via controlled generation of silver nanoparticles and selective recognition. Analyst 144:1147–1152
Hu PY, Wang X, Wei L, Dai R, Yuan X, Huang K, Chen PP (2019) Selective recognition of CdTe QDs and strand displacement signal amplification-assisted label-free and homogeneous fluorescence assay of nucleic acid and protein. J Mater Chem B 7:4778–4783
Chen PP, Wang Y, He YQ, Huang K, Wang X, Zhou RH, Liu TYH, Qu RL, Zhou J, Peng W, Li M, Bai YJ, Chen J, Huang J, Geng J, Xie Y, Hu W, Ying BW (2021) Homogeneous visual and fluorescence detection of circulating tumor cells in clinical samples via selective recognition reaction and enzyme-free amplification. ACS Nano 15:11634–11643
Chen PP, Qu RL, Peng W, Wang X, Huang K, He YQ, Zhang XL, Meng YM, Liu TYH, Chen J, Xie Y, Huang J, Hu Q, Geng J, Ying BW (2021) Visual and dual-fluorescence homogeneous sensor for the detection of pyrophosphatase in clinical hyperthyroidism samples based on selective recognition of CdTe QDs and coordination polymerization of Ce3+. J Mater Chem C 9:4141–4149
Wang X, Chen X, Zhou RH, Hu PY, Huang K, Chen PP (2021) Filter-assisted separation of multiple nanomaterials: mechanism and application in atomic/mass spectrometry/fluorescence label-free multimode bioassays. Anal Chem 93:3889–3897
Chen PP, Yang P, Zhou RX, Yang X, Chen JB, Hou XD (2018) Selective reduction-based, highly sensitive and homogeneous detection of iodide and melamine using chemical vapour generation-atomic fluorescence spectrometry. Chem Commun 54:4696–4699
Ma W, Yin HH, Xu LG, Wu XL, Kuang H, Wang LB, Xu CL (2014) Ultrasensitive aptamer-based SERS detection of PSAs by heterogeneous satellite nanoassemblies. Chem Commun 50:9737–9740
Li FY, Zheng Y, Wu J, Zhao L, Shui LL, Pu QS, Liu SR (2019) Smartphone assisted immunodetection of HIV p24 antigen using reusable, centrifugal microchannel array chip. Talanta 203:83–89
Zhang Y, Yang H, Yu JP, Wei HP (2016) Rapid and sensitive detection of HIV-1 p24 antigen by immunomagnetic separation coupled with catalytic fluorescent immunoassay. Anal Bioanal Chem 408:6115–6121
Gan N, Du XW, Cao YT, Hu FT, Li TH, Jiang QL (2013) An ultrasensitive electrochemical immunosensor for HIV p24 based on Fe3O4@ SiO2 nanomagnetic probes and nanogold colloid-labeled enzyme-antibody copolymer as signal tag. Materials 6:1255–1269
Li ZL, Liu Y, Chen XM, Cao HY, Shen HY, Mou L, Deng XL, Jiang XY, Cong YL (2020) Surface-modified mesoporous nanofibers for microfluidic immunosensor with an ultra-sensitivity and high signal-to-noise ratio. Biosens. Bioelectron. 166:112444.
Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Schumacher RT, Peddada L, Heldebrant C, Smith R, Conrad A, Kleinman SH, Busch MP (2003) Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS 17:1871–1879
Zhu LP, Chen DS, Lu XH, Qi Y, He P, Liu CH, Li ZP (2018) An ultrasensitive flow cytometric immunoassay based on bead surface-initiated template-free DNA extension. Chem Sci 9:6605–6613
Yamashige R, Kimoto M, Okumura R, Hirao I (2018) Visual detection of amplified DNA by polymerase chain reaction using a genetic alphabet expansion system. J Am Chem Soc 140:14038–14041
Liu M, Zhang Q, Kannan B, Botton GA, Yang J, Soleymani L, Brennan JD, Li YF (2018) Self-assembled functional DNA superstructures as high-density and versatile recognition elements for printed paper sensors. Angew Chem Int Ed 57:12440–12443
Xing C, Doi J, Huang YQ, Lin YH, Zhang KL, Lu CH, Yang HH (2019) Active self-assembly of train-shaped DNA nanostructures via catalytic hairpin assembly reactions. Small 15:1901795
Huang P-JJ, de Rochambeau D, Sleiman HF, Liu JW (2020) Target self-enhanced selectivity in metal-specific DNAzymes. Angew Chem Int Ed 59:3573–3577
Acknowledgements
We would like to thank Dr. Shanling Wang of the Analytical & Testing Center of Sichuan University for TEM work.
Funding
This work was supported by the Science and Technology Planning Project of Sichuan (2020YFS0136); National Natural Science Foundation Regional Innovation and Development (U20A20394); Sichuan Science and Technology Program (2021YFS0232); Sichuan Province Health and Family Planning Commission (Sichuan Health Office issued [2017] 70); Science and Technology Project of Chengdu (2017-CY02-00018-GX); China Postdoctoral Science Foundation (2019M653391); Sichuan University Post-Doctor Research Project (2020SCU12051); and Post-Doctor Research Project, West China Hospital, Sichuan University (2020HXBH065).
Author information
Authors and Affiliations
Contributions
Piaopiao Chen, Zhuoyun Tang and Zeliang Wei: performed the experiments and wrote the manuscript;
Piaopiao Chen, Jia Geng, Chuanmin Tao and Binwu Ying: designed the experiments and revised the manuscript;
Ke Huang and Shixin Yan: performed the synthesis and characterization of QDs and data processing;
Yinhao Wei, Dongdong Li and Jin Huang: afford provided the clinical samples and performed clinical experiments.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tang, Z., Wei, Z., Huang, K. et al. Fluorescence and visual immunoassay of HIV-1 p24 antigen in clinical samples via multiple selective recognitions of CdTe QDs. Microchim Acta 188, 422 (2021). https://doi.org/10.1007/s00604-021-05075-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-05075-7